Abstract
Objectives
Historically, chronic pancreatitis (CP) was considered a disease of alcoholic males, but recent data suggest its etiology to be complex. To better understand CP in women, we compared data on women and men with CP in a large, prospectively ascertained multicenter US cohort.
Methods
CP patients enrolled in the NAPS2 Continuation and Validation study (NAPS2-CV) were studied. Information on demographics, etiology, risk factors, phenotype and treatment(s) utilized was obtained from detailed questionnaires completed by patients and physicians. Results: Of 521 cases, 45% were women. Women were significantly (p<0.05) less likely to have alcohol etiology (30 vs. 58.5%) and more likely to have non-alcoholic etiologies (idiopathic 32 vs. 18%, obstructive 12 vs. 2.4%, genetic 12.8 vs. 7.3%). Demographics, pain experience, morphologic findings, exocrine and endocrine insufficiency, CP-related disability, use of medical therapies were mostly similar in both sexes. Sphincterotomy (biliary 33 vs. 24%, pancreatic 38 vs. 28%, p<0.05) was performed more frequently in women, while cyst/pseudocyst operations were more common in men (6.6 vs. 2.6%; p=0.02).
Conclusions
The majority of CP cases in women are from nonalcoholic etiologies. In contrast to many other chronic diseases, clinical phenotype of CP is determined by the disease and is independent of sex.
Keywords: pancreatitis, women, demographics, therapy, evaluation
Introduction
Chronic pancreatitis (CP) has traditionally been considered to be a disease of males who are heavy drinkers. As such, the characteristic of CP in women remain obscure, especially women who do not drink to excess, as they did not fit the profile of a classic CP patient. More recent studies suggest that the prevalence of women among patients with CP may be more common than previously believed1-4. The recognition of genetic predisposing factors, smoking as an independent risk factor and etiologies other than alcohol indicates that clinical recurrent acute (RAP) and CP reflect complex gene-environment interactions in a diverse population rather than the direct effects of excess alcohol in men 3,5,6.
Sex-based differences in clinical presentation, evaluation and management and the use of health care services have been recognized for many diseases7-10. It can therefore be hypothesized that awareness of CP as a possibility in men may bias pancreas specific work-up while multi-organ screening for vague/poorly defined pains in women may lead to detection of milder or earlier forms of CP in women. Attitudes towards medical and surgical therapies may differ by sex, as may attitudes and attention to changes in weight. There could potentially be differences in the use of medical therapies or interventions based on gender due to provider and patient biases.
The clinical presentation and natural history of CP differs based on etiology11-13. While differences in exposure to environmental risk factors (alcohol, smoking) between women and men with CP are recognized6,14, it is unclear whether clinical presentation, diagnosis and therapies used in women with CP differs from men independent of disease etiology. The NAPS2-CV study provides empiric data from a large multicenter prospective cohort to test hypotheses about possible differences in the clinical presentation, morphologic changes in the pancreas and treatment strategies for CP in women and men and potential biases that may exist based on sex.
Methods
NAPS2-CV study
The NAPS2-CV study was conducted to ascertain a replication cohort for a genome-wide association study for the original NAPS2 cohort15,16. The NAPS2-CV study prospectively enrolled patients with established CP (n=521), RAP (n=89) and controls (n=188) from 13 US centers from 2008 - 2011. The details of the definitions and methodologies for the NAPS2 and NAPS2-CV studies have been published previously16,17. The study was approved by the Institutional Review Board at each participating center and all patients provided informed consent prior to enrollment.
Definition of CP and Questionnaires
CP was defined by the presence of definitive changes on imaging studies - computerized tomography [CT] scan, Magnetic resonance imaging [MRI]/Magnetic Resonance Cholangiopancreatography [MRCP], Endoscopic Retrograde Cholangiopancreatography [ERCP] [Cambridge classification] or Endoscopic ultrasound (EUS; presence of 5 or more findings or presence of calcifications) or histology. Two sets of questionnaires were used to collect detailed information, one administered to patients by a trained research coordinator and the other completed by the enrolling physician. The patient questionnaires collected information on demographics, personal and family history, exposure to alcohol and tobacco, pain experience, quality of life, and medication use. The enrolling physician provided information on disease phenotype including information on age at onset and diagnosis of CP, pain patterns, exocrine and endocrine insufficiency, most likely etiology, TIGAR-O risk factors18, findings on imaging studies, treatments tried and their perceived effectiveness.
