Abstract
Ellis-van Creveld (EvC) syndrome is a genetic disorder with mutations in either EVC or EVC2 gene. Previous case studies reported that EvC patients underwent orthodontic treatment, suggesting the presence of craniofacial bone phenotypes. To investigate whether a mutation in EVC2 gene causes a craniofacial bone phenotype, Evc2 knockout (KO) mice were generated and cephalometric analysis was performed. The heads of wild type (WT), heterozygous (Het) and homozygous Evc2 KO mice (1-, 3- and 6-week-old) were prepared and cephalometric analysis based on the selected reference points on lateral X-ray radiographs was performed. The linear and angular bone measurements were then calculated, compared between WT, Het and KO and statistically analyzed at each time point. Our data showed that length of craniofacial bones in KO was significantly lowered by ~20% to that of WT and Het, the growth of certain bones, including nasal bone, palatal length and premaxilla was more affected in KO, and the reduction in these bone length was more significantly enhanced at later postnatal time points (3 and 6 weeks) than early time point (1 week). Furthermore, bone-to-bone relationship to cranial base and cranial vault in KO was remarkably changed, i.e. cranial vault and nasal bone were depressed and premaxilla and mandible were developed in a more ventral direction. Our study was the first to show the cause-effect relationship between Evc2 deficiency and craniofacial defects in EvC syndrome, demonstrating that Evc2 is required for craniofacial bone development and its deficiency leads to specific facial bone growth defect.
Keywords: cephalometric analysis, craniofacial bone, Ellis-van Creveld syndrome, EVC2, knockout (KO) mouse
Introduction
Ellis-van Creveld (EvC) syndrome (OMIM #225500) is an autosomal recessive genetic disorder showing chondro-ectodermal dysplastic dwarfism characterized by short limbs, short ribs and postaxial polydactyly (Kurian et al., 2007). This rare medical condition has been first reported in 1940 by Drs. Richard Ellis and Simon van Creveld (Ellis and van Creveld, 1940). The unique intra-oral clinical manifestations in EvC patients include; a shallow sulcus, the presence of broad/multiple labial frena, missing and dysmorphic teeth, taurodontism and incomplete root formation and malocclusion (Ghosh et al., 2007; Kurian et al., 2007). This syndrome is most prevalent in the Amish population of Lancaster County, Pennsylvania, occurring in 1/5,000 live births, whereas the birth prevalence in non-Amish population is estimated to be 7/1,000,000 (Kurian et al., 2007). Weyers acrofacial dysostosis (WAD; also known as Curry-Hall syndrome, OMIM #193530) is described as an allelic variant of the EvC syndrome with an autosomal dominant inheritance, but shows a milder phenotype (Weyers, 1952; Curry and Hall, 1979). In partnership with the Amish, mutations associated with EvC syndrome were first identified in EVC gene, however, these mutations did not account for all the EvC cases tested (Ruiz-Perez et al., 2000), suggesting the presence of genetic heterogeneity in this syndrome.
Bovine chondrodysplastic dwarfism (bcd) has been reported in many cattle breeds (Jones et al., 1978; Jayo et al., 1987; Agerholm et al., 2004). Affected calves show an autosomal recessive trait and characteristic changes similar to those of chondrodysplasia in human (Julian et al., 1959). The bcd locus in Japanese brown cattle was mapped to the distal end of bovine chromosome 6 by linkage analysis (Yoneda et al., 1999), and disease-specific mutations were identified (Takeda et al., 2002). We previously identified the causative gene for bcd and designated as LIMBIN (LBN) gene (Takeda et al., 2002). Later, a similar mutation has been identified in EvC patients (Galdzicka et al., 2002) where it is designated as EVC2 gene, identified as the human ortholog of LBN, indicating the biological importance of EVC2/LBN protein function in skeletal tissue development.
It is now known that EvC and WAD patients have mutations in either EVC or EVC2 gene, both of which are located on human chromosome 4p16 in a head-to-head configuration (Ruiz-Perez et al., 2000; Galdzicka et al., 2002; Ruiz-Perez et al., 2003; Ye et al., 2006). EvC patients exhibit craniofacial bone growth and developmental phenotypes, i.e. defective skull growth pattern such as enlarged skull, depressed nasal bridge, mandibular prognathism, skeletal class III (maxillary deficiency and mandibular prognathism), and skeletal open bite (Ellis and van Creveld, 1940; Goor et al., 1965; Susami et al., 1999), while some studies described that the face appeared to be normal (Varela and Ramos, 1996; Hanemann et al., 2010). Such inconsistent reports may be in part due to the following reasons; 1) no gene mutation(s) were identified in these earlier case reports and 2) there is little information regarding cephalometric measurements and analysis in these patients. This raises a question whether EVC or EVC2 mutation affects the craniofacial bone development. It has been previously reported that Evc knockout (KO) mice exhibited chondrodysplasia affecting chondrocyte differentiation. Although the report showed the presence of Evc expression in the lateral nasal, maxillary and mandibular processes during prenatal craniofacial development, craniofacial bone phenotype has not been described (Ruiz-Perez et al., 2007). As we previously reported that Evc2 is expressed in cranial bone and facial primordia during mouse development (Takeda et al., 2002), Evc2 likely plays an important role in craniofacial development. To support this notion, more recently, Evc2 KO mice were generated by replacing the first exon with an EGFP cassette and showed cranial base phenotype at embryonic day (E) 18.5 (Caparros-Martin et al., 2013). However, the study was limited to show only the presence of impaired development of the intrasphenoidal synchondrosis and any other craniofacial defects have not been described in detail, partly because these mice do not survive to an appropriate post-natal age (Caparros-Martin et al., 2013). This remains the cause-effect relationship between Evc2 deficiency and craniofacial phenotype(s) unclear and thus, a new animal model by which post-natal craniofacial bone phenotype(s) can be analyzed is warranted. Very recently, we reported that a new mouse model of Evc2/Lbn KO mouse by introducing a premature stop codon in exon 12 mimicking the mutation found in bcd cattle in exon 14 (Takeda et al., 2002). The objective of this study was thus to characterize craniofacial skeletal morphology of the Evc2 KO mouse.
