Abstract
The vesicating agents sulfur mustard (SM) and lewisite (LEW) are potent chemical warfare agents that primarily cause damage to the ocular, skin, and respiratory systems. However, ocular tissue is the most sensitive organ, and vesicant exposure results in a biphasic injury response, including photophobia, corneal lesions, corneal edema, ulceration, and neovascularization, and may cause loss of vision. There are several reports on ocular injury from exposure to SM, which has been frequently used in warfare. However, there are very few reports on ocular injury by LEW, which indicate that injury symptoms appear instantly after exposure and faster than SM. In spite of extensive research efforts, effective therapies for vesicant-induced ocular injuries, mainly to the most affected corneal tissue, are not available. Hence, we have established primary human corneal epithelial (HCE) cells and rabbit corneal organ culture models with the SM analog nitrogen mustard (NM), which have helped to test the efficacy of potential therapeutic agents. These agents will then be further evaluated against in vivo SM- and LEW-induced corneal injury models, which will assist in the development of potential broad-spectrum therapies against vesicant-induced ocular injuries.
Keywords: nitrogen mustard, sulfur mustard, lewisite, corneal injury, therapies
Introduction
Modern chemical warfare started during World War I (WWI) with the use of chlorine by the German army,1 which was followed by the development of more effective chemical weapons. The agents developed comprised nerve agents, vesicants, choking agents, lacrimators, and central nervous system–disabling agents.2 Huge quantities of these agents were synthesized, and at present large stockpiles exist, which pose a continuous risk of accidental exposure to these agents, in addition from their potential use in warfare and terrorism.3 Among these agents, vesicants that form vesicles or blisters upon exposure are of major military importance.2 The vesicants that are considered useful for tactical military weapons as chemical agents are the mustard agents (sulfur and nitrogen mustard), arsenicals (lewisite), and urticant or nettle agent phosgene oxime.2
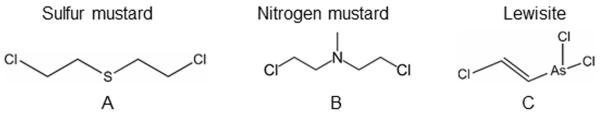
Among the mustard agents, sulfur mustard (SM; 2, 2-dichloroethyl sulfide; H; HD; mustard gas, mustard (Fig. 1A)) and nitrogen mustard (NM; mechlorethamine hydrochloride; Bis (2-chloroethyl) methylamine, HN2; (Fig. 1B)), SM is the most widely used chemical weapon. SM has been responsible for far more casualties during various military conflicts than any other chemical agent, earning it the nickname “king of war gases.”4–6 SM is known to be present at most stockpiles and non-stockpile munitions sites around the world.7 During the Iran–Iraq war, SM was used heavily, and the Iranian victims are still suffering from its late effects.8,9 Compared to its primary targets, the skin and respiratory organs, the eye is the most sensitive organ to SM exposure (~10 times more sensitive compared to the skin tissue).1 SM is a potent alkylating agent, and rapidly reacts and form adducts with all major biomolecules in the cell10 with immediate irritation; however, depending on the concentration and time of exposure, symptoms may manifest 1–12 h postexposure.11,12 The ocular injury from SM is biphasic; an initial acute phase is expressed clinically by photophobia and inflammation, followed by late effects, including epithelial defects, chronic inflammation, corneal erosions, corneal opacity, and corneal neovascularization.12 SM-induced ulcerative keratopathy could take 15–20 years to develop after the initial exposure. This latent onset has been seen in Iranian veterans of the Iran–Iraq war and in WWI veterans.9,13 The events leading to these delayed effects are not well understood. However, loss of limbal stem cells and endothelial cells, persistent inflammation, and neovascularization has been suggested to play roles.14
Figure 1.
Structures of (A) sulfur mustard, (B) nitrogen mustard, and (C) lewisite.
NM, a highly reactive, bifunctional alkylating analog of SM that was developed in WWI but not deployed in warfare, covalently modifies all major cellular biomolecules (DNA, proteins, and lipids) and causes injuries comparable to SM exposure, including histopathological changes and clinical manifestations.15–19 Owing to the limitation of access and use of SM in laboratory settings, NM has been employed to study vesicant-induced ocular injuries and related mechanism of action to identify targeted therapies. This is of importance since the countermeasures currently available are mainly symptomatic and supportive, accentuating an immediate need for the development of therapies.15,16,18–20
Lewisite (dichloro-2-chlorovinylarsine; C2H2AsCl3; L, LEW, (Fig. 1C)) is an organic arsenical vesicant, which is a colorless, odorless, oily liquid at room temperature.2 LEW has been reported to be mixed with SM to achieve greater effectiveness in combat.21,22 LEW may have been deployed by the Japanese army against China (1937–1944).23 LEW-induced ocular damage is characterized by an instant presence of edema and blepharospasm, which can result in massive necrosis and eventual blindness.24 LEW reacts with biological sulfhydryl groups, resulting in injuries similar to other arsenic-containing chemicals.25 In addition, release of hydrochloric acid from LEW reduces the pH of the eye and results in superficial opacity.3 Unlike SM, there are very few reports, mainly from the 1940s, on LEW-induced clinical and pathologic ocular changes, but useful injury biomarkers for mechanistic and efficacy studies are not available.24,26 British Anti-Lewisite (BAL; dimercaprol) has been reported as a useful therapy against LEW-induced injuries; however, due to its toxicity, less toxic variants of BAL were developed.26–29 Nonetheless, the therapeutic efficacy of these agents is also limited by their toxicity, narrow therapeutic window, and difficult administration.26–30 Therefore, to develop safe, mechanism-driven antidotes for LEW and other vesicant-caused ocular injuries, we have developed relevant in vitro, ex vivo culture, and in vivo ocular injury models that are being employed to screen and identify effective therapies against vesicant-induced ocular injuries. These studies are expected to identify novel mechanism-based therapies against vesicant-induced toxic ocular effects, which are summarized in this review.