Data elements for the current study
In this study, we analyzed data on the 521 patients with CP enrolled in the NAPS2-CV study. Comparisons were made between men and women for demographic factors, age of onset of symptoms, diagnosis and enrollment, body mass index (BMI) (maximum during lifetime and at the time of enrollment), physician defined etiology, TIGAR-O risk factors identified by physician, presence and pattern of pain in the year preceding enrollment as reported by the patient, use of pain medication as identified by the enrolling physician, morphological changes in the pancreas on imaging studies, exocrine and endocrine insufficiency and treatment(s) used until the time of enrollment according to the enrolling physician.
Statistics
Descriptive analyses are presented as proportions for categorical data and median and interquartile range (IQR) for continuous data. Bivariate comparisons were performed based on sex using chi-squared or Fischer's exact test for categorical data and Student's-t test or Mann-Whitney-U test for continuous data. As an a-priori, data was analyzed after stratification by physician defined etiology. Due to limited number of significant results on bivariate analyses, mainly within subgroups of patients, stratified analyses by relevant variables was performed and a multivariate model was deferred. Data analysis was performed using SAS version 9.3. Two-tailed p-values <0.05 were considered statistically significant.
Results
Demographics
Of 521 subjects, 235 (45%) were women, and the proportion of women was ≥40% at 8/13 centers (Figure 1). The median age at the onset of symptoms, diagnosis of CP and enrollment was similar in men and women (Table 1). Caucasians were predominant among both sexes (90% in women, 85% in men). The maximum lifetime BMI was at the upper limit of “overweight” range and at enrollment it was close to normal in both sexes. The maximum or current BMI and the difference between these was similar in men and women.
Figure 1. Distribution on women with chronic pancreatitis at participating centers in the NAPS2-CV study.
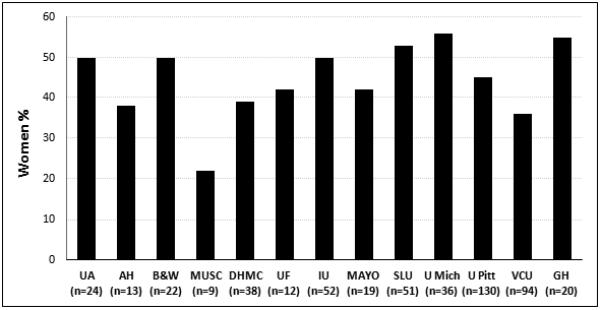
UA – University of Alabama; AH – Aurora Health care; B&H – Brigham & Women's Hospital; MUSC – Medical University of South Carolina; DHMC – Dartmouth-Hitchcock Medical Center; UF – University of Florida; IU – Indiana University; Mayo – Mayo Clinic, Jacksonville, FL; SLU – St. Louis University; U. Mich - University of Michigan; U. Pitt – University of Pittsburgh; VCU – Virginia Commonwealth University; GH – Griffin Hospital
Table 1.
Demographics, age of diagnosis of history of acute pancreatitis in men and women with chronic pancreatitis enrolled in the NAPS2-CV study
| Demographics | Men (N=287) | Women (N=234) |
|---|---|---|
| Age (yrs) at enrollment, median, (Q1-Q3) | 53.1 (43.7-61.1) | 52.9 (41.3-62.6) |
| Age (yrs) distribution at enrollment, n(%): | ||
| <25 | 9 (3) | 14 (6) |
| 25-45 | 75 (26) | 61 (26) |
| 45-65 | 149 (52) | 110 (47) |
| 65+ | 55 (19) | 49 (21) |
| Age (yrs) at diagnosis, median, (Q1-Q3)a | 45 (34-55) | 45 (31-55) |
| Age (yrs) at symptoms, median, (Q1-Q3)b | 41.5 (30-52) | 41 (26-51) |
| Racec (%): | ||
| White | 243 (85) | 210 (90) |
| Others | 44 (15) | 24 (10) |
| BMI at enrollment, median, (Q1-Q3) | 24.7 (22.1-27.7) | 23.7 (20.6-28.8) |
| BMI maximum, median, (Q1-Q3) | 29 (25.7-32.9) | 29 (24.7-34) |
| BMI difference (BMI maximum-BMI at enrollment) | 4.3 | 5.3 |
| Acute pancreatitis everd, n (%) | 180 (72) | 136 (68) |
| Recurrent acute pancreatitise, n (%) | 141 (59) | 113 (57) |
Missing data:
34 patients
7 patients
4 patients
71 patients
86 patients
Proportions/medians shown are based on effective numbers
CP Etiology
As we have reported previously, physician defined etiology of CP varied significantly between men and women3. In men with CP, physicians identified alcohol as the primary etiology when compared with women (58.5 vs. 30.2%, p<0.001). In contrast, physicians identified non-alcohol related etiologies more often in women when compared with men (idiopathic - 31.5 vs. 18.2%, obstructive - 12.3 vs. 2.4%, genetic - 12.8 vs. 7.3%). Racial distribution within etiologies was similar in men and women (i.e. equal proportion of men and women with alcohol and non alcohol-related etiologies were Caucasian). The distribution of risk factors based on TIGAR-O classification in men and women is shown in Table 2. Similar to physician-defined etiology, differences were observed between men and women, with a lower fraction of women having alcohol and tobacco exposure, and a higher fraction of them having idiopathic and obstructive risk factors.
