Summary
Schizophrenia is a complex, heterogeneous behavioural and cognitive syndrome whose origins appear to lie in genetic and/or environmental disruption of brain development. Dysfunction of dopaminergic neurotransmission appears to contribute to the genesis of psychotic symptoms but the evidence also points to a more widespread and variable involvement of brain areas and circuits. There is emerging evidence that disturbances of synaptic function might underlie abnormalities of neuronal connectivity possibly involving interneurons, but the precise nature, location and timing of these events is uncertain. Current treatment consists largely in the administration of antipsychotic drugs combined with psychological therapies, social support and rehabilitation, but there is a pressing need for more effective treatments and for services to be delivered more effectively. Progress in understanding the disorder has been great in recent years with advances in genomics, epidemiology and neuroscience, and the opportunities for further scientific advance are great: but so are the challenges.
Introduction
Schizophrenia is a severe psychiatric disorder that has a profound impact on the individual and society. While outcomes may not be as uniformly negative as is commonly believed, over 50% of those individuals who receive a diagnosis have intermittent but long-term psychiatric problems and around 20% have chronic symptoms and disability.1 Unemployment is staggeringly high at 80–90%2,3 and life expectancy is reduced by 10–20 years.4 In England schizophrenia costs society £11.8 billion per year with around a third of this accounted for by direct expenditure on health and social care, provided both in hospitals and the community.5 Understanding the aetiology and pathogenesis of schizophrenia and developing new more effective and acceptable treatments remains one of the most formidable challenges facing modern medicine. However, the past decade has seen substantial advances in the application of genomics, epidemiology and neuroscience to schizophrenia; while many challenges remain, the opportunities for progress have never been better.
Clinical presentation, signs and symptoms
Schizophrenia is characterised by diverse psychopathology (Box 1); the core features are positive symptoms (delusions and hallucinations; so-called psychotic symptoms in which there is a loss of contact with reality), negative symptoms (in particular impaired motivation, reduction in spontaneous speech, and social withdrawal) and cognitive impairment (as a group patients with schizophrenia perform more poorly than controls over a wide range of cognitive functions though there is much individual variability).6 The positive symptoms tend to relapse and remit, though some patients experience residual long-term psychotic symptoms. The negative and cognitive symptoms tend to be chronic and are associated with long-term effects on social function. The first episode of psychosis usually occurs in late adolescence or early adulthood but is frequently preceded by a prodromal phase or “at risk mental state”7, 8 and in some instances premorbid impairments in cognition and/or social functioning go back many years.9 However, in other instances onset is sudden in previously well-functioning individuals.
Diagnosis and differential diagnosis
Diagnosis is made clinically on the basis of history and by examination of the mental state; there are no diagnostic tests or biomarkers. Schizophrenia usually presents with psychosis and the main differential diagnoses, in DSM510, are affective psychoses (bipolar disorder with psychotic features and major depressive disorder with psychotic features), other, closely related, non-affective psychoses (schizoaffective disorder, schizophreniform disorder, delusional disorder, brief psychotic disorder and psychotic disorder not otherwise specified), substance induced psychotic disorders (alcohol induced, other substance induced) and psychotic disorders due to a general medical condition. Differential diagnosis takes into account the duration of illness, the nature and pattern of associated substance abuse, the co-occurrence of depression or mania and the presence of somatic illness.
Schizophrenia, like the majority of psychiatric diagnoses, remains a syndromic concept. The use of operational criteria, such as those embodied in the Diagnostic or Statistical Manual of the American Psychiatric Association (DSM),11 or the International Classification of Diseases (ICD) of the World Health Organisation12 has provided a reliable approach to making psychiatric diagnoses in the clinic. However, the assumption that the clinical syndromes defined in this way represent valid disease entities with distinct underlying aetiology and pathogenesis is increasingly seen as having impeded research.13–15 Indeed psychiatric diagnoses have the unusual property of being simultaneously too broad and too narrow.15 Individuals with a diagnosis of schizophrenia vary greatly in predominant symptoms, response to treatment, course and outcome. However, attempts to resolve this heterogeneity into valid subtypes has repeatedly failed. On the other hand, many psychiatric diagnoses have symptoms in common (Box 1) and the boundaries between schizophrenia and other disorders are indistinct as are the boundaries between disorder and wellness. With regard to the latter there is an increasing realization that psychotic symptoms, such as auditory hallucinations and paranoid thinking, occur in attenuated form in 5–8% of the healthy population.16 This has led to suggestions that dimensional approaches to diagnosis and classification might replace or enhance current categorical approaches.15,17,18
Genetics
It has long been known on the basis of many genetic epidemiological studies that there is a substantial, but not exclusive, contribution of genetic factors to the aetiology of schizophrenia.19,20 What has changed recently is that, thanks to recent large-scale genomic studies, the contribution of specific variants at the DNA level has begun to emerge and we are beginning to get a clearer picture of how risk alleles (Box 2) of different types contribute to the disorder. We can draw three lessons of general importance from these recent findings.
The first is that schizophrenia is highly polygenic, as predicted many years ago on the basis of genetic epidemiological findings,21 with hundreds, and possibly thousands, of distinct genetic loci (Box 2) involved at the population level. The findings suggest that alleles with a spectrum of population frequencies contribute to risk (Fig 1).22 Genome-wide association studies (GWAS, Box 2) have identified over 100 distinct genetic loci containing relatively common alleles of small effect and the en masse effects of many hundreds of such loci.22,23 Genomic studies have also identified 11 rare, but recurrent, copy number variants (CNVs, Box 2) that individually confer relatively high risk of schizophrenia (Fig 1).24,25 Recent studies have also demonstrated a role of newly occurring (de novo) CNV mutations in schizophrenia.24,26,27 Recent whole exome sequencing studies (Box 2) have implicated rare, inherited and de novo single nucleotide and insertion/deletion variants (indels) in schizophrenia,28,29 though the net contribution of mutations of this type is unknown pending much larger sequencing studies. Bearing in mind that schizophrenia is associated with reduced fecundity,30 the picture that is emerging is one in which alleles that confer high individual risk are rare in the population due to the effects of natural selection,31 whereas those conferring small effects on individual risk can become common due to genetic drift or balancing selection (Fig 1).
Figure 1. The allelic spectrum of schizophrenia.
The figure depicts risk alleles for schizophrenia that have been robustly identified by genomic studies. The x-axis is the allele frequency (AF) in controls and the the y-axis is the odds ratio (genotypic relative risk). For clarity, confidence intervals are not shown. Copy number variants associated with schizophrenia are shown as blue diamonds. Single nucleotide polymorphism that are associated with SCZ and are shown as red diamonds. Alleles that confer high individual risk are rare in the population due to the effects of natural selection, whereas those conferring small effects on individual risk can become common due to genetic drift or balancing selection.
The second major lesson from recent genomic studies is that genetic risk appears to be highly pleiotropic (Box 2) and does not map onto current definitions of disease. Pleiotropy has been observed for common variants at the level of individual risk alleles and en masse effects. A recent study found significant sharing of common risk variants between schizophrenia and bipolar disorder, bipolar disorder and major depressive disorder, schizophrenia and major depressive disorder, ADHD and major depressive disorder, and, to a lesser extent, between schizophrenia and ASD32. Another study found evidence for overlap between schizophrenia and ADHD.33 There is also evidence for pleiotropy with regard to the effects of rare variants; CNVs that confer risk to schizophrenia also confer risk to a range of childhood neurodevelopmental disorders such as ASD, intellectual disability (ID), and ADHD as well generalized epilepsy.24,34,35 There is also emerging evidence that some rare single nucleotide variants and indels are associated with a similar range of outcomes.28 Risk alleles that are relatively non-specific to diagnostic group will be easier to detect than those that confer risk to particular diagnoses or sub-groups and indeed recent work is beginning to identify alleles with relatively specific risk profiles.36–38 However the pleiotropic effects observed to date, along with the lack of clear boundaries between disorders in clinical studies, suggest that there are likely to be overlapping mechanisms at work and that current diagnostic categories may not be optimal for stratifying cases for research into aetiology and pathogenesis.
