Abstract
Superwarfarins were developed following the emergence of warfarin resistance in rodents. Superwarfarins have much longer half-lives and stronger affinity to vitamin K epoxide reductase versus warfarin, and therefore can cause death in warfarin-resistant rodents. By the mid-1970s, the superwarfarins brodifacoum (BDF) and difenacoum (DiF) were the most widely used rodenticides throughout the world. Unfortunately, increased use was accompanied by a rise in accidental poisonings, reaching >16,000 per year in the United States. Risk of exposure has become a concern since large quantities, up to hundreds of kilograms of rodent bait, are applied by aerial dispersion over regions with rodent infestations. Reports of intentional use of superwarfarins in civilian and military scenarios raises the specter of larger incidents or mass casualties. Unlike warfarin overdose, for which 1–2 days of treatment with vitamin K is effective, treatment of superwarfarin poisoning with vitamin K is limited by extremely high cost and can require daily treatment for a year or longer. Furthermore, superwarfarins have actions that are independent of their anticoagulant effects, including both vitamin K–dependent and –independent effects, which are not mitigated by vitamin K therapy. In this review, we will summarize superwarfarin development, biology and pathophysiology, their threat as weapons, and possible therapeutic approaches.
Keywords: superwarfarins, brodifacoum, HPLC, lipid membrane, neuropathology, nephrotoxicity, intralipid
Introduction: the development of superwarfarins
The history of the development of warfarin and other anticoagulants has been comprehensively reviewed.1,2 In brief, dicoumarol (Fig. 1), a product of the plant molecule coumarin, was shown in 19403 to be the agent in sweet clover that induces hemorrhagic illness in cattle. Warfarin (4-hydroxy-3-(3-oxo-1-phenylbutyl) coumarin) was developed in 1948 in a search for more potent anticoagulants and became one of the first widely used commercialized rodenticides (see Fig. 1 for the relevant structures). The mechanism of action of coumarins was first elucidated in 1978, when it was shown to inhibit vitamin K epoxide reductase.4 Warfarin binds to and inhibits the activity of the C1 subunit of vitamin K epoxide reductase (VKORC1), preventing recycling of oxidized vitamin K to its reduced form.5 Vitamin K is an essential cofactor for the enzyme γ-glutamyl carboxylase (GGC) which carboxylates proteins at glutamyl residues,6 leading to their activation. Carboxylation of clotting factors II, VII, IX, and X is required for clotting, therefore accounting for warfarin’s anticoagulant actions. Warfarin is primarily used to decrease thrombosis in patients who have already developed or are at increased risk of developing blood clots. Warfarin has a narrow therapeutic index, since higher dosing can cause excessive bleeding or stroke, while underdosing can result in clot formation or emolus. Warfarin has also been used for decades throughout the world as a rodenticide owing to its ability to induce internal hemorrhage in small rodents,7,8 and in most countries is provided in rodent bait at a concentration of 0.025%. However, mutations in VKORC1 leading to reduced warfarin binding9,10 led to the appearance of warfarin-resistant rodent strains, necessitating the development of more potent “superwarfarins,” also referred to as long-acting anticoagulant rodenticides (LAARs).
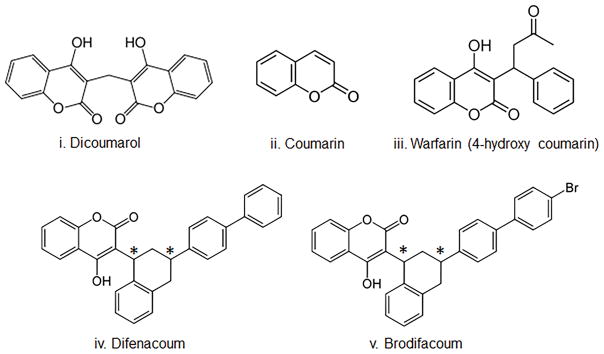
Figure 1.
Chemical structures: (i) dicoumarol; (ii) coumarin; (iii) warfarin; (iv) difenacoum; (v) brodifacoum. * indicates the two chiral centers in BDF and DiF, which lead to four enantiomers.
Superwarfarins are chemically modified forms of warfarin where substituted phenyl rings are attached to the 4-hydroxycoumarin moiety. Of 23 derivatives tested on laboratory rats, difenacoum (DiF, Fig. 1) and brodifacoum (brominated DiF (BDF), Fig. 1) showed the highest toxicity in both warfarin-sensitive (Wistar) and warfarin-resistant (Rattus norvegicus) rats;11 DiF was also effective against warfarin-resistant rats in field tests when provided at 0.005% in bait,12 but was less effective against R. rattus and Mus musculus. In contrast, BDF was efficient against all three species.13 The basis for increased toxicity of superwarfarins is not fully understood. The addition of substituted phenyl rings significantly increases their hydrophobicity, with BDF having a logP value (log of the octanol:water partition coefficient) of 8.5 (compared to a logP value of 2.3 for warfarin). This strong hydrophobicity increases tissue accumulation and retention and likely accounts for the exceedingly long biological half-lives (assessed by plasma drug levels) which are typically greater than 20 days, in contrast to 15–58 h for warfarin.14 Superwarfarins are also up to 100-fold more potent than warfarin in their ability to reduce levels of active vitamin K–dependent coagulation factors, which may be due to their increased liver retention as well as to a 10-fold lower IC50 value (0.15 versus 2.2 μM, BDF and warfarin, respectively) to inhibit VKORC1.15 These properties result in agents with extremely low LD50 values, which for rodents are estimated to be in the range of 0.30–0.70 mg/kg, and approximately 15 mg for fatal toxicity in an adult human. These values are significantly lower than values reported for acute warfarin toxicity, which range from less than 10 mg/kg to over 300 mg/kg for rodents.85
The emerging threat of superwarfarins: unintentional exposure
Despite their extreme toxicity, superwarfarins have been commercially available for use as rodenticides since 1979. Superwarfarins are normally provided in bait at 0.005%, a formulation considered “safe,” since consumption of at least 300 g of bait is needed to deliver a lethal dose to an adult. As a result of its increased use, the incidence of BDF poisoning due to accidental or intentional exposure has risen over the years and represents a growing public health concern. The potential of superwarfarin poisoning (accidental, surreptitious, or deliberate) became evident in the early 1980s following reports of attempted suicide16, and accidental ingestion by children.17,18 A recent meta-review of superwarfarin poisoning19 carried out by analysis of National Poison Data System records revealed that over 300,000 cases were reported in the United States in the 25 years from 1987 to 2012, of which the majority (close to 90%) occurred in children less than 6 years of age. Fortunately, of those only a small percentage (2.3%, or about 7000 cases) had any reported effects, and less than 2000 reported any major effects.