Development of vesicant-induced ocular injury models
Vesicating agents are reported to cause clinical and histopathological changes related to inflammation and vesication in both eye and skin tissues.16,31–35 Studies on SM exposure report histopathological changes of corneal thinning and ulceration, keratocyte death, inflammation, stromal neovascularization, and vesication.16,33,36,37 The mechanisms involved in these SM-induced toxic effects have not been fully elucidated, and efforts are ongoing to understand the multifaceted and complex events leading to its toxicity. Apart from injury to the limbus and other parts of the eye, the outer corneal layer is extremely vulnerable to vesicant-caused injuries and has been the main focus of most reported and our ongoing studies.33,38–40 SM is known to induce DNA damage and cause mutations following erroneous repairs, chromosomal breaks, and chromosomal aberrations.10 Alkylation of DNA and other major molecules, oxidative stress, and lipid peroxidation, and induction of inflammatory responses, matrix metalloproteinases (MMPs), and vascular endothelial growth factor (VEGF) are reported to be involved in its toxicity.10,15,16,41–43
A number of animal models, including mice, rabbits, and rabbit ex vivo corneal cultures, are reported as useful animal models to study vesicant-induced corneal toxicity and related mechanisms.16,32,33,38,41,44 Small animals, such as mouse and rat, have the advantages of easier handling, lower costs, and short life span. However, the difference between human eyes and those of these animal models may present difficulties in translating the results into humans.45 Larger animals, such as cats, dogs, pigs and sheep, have been also reported to be useful for human corneal research but have limitations, such as the lack of inbred strains, higher cost, and difficult handling.45 In addition to larger eyes, the overall pharmacokinetic parameters in rabbit eyes show good correlation with human eyes. Therefore, owing to lesser differences between relevant anatomical and physiological parameters between human and rabbit eyes, rabbit eyes are normally used for ocular pharmacokinetics preclinical studies, as more reliable translation to humans is feasible, and serve as a useful model system to study ocular injuries.45
The ex vivo rabbit corneal organ cultures are reported to be beneficial in primary screening and identification of therapies against vesicant-induced ocular injuries.16,19 Furthermore, this culture system has also been useful to study corneal wound healing, and the injuries have been found to be similar to those occurring in vivo in the rabbit cornea.16,18,19 The rabbit corneas from Pel-freez, a company that sells eyes of rabbits that are used for other purposes, makes it cost effective and reduces the animal use in our studies. This rabbit corneal injury model with NM could serve as an ideal ex vivo system in the laboratory settings to establish injury and to evaluate and optimize therapeutic agents before testing them in in vivo ocular injury rabbit models with vesicating agents like SM and LEW.18,19 In addition, since the corneal epithelial layer is the outermost layer of the eye and is highly susceptible to vesicant-induced injuries,33 human corneal epithelial (HCE) cells are a useful model system to assess the mechanisms of pathogenesis of corneal injury.18 The late complications associated with SM-induced corneal injury cannot be studied in ex vivo corneal organ culture and HCE cell culture. Nevertheless, these provide a valuable tool to understand the pathophysiology and mechanistic aspects of the acute corneal injury phase. To study and address the late complications of vesicant-induced ocular injury, we would employ an in vivo vesicating agent–induced rabbit model.
