Abstract
Primary Sjögren's syndrome (pSS) has a strong female bias. We evaluated an X chromosome dose effect by analyzing 47,XXY (Klinefelter's syndrome, 1 in 500 live male births) among subjects with pSS. 47,XXY was determined by examination of fluorescence intensity of single nucleotide polymorphisms from the X and Y chromosomes. Among 136 pSS men there were 4 with 47,XXY. This was significantly different from healthy controls (1 of 1254 had 47,XXY, p=0.0012 by Fisher's exact test) as well men with rheumatoid arthritis (0 of 363 with 47,XXY), but not different compared to men with systemic lupus erythematosus (SLE) (4 of 136 versus 8 of 306, Fisher's exact test p=NS). These results are consistent with the hypothesis that the number of X chromosomes is critical for the female bias of pSS, a property that may be shared with SLE but not RA.
Keywords: Sjögren's syndrome, X chromosome, Klinefelter's syndrome, sex bias
1. Introduction
1.1. Sjögren's Syndrome
Primary Sjögren's syndrome (SS) characterized by dry eyes and mouth is one of the most common systemic autoimmune diseases with an annual incidence of 3.9 per 100,000 in population [1]. Additionally, SS has a female-to-male sex bias that ranges from 20:1 to 9:1 [2]. Researchers speculate the contributing factor for the observed sexual dimorphism in the pathogenesis of SS can be attributed to the obvious difference in male and female sex steroids. Whether sex hormones plays a major role in causation, severity, or sex bias of the disease is unknown. There are little data concerning sex steroid levels found in patients with Sjögren's syndrome [3] but women are often peri- or post-menopausal during the onset of the disease.
1.2. Sex Bias in Autoimmunity
While anatomy, lifestyle, genetics, sex hormones, among others, have all been suggested as possible culprits, no definite pathological mechanism accounts for the sex bias of Sjögren's syndrome. We suggest an alternate theory: the number of X chromosomes, and not the phenotypic sex, increases the likelihood of developing Sjögren's syndrome.
We have previously shown that men with Klinefelter's syndrome, infertile men characterized genetically by a 47,XXY complement of chromosomes, are overrepresented among men with the SLE [4, 5]. SLE, like SS, has a sex bias, where women are 10 times more like to develop this systemic autoimmune disorder. Despite the worldwide estimated 1 and 500 live births of male infants with KS, only approximately one-fourth of the men are actually diagnosed [6]. Here we show that Klinefelter's syndrome men are also found in excess among men with Sjögren's syndrome, with 15-fold increase over the incidence seen in population, and a 38-fold increase when compared to a healthy control group. Additionally, when comparing Sjögren's syndrome to another autoimmune disorder, rheumatoid arthritis (RA), which has a smaller female to male sex-bias of 3:1 to 6:1, we found no excess Klinefelter's syndrome in RA.
2. Methods
2.1. Patients
Sjögren's syndrome patients and the controls studied herein were collected by the Sjögren's Syndrome Genetic Network (SGENE) collaboration [7], which is organized through the Oklahoma Medical Research Foundation (OMRF). This is an international effort that includes sites in the North America, Europe, South America, and Asia. A majority of subjects were of European ancestry, however. Each participant was shown to fulfill the 2002 American-European Consensus Group Classification criteria for primary Sjögren's syndrome [8]. This included examination by an expert clinician to eliminate the presence of systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis such that secondary Sjögren's syndrome was not included. All subjects gave written informed consent and the procedures were approved by the Institutional Review Boards or equivalent committees at all collaborating sites. Additional men with Sjögren's syndrome were identified via the OMRF Sjögren's Syndrome Research Clinic, which we have previously described in depth [9], for whom complete clinical data were available. Briefly, subjects with dry eye and dry mouth undergo a comprehensive medical/rheumatological, dental and ophthalmological examination at a single clinic visit with collection of biological specimens and a minor salivary gland biopsy. Subjects are classified according to the AECG [8] as well as the American College of Rheumatology Provisional Sjögren's Syndrome Criteria [8, 10]. The OMRF IRB approved the study and all subjects provided written informed consent. Rheumatoid arthritis patients, mostly from South America and of Hispanic ethnicity, were collected and characterized as previously described [11]. All patients met recognized criteria for rheumatoid arthritis [10, 12]. Similar to the Sjögren's syndrome SGENE collaboration, human research approval and written informed consent were conducted at the site of recruitment of the subject.