In 2008, the Environmental Protection Agency (EPA) concluded that BDF should be restricted to use by certified pesticide applicators only and be off-limits to the consumer market. Regulations were passed to limit children’s exposure to second-generation anticoagulants by requiring that all bait products sold for general and home use be limited to bait stations and prohibiting any type of loose bait form.20 The EPA also passed regulations to limit wildlife exposure and ecological risk, prohibiting the general consumer from purchasing bait products that contain the four most toxic rodenticides (BDF, DiF, bromadiolone, and difethialone). In 2014, the EPA reached an agreement with the manufacturers of consumer rat and mouse poison products to cancel production of products that did not meet EPA safety standards.21 However, despite these regulations, it is still possible to purchase large amounts of superwarfarins. Whereas typical bait formulations contain 0.005% BDF (50 mg/kg), concentrated versions containing up to 2.5% (2.5 gm/kg) are commercially available, which would require as little as 500 mg to reach a toxic dose in humans. In this regard, New York street vendors have been arrested for attempting to sell rodent baits containing up to 0.5% BDF.22
There is also a significant risk of unintentional exposure to superwarfarins as a result of their use to eradicate rodents. A recent review of 10 aerial BDF dispersals done in New Zealand and the United States showed that BDF was detected in 11 of 196 (5.6%) marine invertebrate samples tested, and in two of 65 (3.1%) fish samples23 up to 176 days after application. BDF poisoning outbreaks due to accidental leakage is an increasing possibility with potentially devastating consequences. In 2001, close to 20 tons of rodent bait containing 0.002% BDF, for a total of 360 g, was released into the environment owing to a transport accident on the east coast of South Island in New Zealand.24 Measurements were performed immediately after the spill and up to 21 months later on water and sediment samples, marine invertebrates, fish, and birds. While levels in water and sediment declined to below detection limits within 9 days, BDF was detectable in shellfish for up to 21 months after the spill and calculated to require up to 31 months before the levels were acceptable for human consumption. In 2010, 700 kg of similar bait was accidentally dropped into Lake Kirirua in New Zealand.25 It was estimated that 10 bags sank intact, and water and sediment measurements did not detect significant BDF up to 1 month later. The absence of BDF in the few animals tested may be due to the poor solubility of BDF in water, causing it to remain associated with the bait or other particles. Although residual BDF levels are considered safe with respect to effects on anticoagulation, BDF may have actions that occur at significantly lower levels.
Unintentional exposure to superwarfarins was also reported to occur owing to lacing of drugs to enhance and extend euphoric effects.26 The rationale is that saturation of liver enzymes with superwarfarins (or other toxins) reduces metabolism of the drug, thus prolonging its effects. Fortunately, these incidents appear to be sparse.
The emerging threat of superwarfarins: deliberate poisoning
Due to its easy availability, there is a concern that BDF could be used as a chemical weapon for a terrorist attack.27 A joint intelligence bulletin (unclassified, law enforcement sensitive) was released in March of 2011 describing the discovery of a radio-controlled explosive device in which lead weights were coated in BDF, intended to create an antipersonnel device that results in a higher rate of casualties during a parade.28 The same bulletin reported that a similar BDF-laced device was suspected to have been used by Palestinian suicide bombers in the Middle East. BDF poisoning by ingestion was reported in 2015, when 22 Rikers Island prisoners in New York were reported to have eaten meatloaf laced with BDF, and several showed anticoagulant-related symptoms; whether the presence of BDF was accidental or intentional was not determined.29
Mass anticoagulant poisoning as a lethal chemical warfare toxin in human subjects has already been deployed by the Selous Scouts and Special Branch of the all-white Rhodesian military during the 1970s in an attempt to regain control of Rhodesia from rebel forces.30 Dr. Paul Epstein, an American physician practicing in Mozambique at the time, encountered a large number of Zimbabweans being treated for bleeding disorders of unknown etiology.31 Within 2 weeks, 15 of the 35 admitted had died, and about 200 had suffered nonlethal hemorrhagic manifestations. At first, a viral hemorrhagic fever was suspected, but subsequent analysis of a body fat tissue sample carried out by the World Health Organization revealed significant amounts of a warfarin-like compound, and concluded that that was the cause of hemorrhage and death. Subsequent investigations revealed that the Rhodesian military was impregnating clothing, such as jeans, t-shirts and underpants, with warfarin, which were then shipped to rural stores commonly raided by insurgents.32 Wearing these treated articles led to uncontrolled bleeding due to excessive anticoagulation and death within days. Likewise, during the apartheid-era, Project Coast, a chemical and biological weapons program of the South African government, was testing the effects of food articles laced with BDF in primates and human subjects.33 The extent of the program was revealed during hearings held by the Truth and Reconciliation Commission after the abolition of apartheid in South Africa, and the director of the program, Wouter Basson, was suspended from military work and found guilty on several counts of misconduct.34
Identification of superwarfarins
Possible intoxication by superwarfarins requires rapid, sensitive, and specific methods to detect trace amounts in biological and environmental samples. Methods to measure warfarin and its metabolites in biological samples have generally used gas chromatography or high-performance liquid chromatography (HPLC).35 However, owing to their larger mass and lower volatility, superwarfarins such as DiF and BDF are less amenable to analysis using gas chromatography and have been measured instead using thin layer chromatography (TLC) or HPLC.36 One of the first methods developed used a clean-up step that incorporated exclusion chromatography using porous glass beads to eliminate macromolecules, followed by HPLC separation and molecular identification by mass spectrometry.37 Sample preparation for both warfarin and superwarfarins usually uses extraction with organic solvents or, less commonly, solid phase extraction. Because superwarfarins are more hydrophobic than warfarin and form few polar metabolites, nonpolar organic solvents are particularly useful for their extraction. This has included using chloroform to extract BDF from soil samples followed by TLC analysis;38 dichloromethane followed by reversed phase with UV absorbance detection;39 extraction from plasma and liver tissue using acetonitrile and diethyl ether followed by reversed-phase HPLC with fluorescence detection;40 and extraction from whole blood with ethyl acetate before liquid chromatography–mass spectrometry (LC-MS)/MS analysis.41 More recently, solid phase extraction followed by HPLC with UV and fluorescence detection has been used to simultaneously detect multiple anticoagulant rodenticides,42 and current methods that utilize column switching and electrospray ionization mass spectrometric detection allow simultaneous determination of up to 10 or 11 rodenticides.43
A limitation of the above methods has been to separate and identify the distinct isomers of BDF. The chemical structures of superwarfarins DiF and BDF (Fig. 1) show chiral centers at carbons 1′ and 3′, resulting in four stereoisomers.44 These pairs of diastereomers have different chemical and physical properties that might have different biological activities, as has been described for the biological activities and routes of metabolism of the R and S isomers of warfarin.42 Furthermore, DiF and BDF synthesized and purified using different approaches might contain different ratios of these isomers; therefore, enantiomer detection could aid in identification of BDF source or preparation. To address this, we developed a reversed-phase ultra- high-performance liquid chromatography (UHPLC) with tandem mass spectrometric detection methods for the simultaneous measurement of DiF and BDF diastereomers, as well as for vitamin K species.45 This method allows us to rapidly measure BDF enantiomer levels in rodent tissues for the determination of tissue distribution, pharmacokinetics, and metabolism. These methods will advance studies on tissue metabolism of superwarfarins and improve our understanding of mechanisms of toxicity.
