Abstract
Background and aims
The neuropsychological correlates of simultaneous marijuana and tobacco use are largely unknown, which is surprising as both substances have similar neural substrates and have opposing influences on working memory (WM). This study examined the effects of marijuana alone, tobacco alone, and simultaneous marijuana and tobacco use on WM.
Design
Primary aims were tested using a within-subject design, controlling for multiple subject- and momentary-level confounds via ecological momentary assessment (EMA).
Setting
Data collection occurred in the Chicago, USA area in participants' natural environments.
Participants
Participants were 287 community young adults from a larger natural history study, over-sampled for ever smoking, all of whom event-recorded at least one substance use occasion during the study week.
Measurements
Momentary tobacco, marijuana and alcohol use were recorded during multiple EMA across one week of data capture. WM was assessed at the end of each EMA. Contextual variables that may influence WM were recorded via EMA.
Findings
There were main effects for marijuana and tobacco: WM was poorer with marijuana (OR=0.91, 95%CI = 0.84 to 0.99) and better with tobacco (OR=1.11, 95%CI = 1.04 to 1.18). These effects were not qualified by an interaction (OR=1.03, 95%CI = 0.84 to 1.26). Alcohol also reduced WM (OR=0.87, 95%CI = 0.79 to 0.95), and the tobacco by alcohol interaction was significant (OR=0.81, 95%CI = 0.66 to 0.99), indicating that the facilitative effect of tobacco disappeared with concurrent alcohol use.
Conclusions
Relative to when individuals did not use these substances, working memory (WM) decreased with acute marijuana and alcohol use, and increased with acute tobacco use. However, the putative effect of marijuana on WM and the facilitative effect of tobacco on WM were no longer present when used simultaneously with tobacco and alcohol, respectively. Data suggest that tobacco use may compensate for WM decrements from marijuana among young adults and highlight the importance of further investigating the negative impact of alcohol use on cognition.
Marijuana and tobacco are two of the most widely used drugs in the United States (1), and simultaneous marijuana and tobacco use (i.e., use of both substances at the same time or in close temporal sequence) is popular among young adults (2, 3). Simultaneous marijuana and tobacco use typically takes one of two forms: blunt smoking (smoking cigars with tobacco removed and replaced with marijuana and residual tobacco) or “chasing” (smoking cigarettes/cigarillos immediately after marijuana). Simultaneous users experience more deleterious substance use outcomes than non- and co-users (i.e., individuals who use both but not simultaneously), including greater use severity and cessation difficulties. For example, simultaneous users consume marijuana more frequently and have up to 5.1 times greater odds of being marijuana dependent than co-users (4-7). Qualitative reports also suggest that marijuana reinforces cigarette smoking and interferes with quitting (2), with marijuana users less likely to quit tobacco than non-marijuana users (8).
Interactions between cannabinoid and cholinergic systems on neurochemical and behavioral functioning may partially explain rising rates of simultaneous use. Subchronic nicotine exposure results in region-dependent increases in cannabinoid receptor (CB1) hippocampal expression that persists for one month following nicotine cessation (9). Similarly, animals chronically exposed to nicotine have increased endocannabinoid levels in the limbic forebrain and brainstem (10), and cannabinoid agonists produce greater release and lower turnover of acetylcholine in the hippocampus, cortex and striatum (11-14). Behaviorally, co-administration of marijuana and nicotine in vivo results in acute changes in locomotion, heart rate and body temperature (15), with marijuana's depressant effects potentiated even by sub-clinical doses of nicotine (16). Similarly, administration of Δ9-tetrahydrocannabinol, marijuana's primary psychoactive constituent, may mitigate nicotine withdrawal (17), and nicotine's rewarding effects are diminished among CB1 knockout mice (18). Further, administration of CB1 antagonists reduces dopamine release in the nucleus accumbens, nicotine self-administration, and cue-induced nicotine reinstatement (19-21), and has therefore been suggested as a potentially efficacious pharmacological treatment for nicotine addiction (19, 22).
Despite pharmacological interactions between cannabinoid and cholinergic systems as well as greater risk for adverse substance use outcomes among simultaneous users, it remains unknown how simultaneous use impacts executive function capacities such as working memory (WM). Studies have only examined co-use of marijuana and tobacco on neurocognition, finding better verbal memory among co-users than among users of marijuana alone (23) and abnormalities in hippocampal and memory correlations (24). Jacobsen and colleagues (25) have come close in addressing brain-behavior relationships with simultaneous use. They found marijuana users who did not smoke a cigarette compared to ad libitum cigarette smoking had worse delayed recall and WM, and aberrant functional patterns including greater activation in posterior cortical regions and disrupted functional integration of fronto-parietal connectivity (25), which is relevant to efficient verbal WM (26-30). However, this study could not disentangle the mitigating effect of tobacco on neurocognition among marijuana users from the adverse effect of nicotine withdrawal on neurocognition. Therefore, although it is suspected that marijuana disrupts WM and these deficits may be masked in the context of concomitant tobacco use due to independent effects on similar neural substrates that underlie WM, this hypothesis has not been directly tested. Compensatory effects might explain why simultaneous use is reinforcing, elucidate potential barriers to quitting and inform public health efforts aimed at educating young adults on potential risks of marijuana and tobacco co-use.
Associations with alcohol are also important to consider in the context of simultaneous marijuana and tobacco use. Convergent evidence points to probable pharmacokinetic and pharmacodynamic interactions between alcohol and concurrent marijuana or tobacco. This is likely due to overlap in the neurotransmitter systems targeted, including the mesolimbic dopamine pathway for alcohol and marijuana (31-33) and the cholinergic system for alcohol and tobacco (34-36). Studies of co-users of alcohol and marijuana showed worse WM than single substance users (37), though synergistic effects were not found when both substances were acutely administered at low doses (38). With regard to alcohol and tobacco, animal studies have found that pretreatment with nicotine attenuated alcohol's effects on WM (39), and co-administration of sub-clinical doses of alcohol and nicotine resulted in WM impairment (40). Similar effects have been found in humans (41, 42), though synergistic interactions have not been equivocally documented (43, 44). These studies together suggest that alcohol interacts with marijuana and tobacco, but the nature of these effects is incompletely understood. Further, no studies to our knowledge have addressed the neurocognitive consequences of alcohol use when combined with both marijuana and tobacco, which is surprising as alcohol commonly co-occurs with and has overlapping risk and protective factors as marijuana and tobacco use (45).