2.2. Single Nucleotide Polymorphism (SNP) Genotyping
Subjects underwent either genotyping using the Omni-Quad array (Illumina) or a custom genotyping platform designed by a consortium (Immunochip, Illumina), which contained approximately 200,000 SNPs. Quality control protocol was as we have previously described [4, 7].
2.3. Determination of 46,XY and 47,XXY
Using Illumina Genome Studio we examined raw fluorescence data from single nucleotide polymorphisms (SNPs) on the X chromosome, we determined the presence of either one or two X chromosomes in samples from men as previously described for SLE [4]. We have previously confirmed using karyotype and fluorescent in situ hybridization (FISH) that this method using SNP b plot analysis correctly identifies both normal (46,XY) and Klinefelter's (47,XXY) men [4, 5]. Briefly, X chromosome SNP fluorescence was examined in a B plot, that is, at any given SNP the fluorescence of the b allele was plotted over the combined fluorescence of the a plus b the allele. Numbers of X chromosomes can be deduced from SNPs with 3 different B plot values. At any SNP, a value of 1 indicates only a b allele, while a value of 0 indicates only an a allele. Meanwhile, a value of 0.5 indicates heterozygosity (that is, both a and b alleles) at a given SNPs (See Figure 1). Thus, a man with X chromosome SNPs of 1.0, 0.05 and 0.0 values in the b plot has 2 different X chromosomes. A man with only values of 1 and 0 in the X-linked region of X but a 4 band pattern (values of 0.0, 0.33, 0.66, and 1.0) in the pseudoautosomal region of the X chromosome is evidence of two identical X chromosomes and a Y chromosome (See Figure 1). This configuration occurs in about 20% of Klinefelter's syndrome men who have had a meiosis II non-disjunction [13]. Normal men were identified by the presence of only 1 or 0 b plot values for X chromosome SNPs. Examination of Y chromosome B plots confirmed the presence of a Y chromosome in each of the subjects.
Figure 1.
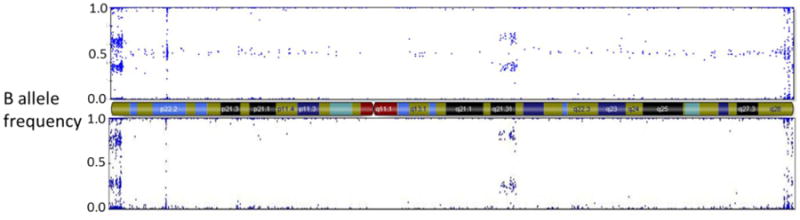
X chromosome B plot of a Klinefelter's syndrome subject with 2 diffferent X chromosomes as demonstrated by heterogeneity of X chromosome single nucleotide markers at 0.5 (top panel), and a Klinefelter's syndrome subject with 2 identical X chromosomes as demonstrated by homozygosity of X chromosome single nucleotide markers at 0.0 and 1.0 with none at 0.5 (bottom panel). Both subjects demonstrate a 4 band pattern (0.0, 0.33, 0.67, 1.0) in the pseudoautosomal region 1 (far left) indicating three total sex chromosomes. The X chromosome centromere and banding patterns are represented in the middle diagram.
2.4. Statistics
We used the Fisher's Exact Test to assess differences in 47,XXY carriage between Sjögren's syndrome men, control men as well as men with rheumatoid arthritis. We calculated odds ratios from these results. We used 95% binomial confidence intervals to compare the incidence of 47,XXY among the Sjögren's syndrome men to the known incidence in the population (1 in 500 live male births). We used Bayes' theorem to calculate the prevalence of Sjögren's syndrome in Klinefelter's men, as previously described [4].