Anticoagulant-independent consequences of superwarfarin poisoning
While the anticoagulant actions of superwarfarins are ultimately responsible for their lethality, additional targets exist that could lead to pathological consequences or contribute to toxicity. In addition to proteins in the coagulation cascade, vitamin K is a cofactor for several other proteins throughout the body, and modeling studies suggest there may be over 100 targets for GGC. Lower vitamin K levels will therefore reduce the GGC-dependent carboxylation of these proteins, including osteocalcin, a hormone involved in energy metabolism, fertility, brain development, and bone remodeling,46 and the matrix protein GLA, important for vascular decalcification.47 As described below, there are also several proteins with important functions within the central nervous system (CNS). Vitamin K is a ligand for the pregnane X receptor (PXR), a transcription factor with multiple roles, including metabolic detoxification, inflammation, cell proliferation, and bone diseases.48 Vitamin K is also an electron carrier and therefore has the potential to modulate mitochondria function; this may account for the ability of vitamin K2 to induce apoptosis in a variety of cancer cell lines.49 As described below, the strong hydrophobic nature of superwarfarins allows these molecules to directly interact with cellular and potentially subcellular membranes, which can lead to membrane disruption, cell activation, and production of oxidative species.
Neuropathology
In addition to their roles in clotting, vitamin K–dependent proteins (VDKPs) are vital to several other processes, including ones that help maintain normal brain function and homeostasis.50 The GGC enzyme is expressed in brain as well as in other tissues; therefore vitamin K deficiency can reduce GGC activity in the CNS and reduce carboxylation of brain proteins. Although only a few GGC targets in the brain have been identified to date, these play critical roles in neuronal and glial cell physiology. One of the first identified was galactosylceramide sulfotransferase, which catalyzes the conversion of galactocerobroside to sulfatide, a necessary component of myelin.51 Sulfatides are critical to the proper formation of the myelin sheath, and warfarin administration has been shown to lead to > 40% decreases in rodent brain sulfatide levels,52 which can be reversed by treatment with vitamin K. Vitamin K regulates other pathways involved in lipid synthesis, which could also contribute to alterations in myelin content.53 A second major VKDP is GAS6, a ligand for members of the tyrosine kinase receptor family including TYRO3, AXL, and MER.54 GAS6 has multiple functions in the CNS, including being a trophic factor for neurons and oligodendrocytes, activating phagocytosis, and suppressing inflammatory activation in glial cells.55,56 The above studies suggest that superwarfarins could have detrimental actions on brain physiology; if confirmed, the causes of the injury, whether due to anticoagulant actions or other effects, will need to be determined.
Nephrotoxicity
That superwarfarins cause other types of pathology is supported by previous reports that warfarin may also have additional coagulant-independent actions. For example, warfarin has been reported to cause changes in capillary permeability and morphology before changes in thrombin levels occurred, and these effects were independent of INR values.57,58 Warfarin was also shown to increase kidney damage in patients with chronic kidney disease. Warfarin-related nephropathy (WRN) is a newly recognized entity and is thought to be the result of excessive anticoagulation that results in glomerular hemorrhage, occlusive tubular red blood cell (RBC) cast formation,59 and acute kidney injury (AKI) in susceptible patients.60
Similar to warfarin, BDF administration also causes AKI in animals with chronic kidney disease (CKD), but not in controls.61 Kidney pathology in animals developing AKI was similar to that in humans and included occlusive tubular red blood cell casts and acute tubular necrosis. Moreover, uncontrolled BDF treatment was also associated with hematuria, which developed 2–3 days after poisoning and was more significant in CKD rats than in controls.61 These BDF renal effects were associated with its anticoagulation properties and were similar to the kidney effects of warfarin in humans and rats.59,61,62
Biomarkers for superwarfarin poisoning
During studies to characterize the effects of BDF on kidney damage, we observed that, within a few hours following BDF administration, animals developed a BDF dose-dependent transient hemoglobinuria, which ceased within 24 hours.63 This was accompanied by a transient decrease in hematocrit, gross hemolysis, and an increase in free hemoglobin in the serum. At later times, animals developed true hematuria with red blood cells in the urine, which was associated with BDF anticoagulation. The basis for these acute effects is not fully known; however, we previously showed an increase in oxidative stress in the kidneys of warfarin-treated rats,64 and warfarin treatment has been associated with oxidative bursts, an increase in circulating IL-6, fibrinogen and haptoglobin, and changes in the activity of the erythrocyte antioxidant enzymes superoxide dismutase and catalase.65 We therefore tested if the acute actions of BDF involved oxidative stress, and found that co-treatment with the antioxidant N-acetylcysteine (NAC) fully prevented the early hemoglobinuria induced by BDF.63 However, NAC did not reduce the late hematuria, did not affect the anticoagulant actions of BDF (assessed by measurement of prothrombin time), and had no significant effect on mortality. These data suggest that the early effects of BDF are mediated via oxidative stress acting on erythrocytes to cause hemolysis and on kidneys, which could induce injury to the glomerular apparatus. In contrast, the delayed BDF effects are presumably related to reductions in vitamin K levels resulting in excessive anticoagulation
Currently, there are no early biomarkers to identify BDF ingestion; patients typically present to a medical facility with severe anticoagulation. Availability of a biomarker would help to rapidly identify poisoned patients, allow early therapeutic intervention, and reduce the number of casualties. We propose that early hemoglobinuria followed by delayed hematuria are novel biomarkers of BDF exposure and toxicity. Specifically, early transient hemoglobinuria indicates BDF ingestion, whereas late hematuria indicates BDF-induced coagulopathy. Monitoring of hematuria/hemoglobinuria is relatively simple, can be easily achieved in the outpatient setting, and is applicable in a mass casualty situation.