In sum, tobacco consumption among marijuana users may improve aspects of cognition, and this compensatory effect may be especially relevant among young adult marijuana users who are at greater risk to experience cognitive decrements due to ongoing neurodevelopment in regions likely impacted by marijuana and tobacco use. However, no studies have directly tested the impact of simultaneous marijuana and tobacco use on neurocognition. This study aimed to isolate the conjoint effects of marijuana and tobacco on WM from 1) the effects of marijuana alone and 2) the effects of tobacco alone using a within-subject design and an ecologically valid, real-time data capture methodology. Given prior research on the independent and opposing effects of marijuana and tobacco on WM, we hypothesized that, compared to randomly sampled times with no substance use, WM would be enhanced with tobacco, impaired with marijuana, and not significantly different during times of simultaneous tobacco and marijuana use. We also examined whether any of our hypothesized effects varied with concomitant alcohol use. Consideration of the potential separate and interactive effects of marijuana, tobacco and alcohol on WM may have significant implications for pharmacological and behavioral treatment interventions.
Methods
Participants
This project was part of a large natural history study of the social-emotional contexts of tobacco smoking (PI: Mermelstein), which followed high-risk adolescents into young adulthood. The parent project recruited adolescents from 16 Chicago-area high schools, over-sampling for students who had ever smoked a cigarette (83% smoked at baseline), and were thus at risk for smoking escalation. Initial recruitment procedures and participant characteristics are detailed in other publications that utilized the parent project cohort (46-49).
Data for the current study came from parent project participants who completed ecological momentary assessments (EMA) during the five-year follow-up, after individuals had graduated from high school, and who recorded at least one episode of marijuana, tobacco, or alcohol use during the EMA assessment week described below (N=287; 94% of parent EMA project).
Overall Design
The EMA protocol involved a seven-day monitoring period using handheld computers programmed with data collection assessments. This interval length ensured both weekday and weekend sampling and provided an adequate sample of events. Participants were individually trained on using the devices and how to complete two types of assessments: 1) random prompts, which were device-initiated (randomly “beeping” the participant, on average 5-7 times/day throughout participants' waking hours); and 2) tobacco smoke events, which were subject-initiated immediately after using tobacco. Each assessment type took approximately 230 seconds to complete, was completed each day and multiple times throughout the day, had similar questions, and concluded with a WM task (detailed below). Entries were password protected, and time and date stamped. Compliance was assessed directly with the random prompts since each prompt was date and time stamped and the device recorded prompts that were not responded to within three minutes. On average, participants had excellent compliance with an average of 92.6% of random prompts completed, consistent with the adequate compliance criterion set forth by Stone and Shiffman (50). Participants received incentive bonuses for completing more than 85% of all random prompts, which helped to maintain high compliance. At the end of the study week, participants were debriefed with structured interviews and were compensated for participation. All procedures were approved by the University of Illinois at Chicago Institutional Review Board.
EMA Measures
EMA contextual covariates
During each EMA assessment, objective and subjective context was queried with a variety of questions using response options in a non-exclusive checklist format. Contextual factors thought to confound WM were included as critical covariates: 1) Proximity to others (0 = with others, 1 = alone); 2) Weekend vs. weekday responding (0 = weekday, 1 = weekend); 3) Watching TV and/or listening to music (0 = no, 1 = yes); 4) At a party (0 = no, 1 = yes); 5) Time of day (1 = 4am-8:59am, 2 = 9am-1:59pm, 3 = 2pm-5:59pm, 4 = 6pm-9:59pm, 5 = 10pm-3:59am); 5) Study day (range: 0-7); 6) Trouble Concentrating (Likert scale item with response options 1-Not at all through 10-Very much); and 7) Negative Affect (average of responses on current feelings of anger, frustration, irritability, sadness and stress, each with continuous response options of 1-Not at all through 10-Very much).
EMA-assessed substance use
In-the-moment use of tobacco was assessed when participants event-recorded a smoking event. Missed episodes of recorded tobacco use were queried during debriefing interviews at the end of the study week. Overall, the agreement between these interviews and the EMA data capture was better than 80%. There were no participants who indicated using tobacco but who did not complete an EMA tobacco use assessment. During both random prompts and smoke events, participants indicated whether they had used marijuana and/or alcohol in the last hour. This design allowed for the capture of occasions of no, single and simultaneous substance use, resulting in eight different drug use categories listed in Table 2. A measure of overall level of tobacco, marijuana and alcohol use was separately derived for each participant based on the proportion of total events across the study week during which use of that substance was reported (0 – 100% of prompts).
Table 2. Percent of Participants by Number of Correctly Recalled Dots in EMA Working Memory Task and EMA Substance Use Type.
| Number of Dots Correctly Recalled | |||||||
|---|---|---|---|---|---|---|---|
| n | 0 | 1 | 2 | 3 | 4 | 5 | |
| Random Prompt | |||||||
| No Substance Use | 9003 | 2% | 5% | 8% | 10% | 17% | 58% |
| Marijuana Only | 851 | 2% | 6% | 10% | 13% | 17% | 52% |
| Alcohol Only | 617 | 1% | 3% | 7% | 10% | 18% | 61% |
| Marijuana and Alcohol | 198 | 3% | 7% | 10% | 11% | 19% | 50% |
| Smoke Prompt | |||||||
| Tobacco Only | 1998 | 2% | 4% | 8% | 10% | 17% | 59% |
| Marijuana and Tobacco | 329 | 3% | 6% | 7% | 12% | 17% | 55% |
| Alcohol and Tobacco | 192 | 2% | 4% | 11% | 12% | 19% | 52% |
| Marijuana, Alcohol and Tobacco | 78 | 3% | 5% | 4% | 10% | 22% | 56% |
EMA WM
Participants completed a brief (∼40 seconds) EMA WM task that was administered at the end of each assessment and that was specifically designed to maximize compatibility with EMA (e.g., was brief to minimize disruptions to the participants' lives and to further promote compliance). Visual WM was assessed for a number of reasons including its well-defined neural correlates (51), established associations with substance use (52-54), and excellent psychometric properties, including high test-retest reliability (55, 56). This task was based on visuospatial simple span tasks (57, 58), with an added spatial processing component involving continuously maintaining and updating dot configurations in memory. Participants were presented with between two and four 4×4 grids in sequence, with each grid displayed for one second and containing a random display of five dots. The number of grids presented and the dot configurations within each grid were random. The software was programmed to minimize the likelihood of duplicate displays during the study week. After the final grid presentation, participants attempted to re-create the pattern of dots from the last grid presented on a blank grid using their stylus. This procedure was repeated one additional time for a total of two trials per EMA assessment, each trial with one test grid.