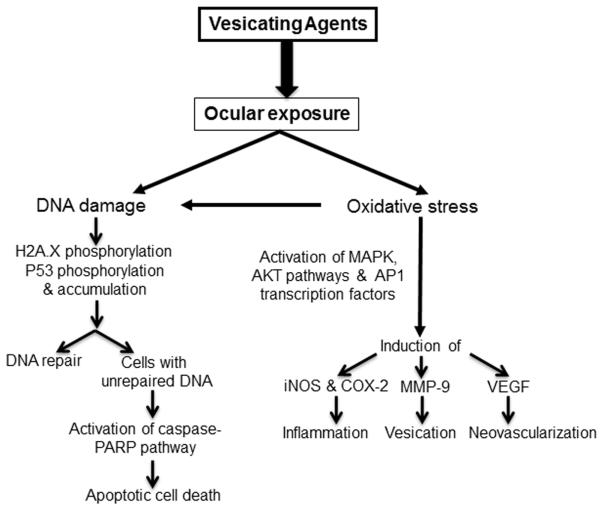
Studies in ex vivo rabbit corneal cultures with NM show an increase in epithelial layer thickness, epithelial detachment/denuding or erosion, separation of epithelial and stromal cells, and apoptotic cell death––events that play important roles in inflammation and the wound-healing process.18,19,34,35,46 In addition, NM exposure is also reported to cause an increase in the expression of cyclooxygenase-2 (COX-2, a key molecule involved in the prostaglandin synthesis and inflammatory and cytotoxic responses),18,47,48 matrix metalloproteinase-9 (MMP-9, plays a major role in the degradation of the basement membrane components), and angiogenic factors, namely vascular endothelial growth factor (VEGF, plays an important role in neovascularization (Fig. 2)).16,18,47,49,50 Further elucidation of mechanisms and pathways in HCE cells related to these lesions in rabbit corneal tissue reveal that DNA damage is an early event, involving p53 and H2A.X phosphorylation, that leads to decreased cell viability and proliferation. NM exposure also induces caspase-3 and poly ADP ribose polymerase (PARP) cleavage, suggesting their involvement in NM-induced apoptotic death in rabbit corneal culture and HCE cells (Fig. 2). Also, NM induces activation of activator protein 1 (AP1) transcription factor proteins and upstream signaling pathways, including mitogen-activated protein kinases (MAPKs; phosphorylation of ERK1/2, p38 and JNK1/2) and Akt protein kinase, signifying that these could be important factors in NM-induced corneal injury (Fig. 2).18 Notably, the MAPK/Akt-AP-1 pathway is activated by growth factors, inflammatory cytokines, stress stimuli, and oxidative stress; oxidative stress is a key initiating event after vesicant exposure. The generation of 4-hydroxynonenal (4-HNE) and 5,5-dimethyl-2-(8-octanoic acid)-1-pyrroline N-oxide (DMPO) adducts indicates that NM-induced oxidative stress could lead to significant lipid peroxidation and nitrone–protein adduct formation (Fig. 2). Apart from oxidative stress, nitrosative stress has also been associated with vesicant toxicity. NO is formed via NO-synthesizing enzymes, including inducible nitric oxide synthase (iNOS), regulated by the MAPK signaling pathway. However, further studies are needed to elaborate on the roles of oxidative or nitrosative stress in vesicant-induced ocular toxicity. These findings have helped in our understanding of mechanisms involved in vesicant-induced ocular injury and suggest the involvement of multiple pathways and possible molecular targets that will help in the identification and the development of targeted therapies (Fig. 2). In addition, these outcomes support the rabbit corneal organ culture injury model and HCE cells with NM as valuable tools to screen and identify therapies to rescue corneal injuries from vesicant exposure.
Figure 2.
Schematic showing possible mechanisms of mustard vesicating agent–induced ocular injury.
Since the gross pathology of vesicant-related rabbit eye lesions is similar to humans and is extensively used for ocular toxicity studies, rabbits offer a relevant ocular injury model with SM and LEW for carrying out in vivo studies to further evaluate the therapies identified in the rabbit corneal culture model.36,44,51–53 Importantly, LEW has not been studied as extensively as SM; therefore, useful end points are required for screening and development of effective therapies. Hence, in collaboration with MRIGlobal®, we have designed an efficient exposure system capable of exposing up to six rabbits at one time, with the ability to adjust both time and dose of vesicants.54 In this exposure system, the left eye of the rabbit serves as a control and is exposed to dilution air, while the right eye is simultaneously exposed to LEW or SM. Modified goggles with flow-through design were used for exposures. Using this consistent and efficient exposure system, we successfully carried out LEW exposures and established clinically relevant end points in a dose- and time-response study.54 Unlike SM, LEW exposure results in an immediate irritation, inflammation of the eyes, and swelling of the eyelids.3,55 The findings from our recent study for the first time showed clinical sequelae of ocular injury following LEW exposure, including corneal wounding, ulceration, inflammation, neovascularization, corneal thickness, iris redness, and redness and swelling of the conjunctiva, and their progression up to 56 days postexposure.54 Importantly, LEW exposure in humans is shown to result in swelling and edema, inflammation to the iris and conjunctiva, and corneal damage;3 the injuries are similar to those observed in the rabbit model in our study, suggesting the relevance of the model developed by us for both mechanistic and efficacy studies in the future. Furthermore, the observed clinical features of LEW injury in our studies may also be useful in diagnosis and further assessment of the histopathological lesions and related ocular toxicity pathways. Additionally, the gross features of LEW-induced ocular injury in our recently developed model were similar to SM-induced lesions in rabbits, except for their early appearance (6 h after LEW exposure compared to 24-48 h after SM exposure).36,56 However, similar to SM exposure, the severity of the clinical lesions peaked 24-72 h postexposure.12 The related histopathology and the mechanisms of acute and long-term corneal injuries by LEW observed in this rabbit corneal injury model are currently being investigated. It is also important to emphasize that, although the ocular lesions from LEW exposure appear to be similar to SM, their pathology and development could be different, as arsenic poisoning and inhibition of carbohydrate metabolism are also involved in LEW-induced injury.29,57,58 Hence, our ongoing efforts aimed at understanding the mechanism of ocular injuries following LEW exposure will help in outlining the additional pathways that could be targeted to develop effective therapies against LEW-induced ocular injuries.