Table 2.
Distribution of select TIGAR-O risk factors in men and women with chronic pancreatitis enrolled in the NAPS2-CV study
| TIGAR-O Factors N (%) | Men (N=287) | Women (N=234) |
|---|---|---|
| Toxic-Metabolic | 239 (83.3) | 142 (60.7)* |
| Alcohol | 190 (66.4) | 84 (35.7)* |
| Tobacco | 192 (67.1) | 114 (48.5)* |
| Hyperlipidemia | 39 (13.6) | 27 (11.5) |
| Hypercalcemia | 0 (0) | 2 (0.9) |
| Medications | 3 (1) | 4 (1.7) |
| Chronic Renal Failure | 8 (2.8) | 2 (0.9) |
| Toxins | 1 (0.3) | 0 (0) |
| Idiopathic | 63 (22) | 86 (36.6)* |
| Early-onset Idiopathic | 29 (10.1) | 24 (10.2) |
| Late-onset Idiopathic | 33 (11.5) | 62 (26.4)* |
| Genetic | 23 (8) | 26 (11.1) |
| Autoimmune | ||
| Autoimmune Pancreatitis | 7 (2.5) | 7 (3) |
| Autoimmune-associated diseases | 6 (2.1) | 12 (5.2) |
| RAP and SAP associated CP | 20 (7.1) | 15 (6.4) |
| Obstructive | 36 (12.7) | 62 (26.7)* |
| Pancreas divisum | 18 (6.4) | 29 (13)** |
| Sphincter of oddi | 4 (1.4) | 16 (7.3)** |
| Post-trauma stricture | 1 (0.4) | 1 (0.5) |
| Duct obstruction | 8 (2.9) | 8 (3.7) |
| PMN | 1 (0.4) | 1 (0.5) |
| Others | 8 (2.9) | 19 (8.6)** |
| Miscellaneous | 24 (8.5) | 26 (11.2) |
RAP=Recurrent Acute Pancreatitis, SAP=Severe Acute Pancreatitis, CP=Chronic Pancreatitis; IPMN=Intra Papillary Mucinous Neoplasm
P value <0.001
P value≤0.01
Morphology, Exocrine and Endocrine insufficiency
Overall, the prevalence of calcifications, exocrine and endocrine insufficiency in CP patients was 55 %, 49 % and 36 % respectively. Men had a higher prevalence of each of these when compared with women, although a significant difference was noted only for the prevalence of calcifications (Table 3). After stratification by etiology, the difference seem to be driven by a higher prevalence of calcifications, exocrine and endocrine insufficiency among men with idiopathic CP, which was not statistically significant due to smaller sample sizes.
Table 3.