Novel molecular actions of superwarfarins
The increased potency of superwarfarins has generally been thought to be related to their increased inhibition of VKORC1, extremely long biological half-lives, and heightened hepatic accumulation. These properties are likely due, at least in part, to the strong hydrophobic nature of superwarfarins, which contributes to both their VKORC1 affinity and their tissue partitioning. In addition, the hydrophobic nature suggests that superwarfarins could also interact with cellular and or subcellular biomembranes, thereby influencing various cellular functions, ranging from metabolic homeostasis to subcellular organelle integrity. Warfarin is thought to undergo passive permeation through membranes, a transport process that governs the anticoagulant’s tissue absorption and accumulation.66,67 Warfarin is also known to induce acute cell damage, independent of its anticlotting actions, including causing disruption of capillary ultrastructure57,58 and increasing kidney damage in patients with chronic kidney disease,59,61 although the latter may be partly a consequence of hemorrhage in kidney glomerular cells. However, the effects warfarin exerts on membrane structure and fluidity have not been examined in detail. The fact that VKORC1 is an integral membrane protein raises the possibility that interactions of superwarfarins with membranes could directly influence VKORC1 activity, providing an additional means by which inhibition is achieved.
We are using two complementary approaches to examine the interactions of superwarfarins, particularly BDF, with membranes: X-ray surface–scattering studies using artificial lipid monolayers and molecular modeling of BDF interactions with lipid interfaces. Model lipid monolayers composed of DPPC (dipalmatidyl phosphatidylcholine, a well-characterized model of biological membranes68) in a Langmuir trough can be studied using surface pressure/molecular area isotherms, and by X-ray reflectivity (XR) and grazing incidence X-ray diffraction (GIXD). Initial studies86 show that superwarfarins, but not warfarin, can intercalate into DPPC monolayers and cause perturbations in the membrane structure. This is likely due to the extremely high hydrophobic character of the superwarfarins, and raises the possibility that membrane disruption contributes to superwarfarin toxicity and tissue damage.
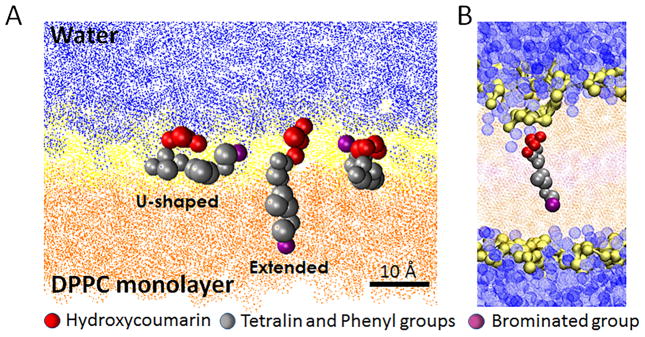
The second approach is to use coarse-grained molecular dynamic simulations of BDF.69 While BDF is a highly lipophilic compound, its molecular structure possesses regions of polarity, such as the 4-hydroxycoumarin moiety that BDF shares with warfarin. The compound is also a weak acid (pKa ≈ 5) and would be expected to adopt a predominately deprotonated, anionic form at physiological pH. Amphiphilic compounds of this nature can interact strongly with the complementary polar/hydrophobic interface of a lipid membrane. Molecular simulations permit a detailed interrogation of the role that such physicochemical interactions play in the association of small molecules with lipid bilayers. Our molecular dynamic simulations of BDF transport from an aqueous phase to a DPPC monolayer at the air–water interface confirmed a preference for BDF to orient with its hydroxycoumarin rings in the hydrated polar head group region (Fig. 2A). The more hydrophobic tetralin and phenyl moieties associate with the hydrophobic tails of the phospholipid layer. Rather than straightforward intercalation, however, multiple possible conformations were observed, with BDF able to adopt either an extended or u-shaped conformation and, in fact, to alternate between these geometries. The associated folding and unfolding action are predicted to produce defects in the interface that can facilitate water permeation (Fig. 2B). BDF folds owing to the presence of a dipole in the bromophenyl ring. This second polar moiety imbues the molecule with a bolaamphiphilic character and creates favorable interactions between the brominated end of the molecule and the polar head group and glycerol backbone of DPPC. We therefore posit that the elevated toxicity of BDF relative to its bromine-free analogue, DiF, may be associated with the membrane-disrupting dynamic conformation of this bolaamphiphilic structure.
Figure 2.
Molecular simulations of BDF interaction with DPPC membranes. (A) The bolaamphiphilic character of brodifacoum allows the compound to adopt both extended (intercalated) and u-shaped conformations at the air–water interface. Depicted are three different BDF molecules coabsorbed at the interface. The region occupied by the polar head group and glycerol backbone of DPPC is rendered in yellow, and the hydrophobic tail region in orange. Water is rendered in blue. (B) The bolamphiphilic nature of BDF is predicted to facilitate water permeation of a DPPC bilayer. Water molecules are depicted as blue spheres. Modified from Ref. 69.
Countermeasures for superwarfarin poisoning: limitations of Vitamin K
Reversing asymptomatic prolongation of INR or prothrombin time caused by warfarin treatment generally requires simply stopping the anticoagulant, and coagulation profiles will return to normal over several days.70 Treatment with oral vitamin K is only needed if prompt reversal is required, for instance in advance of an operation. However, treating a patient with vitamin K depletion logically starts with attention to volume and red cell transfusion plus rapid correction of vitamin K levels by factor replacement with fresh frozen plasma, recombinant activated factor VII (the factor with shortest half-life) or prothrombin complex concentrate (which contains all vitamin K–dependent clotting factors) plus initial intravenous or oral loading of vitamin K1. In the case of warfarin toxicity, follow-up treatment with oral vitamin K is likely required for only a few days. However, the treatment of toxicity caused by superwarfarins is complicated by their potency, long duration of action, and pleiotropic, non-coagulopathic effects. These three factors differentiate superwarfarin toxicity from warfarin overdose such that long-term treatment with oral vitamin K is generally required for months to longer than a year. Moreover, a mass casualty event with large numbers of people poisoned by superwarfarin would require context-specific modifications to optimize the response. A major goal is to develop novel interventions to minimize acute effects and chronic sequelae of severe, protracted vitamin K depletion.