The primary dependent variable was the number of correctly recalled dots in a trial (range 0-5). To account for possible systematic influences that may facilitate associative learning, four parameters of task complexity were created for each pattern to-be-remembered, including the number of interference grids presented, dispersion of the dots on the target grid from a regression line, the number of corner dots in the target grid, and the cumulative distance between dots on the target grid. A full description of the task's development, methods, complexity variables, and psychometric properties can be found in Schuster and colleagues (59). Psychometric evaluation of this task, in brief, indicated that WM performance correlated with laboratory WM measures, particularly visual WM, but not with other laboratory-assessed cognitive capacities.
Analytic Plan
To test for within-person WM differences based on substance use relative to when participants do not use the substance(s) in question, a mixed-effects regression model for repeated ordinal outcomes was fit to the data. This model included random subject effects to account for the correlation between repeated measurements (60). Specifically, a random subject intercept and smoking event effect were included to allow the correlation to vary between the random prompts and the smoking events. The GLIMMIX procedure in SAS 9.2 was used to estimate model parameters by maximum likelihood, and both between- and within-subject factors were entered together to predict WM. Subject-level static covariates including gender and GPA were entered as model covariates. Additionally, the model adjusted for momentary-level covariates that were theoretically linked to WM, including measures of date/time, task complexity, location, social context, affect, and overall level of substance use (tobacco, alcohol, cannabis). Overall substance use levels were created from the momentary-varying reports of substance use based on the decomposition of the between- and within-subjects effects of time-varying covariates as described in Hedeker and Gibbons (61). Specifically, the proportion of total responses in which each of these substances were used represented between-subjects effects, while the deviation of the momentary substance use indicator, relative to a subject's mean, was the within-subjects substance use effect. Primary predictors of interest included the within-subjects main effects for marijuana, tobacco, and alcohol use as well as all two-way and three-way interactions. The model was fit for a six-level ordinal response indicating the number of correctly identified dots in each trial of the WM task. This class of models is useful for analysis of EMA data by allowing multiple observations per subject, multiple levels of nesting (i.e., observations within subjects), multiple subject random effects (e.g., intercept and smoking event indicator), modeling of between-subjects and within-subjects covariates, and extending to non-normal outcomes (e.g., dichotomous, ordinal, counts), which was the case for WM in this study. This approach also allowed for the examination of time trends and practice effects (62, 63).
Results
Descriptive Analyses of Study Sample and EMA Reporting
Sample demographics and substance use are presented in Table 1. This study included 10,669 random prompts and 2,597 smoke assessments, the latter of which came from 86% of the sample (n= 247). The remaining 14% of the sample provided at least one random prompt that indicated current use of alcohol, marijuana, or both concurrently. This resulted in 26,532 ordinal analyzable outcomes (i.e., dots correctly placed in each trial). Participants provided a mean of 37.1 (SD= 7.8) random EMA prompts, during which participants reported an average of 3.7 (SD= 5.9) marijuana use occasions and 2.84 (SD= 3.5) alcohol use occasions (of random prompts: 84% no substance use, 8% marijuana only, 6% alcohol only, 2% concurrent marijuana and alcohol). Participants completed an average of 10.5 (SD= 10.9) smoke assessments, with a mean of 1.7 (SD= 3.5) co-occurring marijuana reports and 1.1 (SD= 1.6) co-occurring alcohol reports (of smoke prompts: 77% tobacco only, 13% concurrent marijuana and tobacco, 7% concurrent alcohol and tobacco, 3% concurrent marijuana, tobacco and alcohol). In the sample of 287 participants, 49% provided at least one marijuana occasion and 66% provided at least one alcohol occasion. Co-occurring marijuana and alcohol use was reported by 35% of the sample.
Table 1. Descriptives of Study Sample.
| N =287 | |
|---|---|
| Demographics | |
| Age | 21.3 (.8) |
| % Female | 54% |
| Ethnicity/Race | |
| Caucasian | 65% |
| Black | 11% |
| Hispanic | 16% |
| Other | 7% |
| Education | |
| Some High School | 4% |
| High School Diploma or GED | 22% |
| Vocational/Technical School | 2% |
| Some College or Beyond | 72% |
| Mental Health | |
| BIS-11 Total Score (M, SD) | 34.7 (7.5) |
| CESD Total Score [Md, IQR] | 12 [7, 21] |
| MASQ Total Score [Md, IQR] | 25 [20, 30] |
| ASRS, % of Scores ≥4 | 20% |
| Tobacco Use | |
| Percent Lifetime Cigarettes (100 or more) | 88% |
| Ever Daily Tobacco Use, % Yes | 81% |
| Current Daily Tobacco Use, % Yes | 41% |
| Number of Days Smoked Cigarettes in the Past Month [Md, IQR] | 25 [7, 30] |
| Number of Cigarettes on Days Smoked [Md, IQR] | 4 [2, 10] |
| mFTQ Total Score [Md, IQR] | 2 [2,4] |
| Marijuana Use | |
| Ever Use, % Yes | 91% |
| Frequency of Use in Past 90 Days | |
| 0 Times | 29% |
| Once a Month or Less | 16% |
| More than Once a Month but Less than Once a Week | 10% |
| One or More Times a Week but Not Every Day | 22% |
| Every Day | 23% |
| CUDIT-R, Total Score [Md, IQR] | 7 [2, 13.71] |
| Alcohol Use | |
| Ever Use, % Yes | 98% |
| Frequency of Use in Past 90 Days | |
| 0 Times | 4% |
| Once a Month or Less | 8% |
| More than Once a Month but Less than Once a Week | 25% |
| One or More Times a Week but Not Every Day | 59% |
| Every Day | 4% |
| Other Substance Use | |
| Ever Use, % Yes | |
| Cocaine | 35% |
| Amphetamines | 24% |
| Hallucinogens | 47% |
| Inhalants | 46% |
| IV Drugs | 2% |
Note. M, Mean; SD, Standard Deviation; Md, Median; IQR, Interquartile range; BIS-11, Barratt Impulsiveness Scale-11th version; CESD, Center for Epidemiological Studies-Depression; MASQ, Mood and Affect Symptom Questionnaire; ASRS, Adult ADHD Self-Report Scale; NDSS, Nicotine Dependence Syndrome Scale; mFTQ, Modified Fagerstrom Tolerance Questionnaire; CUDIT-R, Marijuana Use Disorders Identification Test-Revised
Predicting Momentary Fluctuations in WM Capacity
Table 2 illustrates EMA WM performance during drug use categories derived from raw EMA data.