The third point is that, despite the fact that much of the genetic risk for schizophrenia remains unaccounted for at the DNA level, and the complexity of the picture that has already emerged, there are encouraging signs of convergence on to a set of plausible biological processes. Findings from rare mutations, both CNVs and SNVs/indels, implicate genes encoding a variety of synaptic proteins including components of the post-synaptic density (PSD) and members of the voltage-gated calcium channel family of proteins.39 (Fig 2) Recent large-scale GWAS23 have also implicated common variation at genes encoding glutamate receptors and the voltage-gated calcium channel family of proteins. Recent GWAS have also implicated common variation at the Dopamine receptor D2 (DRD2) gene, which encodes the principal target of antipsychotic drugs (see below). The relationship between glutamatergic dysfunction and abnormalities of dopamine signalling may provide a clue as to how psychosis and cognitive deficits arise in schizophrenia and related disorders. These are very unlikely to be the only mechanisms involved in the aetiology of schizophrenia, and we can expect more possible processes to emerge as we move into the next phase of genomic studies. It is important to note that the most statistically significant association emerging from GWAS of schizophrenia is with multiple highly correlated variants in the major histocompatibility complex (MHC). This contains many genes not involved with immune function, but preliminary data suggest that variants associated with schizophrenia are also enriched in genomic regions outside the MHC that are potentially involved in acquired immunity.23 These findings are in accord with epidemiological and clinical studies implicating immune and inflammatory processes in various developmental stages, such as in utero, adolescence, and adulthood, in psychiatric disorders.40,41
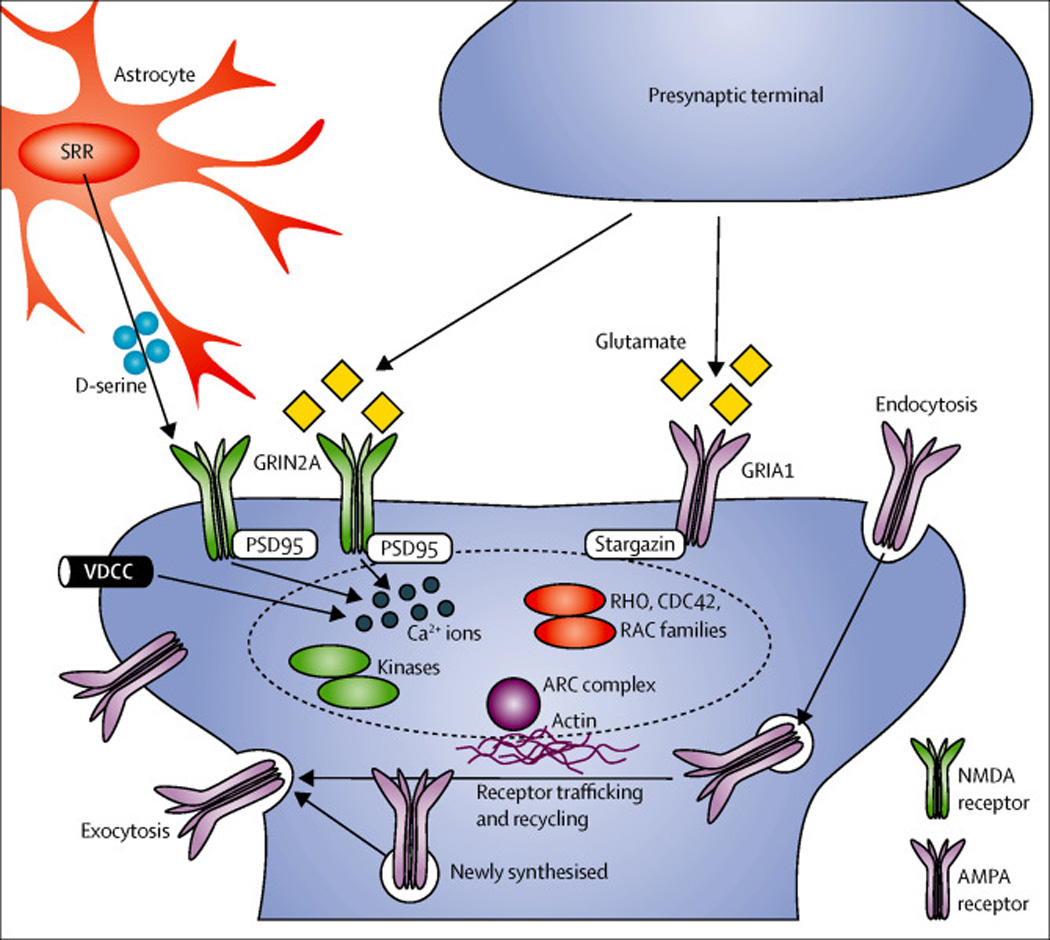
Figure 2. Representative molecular pathway for schizophrenia: fine tuning of the glutamate synapse.
Recent advances in human genetics, from both GWAS and large-scale sequencing, have further supported the significance of fine-tuning of glutamatergic neurotransmission in the pathology of schizophrenia. The genes underscored by these studies include those encoding the glutamate receptor, ionotropic, N-methyl D-aspartate 2A (GRIN2A); glutamate receptor ionotropic, AMPA 1 (GRIA1); serine racemase (SRR); calcium channel, voltage-dependent (VDCC), L type, alpha 1C subunit; the ARC complex, and a number of proteins located in, or associated with, the postsynaptic density of glutamatergic synapses. The N-methyl D-aspartate (NMDA)-type glutamate receptors are fine tuned by a co-agonist D-serine, which is synthesized by SRR. The GRIN2A subunit (in dark green) dimerizes with other type of subunit, forming the NMDA receptors, whereas the GRIA1 subunit (in brown) also forms heterodimers for the AMPA receptors. VDCCs (e.g., the protein encoded by the CACNA1C gene) are also likely to be involved in tuning neural excitability and synaptic transmission via intracellular calcium signaling. Proteins associated with postsynaptic scaffold include PSD95, Stargazin, several kinases, Rho/Cdc42/Rac small G-proteins, and ARC complex. In response to activation of glutamate receptors, these proteins convey intracellular signaling that underlies cytoskeletal regulation and receptor trafficking crucial for synaptic plasticity.
The majority of genetic discoveries in schizophrenia do not yet have direct clinical application. The one potential exception is the finding that certain CNVs are associated with risk of schizophrenia and other neurodevelopmental disorders. Testing for CNVs using chromosomal microarray analysis (CMA) is now a routine, first-line diagnostic test for autism and intellectual disability where 10–20% of affected cases have a clinically relevant deletion or duplication. It has been suggested that we should now introduce CMA in schizophrenia, where the prevalence of clinically relevant CNVs is around 5%.42 A positive test would have implications for genetic counselling and also for medical management since many CNVs are associated with specific patterns of physical morbidities. There are also potential psychological benefits of a genetic diagnosis for individuals with schizophrenia and their families.42
Epidemiology and environmental risk factors
Schizophrenia occurs worldwide, and for decades it was generally believed to have a uniform lifetime morbid risk of 1% across time, geography, and gender. This implied either that environmental factors are not important in conferring risk or that the relevant exposures are ubiquitous across all populations studied. It was not until relatively recently that this uniform view of risk was efficiently dismantled in a series of meta-analyses by McGrath and colleagues.43 They provided central estimates of an incidence per 100.000 population per year of approximately 15 in males and 10 in females, a point prevalence of 4.6 per 1000, and a lifetime morbid risk of app 0.7 %. These rates are based upon relatively conservative diagnostic criteria and, when broader criteria including other psychotic disorders such as delusional disorder, brief psychotic disorder and psychosis not otherwise specified are applied, the rates are 2 – 3 fold higher.44 However, more importantly, McGrath and colleagues documented a large variation across studies, five-fold or more, that could not be ascribed to diagnostic or other methodological differences, but which pointed towards real differences in occurrence and exposure to etiological factors. These findings have revitalized schizophrenia epidemiology and the resulting new wave of studies, together with advances in genetics, have begun to cast light on how the disorder might arise.
The dominant paradigm for understanding the environmental contributions to schizophrenia etiology has for over three decades been the neurodevelopmental hypothesis.45 This directs attention towards established risk factors for schizophrenia affecting early neurodevelopment during pregnancy. These include maternal stress,46 maternal infections,47,48 nutritional deficiencies,47,49 intrauterine growth retardation, and pregnancy and birth complications.50,51 However, socio-economic factors,52–54 childhood adversity,55 and first- and second-generation immigrant background56,57 have also been associated with schizophrenia. There are also consistent reports of higher rates of schizophrenia in individuals born in late winter or early spring,58 in individuals born and/or raised in cities,59 and in individuals where the father was relatively old, but also an association with young parents has been found.60,61 The association with advanced paternal age has been ascribed to the increased rate of de novo mutations in their offspring,62,63 but alternative or complementary explanations have been proposed.64 More recently, evidence has accumulated implicating cannabis use in adolescence, in particular misuse of compounds with high THC content.65,66 Also, several other factors such as head injury,67 epilepsy,68 autoimmune diseases69,70 and severe infections71–73 have been associated with increased risk.