Distribution of imaging findings, exocrine and endocrine insufficiency in men and women with chronic pancreatitis enrolled in the NAPS2-CV study
| N (%) | All | Alcohol | Genetic | Idiopathic | Obstructive | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (N=287) | Women (N=234) | Men (N=167) | Women (N=71) | Men (N=22) | Women (N=29) | Men (N=52) | Women (N=74) | Men (N=7) | Women (N=29) | |
| Calcifications | 174 (61*) | 113 (51) | 102 (62) | 42 (60) | 17 (77) | 14 (61) | 33 (63) | 37 (52) | 2 (29) | 10 (36) |
| Pseudocysts | 105 (40*) | 62 (29) | 73 (47) | 27 (41) | 7 (44) | 5 (23) | 13 (29) | 16 (23) | 1 (14) | 3 (12) |
| Pancreatic atrophy | 171 (65) | 127 (60) | 101 (66) | 44 (66) | 14 (64) | 15 (71) | 32 (71) | 36 (51) | 3 (43) | 13 (50) |
| Pancreatic duct stricture | 84 (34) | 59 (30) | 43 (29) | 19 (30) | 7 (47) | 6 (32) | 17 (40) | 16 (25) | 2 (29) | 10 (40) |
| Pancreatic duct dilatation | 198 (73) | 154 (69) | 119 (74) | 49 (69) | 18 (86) | 20 (77) | 33 (70) | 48 (68) | 5 (71) | 21 (81) |
| Pancreatic duct irregularities | 151 (58) | 115 (56) | 92 (60) | 37 (57) | 11 (65) | 12 (60) | 24 (53) | 35 (51) | 4 (57) | 14 (54) |
| Common bile duct stricture | 42 (16) | 19 (10) | 31 (20) | 10 (17) | 0 (0) | 0 (0) | 5 (11) | 6 (10) | 0 (0) | 1 (4) |
| Common bile duct dilatation | 59 (23) | 34 (17) | 39 (25) | 13 (21) | 0 (0) | 1 (5) | 7 (16) | 12 (19) | 2 (29) | 5 (20) |
| Exocrine insufficiency | 135 (47) | 103 (44) | 75 (45) | 38 (54) | 11 (50) | 11 (38) | 28 (54) | 29 (39) | 2 (29) | 14 (48) |
| Endocrine insufficiency | 113 (39) | 73 (31) | 59 (35) | 24 (34) | 5 (23) | 10 (34) | 19 (37) | 19 (26) | 3 (43) | 8 (28) |
P value<0.05
Pain, Pain Patterns and Narcotic use
Of the 521 CP patients, 84% reported having pain related to CP in the year preceding enrollment. An equal proportion of men and women reported having abdominal pain related to CP (Table 4). The distributions for pain patterns and the use of pain medications was similar in men and women, overall and after stratification by etiology with the exception of obstructive etiology. Women with obstructive etiology were more likely to have constant pain (64% vs. 40%) and severe pain (82% vs. 40%) than men which explains the increased use of pain medications in women in this subgroup (79% vs. 60%). Due to a small sample size for both sexes in this subgroup, especially men, the results should be considered preliminary.
Table 4.
Pain patterns and pain medication use in men and women with chronic pancreatitis enrolled in the NAPS2-CV study
| N (%) | All | Alcohol | Genetic | Idiopathic | Obstructive | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men (N=287) | Women (N=234) | Men (N=167) | Women (N=71) | Men (N=22) | Women (N=29) | Men (N=52) | Women (N=74) | Men (N=7) | Women (N=29) | |
| Abdominal pain | 239 (83) | 201 (86) | 142 (85) | 62 (87) | 21 (95) | 26 (90) | 40 (77) | 61 (82) | 5 (71) | 28 (97) |
| Pain | ||||||||||
| Constant | 146 (61) | 127 (63) | 93 (65) | 42 (68) | 11 (52) | 16 (62) | 23 (57) | 37 (61) | 2 (40) | 18 (64) |
| Intermittent | 93 (40) | 74 (37) | 49 (35) | 20 (32) | 10 (48) | 10 (38) | 17 (43) | 24 (39) | 3 (60) | 10 (36) |
| Severity of pain | ||||||||||
| Mild-moderate | 46 (19) | 46 (23) | 22 (15) | 13 (21) | 6 (29) | 5 (19) | 9 (23) | 15 (25) | 3 (60) | 5 (18) |
| Severe | 193 (81) | 155 (77) | 120 (85) | 49 (79) | 15 (71) | 21 (81) | 31 (77) | 46 (75) | 2 (40) | 23 (82) |
| Pain medications | 186 (78) | 159 (79) | 114 (80) | 52 (84) | 18 (86) | 18 (69) | 28 (70) | 47 (77) | 3 (60) | 22 (79) |
| Non-narcotic | 15 (8) | 22 (14) | 4 (3) | 8 (15*) | 1 (6) | 4 (23) | 6 (22) | 5 (11) | 1 (33) | 3 (13) |
| Narcotic | ||||||||||
| Intermittent | 60 (32) | 62 (39) | 33 (29) | 15 (29) | 11 (61) | 6 (33) | 9 (32) | 25 (53) | 0 (0) | 5 (23) |
| Constant | 111 (60*) | 75 (47) | 77 (68) | 29 (56) | 6 (33) | 8 (44) | 13 (46) | 17 (36) | 2 (67) | 14 (64) |
P value<0.05
Medical, endoscopic and surgical therapy
Medical therapy was commonly used. Oral Pancreatic enzyme replacement therapy (PERT) was used in 69%, vitamins/antioxidants in 28%, octreotide in 2% and celiac plexus block in 5% patients (Table 5). The proportion of patients in whom PERT (67 vs. 70%, p=0.45) vitamins and/or antioxidants (27 vs. 29%, p0.48), Octreotide (1% vs. 3%, p=0.31) and celiac plexus block (6% vs. 4%, p=0.35) was used was similar in women and men (Table 5).