Descriptives of the subject- and momentary-level variables included as model covariates as well as their association with EMA WM performance are in Table 3. Main effects and interactions of within-subject differences in momentary substance use from the mixed effects ordinal regression model predicting the number of correct dots in each task trial are presented in Table 4 and Figure 1. Because the 3-way interaction between marijuana, tobacco and alcohol was not significant (p=0.48), the results of the model including only the two-way interactions are presented. Various momentary permutations of substance use were associated with WM, even after accounting for multiple potential confounds. There were significant main effects for marijuana and tobacco: individuals exhibited worse WM (i.e., lower number of correct dots) when using marijuana, and better WM when using tobacco. These main effects were not qualified by a significant interaction, suggesting that the combined use of marijuana and tobacco resulted in WM performance that approached that exhibited during non-substance using occasions. Alcohol reduced WM, and the tobacco by alcohol interaction was significant, indicating that the facilitative effect of tobacco (when used alone) disappeared when there was concurrent alcohol use. Finally, the marijuana by alcohol interaction was not significant. In summary, participants performed poorly when using alcohol and tobacco together, as well as when using alcohol and/or marijuana.
Table 3. Descriptives of Covariates Included in Random-Effect Ordinal Regression Model and Association with Number Correctly Recalled Dots on EMA Working Memory Task.
| Effect | Descriptives | Estimate | p-value | Marginal OR | 95% CI |
|---|---|---|---|---|---|
| Task Complexity | |||||
| Trial | -- | 0.07 | 0.008 | 1.06 | (1.02,1.11) |
| Number of Grids | -- | ||||
| 2 | 0 | -- | -- | -- | |
| 3 | 0.21 | <0.0001 | 1.20 | (1.13, 1.26) | |
| 4 | 0.30 | <0.0001 | 1.29 | (1.23, 1.36) | |
| Dispersion of Dots from Regression Line | .4 (.2) | 0.37 | <0.0001 | 1.37 | (1.25, 1.50) |
| Number of Corner Dots | 1.3 (.8) | 0.40 | <0.0001 | 1.41 | (1.36, 1.46) |
| Distance between Dots | 21.5 (3. 1) | -0.15 | <0.0001 | 0.88 | (0.87, 0.89) |
| Contextual/Background Factors | |||||
| Gender (B/S) | 54% Female | 0.07 | 0.61 | 1.06 | (0.85, 1.31) |
| GPA | 3.6 (.7) | 0.38 | <0.0001 | 1.39 | (1.20, 1.61) |
| Alone | 52.3% | 0.16 | <0.0001 | 1.15 | (1.09, 1.20) |
| Weekend Responding | 28.2% | -0.04 | 0.12 | 0.96 | (0.92, 1.01) |
| Watching TV/Listening to Music | 34.6% | 0.18 | <0.0001 | 1.17 | (1.11, 1.23) |
| At a Party | 2.1% | -0.17 | 0.06 | 0.86 | (0.74, 1.01) |
| Time of Day | |||||
| 4am-8:59am | 3% | -0.24 | 0.003 | 0.82 | (0.71, 0.93) |
| 9am-1:59pm | 24% | 0 | -- | -- | -- |
| 2pm-5:59pm | 29% | -0.12 | 0.0006 | 0.90 | (0.85, 0.96) |
| 6pm-9:59pm | 31% | -0.08 | 0.03 | 0.93 | (0.88, 0.99) |
| 10pm-3:59am | 13% | -0.09 | 0.05 | 0.93 | (0.85, 1.00) |
| Study Day | 0.30 | <0.0001 | 1.29 | (1.24, 1.34) | |
| 0 | 10% | ||||
| 1 | 16% | ||||
| 2 | 15% | ||||
| 3 | 14% | ||||
| 4 | 14% | ||||
| 5 | 13% | ||||
| 6 | 13% | ||||
| 7 | 5% | ||||
| Study Day (Quadratic) | -- | -0.03 | <0.0001 | 0.97 | (0.97, 0.98) |
| Trouble Concentrating | 3.1 (2.6) | -0.01 | 0.20 | 0.99 | (0.98, 1.00) |
| Negative Affect | 3.0 (2.1) | -0.03 | 0.001 | 0.98 | (0.96, 0.99) |
| Overall Substance Use | |||||
| Proportion Marijuana Prompts (B/S) | .1 (.2) | -0.36 | 0.36 | 0.74 | (0.38, 1.42) |
| Proportion Tobacco Prompts (B/S) | .2 (.2) | 0.62 | 0.14 | 1.71 | (0.84, 3.48) |
| Proportion Alcohol Prompts (B/S) | .1 (.1) | 2.67 | <0.0001 | 9.97 | (3.22, 30.91) |
Note. All descriptive values are means and standard deviations, unless otherwise specified. Between-subject effects (B/S) are specifically denoted. All other covariates represent within-subject effects. Grade point average, GPA (on 4-point scale); Trouble concentrating (Likert scale item with response options 1-Not at all through 10-Very much); Negative affect (average of responses on current feelings of anger, frustration, irritability, sadness and stress, each with continuous response options of 1-Not at all through 10-Very much); Overall substance use separately derived for each participant based on the proportion of total prompts across the study week during which use of that substance was reported.
Table 4. Main Effects and Interactions of Momentary Substance Use From Random-Effect Ordinal Regression Model Predicting Proportion of Correctly Recalled Dots on EMA Working Memory Task.
| Effect | Estimate | p-value | Marginal OR | 95% CI |
|---|---|---|---|---|
| Marijuana | -0.11 | 0.03 | 0.91 | (0.84, 0.99) |
| Alcohol | -0.17 | 0.003 | 0.87 | (0.79, 0.95) |
| Tobacco | 0.12 | 0.002 | 1.11 | (1.04, 1.18) |
| Marijuana*Tobacco | 0.04 | 0.77 | 1.03 | (0.84, 1.26) |
| Marijuana*Alcohol Use | -0.001 | 0.99 | 1.00 | (0.79, 1.27) |
| Tobacco*Alcohol | -0.25 | 0.04 | 0.81 | (0.66, 0.99) |
Note. All predictors represent within-subject effects. This model includes adjustment for all covariates in Table 3. This model was run initially with main effects of marijuana, tobacco and alcohol as well as all two-way and three-way interactions; however, the three-way interaction was not significant (p=.48) and was therefore removed from the model.