Treatment of vesicant-induced ocular injuries
At present, there are no satisfactory or recommended antidotes for vesicant-induced ocular injury, and the symptomatic management to prevent infection and promote healing is the only treatment strategy.59 This is primarily due to the lack of understanding of the underlying mechanisms contributing to the pathological and clinical progression of the injury in suitable animal models. A number of decontamination strategies have been investigated for SM; however, SM is highly lipophilic, and therefore decontamination has to be done immediately after exposure.59 To develop an effective treatment strategy against vesicant-induced ocular injury, a therapeutic approach needs to target multiple pathways involved in injury. It is evident from previous studies that early alkylating effects on macromolecules, inflammation, and corneal damage, as well as the delayed loss of limbal stem cells, are related to the pathogenesis of vesicant-induced ocular injury.38,60 Though the therapeutic potential of the antibiotics doxycycline and ilomastat, glucocorticoid dexamethasone, and the nonsteroidal anti-inflammatory drug (NSAID) diclofenac have been reported, effective and approved, easily deliverable therapies against ocular injury in case of mass exposure to vesicating agents are not available.12,15,16,20,36 Several groups, including ours, have demonstrated the roles of DNA damage, inflammation, and MMP activation in SM-induced injury. Accordingly, various therapeutic approaches targeting these have been investigated to ameliorate SM-induced ocular injury, including PARP inhibitors, MMP inhibitors, and anti-inflammatory agents.12,59,61 The MMP inhibitor doxycycline has been shown to be effective in reducing SM-induced vesication in both live rabbits and corneal culture.16,52 In addition, it is also effective in reducing microbial infections. Dexamethasone, an anti-inflammatory agent, has been also shown to be effective in reducing SM-induced injury in live rabbits and NM-induced injury in corneal culture.19
Therefore, we selected dexamethasone and doxycycline, which have been reported to reduce injury symptoms induced by mustard vesicating agents.12,15,16,36 Our lab has extensively investigated the potential of the flavanone silibinin, a pleotropic agent, as an anti-inflammatory, antioxidant, and cancer chemopreventive agent.62–66 Further studies on the mechanism of action of silibinin revealed that it has pleiotropic effects targeting several pathways related to inflammation, cell death, and vesication.67 As SM-induced injury involves multiple pathways, and use of doxycycline and dexamethasone is accompanied by side effects, we evaluated silibinin, a nontoxic natural flavanone that targets multiple pathways involved in vesicant ocular injury. Our studies using the established ex vivo rabbit corneal culture model have shown the therapeutic potential of doxycycline, dexamethasone, and silibinin in reversing NM-induced corneal damage.19 In addition, the established models and biomarkers, as well as the suggested mechanism of action of vesicating agents from our studies, can help identify new compounds to treat corneal injuries from vesicants or other toxic chemical agents.
Doxycycline
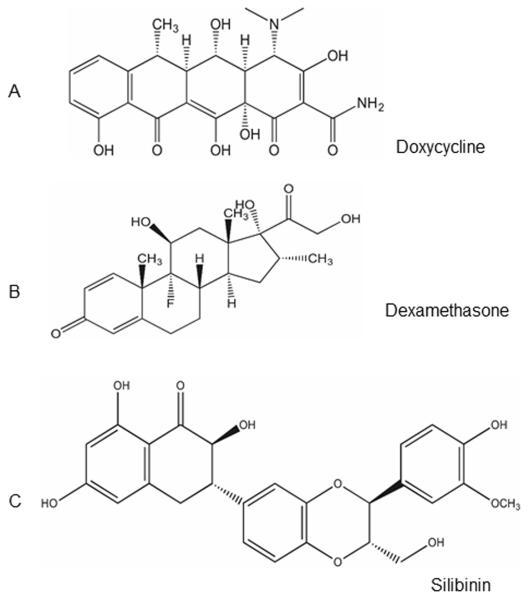
Doxycycline (tetracycline derivative) is a broad-spectrum antibiotic (Fig. 3A) and inhibits MMPs and synthesis of MMPs and IL-1 and is used for the treatment of periodontitis and rosacea.68–70 It also possesses anti-inflammatory, antiapoptotic, and antioxidant properties.71 Topical doxycycline has been shown to be beneficial in ameliorating SM-induced ocular injury in the rabbit model, and was found to be effective in inhibition of corneal neovascularization in animal models.72 In addition, other properties, such as inhibition of interleukin and collagen synthesis, inhibition of activated B cell function, and inhibition of NO synthesis by lipopolysaccharide (LPS) activated macrophage, have also been attributed to doxycycline.69 Doxycycline is also used for the treatment of ocular surface diseases, particularly recurrent epithelial cell erosion, rosacea, and keratitis sicca.68
Figure 3.
Structures of (A) Doxycycline, (B) dexamethasone, and (C) silibinin.