The reported half-life of BDF in humans is as high as 56 days71 but more typically on the order of 20–30 days;72 its pharmacokinetics are variably described as zero73 or first74 order, or initially zero-order and progressing to exponential decay later in the clinical course. Interpatient variability makes the response to treatment unpredictable, accounting for our inability to predict the time course of recovery for any given patient. However, these drugs are highly lipophilic, implying long retention times in fat stores; they are also minimally metabolized and subject to enterohepatic circulation.39 These factors, together with high potency for inhibition of the target enzyme, VCORC1, require that, for all patients, treatment will last for several weeks at a minimum and up to or in excess of a year. Moreover, some patients continue to have biochemical evidence of impaired vitamin K cycling, since the ratio of vitamin K1-2,3-epoxide to vitamin K1, which is typically < 0.1, remained elevated (as high as a ratio of 24) long after initial exposure when BDF blood levels have declined to very low concentrations.75 Treatment of human BDF poisoning with vitamin K1 has included 100 mg daily for 6 months;76 100 mg/day tapering to 10 mg/day over 209 days;71 and 200 mg twice per day for 4 months. Vitamin K1 does not reduce BDF levels; therefore, early tapering or discontinuation can lead to reappearance of symptoms. This is illustrated by a case report77 showing that discontinuing vitamin K after 9 months of successful treatment in an otherwise healthy 36-year-old man led to the recurrence of symptomatic coagulopathy more than a year after the initial BDF exposure, despite contemporaneous, undetectable serum BDF levels.
Absent new methods or strategies in treatment, these factors mean that large-scale exposure to BDF or other superwarfarin exposure is highly problematic given the need, expense, and logistics for both prolonged treatment and continued clinical evaluation. Treatment of BDF toxicity with vitamin K generally requires large doses given orally––current U.S. Food and Drug Administration (FDA)–approved vitamin K tablets contain 5 mg; therefore, dosing could require up to 80 tablets per day––costing thousands of dollars per month. Moreover, follow-up blood draws to monitor coagulation or vitamin K1-2,3-epoxide/vitamin K1 ratios are required on a regular basis for an unspecified albeit prolonged period of time. As described above, BDF also exerts non-anticoagulant effects with both short- and long-term activity, adverse consequences that may involve perturbation of membrane structure, and induction of oxidative stress. Furthermore, vitamin K is required for normal function of many Gla proteins involved in non-coagulation biology, such as normal bone calcification, vascular decalcification, and brain function. Interrupting the BDF toxidrome could prevent chronic pathology from occurring in these organ systems. The key is early intervention.
Countermeasures for superwarfarin poisoning: lipid emulsion
One consideration for rescuing such patients from prolonged superwarfarin toxicity is administering an early infusion of lipid emulsion (ILE), a simple intervention that can be accomplished rapidly, on a large scale and with readily available technology. ILE is a new approach to treating lipophilic drug overdose that was first developed as a treatment for local anesthetic systemic toxicity (LAST).78 It has shown reliable efficacy for this indication in both experimental models and the clinic, where several case reports describe rapid reversal of cardiovascular instability following treatment with lipid emulsion. ILE is now recommended by both the American Society of Regional Anesthesia and Pain Medicine and the American Heart Association for treatment of LAST.79 Recently, ILE has shown promise in treatment of acute cardiovascular toxicity caused by lipophilic drugs of other categories, including calcium channel blockers, tricyclic and atypical antidepressants, antipsychotics, beta blockers, and herbicides.78 The chief mechanism underlying the effects of ILE are thought to be the accelerated redistribution of offending drug away from target organs of toxicity, toward large reservoir organs, such as skeletal muscle, where the drug is harmless, and the liver, where it can be metabolized more quickly.80 The accelerated redistribution results from the lipophilic drug (e.g., local anesthetics with logP values around 3) binding to lipid droplets, which also exert a direct, inotropic effect on the heart.81 The resulting increased cardiac output allows the lipid droplets to more rapidly shuttle bound drug to target organs. It is plausible, in light of this phenomenon, that early treatment with ILE could minimize the effects of BDF or other lipid-soluble superwarfarins by shuttling drug to both skeletal muscle and liver. This could theoretically minimize systemic effects and accelerate its excretion into the gastrointestinal tract.
These observations raised the possibility that ILE could also act as a countermeasure against superwarfarin toxicity, if administered at the appropriate time, frequency, dose, and rate. The potential of a lipid emulsion as a countermeasure may be increased by the composition of commonly used emulsions, which typically are derived from vegetable oils, a natural source of vitamin K1, and can contain up to 30 μg of vitamin K per 100 mL of 10% emulsion.82 In support of this possibility, it was reported that, following induction of mild vitamin K deficiency by dietary restriction and low-dose warfarin, a single intravenous bolus of ILE increased plasma levels of vitamin K as well as serum protein carboxylation within 12–24 h.83 The use of vitamin K–supplemented lipid emulsions could therefore offer further benefit beyond that provided by lipid emulsion alone.
Given that BDF is susceptible to enterohepatic circulation, it is also possible that adding an oral binding resin (e.g., cholestyramine) could capture the drug and increase fecal excretion. This would further shorten its half-life and reduce the need for both prolonged treatment with vitamin K and follow-up monitoring. Evidence for oxidative stress suggests that combining early ILE with oral cholestyramine and an antioxidant such as NAC could provide synergistic benefit in attenuating, shortening, and reducing the costs and clinical consequences of the BDF toxidrome.
Conclusions
Superwarfarins like BDF and DiF represent an emerging threat to human life. The increased use of these agents has raised the risk of accidental bait ingestion or incidental exposure to residual material present in the environment following widespread dispersals.
More urgently, the potential for a devastating mass casualty resulting from terrorist or military actions with these agents demands that we prepare for such an attack. A key factor for mounting an effective response will be the ability to rapidly identify patients as victims of superwarfarin poisoning. Our findings in models of kidney damage provide evidence that the clinical evolution of hemoglobinuria and hematuria shortly after exposure can serve as a simple, rapid, and inexpensive point-of-care biomarker, allowing for prompt diagnosis and treatment by first responders. The recent discovery of additional biomarkers for kidney injury84 may also be useful as early biomarkers for BDF poisoning, although these have not yet been tested. Our ability to detect specific superwarfarins and their distinct isoforms will aid in identifying the source material, as well as the route and time of exposure. It is equally important to recognize that superwarfarins exert severe toxic effects through mechanisms and actions beyond anticoagulation, in both vitamin K–dependent and vitamin K–independent pathways. The damaging effects of these drugs on the kidney and brain are now the subject of intensive study in efforts to understand their mechanisms and develop effective, preventive treatments. In view of the long biological half-lives and diverse pathogenic effects, such treatments could prevent chronic, long-lasting pathological sequelae that will likely occur otherwise. Of critical importance is the idea that these methods must not only replace depleted vitamin K stores to prevent acute morbidity due to hemorrhage, but also sequester and remove superwarfarins from vulnerable tissues and organs to minimize long-term toxicity and morbidity. Initial findings using lipid emulsions are promising, but require optimization the timing, dose, frequency, and duration of administration. It also needs to be determined whether supplementation with antioxidants, vitamin K, or other scavengers and antioxidants provides further benefits. Continued research on the pathogenic mechanisms and effects of superwarfarins is needed to improve our readiness for a catastrophic event surrounding mass exposure to these persistent, pleiotropic poisons.