Figure 1.
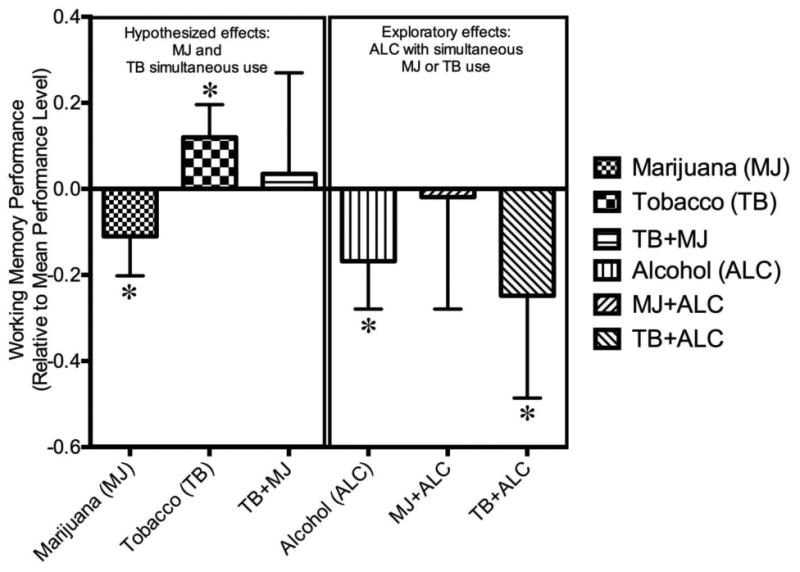
Figure 1 depicts the mean change in working memory performance under different substance use combinations, compared to mean performance levels. Error bars represent 95% confidence intervals. *Represents significant changes from non-use occasions.
Discussion
This study examined how WM, assessed in real-time, varied by momentary use of tobacco and marijuana, along with their combined use as well as in the context of simultaneous alcohol use. Little is known about WM patterns when marijuana and tobacco are used simultaneously, and no research has characterized WM in the actual context in which substance use occurs. Additionally, preliminary work has examined interactions between alcohol and marijuana or tobacco, but results are inconclusive and no studies have examined how all three substances impact WM when used simultaneously. Therefore, this study addressed three primary questions: does WM fluctuate when individuals use marijuana or tobacco; when used together, does tobacco counteract any adverse impact of marijuana on WM; and, how does concurrent alcohol use alter this neurocognitive profile? This study moves beyond previous research by characterizing within-subject WM variability during situations of no use, single substance use and conjoint use using EMA, which enhances the generalizability of findings due to greater emphasis on ecological validity.
Consistent with hypotheses, WM was worse with marijuana and better with tobacco. Findings support previous theory and research suggesting that acute and chronic exposure to marijuana is linked with selective, dose-dependent negative influences on WM in animal (e.g., 64, 65) and human models (e.g., 66, 67), whereas tobacco enhances WM (e.g., 68-70). Additionally and consistent with central hypotheses, marijuana was not associated with diminished WM when used with tobacco. More specifically, although there was an overall effect for marijuana worsening WM, WM was comparable to non-use occasions when marijuana and tobacco were used simultaneously. These findings, alongside data from animal models on functional interactions between cannabinoid and cholinergic systems (11-13, 71), together provide convincing preliminary evidence in favor of a compensatory theory. This theory, which hypothesizes that tobacco counteracts marijuana-induced WM decrements, is based largely on the fact that marijuana and tobacco target similar neuroanatomical structures that are central to WM, namely the hippocampus and prefrontal cortex (72, 73), and exert opposing independent influences on memory and WM (e.g., (66, 67, 74, 75). Although experimental investigations demonstrate that concomitant marijuana and tobacco use is linked with altered behavior (15-18, 21, 23), few studies specifically examine simultaneous use on neurocognition. Therefore, these data provide first steps in understanding whether tobacco mitigates WM impairments from acute marijuana use, and does so using an EMA paradigm allowing for a first-of-its kind real-life replication of laboratory findings.
Associations between acute marijuana and tobacco use and WM persisted after adjusting for multiple potential confounds including task complexity, demographics, background/contextual variables, and overall substance use. Employing this conservative model with multiple controls was critical as many of these factors may adversely influence WM. For instance, Speck and colleagues (76) demonstrated that gender moderated both performance and functional organization during WM tasks. The fact that significant effects were detected above and beyond the influence of multiple confounds speaks to the specificity and the robustness of these associations.
Intriguing effects emerged regarding alcohol's relationship to WM, despite no a priori hypotheses on this relationship. First, people who drank more during the week exhibited better WM than those who drank less (see proportion of alcohol prompts in Table 4). However, the drinking heaviness variable was only a proxy (not absolute) measure of level of alcohol use: the proportion of drinking episodes assessed via EMA was modestly correlated with amount of past month drinking indicated on a single-item retrospective recall question collected through the parent project. (r =0.37), suggesting that this variable may be an adequate albeit not ideal measure of level of alcohol use. In contrast, acute drinking episodes were associated with worse WM, suggesting that the pharmacology of alcohol and/or the contexts surrounding alcohol use are likely adverse contributors to WM. These exploratory findings are consistent with alcohol being associated with decrements in cognition (77-81; however, 43, 82-84) likely due to a narrowing of attentional control and impaired capacity to engage in controlled, effortful processing (85-88). WM decrements with alcohol use may be attributable to the alcohol's depressant effects, such as inhibition of glutamatergic transmission (89). Surprisingly, alcohol was not associated with WM decrements when used in combination with marijuana and tobacco, which may be due to a number of different unmeasured factors including dosing effects, sampling variations, or contexts associated with this three-drug use combination. However, the insignificant three-way interaction should be interpreted with caution given the relatively small number of prompts attained in this substance use category (n=78; 0.06% of all prompts). Further research is warranted to better determine the acute influence of alcohol on WM in real-world settings and in the context of use of other common substances.
