Abstract
Background and Aims
Few randomized controlled trials have evaluated buprenorphine treatment interventions for opioid-dependent youth. Consequently, optimal administration strategies for this cohort are unclear. Our aim was to evaluate the relative efficacy of two different buprenorphine taper lengths in promoting abstinence from illicit opioids and treatment retention among opioid-dependent youth.
Design
A double blind, placebo controlled, multicenter randomized controlled trial.
Setting
Two hospital-based research clinics (Manhattan and Brooklyn) in New York City, USA from 2005 to 2010.
Participants
Volunteer sample of 53 primarily Caucasian participants between the ages of 16 and 24 (n=11 under age 18) who met DSM-IV opioid dependence criteria.
Intervention
Participants were randomly assigned to either a 28-day buprenorphine taper (n=28) or 56-day buprenorphine taper (n=25) via a parallel groups design over a 63-day period. Both groups received behavioral counseling and opioid abstinence incentives. Both taper conditions had a minimum of one week of placebo dosing at the end of the taper.
Measurements
The primary outcome was opioid abstinence measured as percent of scheduled urine toxicology tests documented to be negative for opioids. The secondary outcome was treatment retention, measured as number of days attended scheduled visits.
Findings
Intent-to-treat analyses revealed that participants who received a 56-day buprenorphine taper had a significantly higher percentage of opioid negative scheduled urine tests compared with participants who received a 28-day buprenorphine taper (35% vs 17%, p=.039; Cohen's d=0.57, 95% CI: 0.02,1.13). Participants who received a 56-day buprenorphine taper were retained in treatment significantly longer than participants who received a 28-day buprenorphine taper (37.5 vs 26.4 days, p=.027; Cohen's d=0.63, 95% CI: 0.06, 1.19). Daily attendance requirement was associated with decreased abstinence and shorter retention compared with a 2-3 times weekly attendance requirement, independent of taper duration. Follow-up data were insufficient to report.
Conclusion
Longer (56-day) buprenorphine taper produces better opioid abstinence and retention outcomes than shorter (28-day) buprenorphine taper for opioid-dependent youth.
1. Introduction
Opioid use disorders and overdose continue to be significant international public health problems (1-3). In the United States, close to one million young adults between the ages of 18 and 25, and close to half a million adolescents between the ages of 12 and 17 are current nonmedical users of opioid pain relievers (4). Current nonmedical use of pain relievers is more prevalent among these two groups than among those age 26 and older (4). Lifetime prevalence of misuse of ‘narcotics’ (e.g. Vicodin, OxyContin, Percocet) other than heroin among 12th graders remains alarmingly high at 9.5% (5). Data from the Researched Abuse, Diversion and Addiction-Related Surveillance System (RADARS) indicate that opioids constitute 68% of the types of drugs of intentional exposure among adolescents and three of the five most frequently abused prescription drugs (6). Many youth perceive less risk in using prescription opioids (7) compared with use of other illicit drugs, and early onset of non-medical use of prescription opioids is a significant predictor of an opioid use disorder (8).
Many young people who initiate opioid use with prescription opioids transition to using heroin (9). Additionally, the percent of heroin users initiating opioid use via heroin is rising (10). In 2014, approximately 20% of adolescents (ages 12-17) did not report that they perceived a great risk from using heroin once or twice a week (11) and approximately 20% of 12th graders reported that it would not be very difficult to obtain heroin (5). Many adolescents initiate heroin use intranasally (12) and then progress to injection (13). Among substance-using youth, heroin-using adolescents have the highest rate of injection drug use, (14) which increases their risk of contracting and spreading HIV and other serious diseases (13, 15-17). Young injection drug users also have higher rates of premature mortality compared to the general population (18, 19). From 2008 to 2012, the heroin overdose death rate among those aged 15 to 24 rose to 2.3 per 100,000 (20). The most current Drug Abuse Warning Network data indicate that over 67,000 heroin involved ED visits occurred among youth age 12 to 24 (21).
The prevalence and serious consequences of opioid misuse among youth underscore the importance of effective treatment. In 2012, 12-25 year olds constituted close to 30% of opioid related treatment admissions (22) and yet their rates of retention within the first three months of outpatient opioid addiction treatment are significantly lower than adults (23). Despite this, little research has been conducted to systematically evaluate treatment interventions for young opioid users. The scientific literature indicates that pharmacotherapy is a necessary component of effective opioid withdrawal treatment for adults (24). Buprenorphine is an FDA approved (25) partial mu-opioid receptor agonist medication that is used to treat opioid use disorders (26) by stabilizing the brain neurochemistry (27). While buprenorphine has been established as an effective medication for adults with opioid use disorders, (28, 29) its safety and efficacy for youth is less well understood (26, 30) as research on buprenorphine treatment for youth has been limited. To address this gap, we previously conducted a randomized, double-blind, double-dummy controlled trial comparing the relative efficacy of buprenorphine and the adrenergic blocker clonidine in a 28-day outpatient, medication-assisted withdrawal with opioid-dependent youth (aged 13-18) (31). Retention and opioid abstinence results supported significantly greater efficacy of buprenorphine. Additional results indicated that both medications were safe and did not produce adverse effects. A randomized trial conducted by Woody and colleagues compared the effectiveness of 14-day buprenorphine detoxification to a twelve week buprenorphine maintenance for opioid-dependent youth. Maintenance condition participants had significantly better retention and significantly less opioid positive urine results (32).