Materials and methods
Ethical approval
All experimental animals used in this study had access to food and water ad libitum and housed under regular light cycles. At the end point of the experiment, animals were euthanized by carbon dioxide gas in an uncrowded chamber followed by a physical method to ensure death. All animal procedures were approved by the IACUC at Boston University Medical Campus (Boston, MA, USA) and University of Michigan (Ann Arbor, MI, USA) and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Evc2 knockout (KO) mouse
Generation and genotyping of Evc2 KO mice (129SvEv C57BL/6J mixed strain) were previously reported (Zhang et al., 2015). In brief, a targeting vector was designed for Evc2 KO mice by introducing a premature stop codon in exon 12 followed by Internal Ribosomal Entry Site (IRES) fused to β-galactosidase (LacZ). The targeting cassette was introduced to AB2.2 embryonic stem (ES) cells and the correctly targeted ES cell clones were identified by Southern blot and genomic PCR strategies. After the germline transmission, resulted heterozygous (Het) Evc2 mice were intercrossed to generate homozygous Evc2 KO mice as well as the control wild-type (WT) and Het mice. The deficiency of EVC2 protein in Evc2 KO mouse-derived primary chondrocytes was verified by Western blot analysis (data not shown).
Sample preparation
The heads of Evc2 WT, Het and KO mice were collected, dissected, fixed with 10% formaldehyde and stored in PBS at 4°C until use. The samples were divided into three groups based on the genotypes and age of animals. The 1-week group included WT (n=7), Het (n=5) and KO (n=3) mice at postnatal day from 6 to 8 (P6-P8), the 3-weeks group WT (n=3), Het (n=7) and KO (n=5) at P23-P24, and the 6-weeks group WT (n=5), Het (n=5) and KO (n=3) at P42-P46.
Radiographic imaging
Each head of mice was oriented in a reproducible head position to ensure a true sagittal and horizontal view image by using a specific mold and incorporating a grid background to orient the skulls on a horizontal line that passes through the tip of the nasal bone anteriorly and the most posterior point on the cranial vault (Po) posteriorly. X-ray images of the heads were taken using a standard imaging machine (Prostyle-Intra; PLANMECA, IL, USA) with a digital x-ray sensor (Schick CDR with size 2 sensor; Sirona, NY, USA) at the source to image distance of 18 inches, 60 kV of energy, 8 mA of intensity and 0.1 seconds of exposure time. Object magnification was minimized through direct positioning of the sample onto the film. The radiographic images (900 X 641 dpi digital resolutions) were scanned and used for cephalometric analysis.
Cephalometric analysis
Linear and angular cephalometric parameters were used based on the previous report (Engstrom et al., 1982) with some modifications, i.e. additional linear and angular parameters including nasal bone height and angulation in relation to premaxilla. Based on fourteen reference points identified (Table 1), thirteen linear and twenty-two angular bone measurements (Table 2) were calculated by the CDR DICOM software (Sirona, NY, USA). All data in the graphs were presented as the mean values ± SD. The average of all the linear bone measurements in each genotype was calculated at each time point by the total of linear measurements of all the craniofacial bones divided by the total number of bones and displayed as (average skull linear measurements). Values of linear measurements of each individual bone in KO at each time point were normalized to those in WT and are shown as (KO/WT ratios; %).
Table 1.
List of reference points based on the anatomical landmarks for morphometric measurements
| Abbreviations | Points |
|---|---|
| Po | The most posterior point on the cranial vault. |
| N | A point on the nasofrantal suture. |
| A | The most anterior point on the nasal bone. |
| E | The intersection between the frontal bone and the most superior-anterior point of the posterior limit of the ethmoid bone. |
| So | The intersection between the posterior border of the basisphenoid and the tympanic bulla. |
| Pr | The most inferior and anterior point on the alveolar process of the premaxilla. |
| Bu | A point on the premaxilla between jaw bone and the lingual surface of the upper lingual incisors. |
| Iu | The most prominent point between the incisal edges of the upper incisors. |
| Mu | A point on the intersection between the maxillary bone and the mesial surface of the upper first molar. |
| Ii | The most prominent point between the incisal edges of the lower incisors. |
| Id | The most inferior and anterior point on the alveolar process of the mandible. |
| Mn | A point in the deepest part of the antegonial notch curvature. |
| Ml | A point on the intersection between the mandibular alveolar bone and the mesial surface on the first molar. |
| Bl | A point on the intersection between the lingual surface of the lower incisors and the most anterior part of the lingual alveolar bone. |
Table 2.