Dexamethasone
Dexamethasone is a potent corticosteroid (Fig. 3B) and has been extensively used as an immunosuppressant and anti-inflammatory agent.73 It is already approved for treatment of corneal burns, and it exerts anti-inflammatory and immunosuppressive effects.73,74 Early application of dexamethasone has shown to reduce the inflammatory response in SM-induced injury.12,36,75,76 Although prolonged use of dexamethasone is known to have some adverse side effects, except for a slight delay in corneal erosion healing, it is known to be quite well tolerated, without any clinical consequences.12
Silibinin
Silibinin is a polyphenolic flavanone (Fig. 3C) found in the seeds of the milk thistle plant (Silybum marianum). It has pleiotropic effects and has been used by humans for its strong hepatoprotective activity against a broad range of liver toxicities for several years.67,77 Silibinin is well tolerated in animals and humans, and is shown to be effective against various types of cancers in animal models.78 Silibinin has also been shown to be highly effective in treating SM analog-2-chloroethyl ethyl sulfide (CEES)– and NM-induced skin injuries.65,64
In a recent study, we examined the efficacy of silibinin, doxycycline, and dexamethasone on NM-induced biomarkers of ocular injury when administered after 2 h of NM exposure in cultured rabbit eye cornea.19 The treatments resulted in amelioration of NM-induced epithelial thickness, epithelial-stromal separation, and COX-2 and MMP-9 expression, to varying degrees. Silibinin was found to be more effective in reversing both epithelial thickness and epithelial–stromal separation.19 Most of these drugs come with some side effects; on the other hand, silibinin provides a safer alternative, as it is a nontoxic, natural product and is known to target various signaling pathways, including MAPKs, Akt, AP-1, NF-κB, COX-2, iNOS, and MMP-9.67 Our studies have shown that silibinin treatment significantly reverses NM- and CEES-induced skin and ocular injuries; iNOS, COX-2, and MMP-9 induction; activation of NF-κB and AP-1; and oxidative DNA damage.19,64,65 Importantly, these are critical events in vesicant-induced injuries, and these pathways, directly or via oxidative stress, have been found to play roles in skin and ocular toxicity.18,19,79,80 Owing to the poor water solubility of silibinin, development of an effective, safe, topical formulation with optimal bioavailability is required. In this regard, our efforts are ongoing to develop a safe and effective formulation of silibinin that can be topically applied to the ocular surface. Given the similarity in the injury and pathophysiology parameters in SM- and LEW-induced ocular injury, these agents could be effective against LEW-induced ocular injury. Our ongoing studies include formulation development of these agents and testing their efficacy against SM-, NM-, and LEW-induced ocular injury in live rabbits.
Concluding remarks and future directions
The relative ease of synthesis, devastating effects, and unavailability of effective countermeasures make vesicating chemical agents a weapon of choice for warfare and terrorism. In today’s scenario, possible use of these agents is not a mere speculation––there have been reports of their use in the ongoing conflict in Syria. Therefore, development of effective and safe countermeasures suitable for mass deployment in case of an emergency is the need of the hour. The use of corneal organ culture in our studies has shown the therapeutic efficacy of dexamethasone, doxycycline, and silibinin in reversing NM-induced ocular injury. Accordingly, these agents need to be further studied in LEW- and SM-induced ocular injury models in vivo, which would help in the development of targeted treatments against the severe ocular injury from vesicating agents.
Acknowledgements
This work was supported by the Countermeasures Against Chemical Threats (CounterACT) Program, Office of the Director National Institutes of Health (OD) and the National Eye Institute (NEI) (Grant Number U01EY023143).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Papirmeister B, Feister AJ, Robinson SI, Ford RD. Medical defense against mustard gas, toxic mechanisms and pharmacological implications. CRC press; 1991. [Google Scholar]
- 2.WHO . WHO Guidance: Public health response to biological and chemical weapons. WHO; Geneva Switzerland: 2004. [Google Scholar]
- 3.Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environmental health perspectives. 1992;98:259–280. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clinical and experimental dermatology. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Geraci MJ. Mustard gas: imminent danger or eminent threat? The Annals of pharmacotherapy. 2008;42:237–246. doi: 10.1345/aph.1K445. [DOI] [PubMed] [Google Scholar]
- 6.Smith KJ, Skelton H. Chemical warfare agents: their past and continuing threat and evolving therapies. Part I of II. Skinmed. 2003;2:215–221. doi: 10.1111/j.1540-9740.2003.02509.x. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg MR. Public health, law, and local control: destruction of the US chemical weapons stockpile. American journal of public health. 2003;93:1222–1226. doi: 10.2105/ajph.93.8.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balali-Mood M, Hefazi M, Mahmoudi M, et al. Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundamental & clinical pharmacology. 2005;19:713–721. doi: 10.1111/j.1472-8206.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 9.Mansour Razavi S, Salamati P, Saghafinia M, et al. A review on delayed toxic effects of sulfur mustard in Iranian veterans. Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2012;20:51. doi: 10.1186/2008-2231-20-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacological reviews. 1996;48:289–326. [PubMed] [Google Scholar]
- 11.Javadi MA, Yazdani S, Sajjadi H, et al. Chronic and delayed-onset mustard gas keratitis - Report of 48 patients and review of literature. Ophthalmology. 2005;112:617–625. doi: 10.1016/j.ophtha.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Kadar T, Dachir S, Cohen L, et al. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Ghasemi H, Ghazanfari T, Ghassemi-Broumand M, et al. Long-term ocular consequences of sulfur mustard in seriously eye-injured war veterans. Cutaneous and ocular toxicology. 2009;28:71–77. doi: 10.1080/15569520902913936. [DOI] [PubMed] [Google Scholar]
- 14.Rowell M, Kehe K, Balszuweit F, et al. The chronic effects of sulfur mustard exposure. Toxicology. 2009;263:9–11. doi: 10.1016/j.tox.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Banin E, Morad Y, Berenshtein E, et al. Injury induced by chemical warfare agents: characterization and treatment of ocular tissues exposed to nitrogen mustard. Investigative ophthalmology & visual science. 2003;44:2966–2972. doi: 10.1167/iovs.02-1164. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MK, DeSantis A, Deshmukh M, et al. Doxycycline Hydrogels as a Potential Therapy for Ocular Vesicant Injury. J Ocul Pharmacol Th. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith KJ, Smith WJ, Hamilton T, et al. Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard. The American Journal of dermatopathology. 1998;20:22–28. doi: 10.1097/00000372-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Goswami DG, Tewari-Singh N, Dhar D, et al. Nitrogen Mustard-Induced Corneal Injury Involves DNA Damage and Pathways Related to Inflammation, Epithelial-Stromal Separation, and Neovascularization. Cornea. 2016;35:257–266. doi: 10.1097/ICO.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tewari-Singh N, Jain AK, Inturi S, et al. Silibinin, dexamethasone, and doxycycline as potential therapeutic agents for treating vesicant-inflicted ocular injuries. Toxicology and applied pharmacology. 2012;264:23–31. doi: 10.1016/j.taap.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morad Y, Banin E, Averbukh E, et al. Treatment of ocular tissues exposed to nitrogen mustard: beneficial effect of zinc desferrioxamine combined with steroids. Investigative ophthalmology & visual science. 2005;46:1640–1646. doi: 10.1167/iovs.04-1165. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg G, Runn P, Winter S, et al. Faellman A. Basic Information on Lewisite. A Chemical Warfare Agent with Effects Similar to Mustard Gas (Grundlaeggande Data om Lewisit. Ett Kemiskt Stridsmedel med Egenskaper Liknande Senapsgas) Lindberg, G; Runn, P; Winter, S.