The current trial aimed to build on our earlier work evaluating a 28-day buprenorphine taper and provide new empirical data about optimal models of treatment for opioid-dependent youth by examining whether improved opioid abstinence and retention outcomes could be achieved by lengthening the duration of buprenorphine taper administration. Although buprenorphine maintenance produces better treatment outcomes than buprenorphine tapers among adults with opioid use disorders, we chose to evaluate two different tapers because currently no standardized buprenorphine treatment model exists for this young population. Youth were randomly assigned to receive either a 28-day or 56-day buprenorphine-assisted taper. We hypothesized that over the course of the 63-day trial, adolescents and young adults receiving the 56-day taper would (1) submit a significantly larger percent of opioid negative urines and (2) have a greater mean number of days retained in treatment than adolescents and young adults receiving the 28-day taper.
2. Methods
2.1. Design
Participants were randomly assigned to receive a double-blind buprenorphine and buprenorphine/naloxone-assisted taper (Subutex® and Suboxone® Reckitt Benckiser Pharmaceuticals Inc. Richmond, VA) of either (1) 28 days or (2) 56 days in duration in a parallel groups design on intake day following completion of informed consent procedures. Enrollment occurred at two St. Luke's Roosevelt Hospital clinical research sites in Manhattan and Brooklyn, New York. A minimum allocation procedure was used to randomly assign participants to study conditions (33, 34). This method balances groups on participant characteristics likely to influence treatment outcomes. Participants were balanced on: (1) gender (2) age (13-17 vs. 18-24 years) (3) past month primary route of opioid administration (injection or other), and (4) availability of a parent/guardian or significant other to participate. If a participant failed to: (1) attend the clinic on 3 consecutive, scheduled visits, (2) participate in 3 consecutive, scheduled counseling sessions or (3) provide 3 consecutive, scheduled urine samples, s/he was discharged on the 3rd missed day and referred to a different treatment facility. The study duration was 63 days. To avoid expectancy effects, each participant received the same number of identical looking tablets (provided by the manufacturer) throughout the trial. The amount of active buprenorphine was reduced over the course of the study so that by the end, each participant was taking only placebo tablets. At study inception, participants were required to attend treatment daily. After enrolling 20 participants, we obtained IRB approval to allow participants to earn take home doses of buprenorphine. After a period of stabilization, participants came 2 to 3 times weekly instead of daily. This protocol change was designed to reduce participant attendance burden, and did not alter the study goals. Behavioral therapy and contingency management interventions to incentivize treatment gains were offered to all participants throughout their participation in the trial. Details about these procedures are provided below.
2.2. Participants
Participants were opioid-dependent adolescents (ages 13 to 17) and young adults (ages 18 to 24) (13-24 yrs. eligible) who were recruited from the New York City area. Recruitment strategies included medical network referral, self-referral via advertisements, and community outreach. Eligible participants met DSM-IV criteria for opioid dependence. Additionally, the DSM-IV Checklist for Childhood Disorders(35) and the Global Assessment of Individual Needs (GAIN)(36) scale were used to screen and characterize potential participants. All participants received a standard medical evaluation before study enrollment. Exclusion criteria included: pregnancy; active significant psychiatric disorder (e.g. schizophrenia); or serious medical condition. Adolescents with other co-occurring psychiatric or substance use disorders were not excluded. Ineligible youth were referred to appropriate alternative services. Youth under the age of 18 provided assent with a parent or legal guardian who provided in-person written consent. A study investigator oversaw all informed consent procedures in which a complete description of the study was given to participants and written informed consent was obtained. We obtained institutional review board approval and an investigational new drug (IND) approval from the Food and Drug Administration. This study was conducted before the DSM-V had been published and thus we refer to the DSM-IV diagnostic categories used in this trial.
2.3. Sample Size Calculations
The proposed sample size for the study was 40 participants per group based on the expectation of having sufficient power for the primary outcome measure, opioid abstinence, expressed as the mean percent of scheduled urinalyses documented to be opiate negative. Based on our prior estimate of variability (σ=30%), the estimated power (1-β) was 80% to detect a mean difference of 20% using α=.05. Although we recruited a smaller than initially projected sample size, the post hoc estimated power was higher than initially planned (with approximately 80% power to detect a mean difference of 23%).
2.4. Medication Administration
2.4.1. Induction
Typical buprenorphine inductions lasted approximately 5 to 8 hours. All participants were advised not to use illicit opioids after midnight on the morning of their first buprenorphine dose to prevent antagonist effects (37). Before receiving their first buprenorphine dose, participants must have exhibited observable, clinically significant signs of opioid withdrawal as measured by the standard Clinical Institute Narcotic Assessment Scale (38). Additionally, participants were required to pass breathalyzer and field sobriety tests and provide a urine sample. Physiological data were also obtained (e.g., blood pressure, O2 saturation, pupil diameter).
Subutex® tablets (buprenorphine) were prescribed during the first two days to start with a formulation that did not have the opioid antagonist naloxone. Once stable, participants were prescribed Suboxone® tablets (buprenorphine/naloxone) (37). At intake, participants who weighed < 70 kg and/or reported using < 3 bags of heroin or its prescription opioid equivalent received an initial dose of 6 mg (31, 39). Participants who weighed > 70 kg and/or reported using ≥ 3 bags of heroin or its prescription opioid equivalent received an initial dose of 8 mg (31, 39). Participants held the tablets under their tongue for 5 minutes without speaking, and drank water before and after they ingested the medication.