Results should be examined in the context of several limitations. First, the EMA-based cognitive assessment is novel and it is possible that this was not an accurate WM measure. Yet, substantial work has already been conducted establishing the acceptability and validity of this paradigm. Schuster and colleagues (59) found that even after adjusting for IQ, task performance was associated standardized laboratory WM measures but not processing speed or verbal abilities, providing preliminary evidence supporting the task's validity. Regardless, future studies are warranted that both stringently establish the psychometric properties of this task and implement redundant measures of WM and neurocognition into EMA to determine the sensitivity and specificity of effects observed in this study. Second, different results may have emerged if other unmeasured variables were modeled (e.g., concurrent use of other substances; substance use dose). For example, it is conceivable that participants smoked less marijuana during tobacco-marijuana occasions than with marijuana alone, which would impact the marijuana effects, and this possibility needs to be specifically considered in next step studies. However, we statistically controlled for a multitude of theoretically relevant variables that may have confounded results and rates of past 90-day use of other substances was extremely low (less than 5%). Additionally, studies with specific hypotheses about how real-time negative affect interacts with real-time substance use are warranted, especially as this study found a significant negative relationship between momentary negative affect and WM. Third, there were fewer occasions of (and fewer participants who reported) simultaneous substance use as compared to occasions of no or single substance use, and concerns for possible sampling variations may be minimized by specifically targeting populations that report regular simultaneous marijuana and tobacco use. Fourth, the potential for self-initiated (i.e., tobacco with and without marijuana and/or alcohol) versus randomly prompted (i.e., marijuana and alcohol without tobacco) responses should be considered as a potential cross-substance confound. Fifth, despite the fact that participants were well-trained on EMA data capture (i.e., many had completed prior waves of EMA through the parent study) and the concordance between the debriefing interviews and EMA data was high (>80%), it cannot be completely guaranteed that participants event-recorded tobacco every time used, thereby representing potentially unmeasured confounding. Finally, overall WM performance was negatively skewed and this task was given multiple times a day over one week; therefore, task learning might have influenced findings and/or resulted in a ceiling effect. However, controlling for multiple practice effect parameters minimized this concern.
Despite limitations, this study suggests that tobacco use may compensate for WM decrements from marijuana among young adults. The attenuation of cognitive decrements may be an important mechanism by which tobacco use is reinforced among marijuana users. Particularly given the numerous documented health risks from tobacco, these results may have relevance in informing the development of more tailored and targeted intervention efforts for the growing number of individuals who use both marijuana and tobacco. This may be especially relevant among the average young adult smoker, as studied here, who is a light and non-daily cigarette user. Findings from this study also highlight the importance of further investigating the putative impact of alcohol (particularly in the context of concurrent tobacco use) on WM. Strengths of this study include within-subject comparisons to examine cognitive shifts under different substance use conditions and use of real-time data capture methodology, allowing for simultaneous modeling of contextual factors that may interrupt WM. This is the first study to assess WM under ecologically valid conditions while young adults are using substances. Additionally information on WM fluctuations during marijuana, tobacco and alcohol use occasions supports the sensitivity of the WM task to detect drug effects in an EMA paradigm. Future work will assess whether these effects change as individuals progress to substance dependence and develop tolerance. Additionally, related lines of inquiry will examine whether WM fluctuations impact perceived intoxication, affect and subsequently reinforce continued substance use and serve as a barrier for quitting.
Acknowledgments
We thank Dr. Kathi Diviak and John O'Keefe for their work recruiting and maintaining study participants.
This research was supported by the National Cancer Institute of the National Institutes of Health under award number 5P01CA098262 (PI: Mermelstein) as well as the following fellowships from Massachusetts General Hospital and Harvard Medical School (PI: Schuster): Norman E. Zinberg Fellowship in Addiction Psychiatry, Livingston Fellowship, and Louis V. Gerstner III Research Scholar Award. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCI, the National Institutes of Health, Massachusetts General Hospital or Harvard Medical School.
Footnotes
Declaration of Interest: The authors declare no conflicts of interest.
References
- 1.Miech RA, Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2014: Volume I, Secondary school students. 2015 [Google Scholar]
- 2.Amos A, Wiltshire S, Bostock Y, Haw S, McNeill A. ‘You can't go without a fag…you need it for your hash’--a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99(1):77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 3.Golub A, Johnson BD, Dunlap E. The growth in marijuana use among American youths during the 1990s and the extent of blunt smoking. J Ethn Subst Abuse. 2005;4(3-4):1–21. doi: 10.1300/J233v04n03_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal A, Lynskey MT, Madden PA, Pergadia ML, Bucholz KK, Heath AC. Simultaneous cannabis and tobacco use and cannabis-related outcomes in young women. Drug and alcohol dependence. 2009;101(1-2):8–12. doi: 10.1016/j.drugalcdep.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baggio S, Studer J, Mohler-Kuo M, Daeppen JB, Gmel G. Concurrent and simultaneous polydrug use among young Swiss males: use patterns and associations of number of substances used with health issues. Int J Adolesc Med Health. 2014;26(2):217–24. doi: 10.1515/ijamh-2013-0305. [DOI] [PubMed] [Google Scholar]
- 6.Fairman BJ. Cannabis problem experiences among users of the tobacco-cannabis combination known as blunts. Drug and alcohol dependence. 2015;150:77–84. doi: 10.1016/j.drugalcdep.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ream GL, Benoit E, Johnson BD, Dunlap E. Smoking tobacco along with marijuana increases symptoms of cannabis dependence. Drug and alcohol dependence. 2008;95(3):199–208. doi: 10.1016/j.drugalcdep.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford DE, Vu HT, Anthony JC. Marijuana use and cessation of tobacco smoking in adults from a community sample. Drug Alcohol Depend. 2002;67(3):243–8. doi: 10.1016/s0376-8716(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 9.Marco EM, Granstrem O, Moreno E, Llorente R, Adriani W, Laviola G, et al. Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. European journal of pharmacology. 2007;557(1):37–43. doi: 10.1016/j.ejphar.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez S, Cascio MG, Fernandez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain research. 2002;954(1):73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- 11.Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. European journal of pharmacology. 2000;401(2):179–85. doi: 10.1016/s0014-2999(00)00403-9. [DOI] [PubMed] [Google Scholar]