It has been pointed out that a number of the environmental exposures associated with schizophrenia, particularly those impacting most directly on early brain development, are also associated with a range of other neurodevelopmental outcomes including ID, autism, ADHD and epilepsy.74,35 This is similar to the range of outcomes associated with large, rare CNVs24,35,75 and suggests that schizophrenia might best be conceived as one of a spectrum of clinical outcomes that result from genetically and/or environmentally induced disruption to the developing brain. This points to the need for future epidemiological studies to look more carefully at the range of outcomes associated with particular exposures and not to be constrained by the assumption that current diagnostic approaches delineate disorders with distinct causes and mechanisms.
Many of the associations with environmental risk factors appear robust, and the odds ratios from the cited papers (predominantly the most recent meta-analyses) typically range from 1.5–3.0. However, observational epidemiology suffers from the limitation that is cannot distinguish true causation from association due to confounding, pleiotropy or reverse causation. Thus, at present, caution is required in interpreting these associations and more work is needed before preventive intervention is justified.
Experimental animal studies offer one approach by which evidence can be obtained to support a causative role for environmental risk factors. There is a rapidly growing literature of such studies corroborating epidemiological findings. These include studies of infections, pre-natal maternal inflammation as well as stressors post-natally and onwards, and their impact on behavioural and neurobiological variables that model aspects of schizophrenia.76,77 Interestingly, prenatal factors and pre-pubertal stressors have been found to interact in some of these studies.78 This points to a more complex model in which the impact of environmental exposures is modified by earlier events and suggests the need for a new generation of more sophisticated longitudinal epidemiological studies integrating prenatal and postnatal factors.
A key challenge for observational epidemiological studies of environmental factors has been the inability to control efficiently for confounding due to differences in genetic liability: in other words, are differences associated with, say, infections, due to a higher rate of infections amongst those more genetically predisposed towards schizophrenia? Until now, epidemiology has, at best, only been able to control for this by taking psychiatric family history into account, and, as only a minority of patients have family members with the disease, this is a highly imprecise measure of liability. This situation is now dramatically changing, with the recent advances in identifying common genetic variants associated with schizophrenia risk. Although of no use as an individual predictor of disease risk, a polygenic risk score summarizing associations with approximately 20,000 variants has been shown to reliably predict 5–10 fold risk differences.23 This methodology must be expected to develop and improve rapidly over the next few years, providing researchers with an efficient novel tool in the effort to separate nature from nurture in schizophrenia etiology, as well as facilitating studies of how genetic and environmental factors interact (GxE).79 GxE interactions are very plausibly of substantial importance in schizophrenia as in other complex disorders. The concept of GxE interactions in its broadest sense simply means that the effect of a given environmental factor depends upon single or multiple genetic variants, and vice versa. Although conceptually simple, the study of GxE interactions present a number of challenges, and this emerging literature has taken a number of different approaches.79,80 First, a range of studies has been performed focusing upon a single candidate gene interacting with a specific environmental exposure. These studies have the advantage of a specific prior hypothesis and hence can be performed with a relatively modest sample size. However, as choosing candidate genes must be based upon the existing limited understanding of the genetic architecture of schizophrenia and the likely mechanisms involved in their interactions with the environment, this approach, albeit relevant in its own right, cannot help discover the majority of relevant GxE interactions. However, searching for interactions across the genome in a hypothesis-free manner requires enormous sample sizes. Therefore a number of novel techniques have been developed to perform so-called gene-environment wide interaction studies (GEWIS), and these methods have begun to be applied in schizophrenia research.81,82
Another application of genetic data to enhance the causal interpretation of environmental factors is the Mendelian randomisation design, where genes associated with the level of exposure are used as instrumental variables,83 and applications of methods to assess the extent to which risk factors are mediated through measures of genetic liability.84 For example Agerbo et al.85 showed that a large proportion of the association between a family history of psychosis and schizophrenia risk was mediated through a Polygenic Risk Score, whereas this was not the case as regards socioeconomic risk factors.
In summary, a large body of literature suggests that multiple risk factors, in particular those affecting early neurodevelopment, contribute to the etiology of schizophrenia. There is also emerging evidence that environmental factors, both biological and psychosocial, may impact at later time points (Fig 3). It is certainly possible that the effects on the developing brain of earlier environmental exposures as well as genetic factors increase susceptibility to these later risk factors. However, caution must be observed before inferring causality and there is a need for studies combining measures of both the pre- and post-natal environment with measures of genetic liability. The bringing together of genomics with large-scale epidemiological approaches offers new and exciting ways to better understand the causal role of the environment and this will hopefully lead towards primary prevention.
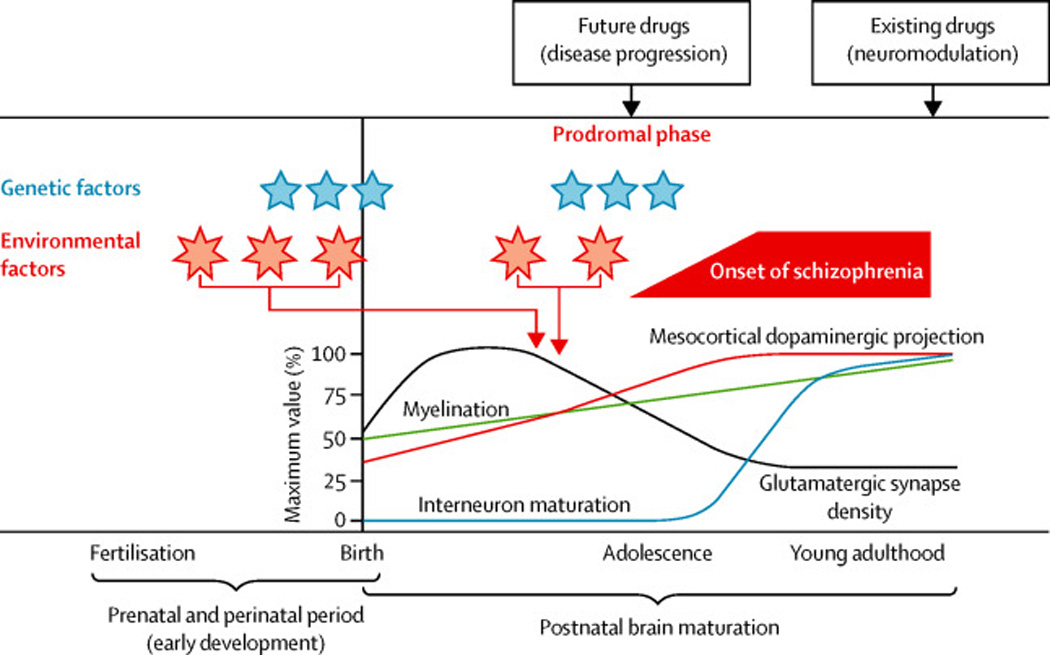
Figure 3. Interaction of genetic and environmental risk factors in the developmental pathology of schizophrenia.
This shows a schematic view of how multiple genetic and environmental risk factors might impact on long-term neurodevelopmental processes leading to schizophrenia. Schizophrenia typically presents when the first episode of psychosis occurs in late adolescence or early adulthood but is frequently preceded by a prodromal phase and in some instances premorbid impairments in cognition and/or social functioning go back many years. It is proposed that disturbances generated by susceptibility genes (indicated by blue stars) and environmental insults (indicated by pink asterisks) during early development and adolescence disturb postnatal brain maturation. These factors are likely to impair some of the crucial processes in early development, including progenitor cell proliferation, neuronal migration and dendritic arborization and outgrowth. Independent of such initial risks/insults, intrinsic disease-associated factors might also directly affect postnatal brain maturation. Accumulation of such deleterious insults results in overall disturbance of proper postnatal brain maturation, including maturation of interneurons and dopaminergic projections, pruning of glutamate synapses and myelination. Therefore, it is crucial to understand the mechanisms that underlie long-term progression to full disease manifestation in young adulthood to facilitate development of novel therapeutic strategies. This contrasts with classic drug discovery that simply modulates disturbed neurotransmission after full onset of the disease. In this figure, interneuron maturation is plotted as an increase in interneuron response to dopamine D2 agonists in the prefrontal cortex, whereas mesocortical dopaminergic projection is based on levels of tyrosine hydroxylase. The relative levels of glutamatergic synapse density and myelination are depicted. Extensively modified from the original figure appeared in Jaaro-Peled et al, TINS 2009.