If participants exhibited clinically significant withdrawal symptoms 1 hour after their initial dose on induction day, an additional 2, 4, 6, or 8 mg was provided. All participants passed a field sobriety test prior to discharge. No trial-specific serious adverse events occurred during this study. Our medical director was on-call every day during the study. Observations from study physicians showed buprenorphine was well tolerated throughout the induction and taper phases. Research staff observed buprenorphine adherence when participants attended the clinic but not when participants took buprenorphine at home. The total number of days of buprenorphine/naloxone use was the same for all participants within a given condition— 28-day or 56-day—despite differences in starting dose.
2.4.2. 28-day Buprenorphine/Naloxone Taper
Participants who received a total of 6 mg during induction received 6, 4, 2, and 0 (placebo) mg on days 1-9, 10-18, 19-27, and 28-63 respectively. Similarly, participants who received a total of 8 mg during induction received 8, 6, 4, 2, and 0 mg on days 1-7, 8-14, 5-21, 22-27, and 28-63 respectively. Similar taper procedures were applied to the other potential starting doses (12 and 16 mg).
2.4.3. 56-day Buprenorphine/Naloxone Taper
Participants who received a total of 6 mg during induction received 6, 4, 2, and 0 mg on days 1-18, 19-37, 38-56, and 57-63 respectively. Similarly, participants who received a total of 8 mg during induction received 8, 6, 4, 2, and 0 mg on days 1-14, 15-28, 29-42, 43-56 and 57-63 respectively. Similar taper procedures were applied to the other potential starting doses (12 and 16 mg). Both taper conditions had a minimum of one week of placebo dosing at the end of the taper.
2.5. Urinalysis
Urine specimens were collected under staff supervision from all participants at intake, and randomly observed on days participants were scheduled to attend the clinic. Samples were screened using semi-quantitative urinalysis procedures (Dade-Behring, VIVA-E) and assessed for opioids (e.g. methadone, morphine, morphine derivatives and other opioids), cocaine, marijuana (1x/wk) and benzodiazepines (1x/wk). Breath alcohol measures conducted during each urine sample collection had to be less than 0.05 g/ml of air to receive medication.
2.6. Behavior Therapy
Behavioral therapy (40) was conducted three times per week for those required to receive daily observed doses of buprenorphine at the clinic. Behavioral therapy was conducted twice per week for those who received take home doses of buprenorphine. The therapy, focused on the unique treatment needs of adolescents (e.g., legal issues, anger management, housing, etc.). The model of therapy was equivalent across groups, and there was no change in this treatment when attendance requirements changed. The therapy was based on a Motivational Interviewing and Community Reinforcement Approach framework (41) and had three components: psychoeducational, cognitive-behavioral, and family systems. Therapists were given study-specific training manuals and trained by study investigators who provided regular supervision. Participant issues were discussed at weekly staff meetings.
2.7. Vouchers
All participants received vouchers (incentives) based on the National Institute on Drug Abuse manual on Community Reinforcement Approach (42) to reinforce opioid abstinence. Participants earned vouchers by providing opioid-negative urine samples. Vouchers were used to help participants increase non-drug use related activities using items such as movie tickets. Vouchers were also used to reinforce clinic attendance and completion of weekly assessments, and were administered at each behavioral therapy session. Participants earned a total of $798.75 in vouchers ($596.25 from opioid-negative urine samples) if they remained opioid abstinent, had perfect clinic attendance, and completed all assessments. The first opioid-negative urine sample was worth $2.50. Vouchers increased in value by $3.00 for each consecutive opioid-negative urine sample. Participants earned a $10.25 bonus voucher for providing a week of opioid-negative urines. Participants who submitted opioid-positive urines earned no voucher for that day and the next voucher for an opioid-negative urine sample was reset to $2.50. If a participant provided 5 consecutive opioid-negative urine samples after the lapse, they returned to their voucher value before the lapse. Among all participants required to attend the clinic daily (n=20), the mean total voucher compensation was $31.73. Earnings ranged from $0 to $517.50. Seventy-five percent (n=15) of these participants earned no vouchers. Among participants required to attend the clinic 2 to 3 times per week (n=33), the mean total voucher compensation was $123.78. Earnings ranged from $0 to $542.25. Approximately 18% (n=6) of these participants earned no vouchers.
2.8. Outcome Measures
2.8.1. Primary Outcome
The primary outcome measure was biochemically documented opioid abstinence as defined by the proportion of all scheduled urinalyses negative for opioids during the 63-day trial. For this intent-to-treat (ITT) approach, all missed urine samples, whether occurring prior to, or after participants dropped out of treatment, were considered opioid positive. Additionally, we conducted sensitivity analyses which examined percent abstinence independent of retention and missed urinalyses. Specifically, we also defined percent abstinence as (a) percent of urine samples documented to be opioid negative during the period participants remained in treatment and (b) percent of urine samples documented to be opioid negative among samples actually obtained from each participant (i.e. missing not assumed to be positive).
2.8.2. Secondary Outcomes
Several secondary outcomes were examined between the two taper conditions including mean number of days retained in treatment, percent of participants that completed treatment, and average of participants’ longest duration of continuous abstinence from opioids.
2.9. Statistical Analyses
Treatment groups were compared on differences in participant characteristics using t-tests for normal-like continuous variables, Wilcoxon Rank Sum tests for non-normal measures, and chi-square tests for nominal variables. Primary analyses were based on all participants randomized to treatment groups independent of early dropout, noncompliance etc., consistent with an intent-to-treat approach to clinical trials. (43) Two-way analyses of variance with fixed factors representing taper condition (28-day and 56-day tapers), attendance schedule (daily vs 2 to 3 times weekly), and their interaction were used to examine primary and secondary outcomes measures of abstinence and retention. The proportion of participants completing treatment was compared using a Cochran-Mantel-Haenszel test comparing taper groups conditioning on attendance schedule. Homogeneity was tested based on a Breslow-Day test. Effect sizes (Cohen's d) were computed using the R subroutine “compute.es”. Statistical analyses were performed using SAS statistical software Version 9.2 (SAS Institute, Cary, NC). Statistical significance was determined at p < 0.05.