3. Results
3.1
A total of 136 men that met the American-European Consensus Group (AECG) criteria for primary Sjögren's syndrome were collected from the OMRF Sjögren's Research Clinic and SGENE. Four of the 136 were identified as Klinefelter's men by B-plot analysis, while only 1 of 1254 healthy control men was found to have 47,XXY (p=0.0012; odds ratio=38, 95% confidence interval 4.2-342.2). Furthermore, using binomial confidence interval calculations, we found that the incidence of 4 of 136 (0.029, or 1 of 34) was statistically significantly different from the known population prevalence of 1 in 500 (or 0.002) live male births (95% binomial confidence interval 0.0736-0.0081, or 1 in 13 to 1 in 123). Thus, there is a 38-fold increase in the number of Sjögren's syndrome men with Klinefelter's syndrome compared to the unaffected, healthy control, where only 1 was determined as having Klinefelter's syndrome. And, there is a 15-fold increase compared to the known birth rate of Klinefelter's syndrome.
3.2
We also compared the incidence of Klinefelter's syndrome in Sjögren's syndrome to that found in rheumatoid arthritis. Rheumatoid arthritis, also a chronic autoimmune disease, disproportionately affects women compared to men, with a female-to-male prevalence of up to 6:1 [14, 15]. However, analyzing approximately three times as many male RA patients samples (n=384) than male pSS (n-136), we found no RA men with Klinefelter's syndrome (47,XXY). The RA and Sjögren's syndrome subjects are not ethnically matched but there is no known ethnic or racial effect on the incidence of Klinefelter's syndrome birth.
3.3
Since we have determined Klinefelter's syndrome among men with Sjögren's syndrome (1 in every 34), this gave us the opportunity to calculate the prevalence of Sjögren's among men with 47XXY. This calculation uses Bayes' theorem along with the known rate of Klinefelter's (17:10000) and calculated frequency of men with Sjögren's syndrome (1:770) in the population from previously published estimates [1]. The former is well known but the latter is less well studied. From our data, we calculate that 1 in 195 men with Klinefelter's syndrome will have Sjögren's syndrome.
3.4
Finally, we compared the clinical manifestations among the 47,XXY Sjögren's syndrome men to those found among normal (46,XY) men as well as among women with Sjögren's syndrome (Tables 2 and 3). The comparison men numbered 32 and were seen and characterized in the OMRF Sjögren's Research Clinic, while the women were also seen and evaluated in this same venue [9]. Of interest, all 4 of the Klinefelter men with Sjögren's had a focus score greater than 1.0 on pathological examination of minor salivary gland biopsy specimens, and all 4 had anti-Ro present in their serum. But, these findings were common among both the 46,XY men and the women (Table 1). When examining extraglandular manifestions, we noted that 3 or 4 Klinefelter men had interstitial lung disease, while none of the 32 men with normal sex chromosomes had lung disease. This difference showed a statistical trend (Fisher exact test p value = 0.11, uncorrected for multiple comparisons), but the small number of 47,XXY men precludes definitive statistical conclusions. Three 47,XXY men with Sjögren's had elevated IgG, but above normal serum levels of IgG has been reported as a feature of Klinefelter's syndrome [16] and is associated with the presence of anti-Ro.
Table 2.
Clinical manifestations of Sjögren's syndrome in the 4 Klinelfelter's syndrome compared to all other 46,XY men available as well as an equal number of randomly selected 46,XX women. Criteria for positive or negative were based on the American-European Classification Criteria for Sjögren's syndrome.