Pathophysiology
Many brain imaging and neuropathological studies have attempted to relate the manifestations of schizophrenia to altered structure or function of particular brain regions and circuits.86,87 There has been progress in relating some aspects of the disorder to specific underlying neurobiology and several lines of evidence implicate the involvement of the prefrontal cortex, in particular, in specific cognitive deficits (e.g., working memory and executive control).88–90 However, subtle reductions in grey matter and abnormalities of white matter have been found across many brain regions and circuits.91 The reduction of grey matter progresses with the duration of illness, especially in the temporal lobe92, and seems to be associated with antipsychotic treatment.93 However, even drug-naïve patients show volume reductions (albeit not as pronounced as treated patients), especially in the caudate nucleus and thalamus.91 Moreover, despite many hundreds of studies, no circumscribed anatomical or functional abnormalities that are specific to the disorder have been identified.87 This is likely to reflect the complexity and heterogeneity of the psychopathology and associated cognitive impairments, and the lack of clear boundaries separating schizophrenia from other disorders or wellness.
There is a coherent body of evidence from pharmacological and brain imaging studies implicating dysfunction of dopaminergic neurotransmission in the genesis of psychotic symptoms such as delusions and hallucinations.94 However, while these occur in the majority of cases of schizophrenia, they are also found in a variety of other psychiatric conditions.94 Moreover, pharmacological, and other, evidence indicates that dopaminergic dysfunction is unlikely to explain the full range of clinical features of the disorder. Evidence from clinical pharmacology, brain imaging, and clinical physiology has suggested that disturbed glutamatergic function may contribute to the biological processes underlying some clinical features, in particular cognitive dysfunction, in schizophrenia.88,95,96 One theory is that glutamatergic dysfunction in schizophrenia is related to dysfunction of parvalbumin-positive interneurons in the cerebral cortex and hippocampus, which are sensitive to alterations in NMDA-type glutamate receptors.89 These fast spiking neurons synchronize the firing of pyramidal neurons and underlie the generation of gamma oscillations, which is critical to proper cognitive function.97 Consequently, dysfunction of this population of neurons may lead to the cognitive deficits observed in schizophrenia. [Fig 4]97
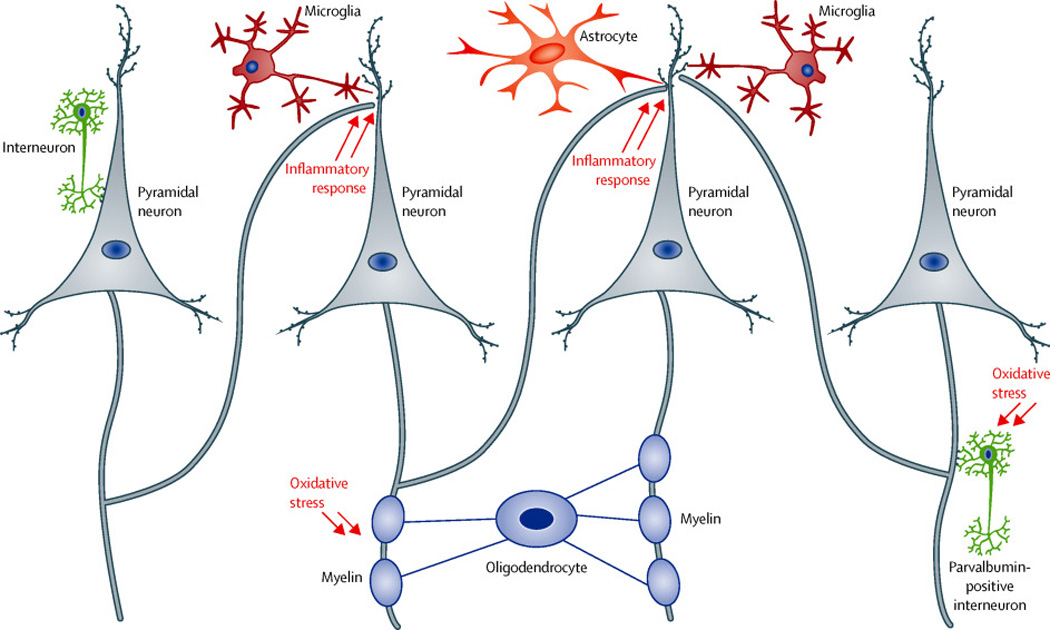
Figure 4. Neuron-glia interactions in the cerebral cortex: key neural substrates for the pathology of schizophrenia.
In the cerebral cortex, interneurons (inhibitory neurons) regulate the output of pyramidal neurons (excitatory neurons). Many studies have reported abnormalities of interneurons (in particular parvalbumin-positive interneurons) and deficits of dendritic spines in the pyramidal neurons in the pathology of schizophrenia. Imbalance of excitatory and inhibitory neurons may be a key feature that underlies the pathology. Recent neurobiology indicates that astrocytes and microglia play key a role in maintenance and pruning of the dendritic spines, in association with immune inflammatory response in the brain. Oligodendrocytes create the myelin sheath, which is crucial for signal transmission inside the axon. Abnormalities of these glial cells have also been reported in schizophrenia.
Recent advances in human genetics, from both GWAS (Box 2) and large-scale sequencing, have further supported the significance of fine-tuning of synaptic transmission, in particular at glutamatergic and dopaminergic synapses in the aetiology and pathophysiology of schizophrenia.23,28,29 The genes underscored by these studies include those encoding the glutamate and dopamine receptors, and those encoding post-synaptic proteins implicated in receptor-mediated signaling. (Fig 2)23,28,29,98,99
Nonetheless, there is a gap between basic information from genetic and other molecular studies and its application in translational research. While psychiatric genetics now convincingly implicates specific sets of genes involved in synaptic function, it does not provide information about the developmental stages, brain regions and circuitries where the molecules play roles in pathogenesis. This will require further studies of brain imaging, post mortem brains, clinical physiology and animal models to build on genetic findings.
At least two types of molecular pathway may be involved. The development and maintenance of normal synaptic function depends upon a large number of molecular pathways, including the molecules highlighted above (Fig 2), and a number of environmental factors will further impact these as the brain develops. Second, stress-associated signaling cascades, in particular those involving inflammatory processes and oxidative stress, are well known to modulate the development and maintenance of synaptic connectivity (Fig 4). For example, microglia (the glial cells that mediate brain inflammation) are involved in synaptic maintenance and deterioration, in particular synaptic pruning in adolescence,100–102 and the major histocompatibility complex I (MHC class I) and complement system have been implicated in synaptic plasticity.102–108 Furthermore, the fast-spiking parvalbumin-positive interneurons referred to above are particularly vulnerable to oxidative stress109,110 and this can also disrupt proper formation and maintenance of myelination.110 Evidence for the involvement of these mechanisms has come from recent studies of preclinical models.111,112
In summary, current understanding of the neurobiology of schizophrenia remains largely incomplete. There is strong evidence implicating dysfunction of dopaminergic neurotransmission in the genesis of psychotic symptoms as well as evidence implicating abnormalities of glutamate signaling which might help account for the negative and cognitive symptoms. There is some evidence linking specific brain areas (e.g. prefrontal cortex) to specific cognitive dysfunctions (working memory impairment) but the evidence also points to a widespread and variable involvement of other brain areas and circuits. These findings are consistent with evidence from genetics and epidemiology that, at least in a proportion of cases, the origins of schizophrenia lie in genetic and/or environmental disruption of early brain development. There is emerging evidence from genetics and neurobiology that disturbances of synaptic function might underlie abnormalities of neuronal connectivity possibly involving interneurons, but the precise nature, location and timing of these events is uncertain. There is also evidence that progression towards schizophrenia can be impacted postnatally by further environmental exposures that may be modulated by genetic factors as well as earlier environmental factors. Recent evidence points to the role, in some cases, of oxidative and inflammatory mechanisms that may themselves impact on synaptic function and modulate connectivity at critical developmental stages (Fig 4).