3. Results
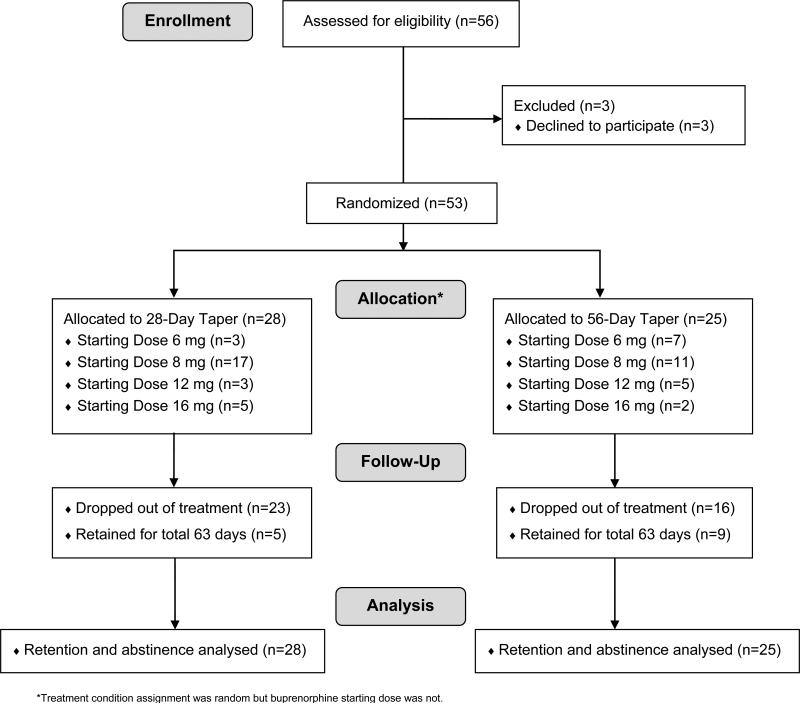
Fifty-six participants provided either consent (≥18 years) or assent (<18 years). Three participants discontinued the study prior to being randomly assignment to treatment. Thus, 53 participants were randomly assigned to a study condition (28-day buprenorphine-taper condition (n=28) and 56-day buprenorphine taper condition (n=25)) (Figure 1).
Figure 1.
CONSORT Diagram
3.1 Participant Characteristics
There were 22 females and 31 males. The mean age of the sample was 20.5 years and most participants were Caucasian (76%). Fourteen percent (n=4) of participants in the 28-day group and 28% (n=7) of participants in the 56-day group were under the age of 18. The youngest participants in the trial were 16 years old. Comparable levels of heroin, prescription opioid, and polysubstance use and co-morbid psychopathology were observed across both conditions. There were no significant differences between treatment groups except for nicotine dependence. Additional participant characteristics are provided in Table 1.
Table 1.
Participant Characteristics by Taper Condition at Treatment Intake*
| 28-day condition (n=28)† | 56-day condition (n=25)† | |||||
|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | n (%) | Mean | SD | n (%) |
| Age (years) | 21.0 | (2.5) | 19.9 | (2.7) | ||
| Male | 15 (54) | 16 (64) | ||||
| Caucasian | 21 (81) | 16 (70) | ||||
| Intravenous opioid user | 16 (57) | 15 (60) | ||||
| Heroin primary opioid used | 24 (86) | 19 (76) | ||||
| Age of first opioid use | 17.0 | (2.1) | 16.0 | (2.1) | ||
| Age of regular opioid use onset | 17.7 | (2.3) | 17.1 | (2.4) | ||
| Years of regular opioid use | 3.6 | (2.2) | 2.9 | (1.6) | ||
| ≥3 Bags of heroin per day | 23 (82) | 15 (60) | ||||
| DSM-IV diagnoses (met criteria at intake) | ||||||
| Mixed anxiety/depression | 20 (71) | 15 (60) | ||||
| Major depressive disorder | 14 (52) | 13 (52) | ||||
| Manic episode disorder | 12 (44) | 9 (36) | ||||
| Oppositional defiant disorder | 9 (32) | 10 (42) | ||||
| Conduct disorder | 8 (29) | 8 (33) | ||||
| Attention-deficit/hyperactivity disorder | 14 (50) | 8 (32) | ||||
| Homeless | 2 (7) | 3 (12) | ||||
| Starting Dose for Taper | ||||||
| 16 mg | 5 (18) | 2 (8) | ||||
| 12 mg | 3 (11) | 5 (20) | ||||
| 8 mg | 17 (61) | 11 (44) | ||||
| 6 mg | 3 (11) | 7 (28) | ||||
| Days used heroin in last 90 days | 67.9 | (30.3) | 59.8 | (33.0) | ||
| Days used prescription opioids in last 90 days | 16.5 | (31.3) | 16.2 | (27.6) | ||
| Prior substance use related ER visits | 0.9 | (2.1) | 0.84 | (1.4) | ||
| Prior outpatient substance use treatment episodes | 0.5 | (0.9) | 0.8 | (1.4) | ||
| Prior inpatient substance use treatment episodes | 0.7 | (1.2) | 2.4 | (5.1) | ||
| Other substance dependence (present at intake) | ||||||
| Alcohol | 7 (25) | 7 (28) | ||||
| Cannabis | 6 (21) | 7 (28) | ||||
| Nicotine | 20 (71) | 8 (32) | ||||
| Cocaine | 7 (25) | 7 (28) | ||||
| Amphetamine | 2 (7) | 1 (4) | ||||
No significant differences were observed between treatment group participant characteristics (p = 0.05) except for nicotine dependence.