| KS1 | KS2 | KS3 | KS4 | pSS men (#/%) | pSS women (#/%) | |
|---|---|---|---|---|---|---|
| (n=32) | (n=32) | |||||
| Oral symptoms | + | + | + | + | 32(100%) | 31(96.8%) |
| Eye symptoms | + | - | + | + | 31(96.8%) | 31(96.8%) |
| WUSF | + | - | + | ND | 22(68.8%) | 20(62.5%) |
| Schirmer's | - | + | - | ND | 17(53.1%) | 17(50%) |
| Eye staining | ND | + | + | ND | 16(50%) | 14(43.8%) |
| Lip Biopsy* | + | + | + | + | 25(78.1%) | 23(71.8%) |
| Anti-Ro | + | + | + | + | 17(53.1%) | 14(43.6%) |
| Anti-La | + | - | - | + | 13(40.6%) | 10(31.3%) |
WUSF = whole unstimulated salivary flow;
Focus of KS1 was 4.0, KS was 5.0, KS was 6.0, while the average focus score of the normal men was 3.42.
Table 3.
Extraglandular clinical manifestations of Sjögren's syndrome in the 4 Klinelfelter's syndrome compared to randomly selected 46,XY men and 46,XX women.
| KS1 | KS2 | KS3 | KS4 | pSS men (#/%) | pSS women | |
|---|---|---|---|---|---|---|
| (n=32) | (n=32) | |||||
| Lymphadenopathy | - | - | - | - | 9(28.1%) | 6(18.8%) |
| Gland swelling | - | + | - | + | 10(31.3%) | 7(21.9%) |
| Arthralgia/Arthritis | - | - | - | + | 20(62.5%) | 24(75%) |
| Vasculitis | - | - | - | - | 0 | 0 |
| Pulmonary | +1 | - | +1 | +1 | 0* | 0* |
| Raynaud's | - | + | + | - | 8(25.0%) | 13(40.6%) |
| Renal | - | - | - | - | 0 | 0 |
| Myositis | - | - | - | - | 0 | 0 |
| PNS‡ | - | - | - | - | 14(43.8%) | 8(25%) |
| CNS | - | - | - | - | 0 | 0 |
| Hepatitis | - | - | - | - | 1(3.1%) | 0 |
| Hematological | - | +2 | - | - | 7(21.9%)† | 4(12.5%) |
| Elevated IgG | - | + | + | + | 10(31.3%) | 5(15.6%) |
| RF | - | - | + | + | 10(31.3%) | 10(31.3%) |
| ANA | - | + | + | + | 26(81.3%) | 22(68.8%) |
ANA = antinuclear antibody, RF=rheumatoid factor, ND=not done or unknown
= All 3 positive have interstitial lung disease
= This patient has both leukopenia and lymphopenia
No patient had interstitial lung disease but 11 men and 9 had chronic cough. Comparing interstitial lung disease among the 47,XXY men and 46,XY men shows a statistical trend (p=0.11 by Fisher's exact test)
1 each with isolated leukopenia or thrombocytopenia, 3 with isolated lymphopenia, 1 with combined lymphopenia and thrombocytopenia, 1 with combined leukopenia and lymphopenia
Peripheral neuropathy was defined as decreased vibratory, pin prick, and light touch, as we have previously reported [27]. No patient was evaluated by electromyography or nerve conduction studies.
Table 1.
Klinefelter's syndrome (47,XXY) among the various subject groups of man, including Sjögren's syndrome, healthy controls, rheumatoid arthritis and systemic lupus erythematosus. The last of these were studied in a previously published paper.
4. Discussion
4.1. Sjögren's Syndrome, SLE and Klinefelter's Sundrome
Primary Sjögren's syndrome has a female-to-male predominance of at least 9:1. This bias is observed in other autoimmune diseases, such as SLE, Hashimoto's thyroiditis, and primary biliary cirrhosis [17]. Although numerous theories have been suggested there has yet to be a clear understanding as to why women are overrepresented in almost all autoimmune diseases. We propose that having two or more X-chromosomes increases susceptibility to pSS. We show here that men with Klinefelter's syndrome (47,XXY) are disproportionately higher (1:34) in pSS compared to healthy controls (1:1254) and what is observed in the population (1:500). Collectively, the affected cases and healthy control men with Klinefelter's (1:280) more closely represent the observed prevalence of Klinefelter's syndrome men found in population compared to the 1:1254 observed among the healthy controls. While we have only studied 4 Klinefelter men with Sjögren's syndrome, we do not find definitive difference in the manifestations of Sjögren's syndrome among these men compared to normal men or women with the disease, although there were differences of interest.