List of abbreviations for linear distances and angles used in this study.
| Distances | Abbreviations | Angles | Abbreviations |
|---|---|---|---|
| Nasal bone length | N-A | Cranial vault to cranial base | PoEL/SoEL |
| Viscerocranial length (anterior to anterior incisors) | E-Pr | Nasal bone to cranial base | ANL/SoEL |
| Viscerocranial length (posterior to anterior incisors) | E-Bu | Nasal bone to cranial vault | ANL/PoEL |
| Nasal bone height* | A-Pr | Nasal bone to premaxilla* | ANL/PrNL |
| Total skull length | Po-A | Premaxilla to cranial base | PrEL/SoEL BuEL/SoEL |
| Palatal length | Mu-Bu | Maxilla-premaxilla to cranial base | MuBuL/SoEL MuPrL/SoEL |
| Cranial base length | So-E | Upper incisors to cranial base | IuEL/SoEL |
| Neurocranial length | Po-E | Premaxilla to cranial vault | PrEL/PoEL BuEL/PoEL |
| Viscerocranial height | E-Mu | Maxilla-premaxilla to cranial vault | MuBuL/PoEL MuPrL/PoEL |
| Mandibular corpus length | Mn-Id | Upper incisors to cranial vault | IuEL/PoEL |
| Mandibular lingual alveolar bone | Ml-Bl | Upper incisor inclination | MuPrL/PrIuL MuBuL/PrIuL |
| Erupted upper incisor length | Pr-Iu | Lower incisor inclination | MlliL/IdliL MIBIL/IdliL |
| Erupted lower incisor length | Ii-Id | Mandibular corpus to cranial vault | MnIdL/PoEL MlBiL/PoEL |
| Mandibular corpus to cranial base | MnIdL/SoEL MIBiL/SoEL |
An asterisk (*) indicates a new linear or angular measurement added to the current study. All other measurements were adapted from Engstrom et al (Engstrom et al., 1982). L; Line.
Statistical analysis
The Mann-Whitney U test was used to identify the significant difference with the p-value of less than 0.05 marking the significance.
Results
Phenotype of the homozygous Evc2 KO mice
Generated Evc2 KO mice had phenotypic changes similar to those seen in EvC patients as well as the Evc knockout mouse model (Ruiz-Perez et al., 2007). While Het Evc2 mice showed no discernible phenotype similar to WT, KO mice demonstrated short body length showing dwarfism (Fig. 1A, left) and signs of ectodermal dysplasia of hair, teeth and nails, some of these phenotypes were briefly described previously (Zhang et al., 2015).
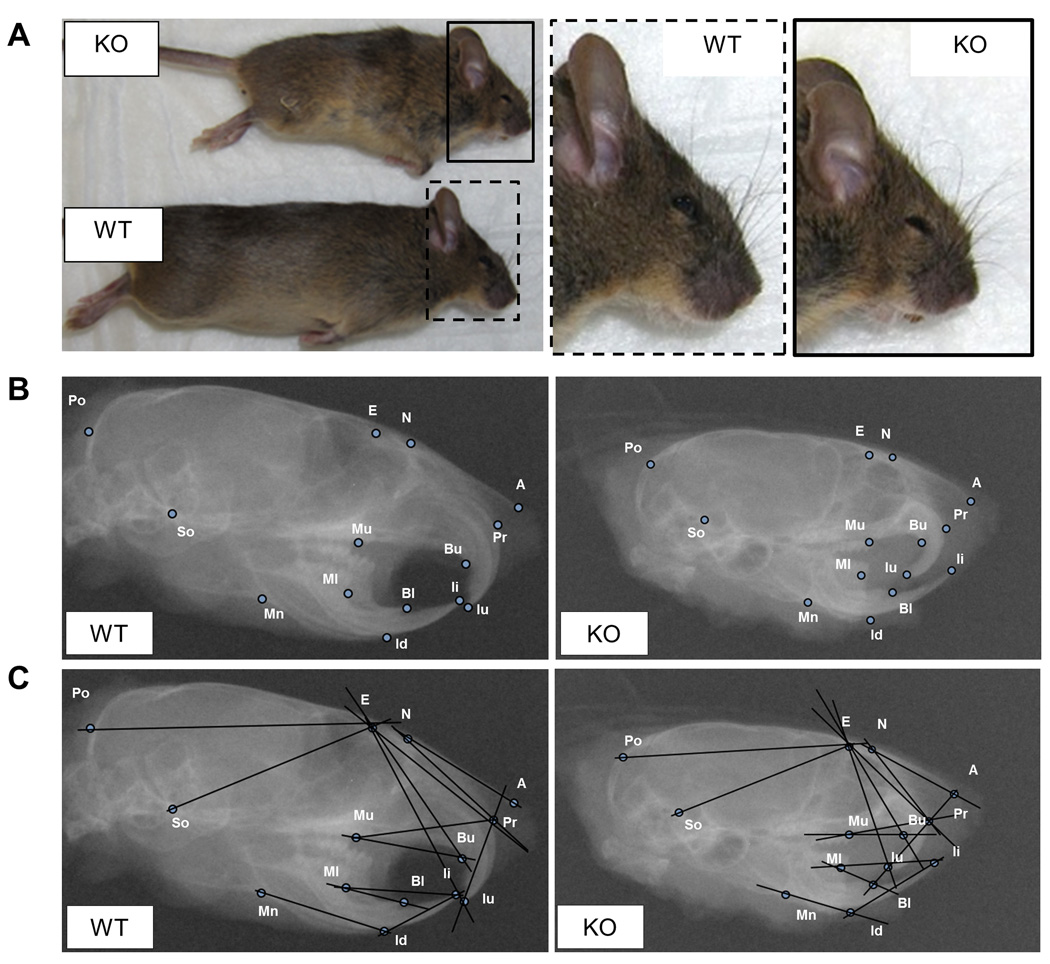
Figure 1. Gross morphology of Evc2 KO mice and cephalometric analysis.