28 or 25 participants were available for all variables in the table with the exception of: Caucasian (n=26 for the 28-day taper & n=23 for the 56-day taper), major depressive disorder (n=27 for the 28-day taper), manic episode disorder (n=27 for the 28-day taper), oppositional defiant disorder (n=24 for the 56-day taper), conduct disorder (n=24 for the 56-day taper).
3.2. Primary Analyses: Opioid Abstinence
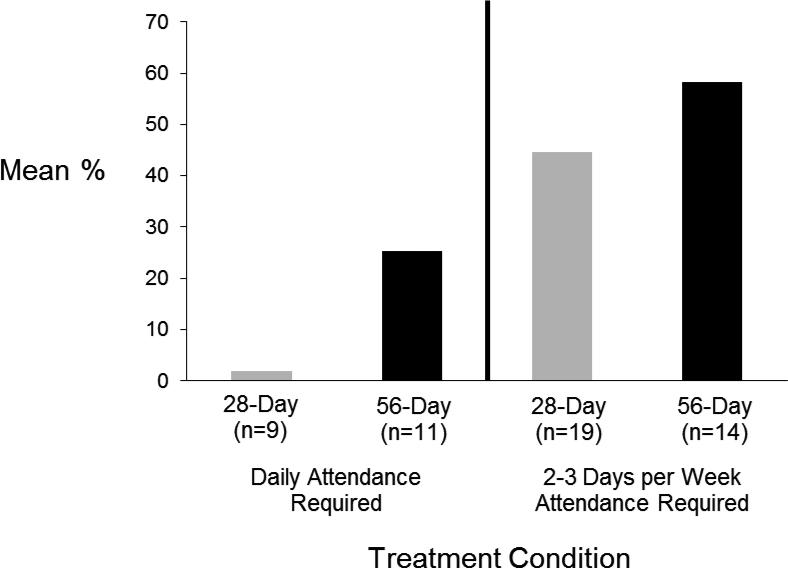
Participants in the 56-day condition achieved significantly greater opioid abstinence compared to those in the 28-day condition. Participants in the 56-day condition had approximately 35% of scheduled urines negative for opioids compared to 17% in the 28-day taper group (Table 2). Similar differences were observed between the two taper groups when percent abstinence was based only on the period in which participants remained in treatment and when based solely on samples obtained (i.e. missing not considered positive). Additionally, attendance schedule had a significant effect on abstinence. Participants who were required to attend 2 to 3 times weekly had a significantly greater percentage of scheduled urine samples documented to be opioid negative compared to participants who were required to attend daily (Table 2, Figure 2). Similar attendance schedule differences were observed for percent abstinence while participants remained in treatment (Table 2) and when considering only samples obtained (Table 2). There was no evidence that treatment differences were dependent on attendance schedule (interaction p ≥ .57 for all measures of abstinence).
Table 2.
Abstinence and Retention Outcomes
| Buprenorphine Taper Group | Clinic Attendance Requirement | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Measure | 28-day (n=28) | 56-day (n=25) | Daily (n=20) | 2-3 days/wk (n=33) | ||||
| Primary Outcome | Mean [95% CI]a | Mean [95% CI] | p-value | Effect Size [95%CI]b | Mean [95% CI]c | Mean [95% CI] | p-value | Effect Size [95%CI]b |
| % opioid negative (all scheduled urinalyses - ITT) | 17.2 [5.8, 28.6] | 34.6 [23.2, 50.0] | .039 | 0.57 [0.02, 1.13] | 8.6 [0.0, 21.6] | 43.2 [33.0, 53.4] | <.001 | 1.20 [0.58, 1.81] |
| % opioid negative (while retained in treatment) | 23.2 [11.4, 34.9] | 41.7 [29.9, 53.5] | .035 | 0.59 [0.03, 1.14] | 13.6 [0.0, 27.1] | 51.4 [40.8, 62.0] | <.001 | 1.25 [0.63, 1.87] |
| % opioid negative (only samples obtained) | 31.0 [17.3, 44.7] | 55.9 [42.2, 69.6] | .017 | 0.68 [0.11, 1.25] | 20.6 [4.7, 36.6] | 66.2 [53.7, 78.7] | <.001 | 1.28 [0.66, 1.90] |
| Secondary Outcomes | ||||||||
| Days retained in treatment | 26.4 [19.6, 32.9] | 37.5 [30.8, 44.3] | .027 | 0.63 [0.06, 1.19] | 17.3 [9.5, 25.0] | 46.7 [40.6, 52.8] | <.001 | 1.70 [1.04, 2.35] |
| Longest Duration of Opioid Abstinence (days) | 7.3 [1.3, 13.6] | 16.3 [10.0, 22.6] | .053 | 0.55 [−0.02, 1.11] | 4.7 [0.0, 11.9] | 19.0 [13.4, 24.6] | .003 | 0.88 [0.30, 1.49] |
Least square means from two-way ANOVA adjusted for attendance schedule
Effect sizes are expressed as Cohen's d.
Least square means from two-way ANOVA adjusted for taper group
Figure 2.