- 12.Revuelta AV, Moroni F, Cheney DL, Costa E. Effect of cannabinoids on the turnover rate of acetylcholine in rat hippocampus, striatum and cortex. Naunyn-Schmiedeberg's archives of pharmacology. 1978;304(2):107–10. doi: 10.1007/BF00495546. [DOI] [PubMed] [Google Scholar]
- 13.Tripathi HL, Vocci FJ, Brase DA, Dewey WL. Effects of cannabinoids on levels of acetylcholine and choline and on turnover rate of acetylcholine in various regions of the mouse brain. Alcohol and drug research. 1987;7(5-6):525–32. [PubMed] [Google Scholar]
- 14.Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(28):9374–84. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pryor GT, Larsen FF, Husain S, Braude MC. Interactions of delta9-tetrahydrocannabinol with d-amphetamine, cocaine, and nicotine in rats. Pharmacology, biochemistry, and behavior. 1978;8(3):295–318. doi: 10.1016/0091-3057(78)90320-9. [DOI] [PubMed] [Google Scholar]
- 16.Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. British journal of pharmacology. 2002;135(2):564–78. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. The European journal of neuroscience. 2004;20(10):2737–48. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- 18.Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43(5):857–67. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- 19.Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30(1):145–55. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- 20.Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002;13(5-6):451–63. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;54(2):438–44. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312(3):875–83. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 23.Schuster RM, Crane NA, Mermelstein R, Gonzalez R. Tobacco May Mask Poorer Episodic Memory Among Young Adult Cannabis Users Neuropsychology. 2015 doi: 10.1037/neu0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filbey FM, McQueeny T, Kadamangudi S, Bice C, Ketcherside A. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res. 2015;293:46–53. doi: 10.1016/j.bbr.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological psychiatry. 2007;61(1):31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24(19):4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage. 2004;21(1):340–51. doi: 10.1016/j.neuroimage.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. NeuroImage. 2004;23(2):604–12. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Karlsgodt KH, Shirinyan D, van Erp TG, Cohen MS, Cannon TD. Hippocampal activations during encoding and retrieval in a verbal working memory paradigm. Neuroimage. 2005;25(4):1224–31. doi: 10.1016/j.neuroimage.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–73. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 31.Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, et al. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49(4):226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- 32.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gessa GL, Casu MA, Carta G, Mascia MS. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. European journal of pharmacology. 1998;355(2-3):119–24. doi: 10.1016/s0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- 34.Hu ZJ, Bai L, Tizabi Y, Southerland W. Computational modeling study of human nicotinic acetylcholine receptor for developing new drugs in the treatment of alcoholism. Interdiscip Sci. 2009;1(4):254–62. doi: 10.1007/s12539-009-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol. 2006;41(5):486–93. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- 36.Korkosz A, Taracha E, Plaznik A, Wrobel E, Kostowski W, Bienkowski P. Extended blockade of the discriminative stimulus effects of nicotine with low doses of ethanol. European journal of pharmacology. 2005;512(2-3):165–72. doi: 10.1016/j.ejphar.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Winward JL, Hanson KL, Tapert SF, Brown SA. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. 2014;20(8):784–95. doi: 10.1017/S1355617714000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballard ME, de Wit H. Combined effects of acute, very-low-dose ethanol and delta(9)-tetrahydrocannabinol in healthy human volunteers. Pharmacology, biochemistry, and behavior. 2011;97(4):627–31. doi: 10.1016/j.pbb.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tracy HA, Jr, Wayner MJ, Armstrong DL. Nicotine blocks ethanol and diazepam impairment of air righting and ethanol impairment of maze performance. Alcohol. 1999;18(2-3):123–30. doi: 10.1016/s0741-8329(98)00074-3. [DOI] [PubMed] [Google Scholar]
- 40.Rezvani AH, Levin ED. Cognitive effects of nicotine. Biological psychiatry. 2001;49(3):258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- 41.Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behavioural pharmacology. 1996;7(2):144–54. [PubMed] [Google Scholar]
- 42.Michel C, Battig K. Separate and combined psychophysiological effects of cigarette smoking and alcohol consumption. Psychopharmacology. 1989;97(1):65–73. doi: 10.1007/BF00443415. [DOI] [PubMed] [Google Scholar]
- 43.Greenstein JE, Kassel JD. The effects of smoking and smoking abstinence on verbal and visuospatial working memory capacity. Experimental and clinical psychopharmacology. 2009;17(2):78–90. doi: 10.1037/a0015699. [DOI] [PubMed] [Google Scholar]
- 44.Ralevski E, Perry EB, Jr, D'Souza DC, Bufis V, Elander J, Limoncelli D, et al. Preliminary findings on the interactive effects of IV ethanol and IV nicotine on human behavior and cognition: a laboratory study. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2012;14(5):596–606. doi: 10.1093/ntr/ntr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latimer W, Zur J. Epidemiologic trends of adolescent use of alcohol, tobacco, and other drugs. Child Adolesc Psychiatr Clin N Am. 2010;19(3):451–64. doi: 10.1016/j.chc.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dierker L, Mermelstein R. Early emerging nicotine-dependence symptoms: a signal of propensity for chronic smoking behavior in adolescents. J Pediatr. 2010;156(5):818–22. doi: 10.1016/j.jpeds.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piasecki TM, Trela CJ, Hedeker D, Mermelstein RJ. Smoking antecedents: separating between- and within-person effects of tobacco dependence in a multiwave ecological momentary assessment investigation of adolescent smoking. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2014;16(Suppl 2):S119–26. doi: 10.1093/ntr/ntt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinstein SM, Mermelstein RJ. Dynamic associations of negative mood and smoking across the development of smoking in adolescence. J Clin Child Adolesc Psychol. 2013;42(5):629–42. doi: 10.1080/15374416.2013.794698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinstein SM, Mermelstein RJ. Influences of mood variability, negative moods, and depression on adolescent cigarette smoking. Psychol Addict Behav. 2013;27(4):1068–78. doi: 10.1037/a0031488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone AA, Shiffman S. Capturing momentary, self-report data: a proposal for reporting guidelines. Ann Behav Med. 2002;24(3):236–43. doi: 10.1207/S15324796ABM2403_09. [DOI] [PubMed] [Google Scholar]