(A) Gross morphology of Evc2 wild type (WT) and knockout (KO) mice at 6 weeks. Note that the body length and skull size in KO are smaller than those in WT mouse. The craniofacial images of these mice were magnified and shown on the right panels (WT; dotted open box, KO; indicated by a black open box). (B) Lateral radiographic images of 6-week-old-WT (left panel) and -KO (right panel) with the selected reference points (see Table 1 for abbreviation). (C) Cephalometric analysis was performed by measuring the linear distances and bone angles (Table 2 for abbreviation) based on the selected reference points.
Impairment of bone growth in Evc2 KO mice during postnatal craniofacial bone development
As Evc2 KO mice appeared to exhibit smaller skull size comparing to WT (Fig. 1A, right boxed panels), the head samples were subjected to lateral cephalometric radiography to measure the craniofacial bone structures. Specific reference points were defined according to the anatomical locations of specific landmarks (Engstrom et al., 1982) (Fig. 1B) and used to analyze the linear bone measurements and the angular bone relations (Fig. 1C) in order to investigate which bones are affected due to Evc2 deficiency during postnatal development.
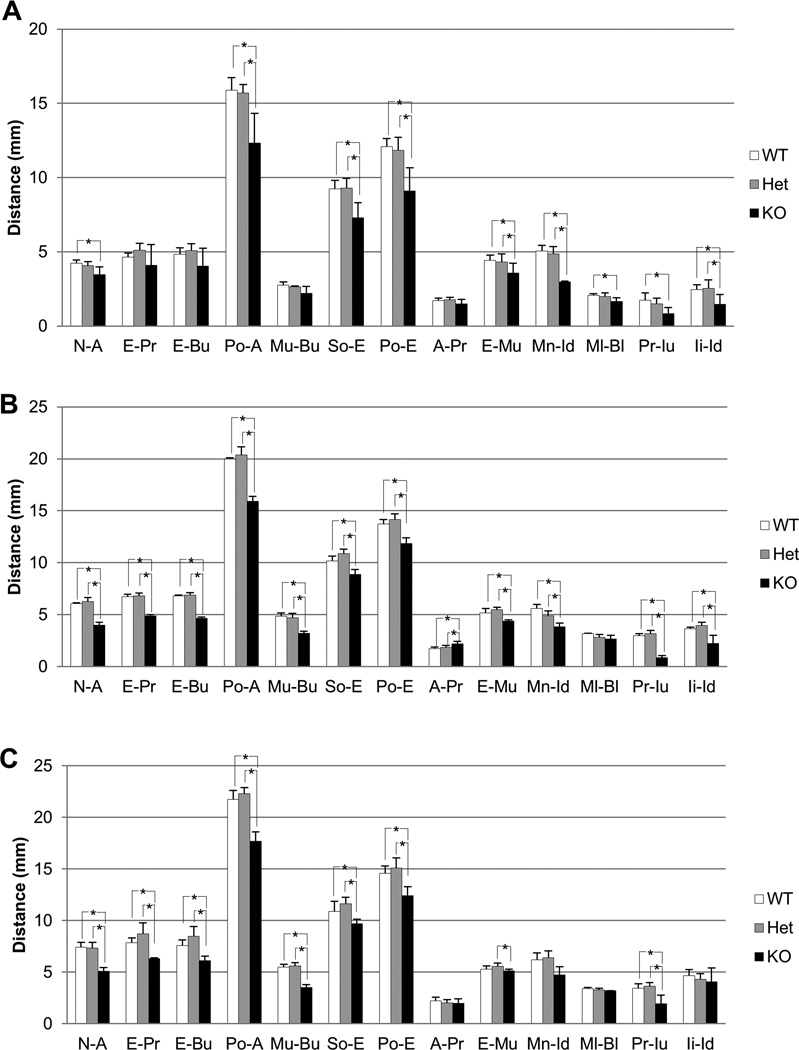
At 1 week, the values of nasal bone length (N-A), total skull length (Po-A), cranial base length (So-E), neurocranial length (Po-E), viscerocranial height (E-Mu), mandibular corpus length (Mn-Id), and mandibular lingual alveolar bone length (Ml-Bl) in KO were statistically smaller than those in WT and/or Het (Fig. 2A). At 3 weeks, in addition to the reduced bone length found at 1 week, almost all of other linear bone measurements in KO were also significantly smaller than those in WT and Het (Fig. 2B). In 3-week-old KO mice, the values of the viscerocranial length (premaxilla); both anterior and posterior to the upper incisors (E-Pr and E-Bu), and palatal length (Mu-Bu) became significantly smaller than those in WT and/or Het (Fig. 2B). However, the value of nasal bone height (A-Pr) was higher in KO than WT and Het, and Ml-Bl showed no difference among the three genotypes (Fig. 2B). At 6 weeks, KO mice had statistically smaller linear bone measurements than WT and Het for N-A, E-Pr, E-Bu, Po-A, Mu-Bu, So-E, and Po-E (Fig. 2C). On the other hand, no significant