Table 5.
Therapies utilized until the time of enrollment in men and women with chronic pancreatitis enrolled in the NAPS2-CV study
| N (%) | All (N=521) | Alcohol (N=238) | Idiopathic (N=126) | Obstructive (N=36) | ||||
|---|---|---|---|---|---|---|---|---|
| Men (N=287) |
Women (N=234) |
Men (N=167) |
Women (N=71) |
Men (N=52) |
Women (N=74) |
Men (N=7) | Women (N=29) |
|
| Medical Therapies | ||||||||
| Oral PERT | 192 (67) | 165 (70) | 110 (66) | 53 (75) | 37 (71) | 48 (65) | 4 (57) | 25 (86) |
| Vitamins/Antioxidants | 76 (27) | 69 (29) | 49 (29) | 20 (28) | 12 (23) | 19 (26) | 2 (29) | 11 (38) |
| Celiac nerve block | 16 (6) | 9 (4) | 8 (5) | 2 (3) | 1 (2) | 5 (7) | 1 (14) | 0 (0) |
| Endoscopic Therapies | ||||||||
| Biliary Sphincterotomy | 69 (24) | 78 (33)* | 33 (20) | 18 (25) | 13 (25) | 33 (45)* | 1 (14) | 11 (38) |
| Pancreatic Sphincterotomy | 79 (28) | 89 (38)* | 40 (24) | 20 (28) | 14 (27) | 36 (49)* | 4 (57) | 17 (59) |
| Bile Duct Stenting | 37 (13) | 23 (10) | 20 (12) | 8 (11) | 7 (14) | 7 (10) | 0 (0) | 4 (14) |
| Pancreatic Duct Stenting | 74 (26) | 78 (33) | 37 (22) | 26 (37)* | 17 (33) | 27 (37) | 1 (14) | 13 (45) |
| Pancreatic duct Stone removal | 40 (14) | 39 (17) | 21 (13) | 14 (20) | 5 (10) | 15 (20) | 1 (14) | 5 (17) |
| Surgical Therapies | ||||||||
| Cholecystectomy | 67 (23) | 89 (38)* | 28 (17) | 22 (31)* | 14 (27) | 32 (43) | 3 (43) | 11 (38) |
| Partial/Complete Pancreatectomy | 32 (11) | 27 (12) | 20 (12) | 5 (7) | 3 (6) | 7 (10) | 0 (0) | 6 (21) |
| Drainage surgery | 19 (7) | 14 (6) | 16 (10) | 3 (4) | 0 (0) | 4 (5) | 0 (0) | 3 (10) |
| Cyst/Pseudocyst Operation | 19 (7)* | 6 (3) | 14 (8)* | 1 (1) | 3 (6) | 2 (3) | 0 (0) | 0 (0) |
PERT: pancreatic enzyme replacement therapy
P-value ≤ 0.05 for differences in utilization of therapy
In contrast to medical therapy, endoscopic therapy was used much less frequently. Any endoscopic therapy was used in 269 (52 %) patients: biliary sphincterotomy in 147 (28 %), pancreatic sphincterotomy in 168 (32 %), bile duct stenting in 60 (12 %), pancreatic duct stenting in 152 (29 %) and pancreatic duct stone removal in 29 (6 %). Biliary and pancreatic sphincterotomy was preformed more frequently in women when compared with men, a difference mainly attributable to its use in patients with idiopathic CP. In idiopathic CP patients, pancreatic duct stenting was used more frequently for RAP in men and was perceived to be more effective for pain in women (Table 5).