Mean Percent of Urine Screens Documented as Opioid Negative by Buprenorphine Treatment Condition Before and After Changes in Attendance Requirement
3.3. Secondary Analyses: Retention and Longest Duration of Continuous Abstinence
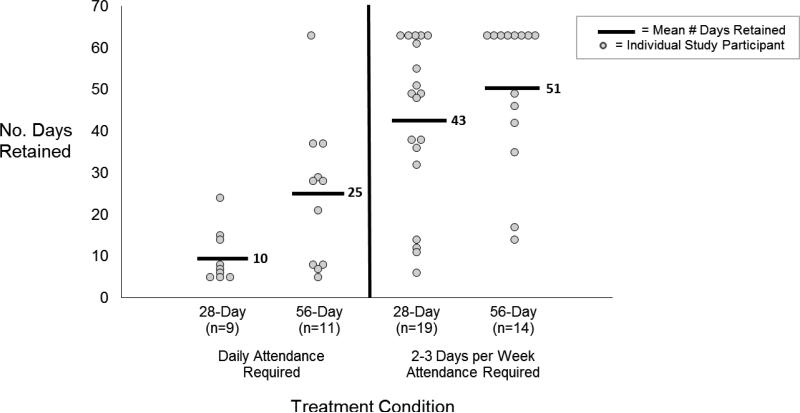
Participants in the 56-day condition were retained in treatment significantly longer than those in the 28-day condition (Table 2). Additionally, participants who were required to attend 2 to 3 times weekly were retained significantly longer than those required to attend daily (Table 2, Figure 3). Differences in retention between taper groups were not dependent on attendance schedule (interaction p=.47). Thirty-six percent of participants in the 56-day compared to 18% of those in the 28-day conditions were retained at the end of the 63-day trial (p = .049). There was evidence that participants in the 56-day taper group had a greater number of days of continuous abstinence from opioids compared to the 28-day taper (Table 2). Additionally, participants who attended 2 to 3 times weekly had a significantly greater number of days of continuous abstinence from opioids than those required to attend daily (Table 2).
Figure 3.
Retention by Buprenorphine Treatment Condition Before and After Changes in Attendance Requirement
4. Discussion
This study contributes novel information to the small but growing body of empirical knowledge regarding the treatment of opioid-dependent youth with buprenorphine. To our knowledge, this is the first study to compare two different buprenorphine-assisted taper lengths with no period of maintenance, among opioid-dependent youth. Results demonstrate that a slower (56-day) buprenorphine taper produces significantly better abstinence and retention outcomes for this population relative to a faster (28-day) taper. This is a novel and important research finding, given the limited treatment research conducted with this population. Providing buprenorphine assisted-treatment to opioid-dependent youth may greatly reduce their likelihood of continued and escalating substance involvement and substance-abusing life trajectories. Currently no effective standard of care exists for this cohort. Thus these data may be useful for developing treatment models that promote greater treatment retention, which may help youth benefit more from behavioral treatment and skills training offered within a treatment setting (44). As noted above, a portion of participants enrolled in the trial were under the age of 18. While these proportions were relatively small - 14% and 28% for the 28-day and 56-day groups respectively - results are encouraging that longer buprenorphine taper strategies will improve abstinence and retention outcomes for adolescents under the age of 18, as well as an older cohort of young adults.
A longer buprenorphine-assisted taper enables a slower opioid reduction in the brains of opioid-dependent youth. This approach may better stabilize brain neurochemistry and control withdrawal symptoms relative to a faster taper. Some concern has been raised that long term opioid replacement therapy may lower an adolescent's likelihood of attaining complete abstinence, (30) and more research is needed to understand the neurological impact that opioid pharmacotherapy can have on youth (45). However, the risks associated with opioid pharmacotherapy must be weighed against the risks associated with discontinuing opioid pharmacotherapy such as overdose (9) and contracting HIV (9, 11-13). While it is likely that buprenorphine maintenance produces better retention and abstinence outcomes than detoxification, if the choice is between differing taper lengths, longer tapers are recommended relative to shorter tapers, as this will likely produce better outcomes.
Prescriptions for up to a month's supply of buprenorphine are routinely provided to patients in the United States (46). Take-home medication was provided to participants in the Woody trial (32) and in this trial. Our initial study design required daily, observed, onsite buprenorphine administration under the rationale that daily attendance would enable daily therapeutic support and observation of medication effects. However, many participants identified this requirement as a significant and counterproductive burden. Participants’ self-report, poor attendance, and high drop-out rates, communicated the logistical infeasibility of a daily attendance regimen. To improve recruitment and retention, and maximize the generalizability of the study results, we changed the protocol to require only 2 to 3 times weekly clinic attendance and offered scripts on intervening days. This change resulted in a more acceptable model of care to adolescents, as evidenced by its impact on opioid abstinence and treatment retention outcomes. This is an important finding because clearly understanding how to engage opioid-dependent youth is essential to shaping models of care for this population.
One limitation of this study was the small sample size. We found that this population of opioid-dependent youth was difficult to recruit for treatment research and a wide variety of recruitment techniques yielded limited benefit. We think that compared to our previous study, obtaining a sample size of 53 was a significant improvement and achievement (31). Notably, this is not an isolated phenomenon. During the Woody et al. clinical trial (32), two study sites had to be closed due to the inability to recruit enough opioid-dependent youth willing to participate. An additional limitation of this study is the fact that participants were self-selected. Youth who self-identify as having a substance use problem and seek treatment may be distinct from youth who have no desire to seek treatment or who are mandated to treatment by criminal justice systems.