Acknowledgments
This work was supported by NIH Grant U01NS083457, VA Merit grants (I.R. and G.W.) and a Research Career Scientist award (D.L.F.).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Mueller RL, Scheidt S. History of drugs for thrombotic disease. Discovery, development, and directions for the future. Circulation. 1994;89:432–449. doi: 10.1161/01.cir.89.1.432. [DOI] [PubMed] [Google Scholar]
- 2.Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. British journal of haematology. 2008;141:757–763. doi: 10.1111/j.1365-2141.2008.07119.x. [DOI] [PubMed] [Google Scholar]
- 3.Stahman MA, Huebner CF, Link KP. Studies on the hemorrhagic sweet clover disease: V. Identification and synthesis of the hemorrhagic agent. Journal Biological Chemistry. 1941;138:513–527. [Google Scholar]
- 4.Whitlon DS, Sadowski JA, Suttie JW. Mechanism of coumarin action: significance of vitamin K epoxide reductase inhibition. Biochemistry. 1978;17:1371–1377. doi: 10.1021/bi00601a003. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg J, et al. Vitamin K epoxide reductase complex subunit 1 (VKORC1): the key protein of the vitamin K cycle. Antioxid Redox Signal. 2006;8:347–353. doi: 10.1089/ars.2006.8.347. [DOI] [PubMed] [Google Scholar]
- 6.Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3:1873–1878. doi: 10.1111/j.1538-7836.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayes WJJ. Control of Norway rats with residual rodenticide warfarin. Public Health Rep. 1950;65:1537–1555. [PMC free article] [PubMed] [Google Scholar]
- 8.D’Ambrosio G. Warfarin as rodenticide. Riv Ital Ig. 1952;12:367–386. [PubMed] [Google Scholar]
- 9.Oldenburg J, et al. Comparative genetics of warfarin resistance. Hamostaseologie. 2014;34:143–159. doi: 10.5482/HAMO-13-09-0047. [DOI] [PubMed] [Google Scholar]
- 10.Rost S, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 11.Hadler MR, Shadbolt RS. Novel 4-hydroxycoumarin anticoagulants active against resistant rats. Nature. 1975;253:275–277. doi: 10.1038/253275a0. [DOI] [PubMed] [Google Scholar]
- 12.Rennison BD, Hadler MR. Field trials of difenacoum against warfarin-resistant infestations of Rattus norvegicus. J Hyg (Lond) 1975;74:449–455. doi: 10.1017/s0022172400046969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfern R, Gill JE, Hadler MR. Laboratory evaluation of WBA 8119 as a rodenticide for use against warfarin-resistant and non-resistant rats and mice. J Hyg (Lond) 1976;77:419–426. doi: 10.1017/s0022172400055807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Reilly RA, Aggeler PM, Leong LS. Studies on the coumarin anticoagulant drugs: The pharmacodynamics of warfarin in man. The Journal of clinical investigation. 1963;42:1542–1551. doi: 10.1172/JCI104839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebauer M. Synthesis and structure-activity relationships of novel warfarin derivatives. Bioorg Med Chem. 2007;15:2414–2420. doi: 10.1016/j.bmc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Lipton RA, Klass EM. Human ingestion of a ‘superwarfarin’ rodenticide resulting in a prolonged anticoagulant effect. JAMA. 1984;252:3004–3005. [PubMed] [Google Scholar]
- 17.Watts RG, Castleberry RP, Sadowski JA. Accidental poisoning with a superwarfarin compound (brodifacoum) in a child. Pediatrics. 1990;86:883–887. [PubMed] [Google Scholar]
- 18.Greeff MC, Mashile O, MacDougall LG. “Superwarfarin” (bromodialone) poisoning in two children resulting in prolonged anticoagulation. Lancet. 1987;2:1269. doi: 10.1016/s0140-6736(87)91876-9. [DOI] [PubMed] [Google Scholar]
- 19.King N, Tran MH. Long-Acting Anticoagulant Rodenticide (Superwarfarin) Poisoning: A Review of Its Historical Development, Epidemiology, and Clinical Management. Transfusion medicine reviews. 2015;29:250–258. doi: 10.1016/j.tmrv.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury S. Risk Mitigation Decision for Ten Rodenticides. US Environmental Protection Agency; 2008. www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2006-0955-0764. [Google Scholar]
- 21.Environmental_Protection_Agency. Canceling Some d-CON Mouse and Rat Control Products. 2014 www.epa.gov/rodenticides/canceling-some-d-con-mouse-and-rat-control-products.
- 22.Environmental_Protection_Agency. Twelve Defendants Arrested for Involvement in the Illegal Distribution and Sale of Pesticides in New York’s Chinatown. 2011 yosemite.epa.gov/opa/admpress.nsf/d0cf6618525a9efb85257359003fb69d/79cae83797bd87a3852579100073d90e!opendocument.
- 23.Masuda BM, Fisher P, Beaven B. Residue profiles of brodifacoum in coastal marine species following an island rodent eradication. Ecotoxicol Environ Saf. 2015;113:1–8. doi: 10.1016/j.ecoenv.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Primus T, Wright G, Fisher P. Accidental discharge of brodifacoum baits in a tidal marine environment: a case study. Bulletin of environmental contamination and toxicology. 2005;74:913–919. doi: 10.1007/s00128-005-0668-1. [DOI] [PubMed] [Google Scholar]
- 25.Fisher P, et al. Accidental discharge of brodifacoum baits into a freshwater lake: a case study. Bull Environ Contam Toxicol. 2012;88:226–228. doi: 10.1007/s00128-011-0470-1. [DOI] [PubMed] [Google Scholar]
- 26.Spahr JE, Maul JS, Rodgers GM. Superwarfarin poisoning: a report of two cases and review of the literature. Am J Hematol. 2007;82:656–660. doi: 10.1002/ajh.20784. [DOI] [PubMed] [Google Scholar]
- 27.Patocka J, Kuca K. Toxic potential of superwarfarin: Brodifacoum. Mil Med Sci Lett. 2013;82:9. [Google Scholar]
- 28.Federal_Bureau_Investigation. Arrest of Suspect in Connection with Viable Improvised Explosive Device Found in Spokane. Washington: 2011. [Accessed April 8, 2016]. Joint Intelligence Bulletin March 9, 2011 info.publicintelligence.net/FBI-SpokaneIED.pdf. [Google Scholar]
- 29.Lupkin S. [Accessed April 8, 2016];22 Rikers Island Prisoners Sickened By Rat Poison in Meatloaf, Lawyer Says. 2015 abcnews.go.com/Health/22-rikers-island-prisoners-sickened-rat-poison-meatloaf/story?id=30676137.