Similar to endoscopic therapy, surgery was performed less often in CP patients when compared with medical therapy (Table 5). The most commonly performed operation was cholecystectomy (30%) while specific operations for the pancreas (pancreas resection 11%, drainage 6%, cyst operation 5%, surgical sphincterotomy 3%) were performed less frequently. Cholecystectomy was performed more frequently in women than men (38 vs. 23%, p<0.001), overall and in subsets of patients with alcoholic and idiopathic CP. Cyst/pseudocyst operations were more common in men than in women (6.6 vs. 2.6%; p=0.02), a difference mainly seen in patients with alcoholic CP.
Discussion
The NAPS2 cohort represents the largest US study of prospectively ascertained patients comparing the clinical phenotype, etiology and treatment strategies utilized in men and women with established CP. Our primary finding is that the demographic profile, symptomatology, morphologic findings, disease-related morbidity and disability in women with CP are generally similar to men. The major difference noted was in the etiology of CP, with alcohol and tobacco being predominant in men, whereas idiopathic and obstructive etiologies predominated in women. We noted some differences in the use of pain medication, treatments tried, but these were related more to the etiology of CP rather than sex.
The prevalence of women with CP in the NAPS2-CV study replicates our initial report from the NAPS2 dataset 3. It is reassuring that the prevalence of women in the NAPS2 cohort was not much different than the results of three recent population studies from the US1,2,19. We have previously discussed potential reasons for a lower prevalence of patients with alcohol etiology in the NAPS2 studies14 when compared with several cohorts from other regions of the world11-13,20-26. While these reasons are also the likely explanations for a higher prevalence of women in the NAPS2 cohort, future studies should evaluate whether there are other potential explanations for these observation.
Our primary finding was that the demographic distribution of onset of symptoms, diagnosis, history of acute or recurrent acute pancreatitis, pain in the year preceding enrollment, pancreatic exocrine and endocrine insufficiency and morphological findings are similar in men and women, suggesting an unbiased approach to the diagnosis and management of CP among expert physicians. Although the proportion of men who were on constant narcotics for pain was somewhat higher, the presence and pattern of pain and the need for pain medications was similar in women when compared with men. Moreover, CP related disability was also similar in both sexes. These findings suggest that the clinical phenotype of CP is related to disease etiology and complications rather than sex.
Alcohol remained the primary etiology men, but not women. Contributing factors may include a higher proportion of men in the population at-risk and heavy drinkers compared to women27. Although the lifetime risk of pancreatitis in very heavy drinkers is relatively low 28,29, additional factors appear to markedly increase risk in drinkers. We previously identified the high-risk CLDN2 locus on the X chromosome, which increases the risk of alcoholic men who are hemizygous for the risk allele, while the risk in woman, with two chromosomes, is significantly lower15. Cigarette smoking is positively correlated with alcohol drinking, and the risk of CP in subjects who both smoke and drink appear to be higher than either factor alone14. Finally, the risk of CP in subjects with both habits is also increased with variants in the chymotrypsinogen C gene (CTRC)30.
Non-alcohol etiologies, mainly idiopathic, and to some extent obstructive and genetic, were predominant in women. One potential explanation for this would be a lower prevalence of exposure to traditional risk factors associated with CP, i.e. alcohol and tobacco smoking6,14. Consequently, physicians may be more likely to perform additional testing, e.g. genetic testing or MRCP than men to uncover potential etiologies. With the exception of established genetic causes of CP, the definitive role of certain risk factors, e.g. pancreas divisum or sphincter of oddi remains uncertain. Previous studies reported no gender differences in distribution of early and late onset idiopathic CP11. In contrast, we found more women to have late-onset idiopathic CP. This could potentially be related to the onset and diagnosis of CP between 40-60 years of age in general and to a higher prevalence of women in our study.