Although results were promising overall, less than half of participants enrolled in both conditions completed the entire 63 days of the study. This finding underscores the need to increase the potency of treatment models for youth with opioid use disorders. A recent systematic review using buprenorphine for opioid detoxification among adults with opioid use disorders concluded that longer taper duration is associated with better abstinence outcomes (28). Our data among youth parallel this trend. However, buprenorphine maintenance treatment reliably produces better treatment outcomes relative to buprenorphine tapers among adults (47, 48). Additional trials with larger sample sizes of adolescents and young adults should evaluate the relative effectiveness of varying taper lengths in combination with varying lengths of antecedent maintenance periods. These data would allow clinicians to make more informed decisions when trying to determine the best treatment for an individual opioid-dependent youth.
Clinical Trial Registration.
Trial registration occurred prior to data collection.
Science-Based Treatment for Opioid-Dependent Adolescents
ClinicalTrials.gov Identifier: NCT00182572
https://clinicaltrials.gov/ct2/show/NCT00182572?term=marsch&rank=2
Acknowledgments
This study was funded by the National Institute of Drug Abuse: Grant Number: R01 DA018297 and R01 DA018297-S1 (PI: Marsch). Portions of this work were previously presented at the annual conference of the College on Problems of Drug Dependence (CPDD) in Hollywood, FL (June 2011).
Footnotes
Declarations of interest:
All authors report no conflict of interest.
References
- 1.Lifestyles Statistics Health and Social Care Information Centre Statistics on Drug Misuse England 2014. 2014:1. [Google Scholar]
- 2.United Nations Office on Drugs and Crime Organization WH, editor. Opioid overdose: preventing and reducing opioid overdose mortality. 2013 [Google Scholar]
- 3.United Nations Office on Drugs and Crime . World Drug Report. United Nations publication; 2015. Sales No E15XI6. [Google Scholar]
- 4.Center for Behavioral Health Statistics and Quality Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 [Google Scholar]
- 5.Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975-2014: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research, The University of Michigan; Ann Arbor: 2015. [Google Scholar]
- 6.Zosel A, Bartelson BB, Bailey E, Lowenstein S, Dart R. Characterization of adolescent prescription drug abuse and misuse using the Researched Abuse Diversion and Addiction- related Surveillance (RADARS((R))) System. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(2):196–204. e2. doi: 10.1016/j.jaac.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drugfree.org TPa 2012 Partnership Attitude Tracking Study: Teens and Parents. 2013 [Google Scholar]
- 8.McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction (Abingdon, England) 2007;102(12):1920–30. doi: 10.1111/j.1360-0443.2007.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. The CBHSQ Data Review. 2013 [Google Scholar]
- 10.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA psychiatry. 2014;71(7):821–6. doi: 10.1001/jamapsychiatry.2014.366. [DOI] [PubMed] [Google Scholar]
- 11.Lipari R, Kroutil LA, Pemberton MR. Risk and Protective Factors and Initiation of Substance Use: Results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. 2015 [PubMed] [Google Scholar]
- 12.Substance Abuse Mental Health Services Administration Office of Applied Studies . In: National Household Survey on Drug Abuse. Services USDoHH, editor. Inter-university Consortium for Political and Social Research (ICPSR); 2000. 2013. distributor. [Google Scholar]
- 13.Neaigus A, Atillasoy A, Friedman SR, Andrade X, Miller M, Ildefonso G, et al. Trends in the noninjected use of heroin and factors associated with the transition to injecting. 1998 [Google Scholar]
- 14.Hopfer CJ, Mikulich SK, Crowley TJ. Heroin use among adolescents in treatment for substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(10):1316–23. doi: 10.1097/00004583-200010000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Curran JW, Jaffe HW, Hardy AM, Morgan WM, Selik RM, Dondero TJ. Epidemiology of HIV infection and AIDS in the United States. Science (New York, NY) 1988;239(4840):610–6. doi: 10.1126/science.3340847. [DOI] [PubMed] [Google Scholar]
- 16.Deas-Nesmith D, Brady KT, White R, Campbell S. HIV-risk behaviors in adolescent substance abusers. Journal of substance abuse treatment. 1999;16(2):169–72. doi: 10.1016/s0740-5472(98)00040-3. [DOI] [PubMed] [Google Scholar]
- 17.Rice DP, Kelman S. Measuring comorbidity and overlap in the hospitalization cost for alcohol and drug abuse and mental illness. Inquiry : a journal of medical care organization, provision and financing. 1989;26(2):249–60. [PubMed] [Google Scholar]
- 18.Miller CL, Kerr T, Strathdee SA, Li K, Wood E. Factors associated with premature mortality among young injection drug users in Vancouver. Harm reduction journal. 2007;4:1. doi: 10.1186/1477-7517-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JL, Tsui JI, Hahn JA, Davidson PJ, Lum PJ, Page K. Mortality among young injection drug users in San Francisco: a 10-year follow-up of the UFO study. American journal of epidemiology. 2012;175(4):302–8. doi: 10.1093/aje/kwr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudd RA, Paulozzi LJ, Bauer MJ, Burleson RW, Carlson RE, Dao D, et al. Increases in heroin overdose deaths - 28 States, 2010 to 2012. MMWR Morbidity and mortality weekly report. 2014;63(39):849–54. [PMC free article] [PubMed] [Google Scholar]