differences were seen for A-Pr, E-Mu, Mn-Id, and Ml-Bl lengths between KO and both WT and Het (Fig. 2C), although the mandibular length (Mn-Id, Ml-Bl) in KO was shorter than WT and Het. Linear measurements for the incisors showed that the erupted upper incisors length (Pr-Iu) was significantly shorter in KO than WT and/or Het at any time points tested (Fig. 2). The erupted lower incisors length (Ii-Id) was significantly shorter in KO than WT and Het at 1 and 3 weeks (Figs. 2A&B), while there was no significant difference at 6 weeks (Fig. 2C). There were no statistical differences in linear bone measurements between WT and Het at any time points tested.
Figure 2. Linear bone measurements of Evc2 mice.
Comparison of linear bone measurements between WT, Het and KO mice at 1, 3 and 6 weeks. (A) At 1 week, most bone measurements were significantly smaller in KO, except for the premaxilla (E-Pr, E-Bu), palate (Mu-Bu) and nasal bone height (A-Pr) comparing to WT and/or Het. *p < 0.05. (B) At 3 weeks, all the linear bone measurements in KO were significantly smaller than WT and/or Het, except for nasal bone height (A-Pr) and mandibular lingual measurement (Ml-Bl). *p < 0.05. (C) At 6 weeks, KO mice showed significant decrease in most linear bone measurements comparing to WT and Het, except for nasal bone height (A-Pr) and mandibular bone measurements (Mn-Id and Ml-Bl). *p < 0.05.
Abnormal bone-to-bone relation in Evc2 KO mice
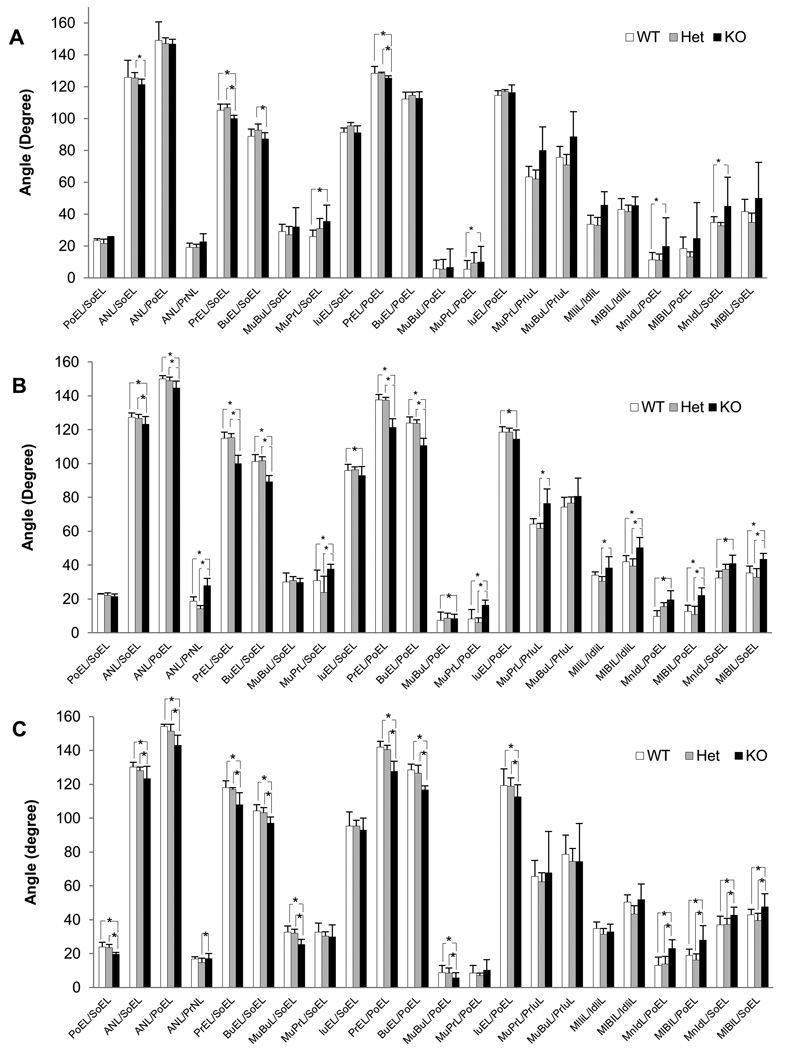
Angular bone relations demonstrate bone-to-bone relationships and establish the spatial relations and positions of the facial bones to those of the cranial base and cranial vault. We next compared angular bone relations between Evc2 WT, Het and KO mice at three different ages. At 1 week, the values of angular bone relations of nasal bone to cranial base (ANL/SoEL), premaxilla to cranial base (PrEL/SoEL, BuEL/SoEL), and premaxilla to cranial vault (PrEL/PoEL) in KO were significantly smaller than those in WT and/or Het, and those of maxilla-premaxilla to cranial base (MuPrL/SoEL), maxilla-premaxilla to cranial vault (MuPrL/PoEL), mandibular corpus to cranial vault (MnIdL/PoEL), and mandibular corpus to cranial base (MnIdL/SoEL) in KO were significantly higher than those in WT (Fig. 3A). The differences became significantly obvious at 3 and 6 weeks (Figs. 3B & 3C).