- 22.Sawyer TW, Nelson P. Hypothermia as an Adjunct Therapy to Vesicant-induced Skin Injury. Eplasty. 2008;8:e25. [PMC free article] [PubMed] [Google Scholar]
- 23.Beebe GW. Lung Cancer in World War I Veterans: Possible Relation to Mustard-Gas Injury and 1918 Influenza Epidemic. Journal of the National Cancer Institute. 1960;25:1231–1252. [PubMed] [Google Scholar]
- 24.Mann I, Pirie A, Pullinger BD. A Study of Lewisite Lesions of the Eyes of Rabbits. American journal of ophthalmology. 29:1223–1227. doi: 10.1016/0002-9394(46)91629-7. [DOI] [PubMed] [Google Scholar]
- 25.Lindsay CD, Hambrook JL, Brown RFR, et al. Examination of changes in connective tissue macromolecular components of large white pig skin following application of lewisite vapour. Journal of Applied Toxicology. 2004;24:37–46. doi: 10.1002/jat.942. [DOI] [PubMed] [Google Scholar]
- 26.Hughes WF. CLINICAL USES OF 2,3-DIMERCAPTOPROPANOL (BAL). IX. THE TREATMENT OF LEWISITE BURNS OF THE EYE WITH BAL. Journal of Clinical Investigation. 1946;25:541–548. doi: 10.1172/JCI101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes WF., Jr. Treatment of lewisite burns of the eye with dimercaprol (BAL) Arch Ophthal. 1947;37:25–41. doi: 10.1001/archopht.1947.00890220030004. [DOI] [PubMed] [Google Scholar]
- 28.Boyd VL, Harbell JW, O'Connor RJ, et al. 2,3-Dithioerythritol, a possible new arsenic antidote. Chemical research in toxicology. 1989;2:301–306. doi: 10.1021/tx00011a006. [DOI] [PubMed] [Google Scholar]
- 29.Mouret S, Wartelle J, Emorine S, et al. Topical efficacy of dimercapto-chelating agents against lewisite-induced skin lesions in SKH-1 hairless mice. Toxicology and applied pharmacology. 2013;272:291–298. doi: 10.1016/j.taap.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Sahu C, Pakhira S, Sen K, et al. A computational study of detoxification of lewisite warfare agents by British anti-lewisite: catalytic effects of water and ammonia on reaction mechanism and kinetics. The journal of physical chemistry. A. 2013;117:3496–3506. doi: 10.1021/jp312254z. [DOI] [PubMed] [Google Scholar]
- 31.Black AT, Hayden PJ, Casillas RP, et al. Expression of proliferative and inflammatory markers in a full-thickness human skin equivalent following exposure to the model sulfur mustard vesicant, 2-chloroethyl ethyl sulfide. Toxicology and applied pharmacology. 2010;249:178–187. doi: 10.1016/j.taap.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon MK, Enzenauer RW, Babin MC. Ocular toxicity of sulfur mustard. In: Gupta RC, editor. Handbook of toxicology of chemical warfare agents. Elsevier Inc; 2009. pp. 575–594. [Google Scholar]
- 33.Milhorn D, Hamilton T, Nelson M, et al. Progression of ocular sulfur mustard injury: development of a model system. Annals of the New York Academy of Sciences. 2010;1194:72–80. doi: 10.1111/j.1749-6632.2010.05491.x. [DOI] [PubMed] [Google Scholar]
- 34.Joseph LB, Gerecke DR, Heck DE, et al. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Experimental and molecular pathology. 2011;91:515–527. doi: 10.1016/j.yexmp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain AK, Tewari-Singh N, Gu M, et al. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicology letters. 2011;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amir A, Turetz J, Chapman S, et al. Beneficial effects of topical anti-inflammatory drugs against sulfur mustard-induced ocular lesions in rabbits. Journal of applied toxicology : JAT. 2000;20(Suppl 1):S109–114. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat669>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Javadi MA, Yazdani S, Kanavi MR, et al. Long-term Outcomes of Penetrating Keratoplasty in Chronic and Delayed Mustard Gas Keratitis. Cornea. 2007;26:1074–1078. doi: 10.1097/ICO.0b013e3181334752. 1010.1097/ICO.1070b1013e3181334752. [DOI] [PubMed] [Google Scholar]
- 38.McNutt P, Hamilton T, Nelson M, et al. Pathogenesis of acute and delayed corneal lesions after ocular exposure to sulfur mustard vapor. Cornea. 2012;31:280–290. doi: 10.1097/ICO.0B013E31823D02CD. [DOI] [PubMed] [Google Scholar]
- 39.Kadar T, Amir A, Cohen L, et al. Anti-VEGF therapy (bevacizumab) for sulfur mustard-induced corneal neovascularization associated with delayed limbal stem cell deficiency in rabbits. Current eye research. 2014;39:439–450. doi: 10.3109/02713683.2013.850098. [DOI] [PubMed] [Google Scholar]
- 40.Kadar T, Cohen M, Cohen L, et al. Endothelial cell damage following sulfur mustard exposure in rabbits and its association with the delayed-onset ocular lesions. Cutaneous and ocular toxicology. 2013;32:115–123. doi: 10.3109/15569527.2012.717571. [DOI] [PubMed] [Google Scholar]