Management and outcome
Since the serendipitous discovery of chlorpromazine over 50 years ago, almost all antipsychotic drugs available in the clinical settings for schizophrenia derive their effectiveness through DRD2 blockade.113,114 Clozapine is the most potent in efficacy, and a group of antipsychotics including clozapine binds and influences not only DRD2 but also other neurotransmitter receptors, such as serotonin receptors 2 (5HT-2R).115 In the UK Clozapine is only licensed for use in those who have failed to respond to other antispsychotics due to the risk of agranulocytosis and neutropenia (1–3%) requiring ongoing blood monitoring. Antipsychotic drugs are relatively effective in reducing positive symptoms, such as auditory hallucinations and delusions, and remain the mainstay of both acute and long-term pharmacological treatment. However they are not effective for other important clinical features of schizophrenia, such as negative symptoms and cognitive dysfunction, which are more strongly associated with functional impairment than positive symptoms. There is evidence that long-term, maintenance treatment with antipsychotic drugs is effective in preventing relapse of psychotic symptoms, but troublesome side effects such as weight gain, movement disorders and sedation are common and contribute to poor adherence.116 Moreover, a substantial number of patients show no, or at best partial, response in positive symptoms with current antipsychotic drugs.117–119 Individual response is often idiosyncratic and difficult to predict. Newer, so-called second-generation, antipsychotic drugs can be effective in treating psychotic symptoms with fewer movement disorders, but carry a higher risk of cardio-metabolic side effects. Choice of the optimal antipsychotic is therefore usually pragmatic and balances individual benefits with costs and risks. Clozapine is effective in around 60% of previously treatment refractory cases127, but there is evidence that it is underprescribed5.
While antipsychotic medication remains the cornerstone of treatment, the effective management of schizophrenia requires pharmacotherapy to be embedded within a framework of strong psychological and social support. These include approaches aimed at improving adherence as well as vocational and educational support, and rehabilitation. This requires a multi-disciplinary approach involving a variety of health-care professionals and agencies delivered in a community-care setting. Specialist early intervention services, which focus on those who are experiencing their first psychotic episode and the following three years, are available in many developed countries, and are popular with service users and carers5. These have beneficial effects on outcome in the first few years, but their long-term impact remains uncertain120. Psychological treatments have been mandated by current UK NICE guidelines, which recommend that everyone with schizophrenia should be offered cognitive behavioral therapy (CBT) and family intervention as well as antipsychotic medication. A role for CBT is justified by evidence that various potentially mutable psychological mechanisms increase the risk of specific symptoms.121 However, the degree of efficacy and cost-effectiveness of CBT in schizophrenia is controversial122 and there is little evidence that it impacts on underlying psychological mechanisms. One possibility is that the efficacy of CBT depends upon non-specific factors such as the quality of the relationship between the therapist and the patient (therapeutic alliance) and there is evidence to support this in regard to schizophrenia.123 Medical management also focuses upon physical health: in particular preventative measures such as dietary advice, drug abuse, exercise and smoking cessation; and monitoring of cardiovascular and metabolic risk factors. In many countries care is provided by a multidisciplinary team of mental health professionals in primary, secondary and community settings and focuses on both health and social care.44
In recent years the perception that outcome is necessarily poor has been challenged by results of prospective studies and there is clearly great heterogeneity with relatively good outcome seen in 20–50% of cases.44 However, while the majority of those with schizophrenia live independently outside hospital, many require continuing support either from services or relatives. Moreover, mortality from all causes of death is substantially increased.124,125 The relative risk for suicide is increased 12-fold with a lifetime risk of approximately 6.5%,126 but mortality from most natural causes, and in particular cardiovascular disorders, is the strongest contributor to the 10–20 year reduction in life expectancy. The causes of this are believed to include smoking and other life-style factors, and sub-optimal treatment of physical disorders in schizophrenia patients124,127 but also side effects of pharmacological treatment in particular cardio-metabolic. Several trials to reduce the excess mortality are ongoing.
Outstanding research questions
It should be apparent from the forgoing that while progress has been great in the past 5–10 years, we still have much to learn about what causes schizophrenia and how to treat it more effectively. It should also hopefully be clear that the opportunities for progress have never been better.
Genomic studies have begun to reveal the complex genetic architecture of schizophrenia and to converge on some interesting and tractable areas of biology. The focus of genetics for the next few years will be to be to deepen our understanding by identifying more rare as well as common risk alleles. This should allow us to identify other areas of relevant biology and reagents, in the form of more highly penetrant mutations, for animal and cellular studies. Another important question is the extent to which somatic de novo mutations might play a role in schizophrenia. There is evidence that these contribute to some neurodevelopmental disorders128 but this issue, which requires deep sequencing of brain tissue, has not been addressed yet in schizophrenia. There is also the challenge of how to deepen our insights into the relationships between genetic risk and phenotypic outcome. Genetic studies have undermined our current categorical notions of classification by showing extensive pleiotropy, but the large samples required for robust studies have come at the expense of detailed phenotype data. A major challenge for the future will be to conduct large scale genetic studies with more detailed clinical and endophenotype data. These will be required to understand how genetic risk impacts on brain mechanisms leading to particular clinical outcomes and to develop new approaches to diagnosis and classification that better reflect the underlying disturbances in brain function.
While the recent advances in genetics may have been impressive, epidemiological studies have also been highly productive implicating a number of biological and psychosocial risk factors. However, as noted already, there are limitations to the explanatory power of observational epidemiology and a key challenge for research in the next decade will be to understand the relevance of environmental risk exposures to disease causation. This will benefit from the fact that we can now bring together genomics and epidemiological studies of environmental risk factors and there is a need for longitudinal epidemiological research addressing how environment exposures impacting at different time points interact with each other and with genetic risk to produce clinically relevant outcomes. Such studies will likely benefit from access to routinely collected electronic clinical data though ethical and other challenges remain. This should also allow the identification of protective factors and guide the implementation of public health measures.
What can we expect in the next five to ten years in the biological understanding of schizophrenia? Findings from ongoing and future genetic studies will continue to drive mechanistic studies in patients and model systems. Rare variants conferring high individual risk will be of particular importance, especially for designing cellular and animal models. Bioinformatic analyses of genetic data should also become increasingly informative as study sizes are increasing. However, the further challenge will be to determine how, where and when genetic risk impacts on brain development and function.
Genetically engineered cells obtained directly from patients, such as induced pluripotent stem cells (iPS cells) and induced neuronal cells (iN cells), provide an opportunity to investigate neuronal mechanisms in vitro.129 It is also possible to introduce risk alleles, and combinations thereof, into human stem cell lines using new genome engineering approaches such as the Clustered Regularly Interspaced Short Palindromic Repeat (CRISPR) system. We can now differentiate cells into many types of central nervous system cells, including different subtypes of neurons (e.g., glutamatergic and dopaminergic neurons) and glial cells, and partially recapitulate neurodevelopmental processes in vitro.130 It is still unclear whether they capture features of mature neural networks in vivo. Nonetheless, such patient-derived neuronal cells will be useful for addressing cell-autonomous intrinsic susceptibility to the disease. Moreover, if such cellular susceptibility provides valid drug targets, human cell culture may be used for compound screening.131
The study of animal models, particularly rodents in combination with human brain imaging will be needed to address circuitry mechanisms. The validity of many animal models can be questioned, but the recent advances in genetics allow models of human risk mutations to be developed. The polygenic basis of psychiatric conditions currently limits the utility of genetic rodent models, which often include a single specific mutation, and, at the present time, rare high penetrance alleles offer the best approach to generating models with high construct validity. However, the recently introduced CRISPR system may facilitate the introduction of genetic variations at multiple sites.132 We need to examine neural circuits in genetic animal models with respect to behavioral changes, with particular attention to the pathological trajectory from early development to full onset of disease in adulthood. Such studies will benefit from recent novel technologies and methodologies to intervene with specific neural cells and circuits.133 Given that environmental stressors play key roles in the aetiology of schizophrenia,134 studying gene- environment interactions in cell and animal models will become increasingly important.. Such studies will help us answer important questions such as which biological contexts, cells, and mechanisms are the key sites of convergence of genetic and environmental stressors.