- 21.Substance Abuse and Mental Health Services Administration Drug Abuse Warning Network . National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39 Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- 22.Treatment episode data set (TEDS) 2002 - 2012 [Internet] US Department of Health and Human Services; 2012. [Google Scholar]
- 23.Schuman-Olivier Z, Weiss RD, Hoeppner BB, Borodovsky J, Albanese MJ. Emerging adult age status predicts poor buprenorphine treatment retention. Journal of substance abuse treatment. 2014;47(3):202–12. doi: 10.1016/j.jsat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woody EG, O'Brien CP. Update in methadone maintenance. In: Miller NS, editor. Comprehensive handbook of drug and alcohol addiction. Marcal Dekker; New York: 1991. pp. 1113–26. [Google Scholar]
- 25.McCormick Cynthia G. In: Young AN, editor. Food and Drug Administration Dearptment of Health and Human Services; Rockville, MD: 2002. NDA 20-732, NDA 20-733. [Google Scholar]
- 26.U.S. Food and Drug Administration Buprenorphine Drug Label. NDA 20-732 & NDA 20-7332002. [Google Scholar]
- 27.Fiellin DA, Friedland GH, Gourevitch MN. Opioid dependence: rationale for and efficacy of existing and new treatments. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43(Suppl 4):S173–7. doi: 10.1086/508180. [DOI] [PubMed] [Google Scholar]
- 28.Dunn KE, Sigmon SC, Strain EC, Heil SH, Higgins ST. The association between outpatient buprenorphine detoxification duration and clinical treatment outcomes: a review. Drug Alcohol Depend. 2011;119(1-2):1–9. doi: 10.1016/j.drugalcdep.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2015:1–14. doi: 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiellin DA. Treatment of adolescent opioid dependence: no quick fix. Jama. 2008;300(17):2057–9. doi: 10.1001/jama.2008.567. [DOI] [PubMed] [Google Scholar]
- 31.Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C, et al. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Archives of general psychiatry. 2005;62(10):1157–64. doi: 10.1001/archpsyc.62.10.1157. [DOI] [PubMed] [Google Scholar]
- 32.Woody GE, Poole SA, Subramaniam G, Dugosh K, Bogenschutz M, Abbott P, et al. Extended vs short-term buprenorphine-naloxone for treatment of opioid-addicted youth: a randomized trial. Jama. 2008;300(17):2003–11. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephen Evans PRaSD. Minim: allocation by minimisation in clinical trials [updated 3/14/20131/20/16] Available from: http://www.webcitation.org/query?url=https%3A%2F%2Fwww-users.york.ac.uk%2F%7Emb55%2Fguide%2Fminim.htm&date=2016-01-20.
- 34.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330(7495):843. doi: 10.1136/bmj.330.7495.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hudziak J. DSM-IV Checklist for childhood disorders. Research Center for Children, Youth, and Families, University of Vermont; Burlington, VT: 1998. [Google Scholar]
- 36.Dennis ML, Titus JC, White MK, Unsicker JI, Hodgkins D. Global appraisal of individual needs: Administration guide for the GAIN and related measures. Chestnut Health Systems; Bloomington, IL: 2003. [Google Scholar]
- 37.Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction, Treatment Improvement Protocol (TIP) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2004. Series 40. ed. [PubMed] [Google Scholar]
- 38.Peachey JE, Lei H. Assessment of opioid dependence with naloxone. British journal of addiction. 1988;83(2):193–201. doi: 10.1111/j.1360-0443.1988.tb03981.x. [DOI] [PubMed] [Google Scholar]
- 39.Bickel WK, Amass L. Buprenorphine treatment of opioid dependence: A review. Exp Clin Psychopharm. 1995;3(4):477. [Google Scholar]
- 40.Azrin NH, McMahon PT, Donohue B, Besalel VA, Lapinski KJ, Kogan ES, et al. Behavior therapy for drug abuse: a controlled treatment outcome study. Behav Res Ther. 1994;32(8):857–66. doi: 10.1016/0005-7967(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 41.Miller WR, Yahne CE, Tonigan JS. Motivational interviewing in drug abuse services: a randomized trial. Journal of consulting and clinical psychology. 2003;71(4):754–63. doi: 10.1037/0022-006x.71.4.754. [DOI] [PubMed] [Google Scholar]
- 42.Budney A, Higgins ST. A community reinforcement plus vouchers approach: Treating cocaine addiction. National Institute on Drug Abuse; Rockville, MD: 1998. [Google Scholar]
- 43.P A. Exclusions, losses to follow-up and withdrawals in clinical trials. In: SHAPIRO LT, editor. Clinical Trials, Issues and Approaches. Marcel Dekker, Inc; New York: 1983. pp. 99–113. [Google Scholar]
- 44.Moore SK, Marsch LA, Badger GJ, Solhkhah R, Hofstein Y. Improvement in psychopathology among opioid-dependent adolescents during behavioral-pharmacological treatment. Journal of addiction medicine. 2011;5(4):264–71. doi: 10.1097/ADM.0b013e3182191099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Current psychiatry reports. 2009;11(5):360–3. doi: 10.1007/s11920-009-0054-5. [DOI] [PubMed] [Google Scholar]
- 46.Dupont HB, Kaplan CD, Braam RV, Verbraeck HT, de Vries NK. The application of the rapid assessment and response methodology for cannabis prevention research among youth in the Netherlands. International Journal of Drug Policy. 2014 doi: 10.1016/j.drugpo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Parran TV, Adelman CA, Merkin B, Pagano ME, Defranco R, Ionescu RA, et al. Long-term outcomes of office-based buprenorphine/naloxone maintenance therapy. Drug Alcohol Depend. 2010;106(1):56–60. doi: 10.1016/j.drugalcdep.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldiero RM, Parran TV, Jr, Adelman CL, Piche B. Inpatient initiation of buprenorphine maintenance vs. detoxification: can retention of opioid-dependent patients in outpatient counseling be improved? American Journal on Addictions. 2006;15(1):1–7. doi: 10.1080/10550490500418989. [DOI] [PubMed] [Google Scholar]