- 41.Ruff AL, Jarecke AJ, Hilber DJ, et al. Development of a mouse model for sulfur mustard-induced ocular injury and long-term clinical analysis of injury progression. Cutaneous and ocular toxicology. 2013;32:140–149. doi: 10.3109/15569527.2012.731666. [DOI] [PubMed] [Google Scholar]
- 42.Zheng R, Po I, Mishin V, et al. The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicology and applied pharmacology. 2013;272:345–355. doi: 10.1016/j.taap.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruff AL, Dillman JF., 3rd Sulfur mustard induced cytokine production and cell death: investigating the potential roles of the p38, p53, and NF-kappaB signaling pathways with RNA interference. Journal of biochemical and molecular toxicology. 2010;24:155–164. doi: 10.1002/jbt.20321. [DOI] [PubMed] [Google Scholar]
- 44.Petrali JP, Dick EJ, Brozetti JJ, et al. Acute ocular effects of mustard gas: ultrastructural pathology and immunohistopathology of exposed rabbit cornea. Journal of applied toxicology : JAT. 2000;20(Suppl 1):S173–175. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat679>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 45.del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Experimental eye research. 2015;137:111–124. doi: 10.1016/j.exer.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Tewari-Singh N, Gu M, Agarwal C, et al. Biological and molecular mechanisms of sulfur mustard analogue-induced toxicity in JB6 and HaCaT cells: possible role of ataxia telangiectasia-mutated/ataxia telangiectasia-Rad3-related cell cycle checkpoint pathway. Chemical research in toxicology. 2010;23:1034–1044. doi: 10.1021/tx100038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kehe K, Balszuweit F, Steinritz D, et al. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Shakarjian MP, Heck DE, Gray JP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 50.Jain AK, Tewari-Singh N, Orlicky DJ, et al. 2-Chloroethyl ethyl sulfide causes microvesication and inflammation-related histopathological changes in male hairless mouse skin. Toxicology. 2011;282:129–138. doi: 10.1016/j.tox.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mann I, Pirie A, Pullinger BD. The treatment of lewisite and other arsenical vesicant lesions of the eyes of rabbits with British anti-lewisite (BAL) American journal of ophthalmology. 1947;30:421–435. doi: 10.1016/0002-9394(47)91183-5. [DOI] [PubMed] [Google Scholar]
- 52.Anumolu SS, DeSantis AS, Menjoge AR, et al. Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds. Biomaterials. 2010;31:964–974. doi: 10.1016/j.biomaterials.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadar T, Turetz J, Fishbine E, et al. Characterization of acute and delayed ocular lesions induced by sulfur mustard in rabbits. Current eye research. 2001;22:42–53. doi: 10.1076/ceyr.22.1.42.6975. [DOI] [PubMed] [Google Scholar]
- 54.Tewari-Singh N, Croutch CR, Tuttle R, et al. Clinical progression of ocular injury following arsenical vesicant lewisite exposure. Cutaneous and ocular toxicology. 2016:1–10. doi: 10.3109/15569527.2015.1127255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Olajos EJ, Olson CT, Salem H, et al. Evaluation of neutralized Chemical Agent Identification Sets (CAIS) for skin injury with an overview of the vesicant potential of agent degradation products. Journal of Applied Toxicology. 1998;18:409–420. doi: 10.1002/(sici)1099-1263(199811/12)18:6<409::aid-jat515>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 56.McNutt P, Tuznik K, Nelson M, et al. Structural, morphological, and functional correlates of corneal endothelial toxicity following corneal exposure to sulfur mustard vapor. Investigative ophthalmology & visual science. 2013;54:6735–6744. doi: 10.1167/iovs.13-12402. [DOI] [PubMed] [Google Scholar]
- 57.Kehe K, Flohe S, Krebs G, et al. Effects of Lewisite on cell membrane integrity and energy metabolism in human keratinocytes and SCL II cells. Toxicology. 2001;163:137–144. doi: 10.1016/s0300-483x(01)00389-4. [DOI] [PubMed] [Google Scholar]
- 58.Nelson P, Hancock JR, Sawyer TW. Therapeutic effects of hypothermia on Lewisite toxicity. Toxicology. 2006;222:8–16. doi: 10.1016/j.tox.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 59.Graham JS, Schoneboom BA. Historical perspective on effects and treatment of sulfur mustard injuries. Chemico-biological interactions. 2013;206:512–522. doi: 10.1016/j.cbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Kadar T, Horwitz V, Sahar R, et al. Delayed Loss of Corneal Epithelial Stem Cells in a Chemical Injury Model Associated with Limbal Stem Cell Deficiency in Rabbits. Current eye research. 2011;36:1098–1107. doi: 10.3109/02713683.2011.609305. [DOI] [PubMed] [Google Scholar]