There is a clear need to develop antipsychotic compounds with fewer side effects, in particular those affecting metabolism that result in adverse cardiovascular outcomes.135 There is also interest in using pharmacogenetics to identify patients at particular risk of specific side-effects.136,137 Furthermore, efforts are being made to develop compounds that show efficacy not only for positive symptoms but also for negative and cognitive symptoms by modulating neurotransmission beyond the dopamine receptor. These efforts include modulation of glutamate and acetylcholine neurotransmission by interfering with glycine transporter 1 and alpha7 nicotinic acetylcholine receptor.138–140 There is also interest in using pharmacogenetics in combination with other biomarkers to identify patients who might respond differentially to drugs with different modes of action141 and this follows a more general trend to investigate the potential of stratified medicine in which drugs are targeted more efficiently to specific subgroups of patients. It seems likely that the road from recent progress in genetics and biology to the discovery of new treatments will be long one and will depend upon insights from cellular and animal modeling as well as human studies.142 This work will need to address the key issues of cell type- and circuit-specificity and of the timing of critical events. We will also need advances in high-throughput methodology as well as advances in our ability to model cell circuits in vitro.142
Recent years have seen burgeoning interest in the possibility of treating those at high risk to prevent the development of full-blown psychosis and to reduce functional impairment. Meta–analyses of randomized controlled trials suggest a positive, if modest, impact despite the wide variety of interventions employed (psychological, pharmacological, nutritional)140, 141, but there is a clear need for further, well-controlled trials143,144,145. As described above, stress-associated signaling cascades are likely to impact upon synaptic pruning and the maturation of neural networks in adolescence. Compounds that can regulate inflammation and oxidative stress are being tested. For example, omega-3 fatty acids displayed beneficial effects in a clinical trial.146 Preclinical studies have demonstrated that application of antioxidants, including N-acetyl cysteine, ameliorate physiological and behavioral deficits associated with schizophrenia.111 Encouraged by such preclinical studies, increasing numbers of investigator-initiated clinical trials with antioxidants are ongoing. Future applications of genetic and other biomarkers, together with early neuropsychological, developmental and behavioural risk markers, to identify high risk groups as relevant recipients of preventive interventions, as well as controlled studies of the interventions in high risk children remain high research priorities.
Arguably the greatest challenge facing future research into aetiology, pathogenesis and treatment is the failure of current syndromic definitions to delineate a valid disease entity. If research is to progress, we will need new approaches to patient stratification that recognize the varying degree of overlap between syndromes and which bring modern neuroscience to bear on intermediate phenotypes that index the pathophysiology underlying the various clinical features and impairments.15 Given the complex and variable clinical features and cognitive impairments associated with the disorder it seems likely that multiple brain systems are impacted to varying extents in different individuals and it would be unwise to expect to implicate dysfunction of a single brain region or circuit that accounts for the full range of features of the disorder and which distinguishes it from other disorders. Rather we should expect that different features of the disorder reflect underlying disturbances of different brain functions and moreover that these will cross current diagnostic boundaries. These considerations also imply that we should expect that novel treatments will target particular symptoms or groups of symptoms sharing common underlying mechanisms and that they will be applicable across diagnostic groups.
Controversies and uncertainties
Schizophrenia has long divided opinion: nature versus nurture, psychosocial versus biological, a myth or an illness, or a sane response to an insane society. It is perhaps a sign of increased knowledge that current controversies are less polarized. But, as should be clear from this seminar, many controversies and uncertainties do remain. For example: How should we diagnose schizophrenia? Should we use categories or dimensions? What clinical features, or combination of features will map best onto underlying neurobiological disturbances? Which environmental risk factors are truly casual and which are secondary to illness or genetic confounding? Is it better viewed a disorder of circumscribed brain regions and circuits, or a disorder of the whole brain? Is there a progressive, neurodegenerative component as well as a neurodevelopmental one? Is it one disorder, several disorders, or part of a continuous landscape of psychopathology analogous to say metabolic syndrome? What is the relationship between schizophrenia and disorders, such as autism and ADHD, with which it shares a number several clinical features and risk factors?
One area, which we have not touched on, which is perhaps of greatest immediate concern, is the poor quality of care for schizophrenia even in developed countries. The Schizophrenia Commission recently reviewed this issue and described current services in the UK as “broken and demoralized”5. They not only documented in uncompromising terms the shortcomings of our current approaches to managing schizophrenia but also made a large number of recommendations for improving policy and practice5. There seems little doubt that the implementation of these changes would do a great deal to improve the lives of those with schizophrenia and we should all do all that we can to ensure that this happens.
Given the need for an overhaul in the ways in which care is provided, it might seem inappropriate to point out the lack of investment in research in mental disorders relative to burden.147 It is sometimes argued that this is a consequence rather than a cause of lack of progress and capacity in this area. However, a counterview would be that this is yet another example of the way in which mental disorders fail to achieve parity of esteem with physical illnesses. Whatever the explanation, it is to be hoped that the recent advances and unprecedented opportunities documented in this article will help redress this imbalance.
Acknowledgments
Profs David Linden and Richard Bentall made valuable suggestions. Ruth Sellars, Yukiko Lema, Annette Rand Madsen and Victoria Hirst helped prepare the paper and display items.
Conflicts of Interest
MO receives funding from the MRC, Wellcome Trust, NIMH, EU, and NISCHR. He has been paid a speaker’s fee by Janssen Pharmaceutical Companies. PM receives research funding from the Lundbeck Foundation, the Stanley Medical research Institute, and the ERC. AS receives research funding from NIH, NARSAD/BBRF, Stanley foundation, RUSK foundation, and Maryland Stem Cell Research Funds. Role of the funding sources: None of the funding bodies had any influence on any part of the work on this paper. None of the authors received any payment from any pharmaceutical company or other agency to write this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
MO, AS and PM were involved in the development of the structure of the review, literature searches, writing the article, constructing the graphics and approving the final manuscript.
Search Strategy and Selection Criteria.
We searched publications in PubMed using the search term “schizophrenia” for reviews and meta-analyses published in the past 5 years. These were assessed for relevance to the topics selected. Further focused searches on PubMed were then done on the selected topics.
Contributor Information
Prof. Michael J Owen, MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, Cardiff, UK.
Prof. Akira Sawa, Department of Psychiatry, Johns Hopkins University School of Medicine, USA.
Prof. Preben B Mortensen, Department of Economics, School of Business and Social Science, Aarhus University, Denmark.
References
- 1.Barbato A. Psychiatry in transition: outcomes of mental health policy shift in Italy. Aust N Z J Psychiatry. 1998;32(5):673–679. doi: 10.3109/00048679809113122. [DOI] [PubMed] [Google Scholar]
- 2.Kooyman I, Dean K, Harvey S, Walsh E. Outcomes of public concern in schizophrenia. Br J Psychiatry. 2007;194:s29–s36. doi: 10.1192/bjp.191.50.s29. [DOI] [PubMed] [Google Scholar]
- 3.Marwaha S, Johnson S. Schizophrenia and employment. Social Psychiatry and Psychiatric Epidemiology. 2004;39(5):337–349. doi: 10.1007/s00127-004-0762-4. [DOI] [PubMed] [Google Scholar]
- 4.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153–160. doi: 10.1002/wps.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schizophrenia Commission. The abandoned illness: a report from the Schizophrenia Commission. London: Rethink Mental Illness. 2012 [Google Scholar]
- 6.Joyce EM, Roiser JP. Cognitive heterogeneity in schizophrenia. Curr Opin Psychiatry. 2007;20(3):268–272. doi: 10.1097/YCO.0b013e3280ba4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biological psychiatry. 2001;50(11):884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 8.Addington J, Heinssen R. Prediction and prevention of psychosis in youth at clinical high risk. Annu Rev Clin Psychol. 2012;8:269–289. doi: 10.1146/annurev-clinpsy-032511-143146. [DOI] [PubMed] [Google Scholar]
- 9.Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;2:225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- 10.Association AP. Diagnostic and statistical manual of mental disorders : DSM-5. Fifth. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 11.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders - Fifth Edition: DSM-5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 12.World Health Organisation. The ICD-10 classification of mental and behavioural disorders : clinical descriptions and diagnostic guidelines. Geneva: World Health Organisation; 1992. [Google Scholar]
- 13.Craddock N, Owen MJ. The Kraepelinian dichotomy - going, going… but still not gone. Br J Psychiatry. 2010;196(2):92–95. doi: 10.1192/bjp.bp.109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155–179. doi: 10.1146/annurev.clinpsy.3.022806.091532. [DOI] [PubMed] [Google Scholar]