Figure 3. Angular bone measurements of Evc2 mice.
Comparison of angular bone measurements between WT, Het and KO mice at 1, 3 and 6 weeks. (A) At 1 week, the nasal bone (ANL/SoEL), premaxilla (PrEL/SoEL, BuEL/SoEL, and PrEL/PoEL), palatal plane (MuPrL/PoEL), and mandible relations in KO were different from controls. *p < 0.05. (B,C) At 3 and 6 weeks, differences in bone relations between KO and WT and/or Het were enhanced. Major changes in the bone relations included nasal bone (ANL/SoEL, ANL/PoEL), premaxilla (PrEL/SoEL, BuEL/SoEL, PrEL/PoEL, BuEL/PoEL), palatal plane (MuPrL/SoEL, MuPrL/PoEL), and mandible (MnIdL/PoEL, MlBlL/PoEL, MnIdL/SoEL, MlBlL/SoEL) to the cranial base and/or vault in KO as compared to WT and Het. In addition, at 6 weeks there was a decrease in the relation of the cranial vault to cranial base (PoEL/SoEL) in KO. No differences in morphometric bone relations between WT and Het. *p < 0.05.
At 3 weeks (Fig. 3B), the values of angular measurement in nasal bone to cranial base and to cranial vault (ANL/SoEL and ANL/PoEL) in KO were significantly decreased when compared to those in WT and Het. Moreover, the value of nasal bone to premaxilla (ANL/PrNL) was increased in KO comparing to WT and Het. These two altered relations demonstrated that the nasal bone was depressed. The values of premaxilla to cranial base and to cranial vault (PrEL/SoEL, BuEL/SoEL, PrEL/PoEL, and BuEL/PoEL) in KO were more significantly decreased than those in WT and Het, demonstrating a rotation of the premaxilla in a ventral direction. Moreover, the values of palatal plane to cranial base and to cranial vault (MuPrL/SoEL, MuBuL/PoEL and MuPrL/PoEL) in KO were significantly increased compared to WT and/or Het, indicating the ventral rotation of this plane consistent with the rotation of the premaxilla. The values of mandibular corpus to cranial vault and to cranial base (MnIdL/PoEL, MlBlL/PoEL, MnIdL/SoEL, and MlBlL/SoEL) were markedly increased in KO comparing to WT and/or Het, indicating rotation in a clockwise/ventral direction. Dental relations showed that the upper incisor to cranial vault relationship (IuEL/PoEL) in KO was decreased compared to WT (Fig. 3B) showing a rotation in a clockwise/ventral direction. In addition, the upper incisors were more anteriorly inclined (MuPrL/PrIuL) (less curved), while the lower incisors were more posteriorly inclined (MlIiL/IdliL and MIBIL/IdliL) in KO than WT and/or Het.
At 6 weeks, the results of angular bone relations were mostly consistent with those found at 3 weeks, i.e. the values of cranial vault to cranial base (PoEL/SoEL), nasal bone to cranial bone and to cranial vault (ANL/SoEL, ANL/PoEL), and maxilla-premaxilla to cranial vault (MuBuL/PoEL) in KO was significantly decreased as compared to WT and Het. Similar to the data at 3 weeks, the values of premaxilla to cranial base and to cranial vault (PrEL/SoEL, BuEL/SoEL, PrEL/PoEL, and BuEL/PoEL) in KO were significantly decreased as compared to those in WT and Het, indicating ventral (downward and backward) rotation of the premaxilla. The value of maxilla-premaxilla to cranial base (MuBuL/SoEL) became significantly smaller at 6 weeks. In addition, the value of nasal bone to premaxilla (ANL/PrNL) was significantly increased in KO comparing to Het. On the other hand, there was no statistical difference of maxilla-premaxilla to cranial base and to cranial vault, upper incisors to cranial base, and upper and lower incisor inclination (MuPrL/SoEL and MuPrL/PoEL, IuEL/SoEL, MuPrL/PrIuL and MlIiL/IdIiL) between KO, WT and Het. The values of mandible to cranial vault (MnIdL/PoEL and MlBlL/PoEL) were markedly increased in KO as compared to WT and Het, indicating rotation in a ventral direction/diverge relation. The values of mandibular corpus to cranial vault and to cranial base (MnIdL/PoEL, MlBlL/PoEL, MnIdL/SoEL, and MlBlL/SoEL) were markedly increased in KO comparing to WT and Het, indicating rotation in a ventral direction.