- 30.Brickhill J. Zimbabwe’s poisoned legacy: Secret war in Southern Africa. Covert Action. 1992;43:58–61. [Google Scholar]
- 31.Epstein PR, Ferber D, Sachs J. Changing Planet, Changing Health: How the Climate Crisis Threatens Our Health and What We Can Do about It. University of California Press; 2011. [Google Scholar]
- 32.White L. Poisoned food, poisoned uniforms, and anthrax: or, how guerillas die in war. Osiris. 2004;19:220–233. doi: 10.1086/649403. [DOI] [PubMed] [Google Scholar]
- 33.United_Nations. Project Coast: Apartheid’s Chemical and Biological Warfare Programme. United Nations Institute for Disarmament Research; 2003. [Google Scholar]
- 34.Gould C, Burger M. Secrets and Lies: Wouter Basson and South Africa’s Chemical and Biological Warfare Programme. Struik; 2002. [Google Scholar]
- 35.Jones DR, Miller GP. Assays and applications in warfarin metabolism: what we know, how we know it and what we need to know. Expert opinion on drug metabolism & toxicology. 2011;7:857–874. doi: 10.1517/17425255.2011.576247. [DOI] [PubMed] [Google Scholar]
- 36.Rengel I, Friedrich A. Detection of anticoagulant rodenticides (4-hydroxycoumarins) by thin-layer chromatography and reversed-phase high-performance liquid chromatography with fluorescence detection. Veterinary research communications. 1993;17:421–427. doi: 10.1007/BF01839210. [DOI] [PubMed] [Google Scholar]
- 37.Mundy DE, Machin AF. Determination of the rodenticide difenacoum in biological materials by high-pressure liquid chromatography with confirmation of identity by mass spectrometry. Journal of chromatography. 1977;139:321–329. doi: 10.1016/s0021-9673(00)89327-9. [DOI] [PubMed] [Google Scholar]
- 38.Benchev I, Vasileva R. Derivative spectroscopy and its use in sanitary hygiene control. Problemi na khigienata. 1990;15:102–111. [PubMed] [Google Scholar]
- 39.Bachmann KA, Sullivan TJ. Dispositional and pharmacodynamic characteristics of brodifacoum in warfarin-sensitive rats. Pharmacology. 1983;27:281–288. doi: 10.1159/000137881. [DOI] [PubMed] [Google Scholar]
- 40.O’Bryan SM, Constable DJ. Quantification of brodifacoum in plasma and liver tissue by HPLC. Journal of analytical toxicology. 1991;15:144–147. doi: 10.1093/jat/15.3.144. [DOI] [PubMed] [Google Scholar]
- 41.Yan H, et al. Determination of bromadiolone and brodifacoum in human blood using LC-ESI/MS/MS and its application in four superwarfarin poisoning cases. Forensic science international. 2012;222:313–317. doi: 10.1016/j.forsciint.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Vudathala D, Cummings M, Murphy L. Analysis of multiple anticoagulant rodenticides in animal blood and liver tissue using principles of QuEChERS method. Journal of analytical toxicology. 2010;34:273–279. doi: 10.1093/jat/34.5.273. [DOI] [PubMed] [Google Scholar]
- 43.Marsalek P, et al. Simultaneous determination of ten anticoagulant rodenticides in tissues by column-switching UHPLC-ESI-MS/MS. Analytical and bioanalytical chemistry. 2015;407:7849–7854. doi: 10.1007/s00216-015-8954-1. [DOI] [PubMed] [Google Scholar]
- 44.Cort JR, Alperin PJ, Cho H. Measurement and analysis of diastereomer ratios for forensic characterization of brodifacoum. Forensic science international. 2012;214:178–181. doi: 10.1016/j.forsciint.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Hauck ZZ, Feinstein DL, van Breeman RB. LC-MS/MS analysis of brodifacoum isomers in rat tissue. J Anal Tox. 2016 doi: 10.1093/jat/bkw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, et al. An overview of osteocalcin progress. Journal of bone and mineral metabolism. 2016 doi: 10.1007/s00774-015-0734-7. [DOI] [PubMed] [Google Scholar]
- 47.Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends in molecular medicine. 2013;19:217–226. doi: 10.1016/j.molmed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Ma X, Chen J, Tian Y. Pregnane X receptor as the “sensor and effector” in regulating epigenome. Journal of cellular physiology. 2015;230:752–757. doi: 10.1002/jcp.24838. [DOI] [PubMed] [Google Scholar]
- 49.Shibayama-Imazu T, Aiuchi T, Nakaya K. Vitamin K2-mediated apoptosis in cancer cells: role of mitochondrial transmembrane potential. Vitamins and hormones. 2008;78:211–226. doi: 10.1016/S0083-6729(07)00010-6. [DOI] [PubMed] [Google Scholar]
- 50.Ferland G. Vitamin K and the nervous system: an overview of its actions. Adv Nutr. 2012;3:204–212. doi: 10.3945/an.111.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckhardt M. The role and metabolism of sulfatide in the nervous system. Molecular neurobiology. 2008;37:93–103. doi: 10.1007/s12035-008-8022-3. [DOI] [PubMed] [Google Scholar]
- 52.Sundaram KS, Lev M. Warfarin administration reduces synthesis of sulfatides and other sphingolipids in mouse brain. J Lipid Res. 1988;29:1475–1479. [PubMed] [Google Scholar]
- 53.Crivello NA, et al. Age- and brain region-specific effects of dietary vitamin K on myelin sulfatides. J Nutr Biochem. 2010;21:1083–1088. doi: 10.1016/j.jnutbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp Neurol. 2000;425:295–314. [PubMed] [Google Scholar]
- 55.Binder MD, et al. Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. J Neurosci. 2008;28:5195–5206. doi: 10.1523/JNEUROSCI.1180-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng T, Chen Q, Han D. The roles of TAM receptor tyrosine kinases in the mammalian testis and immunoprivileged sites. Frontiers in bioscience (Landmark edition) 2016;21:316–327. doi: 10.2741/4390. [DOI] [PubMed] [Google Scholar]
- 57.Kahn RA, Johnson SA, DeGraff AF. Effects of sodium warfarin on capillary ultrastructure. The American journal of pathology. 1971;65:149–156. [PMC free article] [PubMed] [Google Scholar]
- 58.Leithauser B, et al. Capillary bleeding under oral anticoagulation. Clinical hemorheology and microcirculation. 2009;43:167–171. doi: 10.3233/CH-2009-1231. [DOI] [PubMed] [Google Scholar]