We have shown the same phenomena of increased Klinefelter's syndrome in men with SLE [4], another sex-bias autoimmune disorder. In fact, the present finding in Sjögren's syndrome is not statistically different our previous finding in SLE [4, 5] (4 in 136 Sjögren's men versus 8 in 316 SLE men (χ2=0.0, p=1.0). Conversely, Klinefelter men were not found in our RA cohort. Other data from our group also show that SS and SLE women with three X chromosomes (47,XXX) are increased in numbers compared to healthy controls [18]. Taken together these data suggest a chromosome dosage effect in these two sex-bias autoimmune disorders; namely, Sjögren's syndrome and systemic lupus erythematosus.
4.2. Other Autoimmune Diseases
We have not found an X chromosome effect for men with RA, nor have we found excess 47,XXX among women with either RA or primary biliary cirrhosis [18]. In addition, Turner's syndrome women (45,XO) are at increased risk for type 1 diabetes, autoimmune thyroid disease, and celiac disease, but not SLE [19]. Also, other X chromosome abnormalities such as acquired X monosomy are found in patients with autoimmune diseases such as primary biliary cirrhosis, autoimmune thyroid disease, and systemic sclerosis [20-22], but not in SLE [23]. Therefore, there may be multiple mechanisms, including more than one involving the X chromosome, that lead to strong female bias in autoimmune disease.
4.3. Possible Mechanisms
Of course, sex hormones differ between men and women, and Klinefelter men have abnormalities in both estrogens and androgens compared to normal men. Thus, difference in sex hormones induced by the presence of two X chromosomes may underlie the X chromosome dose effect. However, 47,XXX women have normal sex hormone levels when compared to 46,XX women, but are found in excess among women with either SLE or Sjögren's syndrome [18]. We propose that these data argue against a sex hormone effect. In order to equalize the phenotypic expression between females and males, one female X chromosome genes randomly undergoes X-inactivation so that human XY male and the XX female genes are equally expressed. However, genes that escape that X-inactivation, which may be up to 15% of X genes in humans [24], may increase the gene dose, thus possibly predisposing women (46,XX or 47,XXX) or men with more than one X chromosome (47,XXY) to differential expression of these genes. X chromosome microRNAs may also escape X inactivation [25]. We hypothesize that genes escaping X inactivation underlie the X chromosome dose effect we have demonstrated for both Sjögren's syndrome and SLE.
4.4. Conclusions
We have shown that 47,XXY is found in excess among men with Sjögren's syndrome. This excess is similar to that found in men with systemic lupus erythematosus. In addition, 47,XXX is increased among women with these same illnesses. We contend these findings for uncommon X chromosome aneuploidies inform greatly about the marked over-representation of normal (that is 46,XX) women compared to normal (that is 46,XY) men in these diseases, and indicate that an X chromosome dose effect is in part responsible for the observed sex bias.
Highlights.
-
-
Sjögren's syndrome has a strong sex bias
-
-
47,XXY (Klinefelter's syndromes) is found in 1 of 500 live male births
-
-
We have assembled a large cohort of men with Sjögren's syndrome
-
-
Four of 126 Sjögren's men had 47,XXY
-
-
This result is statistically higher than that found in controls or men with RA
-
-
This result is not statistically different than our previous results among men with SLE.
-
-
An X chromosome dose effect may mediate sex bias in Sjögren's syndrome.