- 15.Owen MJ. New approaches to psychiatric diagnostic classification. Neuron. 2014;84(3):564–571. doi: 10.1016/j.neuron.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 16.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 17.Craddock N, Owen MJ. Rethinking psychosis: the disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry. 2007;6(2):84–91. [PMC free article] [PubMed] [Google Scholar]
- 18.van Os J, Kenis G, Rutten BPF. The environment and Schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 19.Gottesman II. A series of books in psychology. New York US: Freeman/Times Books/ Henry Holt & Co.; 1991. Schizophrenia genesis: The origins of madness. [Google Scholar]
- 20.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a Complex Trait: Evidence From a Meta-analysis of Twin Studies. Arch Gen Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 21.Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci U S A. 1967;58(1):199–205. doi: 10.1073/pnas.58.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13(8):537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rees E, Walters JT, Chambert KD, et al. CNV analysis in a large schizophrenia sample implicates deletions at 16p12.1 and SLC1A1 and duplications at 1p36.33 and CGNL1. Hum Mol Genet. 2014;23(6):1669–1676. doi: 10.1093/hmg/ddt540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirov G, Pocklington AJ, Holmans P, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Molecular psychiatry. 2012;17(2):142–153. doi: 10.1038/mp.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nature genetics. 2008;40(7):880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- 28.Fromer M, Pocklington AJ, Kavanagh DH, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purcell SM, Moran JL, Fromer M, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–190. doi: 10.1038/nature12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bundy H, Stahl D, MacCabe JH. A systematic review and meta-analysis of the fertility of patients with schizophrenia and their unaffected relatives. Acta Psychiatrica Scandinavica. 2011;123(2):98–106. doi: 10.1111/j.1600-0447.2010.01623.x. [DOI] [PubMed] [Google Scholar]
- 31.Rees E, Moskvina V, Owen MJ, O'Donovan MC, Kirov G. De Novo Rates and Selection of Schizophrenia-Associated Copy Number Variants. Biological Psychiatry. 2011;70(12):1109–1114. doi: 10.1016/j.biopsych.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Cross-Disorder Group of the Psychiatric Genomics C. Lee SH, Ripke S, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature genetics. 2013;45(9):984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamshere ML, Stergiakouli E, Langley K, et al. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. Br J Psychiatry. 2013 doi: 10.1192/bjp.bp.112.117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirov G, Rees E, Walters JTR, et al. The Penetrance of Copy Number Variations for Schizophrenia and Developmental Delay. Biological Psychiatry. 2014;75(5):378–385. doi: 10.1016/j.biopsych.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen MJ. Implications of genetic findings for understanding schizophrenia. Schizophr Bull. 2012;38(5):904–907. doi: 10.1093/schbul/sbs103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruderfer DM, Fanous AH, Ripke S, et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craddock N, Owen MJ. The Kraepelinian dichotomy – going, going… but still not gone. Br J Psychiatry. 2010;196:92–95. doi: 10.1192/bjp.bp.109.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grozeva D, Kirov G, Ivanov D, et al. Rare Copy Number Variants: A Point of Rarity in Genetic Risk for Bipolar Disorder and Schizophrenia. Arch Gen Psychiatry. 2010;67(4):318–327. doi: 10.1001/archgenpsychiatry.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall J, Trent S, Thomas KL, O’Donovan MC, Owen MJ. Genetic Risk for Schizophrenia: Convergence on Synaptic Pathways Involved in Plasticity. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Benros ME, Mortensen PB, Eaton W. Autoimmune diseases and infections as risk factors for schizophrenia. Ann N Y Acad Sci. 2012;1262:56–66. doi: 10.1111/j.1749-6632.2012.06638.x. [DOI] [PubMed] [Google Scholar]
- 41.Smyth AM, Lawrie SM. The Neuroimmunology of Schizophrenia. Clinical Psychopharmacology and Neuroscience. 2013;11(3):107–117. doi: 10.9758/cpn.2013.11.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker K, Costain G, Fung WLA, Bassett AS. Chromosomal microarray analysis-a routine clinical genetic test for patients with schizophrenia. Lancet Psychiat. 2014;1(5):329–331. doi: 10.1016/S2215-0366(14)70308-6. [DOI] [PubMed] [Google Scholar]
- 43.McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- 44.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- 45.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35(3):528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khashan AS, Abel KM, McNamee R, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure ot severe adverse life events. Arch Gen Psychiatry. 2008;65(2):146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 47.Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72(10):1272–1276. doi: 10.1002/dneu.22024. Epub 2012 Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med. 2013;43(2):239–257. doi: 10.1017/S0033291712000736. Epub 2012 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGrath JJ, Eyles DW, Pedersen CB, et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. ArchGenPsychiatry. 2010;67(9):889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- 50.Brown AS. The environment and susceptibility to schizophrenia. ProgNeurobiol. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cannon M, Kendell R, Susser E, Jones P. Prenatal and perinatal risk factors for schizophrenia. In: Murray RM, Jones PB, Susser E, Van Os J, Cannon M, editors. The epidemiology of schizophrenia. Cambridge: Cambridge University Press; 2003. pp. 74–99. [Google Scholar]
- 52.Allardyce J, Boydell J. Review: the wider social environment and schizophrenia. Schizophr Bull. 2006;32(4):592–598. doi: 10.1093/schbul/sbl008. Epub 2006 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrne M, Agerbo E, Eaton WW, Mortensen PB. Parental socio-economic status and risk of first admission with schizophrenia- a Danish national register based study. SocPsychiatry PsychiatrEpidemiol. 2004;39(2):87–96. doi: 10.1007/s00127-004-0715-y. [DOI] [PubMed] [Google Scholar]
- 54.Paksarian D, Eaton WW, Mortensen PB, Pedersen CB. Childhood Residential Mobility, Schizophrenia, and Bipolar Disorder: A Population-based Study in Denmark. Schizophr Bull. 2015;41(2):346–354. doi: 10.1093/schbul/sbu074. Epub 2014 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varese F, Smeets F, Drukker M, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38(4):661–671. doi: 10.1093/schbul/sbs050. Epub 2012 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cantor-Graae E, Pedersen CB. Full spectrum of psychiatric disorders related to foreign migration: a Danish population-based cohort study. JAMA Psychiatry. 2013;70(4):427–435. doi: 10.1001/jamapsychiatry.2013.441. [DOI] [PubMed] [Google Scholar]
- 57.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 58.Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29(3):587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 59.Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull. 2012;38(6):1118–1123. doi: 10.1093/schbul/sbs096. Epub 2012 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA psychiatry (Chicago, Ill) 2014;71(3):301–309. doi: 10.1001/jamapsychiatry.2013.4081. [DOI] [PubMed] [Google Scholar]
- 61.Miller B, Messias E, Miettunen J, et al. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull. 2011;37(5):1039–1047. doi: 10.1093/schbul/sbq011. Epub 2010 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong A, Frigge ML, Masson G, et al. Rate of de novo mutations and the importance of father's age to disease risk. Nature. 2012;488(7412):471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malaspina D, Harlap S, Fennig S, et al. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58(4):361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- 64.Pedersen CB, McGrath J, Mortensen PB, Petersen L. The importance of father's age to schizophrenia risk. Molecular psychiatry. 2014;19(5):530–531. doi: 10.1038/mp.2013.69. [DOI] [PubMed] [Google Scholar]
- 65.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 66.Radhakrishnan R, Wilkinson ST, D'Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Front Psychiatry. 2014;5(54) doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orlovska S, Pedersen MS, Benros ME, Mortensen PB, Agerbo E, Nordentoft M. Head injury as risk factor for psychiatric disorders: a nationwide register-based follow-up study of 113,906 persons with head injury. Am J Psychiatry. 2014;171(4):463–469. doi: 10.1176/appi.ajp.2013.13020190. [DOI] [PubMed] [Google Scholar]
- 68.Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 2014;14(75):14–75. doi: 10.1186/1471-244X-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benros ME, Eaton WW, Mortensen PB. The Epidemiologic Evidence Linking Autoimmune Diseases and Psychosis. Biological psychiatry. 2014;75:300–306. doi: 10.1016/j.biopsych.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller N. Immunology of schizophrenia. Neuroimmunomodulation. 2014;21(2–3):109–116. doi: 10.1159/000356538. Epub 2014 Feb 14. [DOI] [PubMed] [Google Scholar]
- 71.Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia. A 30-year population-based register study. Am J Psychiatry. 2011;168(12):1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- 72.Khandaker GM, Zimbron J, Dalman C, Lewis G, Jones PB. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr Res. 2012;139(1–3):161–168. doi: 10.1016/j.schres.2012.05.023. Epub Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nielsen PFR, Benros ME, Mortensen PB. Hospital Contacts With Infection and Risk of Schizophrenia. A Population-Based Cohort Study With Linkage of Danish National Registers. 2014;40(6):1526–1532. doi: 10.1093/schbul/sbt200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasamanick B, Rogers ME, Lilienfeld AM. Pregnancy experience and the development of behavior disorders in children. Am J Psychiatry. 1956;112(8):613–618. doi: 10.1176/ajp.112.8.613. [DOI] [PubMed] [Google Scholar]