- 61.Tianyi G. Mechanism and treatment of sulfur mustard-induced cutaneous injury. Chinese Journal of Traumatology. 2014;17:345–350. [PubMed] [Google Scholar]
- 62.Kumar R, Deep G, Agarwal R. An Overview of Ultraviolet B Radiation-Induced Skin Cancer Chemoprevention by Silibinin. Current pharmacology reports. 2015;1:206–215. doi: 10.1007/s40495-015-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deep G, Agarwal R. Targeting tumor microenvironment with silibinin: promise and potential for a translational cancer chemopreventive strategy. Current cancer drug targets. 2013;13:486–499. doi: 10.2174/15680096113139990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jain AK, Tewari-Singh N, Inturi S, et al. Flavanone silibinin treatment attenuates nitrogen mustard-induced toxic effects in mouse skin. Toxicology and applied pharmacology. 2015;285:71–78. doi: 10.1016/j.taap.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tewari-Singh N, Jain AK, Inturi S, et al. Silibinin attenuates sulfur mustard analog-induced skin injury by targeting multiple pathways connecting oxidative stress and inflammation. PloS one. 2012;7:e46149. doi: 10.1371/journal.pone.0046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ting H, Deep G, Agarwal R. Molecular mechanisms of silibinin-mediated cancer chemoprevention with major emphasis on prostate cancer. The AAPS journal. 2013;15:707–716. doi: 10.1208/s12248-013-9486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer metastasis reviews. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bian F, Pelegrino FS, Tukler Henriksson J, et al. Differential Effects of Dexamethasone and Doxycycline on Inflammation and MMP Production in Alkali-Burned Corneas Associated with Dry Eye. The ocular surface. 2016 doi: 10.1016/j.jtos.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith VA, Cook SD. Doxycycline—a role in ocular surface repair. The British journal of ophthalmology. 2004;88:619–625. doi: 10.1136/bjo.2003.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solomon A, Rosenblatt M, Li D, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. American journal of ophthalmology. 2000;130:688. doi: 10.1016/s0002-9394(00)00755-8. [DOI] [PubMed] [Google Scholar]
- 71.Yeh YC, Lai HC, Ting CT, et al. Protection by doxycycline against doxorubicin-induced oxidative stress and apoptosis in mouse testes. Biochemical pharmacology. 2007;74:969–980. doi: 10.1016/j.bcp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 72.Horwitz V, Dachir S, Cohen M, et al. The Beneficial Effects of Doxycycline, An Inhibitor of Matrix Metalloproteinases, on Sulfur Mustard-Induced Ocular Pathologies Depend on the Injury Stage. Current eye research. 2014 doi: 10.3109/02713683.2013.874443. [DOI] [PubMed] [Google Scholar]
- 73.Kimura K, Teranishi S, Kawamoto K, et al. Protective effect of dexamethasone against hypoxia-induced disruption of barrier function in human corneal epithelial cells. Experimental eye research. 2011;92:388–393. doi: 10.1016/j.exer.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Chung JH, Paek SM, Choi JJ, et al. Effect of topically applied 0.1% dexamethasone on endothelial healing and aqueous composition during the repair process of rabbit corneal alkali wounds. Current eye research. 1999;18:110–116. doi: 10.1076/ceyr.18.2.110.5375. [DOI] [PubMed] [Google Scholar]
- 75.Dachir S, Fishbeine E, Meshulam Y, et al. Amelioration of sulfur mustard skin injury following a topical treatment with a mixture of a steroid and a NSAID. Journal of applied toxicology : JAT. 2004;24:107–113. doi: 10.1002/jat.955. [DOI] [PubMed] [Google Scholar]
- 76.Dachir S, Fishbeine E, Meshulam Y, et al. Potential anti-inflammatory treatments against cutaneous sulfur mustard injury using the mouse ear vesicant model. Human & experimental toxicology. 2002;21:197–203. doi: 10.1191/0960327102ht229oa. [DOI] [PubMed] [Google Scholar]
- 77.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocrine-related cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 78.Cheung CW, Gibbons N, Johnson DW, et al. Silibinin--a promising new treatment for cancer. Anti-cancer agents in medicinal chemistry. 2010;10:186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- 79.Inturi S, Tewari-Singh N, Gu M, et al. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free radical biology & medicine. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar D, Tewari-Singh N, Agarwal C, et al. Nitrogen mustard exposure of murine skin induces DNA damage, oxidative stress and activation of MAPK/Akt-AP1 pathway leading to induction of inflammatory and proteolytic mediators. Toxicology letters. 2015;235:161–171. doi: 10.1016/j.toxlet.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]