Acknowledgments
This work has been supported in part by NIH grants AR053483, AI082714, AR053734 and GM104938 as well as by a Merit Review Award from the US Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Patel R, Shahane A. The epidemiology of Sjogren's syndrome. Clin Epidemiol. 2014;6:247–55. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmunity Reviews. 2007;6:537–42. doi: 10.1016/j.autrev.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, et al. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–7. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillon S, Aggarwal R, Harding JW, Li LJ, Weissman MH, Li S, et al. Klinefelter's syndrome (47,XXY) among men with systemic lupus erythematosus. Acta Paediatr. 2011;100:819–23. doi: 10.1111/j.1651-2227.2011.02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17:363–8. doi: 10.1002/(sici)1097-0223(199704)17:4<363::aid-pd79>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet. 2013;45:1284–92. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen A, Ice JA, Li H, Grundahl K, Kelly JA, Radfar L, et al. Comparison of the American-European Consensus Group Sjogren's syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2013;73:31–8. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res. 2012;64:475–87. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Herraez D, Martinez-Bueno M, Riba L, Garcia de la Torre I, Sacnun M, Goni M, et al. Rheumatoid arthritis in Latin Americans enriched for Amerindian ancestry is associated with loci in chromosomes 1, 12, and 13, and the HLA class II region. Arthritis Rheum. 2013;65:1457–67. doi: 10.1002/art.37923. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs PA, Bacino C, Hassold T, Morton NE, Keston M, Lee M. A cytogenetic study of 47,XXY males of known origin and their parents. Ann Hum Genet. 1988;52:319–25. doi: 10.1111/j.1469-1809.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:269–81. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 15.Barragan-Martinez C, Amaya-Amaya J, Pineda-Tamayo R, Mantilla RD, Castellanos-de la Hoz J, Bernal-Macias S, et al. Gender differences in Latin-American patients with rheumatoid arthritis. Gend Med. 2012;9:490–510.e5. doi: 10.1016/j.genm.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Kocar IH, Yesilova Z, Ozata M, Turan M, Sengul A, Ozdemir I. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter's syndrome. Clin Exp Immunol. 2000;121:448–52. doi: 10.1046/j.1365-2249.2000.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockshin MD. Sex differences in autoimmune disease. Lupus. 2006;15:753–6. doi: 10.1177/0961203306069353. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Kurien BT, Zimmerman SL, Kaufman KM, Taft DH, Kottyan LC, et al. X Chromosome Dose and Sex Bias in Autoimmune Diseases: Increased 47,XXX in Systemic Lupus Erythematosus and Sjogren's Syndrome. Arthritis & Rheumatol. 2015 doi: 10.1002/art.39560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooney CM, Bruner GR, Aberle T, Namjou-Khales B, Myers LK, Feo L, et al. 46,X,del(X)(q13) Turner's syndrome women with systemic lupus erythematosus in a pedigree multiplex for SLE. Genes Immun. 2009;10:478–81. doi: 10.1038/gene.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363:533–5. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 21.Invernizzi P. The X chromosome in female-predominant autoimmune diseases. Ann N Y Acad Sci. 2007;1110:57–64. doi: 10.1196/annals.1423.007. [DOI] [PubMed] [Google Scholar]
- 22.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. J Immunol. 2005;175:575–8. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 23.Invernizzi P, Miozzo M, Oertelt-Prigione S, Meroni PL, Persani L, Selmi C, et al. X monosomy in female systemic lupus erythematosus. Ann N Y Acad Sci. 2007;1110:84–91. doi: 10.1196/annals.1423.010. [DOI] [PubMed] [Google Scholar]
- 24.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–4. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 25.Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet. 2009;41:488–93. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dillon SP, Kurien BT, Li S, Bruner GR, Kaufman KM, Harley JB, et al. Sex chromosome aneuploidies among men with systemic lupus erythematosus. J Autoimmun. 2012;38:J129–34. doi: 10.1016/j.jaut.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scofield AK, Radfar L, Ice JA, Vista E, Anaya JM, Houston G, et al. Relation of sensory peripheral neuropathy in Sjogren syndrome to anti-Ro/SSA. JCR: Journal of Clinical Rheumatology. 2012;18:290–3. doi: 10.1097/RHU.0b013e3182675e4f. [DOI] [PMC free article] [PubMed] [Google Scholar]