The use of vitamins and antioxidants was reported to be higher and for biliary stenting lower in the NAPS2-CV study when compared with the original NAPS2 study. Otherwise, the utilization of different therapies was generally similar in the NAPS2-CV study validating findings of the original NAPS2 study31,32. We did not find any significant differences in the use of medical therapies between women and men. However, a significantly higher fraction of women were treated with endotherapy (biliary and pancreatic sphincterotomy) when compared with men. After stratification, this observation was attributable to differences in patients with idiopathic CP. The reasons for increased use of these endoscopic therapies in women are unclear and should be explored in future studies. Potential explanations may include physician bias with regard to expected benefits, lack of other definitive treatment options and a lack of behavior modification approaches that can be suggested to women. Cholecystectomy was performed more commonly in women. Although the reason is unknown, this finding may be independent of CP. The prevalence of cholelithiasis is higher in women when compared with men33 and cholecystectomy is often performed after the first episode of acute pancreatitis when no other etiology is identified to prevent recurrences34. Significant differences in pancreas specific operations were limited to a higher fraction of men undergoing cyst/pseudocyst operations, mainly due to alcoholic CP.
The limitation of recruitment of study subjects mainly from referral centers has already been discussed earlier. Being a cross-sectional evaluation, we do not have longitudinal data on patients to determine if the natural history of CP would be similar during longer periods of follow up. Our study was also not designed to determine gender based differences in the work up leading to diagnosis of CP, probability of getting a diagnosis of CP after evaluation or differences in the risk of developing CP. We also did not collect information on resource utilization in CP patients, i.e. rates of hospitalizations, outpatient clinic visits, number of imaging studies, etc. during care for CP. These are important and relevant questions which need to be evaluated in future studies.
In summary, physicians should be aware that demographic profile, clinical phenotype, morphologic findings, disease-related morbidity and disability in patients with CP are similar in women and men. Heavy alcohol consumption usually in combination with tobacco smoking is the predominant cause in ~60% men but in only ~30% women. A careful history of exposure to environmental factors is therefore necessary to identify men and women who will benefit from behavior modification strategies. Women are more likely to have unexplained CP or from presumed obstructive etiologies which may lead to higher rates of endoscopic interventions. Future studies should focus on identifying factors that increase the risk of CP in women and whether there are gender based differences exist in the work up of CP, probability of getting a diagnosis of CP and resource utilization in CP patients.
Acknowledgement
Presented (by JR) and published as abstract at DDW 2013.
Funding: The study was supported by R01DK061451 (DCW), R01 DK077906 (DY) and UL1 RR024153 and UL1TR000005 (PI – Steven E Reis, MD), ASGE Senior Investigator Mentoring Award (JR).
Footnotes
Conflict of Interest: The authors report no conflict of interest relevant to this manuscript.
Clinicaltrials.gov Registration: NA
Author Contributions:
Study design: JR, JT, DY, DCW
Writing: JR, JT, DY, DCW
Ascertained and phenotyped patients: SA, MAA, PAB, REB, GC, DLC, CEF, NG, TBG, AG, ML, TM, JR, BS, SS, AS, CMW, DY,
Statistical Analysis: EK, YT, SRW, JR, JT, DY
All authors reviewed and approved the manuscript.
References
- 1.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol. 2011;106:2192–2199. doi: 10.1038/ajg.2011.328. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Muddana V, O'Connell M. Hospitalizations for chronic pancreatitis in allegheny county, pennsylvania, USA. Pancreatology. 2011;11:546–552. doi: 10.1159/000331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–273. doi: 10.1016/j.cgh.2010.10.015. quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frulloni L, Gabbrielli A, Pezzilli R, et al. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis. 2009;41:311–317. doi: 10.1016/j.dld.2008.07.316. [DOI] [PubMed] [Google Scholar]
- 5.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology. 2013;144:1292–1302. doi: 10.1053/j.gastro.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andriulli A, Botteri E, Almasio PL, et al. Smoking as a cofactor for causation of chronic pancreatitis: a meta-analysis. Pancreas. 2010;39:1205–1210. doi: 10.1097/MPA.0b013e3181df27c0. [DOI] [PubMed] [Google Scholar]