Abnormal bone-to-bone relations are not due to decreased skull size in Evc2 KO mice
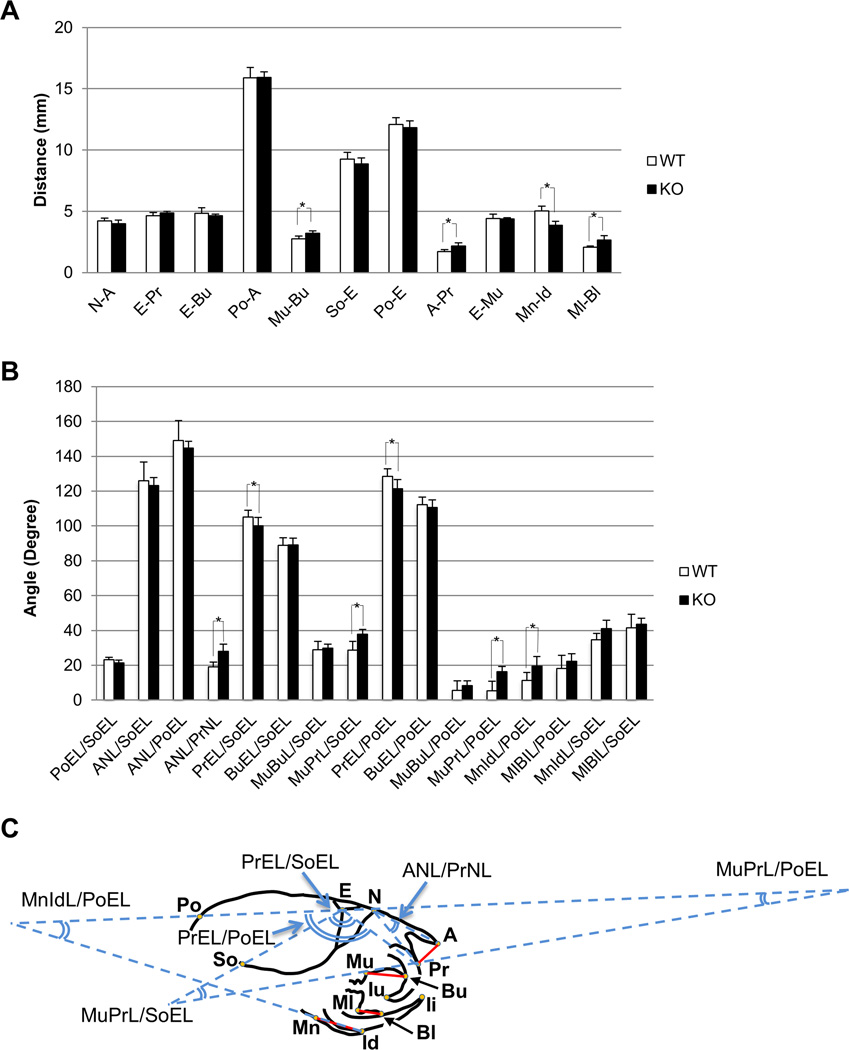
The Evc2 KO mice were smaller (Fig. 1), thus one possible argument is that the differences in angular bone relations described in Fig. 3 are simply due to the reduction in bone length as observed by linear measurement (Fig. 2). Thus, we normalized the linear measurements as follows. We compared the values of linear measurement between 3-week-old-KO and 1-week-old-WT only in bone length, but not incisor length (thus, Pr-Iu and Ii-Id were omitted in Fig. 4A and angle measurements including these two length were also eliminated in Fig. 4B) as incisor length is likely affected by both bone and tooth developmental processes. Most of their linear measurements such as the length of nasal bone (N-A), viscerocraial bone (E-Pr, E-Bu), total skull (Po-A), cranial base (So-E), neurocranial bone (Po-E) and viscerocranial height (E-Mu) were not statistically different between 3-week-old-KO and 1-week-old-WT (Fig. 4A). The length of palatal length (Mu-Bu), nasal bone height (A-Pr), and mandibular lingual alveolar bone (Ml-Bl) in 3-week-old-KO was greater than that of 1-week-old-WT, and the length of mandibular corpus length in 3-week-old-KO was smaller than that of 1-week-old-WT (Mn-Id) (Fig. 4C, indicated by red lines). Angular bone relations were then compared between these mice. As shown in Fig. 4B, although 3-week-old-KO had the same size of the nasal bone (N-A) and premaxilla (E-Pr and E-Bu) as 1-week-old-WT, the angles of nasal bone to premaxilla (ANL/PrNL), and premaxilla-maxilla to cranial base and to cranial vault (MuPrL/SoEL, MuPrL/PoEL) were significantly larger in 3-week-old-KO, and those of premaxilla to the cranial base and to cranial vault (PrEL/SoEL, PrEL/PoEL) were significantly smaller in 3-week-old-KO comparing to 1-week-old-WT (smaller/larger angle measurements were indicated by blue lines in Fig. 4C), indicating the ventral rotation or clockwise depression of the premaxilla and palatal plane. These data clearly demonstrate that Evc2 deficiency lead to defective bone development not only in size but also spatial bone-to-bone relations particularly affecting premaxilla and maxilla.