- 59.Brodsky SV, et al. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009;54:1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 60.Brodsky SV, et al. Warfarin therapy that results in an International Normalization Ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clinical practice. 2010;115:c142–146. doi: 10.1159/000312877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ware K, et al. Warfarin-related nephropathy modeled by nephron reduction and excessive anticoagulation. J Am Soc Nephrol. 2011;22:1856–1862. doi: 10.1681/ASN.2010101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozcan A, et al. 5/6 nephrectomy as a validated rat model mimicking human warfarin-related nephropathy. American journal of nephrology. 2012;35:356–364. doi: 10.1159/000337918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ware KM, et al. Brodifacoum induces early hemoglobinuria and late hematuria in rats: novel rapid biomarkers of poisoning. American journal of nephrology. 2015;41:392–399. doi: 10.1159/000433568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ware K, et al. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. American journal of physiology Renal physiology. 2013;304:F1421–1427. doi: 10.1152/ajprenal.00689.2012. [DOI] [PubMed] [Google Scholar]
- 65.Belij S, et al. Effects of subacute oral warfarin administration on peripheral blood granulocytes in rats. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2012;50:1499–1507. doi: 10.1016/j.fct.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 66.Karlsson BC, et al. How warfarin’s structural diversity influences its phospholipid bilayer membrane permeation. J Phys Chem B. 2013;117:2384–2395. doi: 10.1021/jp400264x. [DOI] [PubMed] [Google Scholar]
- 67.Velicky M, et al. In situ artificial membrane permeation assay under hydrodynamic control: permeability-pH profiles of warfarin and verapamil. Pharmaceutical research. 2010;27:1644–1658. doi: 10.1007/s11095-010-0150-6. [DOI] [PubMed] [Google Scholar]
- 68.MacDonald RC, Simon SA. Lipid monolayer states and their relationships to bilayers. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4089–4093. doi: 10.1073/pnas.84.12.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ayee MA, Roth CW, Akpa BS. Structural perturbation of a dipalmitoylphosphatidylcholine (DPPC) bilayer by warfarin and its bolaamphiphilic analogue: A molecular dynamics study. J Colloid Interface Sci. 2016;468:227–237. doi: 10.1016/j.jcis.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baglin T. Management of warfarin (coumarin) overdose. Blood reviews. 1998;12:91–98. doi: 10.1016/s0268-960x(98)90020-0. [DOI] [PubMed] [Google Scholar]
- 71.Olmos V, Lopez CM. Brodifacoum poisoning with toxicokinetic data. Clin Toxicol (Phila) 2007;45:487–489. doi: 10.1080/15563650701354093. [DOI] [PubMed] [Google Scholar]
- 72.Pavlu J, et al. Superwarfarin poisoning. Lancet. 2005;365:628. doi: 10.1016/S0140-6736(05)17916-1. [DOI] [PubMed] [Google Scholar]
- 73.Bruno GR, et al. Long-acting anticoagulant overdose: brodifacoum kinetics and optimal vitamin K dosing. Ann Emerg Med. 2000;36:262–267. doi: 10.1067/mem.2000.108317. [DOI] [PubMed] [Google Scholar]
- 74.Hollinger BR, Pastoor TP. Case management and plasma half-life in a case of brodifacoum poisoning. Archives of internal medicine. 1993;153:1925–1928. [PubMed] [Google Scholar]
- 75.Weitzel JN, et al. Surreptitious ingestion of a long-acting vitamin K antagonist/rodenticide, brodifacoum: clinical and metabolic studies of three cases. Blood. 1990;76:2555–2559. [PubMed] [Google Scholar]
- 76.Gunja N, Coggins A, Bidny S. Management of intentional superwarfarin poisoning with long-term vitamin K and brodifacoum levels. Clin Toxicol (Phila) 2011;49:385–390. doi: 10.3109/15563650.2011.587126. [DOI] [PubMed] [Google Scholar]
- 77.Underwood EL, et al. Prolonged coagulopathy after brodifacoum exposure. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2014;71:639–642. doi: 10.2146/ajhp130537. [DOI] [PubMed] [Google Scholar]
- 78.Fettiplace MR, Weinberg G. Past, Present, and Future of Lipid Resuscitation Therapy. JPEN Journal of parenteral and enteral nutrition. 2015;39:72s–83s. doi: 10.1177/0148607115595979. [DOI] [PubMed] [Google Scholar]
- 79.Neal JM, et al. ASRA practice advisory on local anesthetic systemic toxicity. Regional anesthesia and pain medicine. 2010;35:152–161. doi: 10.1097/AAP.0b013e3181d22fcd. [DOI] [PubMed] [Google Scholar]
- 80.Fettiplace MR, et al. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015;198:62–70. doi: 10.1016/j.jconrel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fettiplace MR, et al. Rapid cardiotonic effects of lipid emulsion infusion*. Crit Care Med. 2013;41:e156–162. doi: 10.1097/CCM.0b013e318287f874. [DOI] [PubMed] [Google Scholar]
- 82.Lennon C, et al. The vitamin K content of intravenous lipid emulsions. JPEN Journal of parenteral and enteral nutrition. 1993;17:142–144. doi: 10.1177/0148607193017002142. [DOI] [PubMed] [Google Scholar]
- 83.Camilo ME, et al. Bioavailability of phylloquinone from an intravenous lipid emulsion. The American journal of clinical nutrition. 1998;67:716–721. doi: 10.1093/ajcn/67.4.716. [DOI] [PubMed] [Google Scholar]
- 84.Medic B, et al. Evaluation of novel biomarkers of acute kidney injury: the possibilities and limitations. Current medicinal chemistry. 2016 doi: 10.2174/0929867323666160210130256. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 85.United States Environmental Protection Agency. Warfarin and Its Sodium Salt: Pesticide Registration Standard. USEPA, Office of Pesticides and Toxic Substances; Washington, DC: 1981. p. 193. [Google Scholar]
- 86.Marangoni MN, et al. Membrane cholesterol modulates superwarfarin toxicity. Biophysical Journal. 2016 doi: 10.1016/j.bpj.2016.03.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]