- 51.Ventre-Dominey J, Bailly A, Lavenne F, Lebars D, Mollion H, Costes N, et al. Double dissociation in neural correlates of visual working memory: a PET study. Brain research Cognitive brain research. 2005;25(3):747–59. doi: 10.1016/j.cogbrainres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Anderson BA, Faulkner ML, Rilee JJ, Yantis S, Marvel CL. Attentional bias for nondrug reward is magnified in addiction. Experimental and clinical psychopharmacology. 2013;21(6):499–506. doi: 10.1037/a0034575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(13):4926–34. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65(6):692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- 55.Johnson MK, McMahon RP, Robinson BM, Harvey AN, Hahn B, Leonard CJ, et al. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27(2):220–9. doi: 10.1037/a0032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyllingsbaek S, Bundesen C. Changing change detection: improving the reliability of measures of visual short-term memory capacity. Psychon Bull Rev. 2009;16(6):1000–10. doi: 10.3758/PBR.16.6.1000. [DOI] [PubMed] [Google Scholar]
- 57.Ichikawa SI. Verbal memory span, visual memory span, and their correlations with cognitive tasks. Japanese Psychological Research. 1983;25:173–80. [Google Scholar]
- 58.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971;27(3):272–7. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 59.Schuster RM, Mermelstein RJ, Hedeker D. Acceptability and Feasibility of a Visual Working Memory Task in an Ecological Momentary Assessment Paradigm. Psychological assessment. 2015 doi: 10.1037/pas0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hedeker D, Gibbons RD. A random-effects ordinal regression model for multilevel analysis. Biometrics. 1994;50(4):933–44. [PubMed] [Google Scholar]
- 61.Hedeker D, Gibbons RD. MIXOR: a computer program for mixed-effects ordinal regression analysis. Computer methods and programs in biomedicine. 1996;49(2):157–76. doi: 10.1016/0169-2607(96)01720-8. [DOI] [PubMed] [Google Scholar]
- 62.Hedeker D, Mermelstein RJ, Demirtas H. An application of a mixed-effects location scale model for analysis of Ecological Momentary Assessment (EMA) data. Biometrics. 2008;64(2):627–34. doi: 10.1111/j.1541-0420.2007.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hedeker D, Mermelstein RJ, Berbaum ML, Campbell RT. Modeling mood variation associated with smoking: an application of a heterogeneous mixed-effects model for analysis of ecological momentary assessment (EMA) data. Addiction. 2009;104(2):297–307. doi: 10.1111/j.1360-0443.2008.02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mishima K, Egashira N, Matsumoto Y, Iwasaki K, Fujiwara M. Involvement of reduced acetylcholine release in Delta9-tetrahydrocannabinol-induced impairment of spatial memory in the 8-arm radial maze. Life sciences. 2002;72(4-5):397–407. doi: 10.1016/s0024-3205(02)02274-9. [DOI] [PubMed] [Google Scholar]
- 65.Egerton A, Allison C, Brett RR, Pratt JA. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neuroscience and biobehavioral reviews. 2006;30(5):680–95. doi: 10.1016/j.neubiorev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9(5):679–89. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 67.Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970–6. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25(3):313–9. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 69.Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology. 2002;162(2):129–37. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- 70.Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123(1):88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- 71.Viveros MP, Marco EM, Llorente R, Lamota L. The role of the hippocampus in mediating emotional responses to nicotine and cannabinoids: a possible neural substrate for functional interactions. Behavioural pharmacology. 2007;18(5-6):375–89. doi: 10.1097/FBP.0b013e3282d28fb4. [DOI] [PubMed] [Google Scholar]
- 72.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184(3-4):523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 73.Yucel M, Lubman DI, Solowij N, Brewer WJ. Understanding drug addiction: a neuropsychological perspective. The Australian and New Zealand journal of psychiatry. 2007;41(12):957–68. doi: 10.1080/00048670701689444. [DOI] [PubMed] [Google Scholar]
- 74.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210(4):453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleykamp BA, Jennings JM, Blank MD, Eissenberg T. The effects of nicotine on attention and working memory in never-smokers. Psychol Addict Behav. 2005;19(4):433–8. doi: 10.1037/0893-164X.19.4.433. [DOI] [PubMed] [Google Scholar]
- 76.Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11(11):2581–5. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- 77.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism, clinical and experimental research. 2000;24(2):164–71. [PubMed] [Google Scholar]
- 78.Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism, clinical and experimental research. 1994;18(1):159–63. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 79.Casbon TS, Curtin JJ, Lang AR, Patrick CJ. Deleterious effects of alcohol intoxication: diminished cognitive control and its behavioral consequences. Journal of abnormal psychology. 2003;112(3):476–87. doi: 10.1037/0021-843x.112.3.476. [DOI] [PubMed] [Google Scholar]
- 80.Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146(4):465–72. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- 81.Grattan-Miscio KE, Vogel-Sprott M. Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology. 2005;181(1):188–96. doi: 10.1007/s00213-005-2226-2. [DOI] [PubMed] [Google Scholar]
- 82.Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcoholism, clinical and experimental research. 2006;30(8):1363–71. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schweizer TA, Vogel-Sprott M, Danckert J, Roy EA, Skakum A, Broderick CE. Neuropsychological profile of acute alcohol intoxication during ascending and descending blood alcohol concentrations. Neuropsychopharmacology. 2006;31(6):1301–9. doi: 10.1038/sj.npp.1300941. [DOI] [PubMed] [Google Scholar]
- 84.Weissenborn R, Duka T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology. 2003;165(3):306–12. doi: 10.1007/s00213-002-1281-1. [DOI] [PubMed] [Google Scholar]
- 85.Josephs RA, Steele CM. The two faces of alcohol myopia: attentional mediation of psychological stress. Journal of abnormal psychology. 1990;99(2):115–26. doi: 10.1037//0021-843x.99.2.115. [DOI] [PubMed] [Google Scholar]
- 86.Steele CM, Josephs RA. Alcohol myopia. Its prized and dangerous effects. The American psychologist. 1990;45(8):921–33. doi: 10.1037//0003-066x.45.8.921. [DOI] [PubMed] [Google Scholar]
- 87.Colflesh GJ, Wiley J. Drunk, but not blind: the effects of alcohol intoxication on change blindness. Consciousness and cognition. 2013;22(1):231–6. doi: 10.1016/j.concog.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Saults JS, Cowan N, Sher KJ, Moreno MV. Differential effects of alcohol on working memory: distinguishing multiple processes. Experimental and clinical psychopharmacology. 2007;15(6):576–87. doi: 10.1037/1064-1297.15.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19(12):491–5. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]