- 7.Durazzo M, Belci P, Collo A, et al. Gender specific medicine in liver diseases: a point of view. World J Gastroenterol. 2014;20:2127–2135. doi: 10.3748/wjg.v20.i9.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedeschi SK, Bermas B, Costenbader KH. Sexual disparities in the incidence and course of SLE and RA. Clin Immunol. 2013;149:211–218. doi: 10.1016/j.clim.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 11.Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–1487. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 12.Lankisch PG, Lohr-Happe A, Otto J, et al. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54:148–155. doi: 10.1159/000201029. [DOI] [PubMed] [Google Scholar]
- 13.Ammann RW, Buehler H, Muench R, et al. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas. 1987;2:368–377. doi: 10.1097/00006676-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet. 2012;44:1349–1354. doi: 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology. 2008;8:520–531. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox MC, Yadav D, Ye T, et al. Chronic Pancreatitis Pain Pattern and Severity Are Independent of Abdominal Imaging Findings. Clin Gastroenterol Hepatol. 2015;13:552–60. doi: 10.1016/j.cgh.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- 19.Yang AL, Vadhavkar S, Singh G, et al. Epidemiology of alcohol-related liver and pancreatic disease in the United States. Arch Intern Med. 2008;168:649–656. doi: 10.1001/archinte.168.6.649. [DOI] [PubMed] [Google Scholar]
- 20.Ammann RW, Akovbiantz A, Largiader F, et al. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–828. [PubMed] [Google Scholar]
- 21.Cavallini G, Frulloni L, Pederzoli P, et al. Long-term follow-up of patients with chronic pancreatitis in Italy. Scand J Gastroenterol. 1998;33:880–889. doi: 10.1080/00365529850171567. [DOI] [PubMed] [Google Scholar]
- 22.Dani R, Mott CB, Guarita DR, et al. Epidemiology and etiology of chronic pancreatitis in Brazil: a tale of two cities. Pancreas. 1990;5:474–478. doi: 10.1097/00006676-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Durbec JP, Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18:337–350. doi: 10.1159/000198221. [DOI] [PubMed] [Google Scholar]
- 24.Robles-Diaz G, Vargas F, Uscanga L, et al. Chronic pancreatitis in Mexico City. Pancreas. 1990;5:479–483. doi: 10.1097/00006676-199007000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Hirota M, Shimosegawa T, Masamune A, et al. The seventh nationwide epidemiological survey for chronic pancreatitis in Japan: clinical significance of smoking habit in Japanese patients. Pancreatology. 2014;14:490–496. doi: 10.1016/j.pan.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion. 1973;9:447–453. doi: 10.1159/000197473. [DOI] [PubMed] [Google Scholar]
- 27.McCullough PA, Ahmed AB, Zughaib MT, et al. Treatment of hypertriglyceridemia with fibric acid derivatives: impact on lipid subfractions and translation into a reduction in cardiovascular events. Rev Cardiovasc Med. 2011;12:173–185. doi: 10.3909/ricm0619. [DOI] [PubMed] [Google Scholar]
- 28.Yadav D, Eigenbrodt ML, Briggs MJ, et al. Pancreatitis: prevalence and risk factors among male veterans in a detoxification program. Pancreas. 2007;34:390–398. doi: 10.1097/mpa.0b013e318040b332. [DOI] [PubMed] [Google Scholar]
- 29.Lankisch PG, Lowenfels AB, Maisonneuve P. What is the risk of alcoholic pancreatitis in heavy drinkers? Pancreas. 2002;25:411–412. doi: 10.1097/00006676-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 30.LaRusch J, Lozano-Leon A, Stello K, et al. The Common Chymotrypsinogen C (CTRC) Variant G60G (C.180T) Increases Risk of Chronic Pancreatitis But Not Recurrent Acute Pancreatitis in a North American Population. Clin Transl Gastroenterol. 2015;6:e68. doi: 10.1038/ctg.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glass LM, Whitcomb DC, Yadav D, et al. Spectrum of use and effectiveness of endoscopic and surgical therapies for chronic pancreatitis in the United States. Pancreas. 2014;43:539–543. doi: 10.1097/MPA.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burton F, Alkaade S, Collins D, et al. Use and perceived effectiveness of non-analgesic medical therapies for chronic pancreatitis in the United States. Aliment Pharmacol Ther. 2011;33:149–159. doi: 10.1111/j.1365-2036.2010.04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Trna J, Vege SS, Pribramska V, et al. Lack of significant liver enzyme elevation and gallstones and/or sludge on ultrasound on day 1 of acute pancreatitis is associated with recurrence after cholecystectomy: a population-based study. Surgery. 2012;151:199–205. doi: 10.1016/j.surg.2011.07.017. [DOI] [PubMed] [Google Scholar]