- 75.Kirov G, Rees E, Walters JTR, et al. The penetrance of copy number variations for schizophrenia and developmental delay. Biological psychiatry. 2014;75(5):378–385. doi: 10.1016/j.biopsych.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knuesel I, Chicha L, Britschgi M, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643–660. doi: 10.1038/nrneurol.2014.187. Epub Oct 14. [DOI] [PubMed] [Google Scholar]
- 77.Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. ProgNeurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 78.Giovanoli S, Engler H, Engler A, et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 79.Iyegbe C, Campbell D, Butler A, Ajnakina O, Sham P. The emerging molecular architecture of schizophrenia, polygenic risk scores and the clinical implications for GxE research. Soc Psychiatry Psychiatr Epidemiol. 2014;49(2):169–182. doi: 10.1007/s00127-014-0823-2. Epub 2014 Jan 17. [DOI] [PubMed] [Google Scholar]
- 80.Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry. 2014;5(48) doi: 10.3389/fpsyt.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borglum AD, Demontis D, Grove J, et al. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Molecular psychiatry. 2014;19(3):325–333. doi: 10.1038/mp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas D. Gene--environment-wide association studies: emerging approaches. NatRevGenet. 2010;11(4):259–272. doi: 10.1038/nrg2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Davey-Smith G, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 84.VanderWeele TJ, Vansteelandt S. Mediation Analysis with Multiple Mediators. Epidemiol Method. 2014;2(1):95–115. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agerbo E, Sullivan PF, Vilhjalmsson BJ, et al. Polygenic Risk Score, Parental Socioeconomic Status, Family History of Psychiatric Disorders and the Risk of Schizophrenia: A Danish Population-Based Study. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0346. In press. [DOI] [PubMed] [Google Scholar]
- 86.Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: new pathological insights and therapies. Annual review of medicine. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- 87.Linden DE. The challenges and promise of neuroimaging in psychiatry. Neuron. 2012;73(1):8–22. doi: 10.1016/j.neuron.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in cognitive sciences. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewis DA. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. The European journal of neuroscience. 2012;35(12):1871–1878. doi: 10.1111/j.1460-9568.2012.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meyer-Lindenberg A, Tost H. Neuroimaging and plasticity in schizophrenia. Restorative neurology and neuroscience. 2014;32(1):119–127. doi: 10.3233/RNN-139014. [DOI] [PubMed] [Google Scholar]
- 91.Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Translational psychiatry. 2012;2:e190. doi: 10.1038/tp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fusar-Poli P, Smieskova R, Kempton MJ, Ho BC, Andreasen NC, Borgwardt S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neuroscience and biobehavioral reviews. 2013;37(8):1680–1691. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383(9929):1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain research bulletin. 2010;83(3–4):108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(1):4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature reviews Neuroscience. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 98.Ting JT, Peca J, Feng G. Functional consequences of mutations in postsynaptic scaffolding proteins and relevance to psychiatric disorders. Annual review of neuroscience. 2012;35:49–71. doi: 10.1146/annurev-neuro-062111-150442. [DOI] [PubMed] [Google Scholar]
- 99.Hall J, Trent S, Thomas KL, O'Donovan MC, Owen MJ. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biological psychiatry. 2015;77(1):52–58. doi: 10.1016/j.biopsych.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 100.Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural plasticity. 2013;2013:627325. doi: 10.1155/2013/627325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 102.Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64(1):93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nature reviews Neuroscience. 2004;5(7):521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 105.Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci U S A. 2010;107(51):22278–22283. doi: 10.1073/pnas.0914064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290(5499):2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee H, Brott BK, Kirkby LA, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509(7499):195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annual review of neuroscience. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 109.Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318(5856):1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 110.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Current opinion in neurobiology. 2009;19(2):220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 111.Cabungcal JH, Counotte DS, Lewis EM, et al. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83(5):1073–1084. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sawa A, Seidman LJ. Is prophylactic psychiatry around the corner? combating adolescent oxidative stress for adult psychosis and schizophrenia. Neuron. 2014;83(5):991–993. doi: 10.1016/j.neuron.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 114.Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261(5562):717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- 115.Meltzer HY. Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia. Psychopharmacology. 1989;99(Suppl):S18–S27. doi: 10.1007/BF00442554. [DOI] [PubMed] [Google Scholar]
- 116.Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- 117.Berrios GE. Positive and negative symptoms and Jackson. A conceptual history. Arch Gen Psychiatry. 1985;42(1):95–97. doi: 10.1001/archpsyc.1985.01790240097011. [DOI] [PubMed] [Google Scholar]
- 118.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 119.Meltzer HY. Update on typical and atypical antipsychotic drugs. Annual review of medicine. 2013;64:393–406. doi: 10.1146/annurev-med-050911-161504. [DOI] [PubMed] [Google Scholar]
- 120.Secher RG, Hjorthoj CR, Austin SF, et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull. 2015;41(3):617–626. doi: 10.1093/schbul/sbu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bentall RP. Madness explained: Psychosis and human nature. London: Penguin; 2003. [Google Scholar]
- 122.Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry. 2014;204(1):20–29. doi: 10.1192/bjp.bp.112.116285. [DOI] [PubMed] [Google Scholar]
- 123.Goldsmith LP, Lewis SW, Dunn G, Bentall RP. Psychological treatments for early psychosis can be beneficial or harmful, depending on the therapeutic alliance: an instrumental variable analysis. Psychological medicine. 2015:1–9. doi: 10.1017/S003329171500032X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Laursen TM, Nordentoft M, Mortensen PB. Excess Early Mortality in Schizophrenia. Annual Review of Clinical Psychology. 2014;10:425-. doi: 10.1146/annurev-clinpsy-032813-153657. [DOI] [PubMed] [Google Scholar]
- 125.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 126.Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry. 2011;68(10):1058–1064. doi: 10.1001/archgenpsychiatry.2011.113. [DOI] [PubMed] [Google Scholar]
- 127.Laursen TM, Mortensen PB, MacCabe JH, Cohen D, Gasse C. Cardiovascular drug use and mortality in patients with schizophrenia or bipolar disorder: a Danish population-based study. Psychological medicine. 2014;44(8):1625–1637. doi: 10.1017/S003329171300216X. [DOI] [PubMed] [Google Scholar]
- 128.Poduri A, Evrony GD, Cai X, Walsh CA. Somatic mutation, genomic variation, and neurological disease. Science. 2013;341(6141):1237758. doi: 10.1126/science.1237758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gamo NJ, Sawa A. Human stem cells and surrogate tissues for basic and translational study of mental disorders. Biological psychiatry. 2014;75(12):918–919. doi: 10.1016/j.biopsych.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145(6):831–834. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nature reviews Molecular cell biology. 2012;13(11):713–726. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
- 132.Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC. Illuminating circuitry relevant to psychiatric disorders with optogenetics. Current opinion in neurobiology. 2014;30C:9–16. doi: 10.1016/j.conb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 135.Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Molecular psychiatry. 2012;17(12):1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 136.Adkins DE, Aberg K, McClay JL, et al. Genomewide pharmacogenomic study of metabolic side effects to antipsychotic drugs. Molecular psychiatry. 2011;16(3):321–332. doi: 10.1038/mp.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Malhotra AK, Correll CU, Chowdhury NI, et al. Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Arch Gen Psychiatry. 2012;69(9):904–912. doi: 10.1001/archgenpsychiatry.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Field JR, Walker AG, Conn PJ. Targeting glutamate synapses in schizophrenia. Trends in molecular medicine. 2011;17(12):689–698. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harvey RJ, Yee BK. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nature reviews Drug discovery. 2013;12(11):866–885. doi: 10.1038/nrd3893. [DOI] [PubMed] [Google Scholar]
- 140.Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochemical pharmacology. 2013;86(8):1122–1132. doi: 10.1016/j.bcp.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Howes OD, Kapur S. A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic) Br J Psychiatry. 2014;205(1):1–3. doi: 10.1192/bjp.bp.113.138578. [DOI] [PubMed] [Google Scholar]
- 142.McCarroll SA, Hyman SE. Progress in the genetics of polygenic brain disorders: significant new challenges for neurobiology. Neuron. 2013;80(3):578–587. doi: 10.1016/j.neuron.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heinssen RK, Insel TR. Preventing the onset of psychosis: not quite there yet. Schizophr Bull. 2015;41(1):28–89. doi: 10.1093/schbul/sbu161. Epub 2014 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 145.Uher R, Cumby J, MacKenzie LE, et al. A familial risk enriched cohort as a platform for testing early interventions to prevent severe mental illness. BMC Psychiatry. 2014;14(1):344. doi: 10.1186/s12888-014-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 147.MQ. UK Mental health research funding. London: MQ; 2015. [Google Scholar]