Figure 4. Abnormal Bone-to-bone Relations are not due to Decreased Skull Size in Evc2 KO Mice.
Comparison of linear bone measurements between 1-week-old-WT and 3-week-old-KO mice. (A) No statistical difference was found in the linear bone measurements of N-A, E-Pr, E-Bu, Po-A, So-E, Po-E and E-Mu between WT and KO, while the length of Mu-Bu, A-Pr, and Ml-Bl was significantly higher in KO. * p<0.05. (B) The value of premaxilla relation (PrEL/PoEL) in KO was significantly decreased from that in WT, although the bone measurements in (E-Pr) showed no differences, indicating that the changes in angular bone relation was not entirely due to the bone size. (C) Illustration of the Evc2 KO mouse with reference points and lines in the cephalometric analysis. Red solid lines indicate the linear measurements which were significantly different between 3-week-old-KO and 1-week-old-WT identified in Fig. 4A. Angles in blue lines indicate the angular measurements which were significantly different between 3-week-old-KO and 1-week-old-WT identified in Fig. 4B.
Impairment of nasal and palatal length growth in Evc2 KO mice
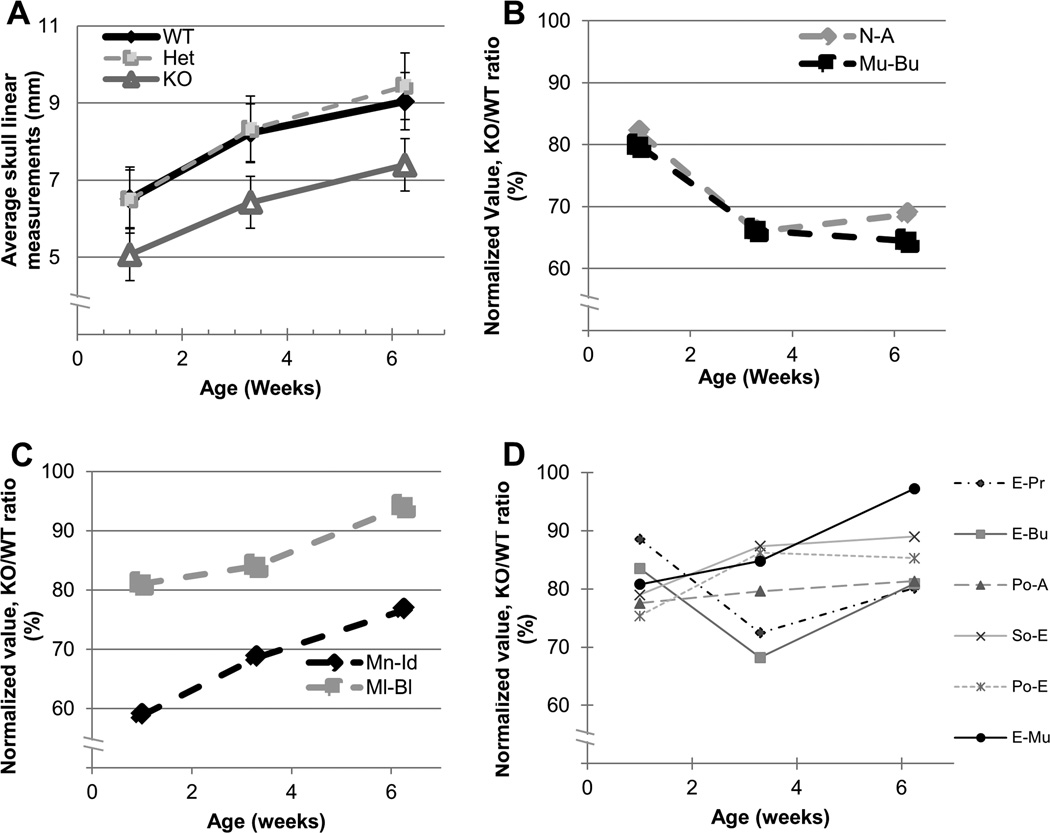
To further identify postnatal bone growth phenotype in Evc2 KO mice, we first analyzed the average skull linear measurements (Fig. 5A). The data demonstrated the high resemblance of profile of values between WT, Het and KO with a constant decrease of ~20% in KO during the postnatal development (Fig. 5A). Secondly, KO/WT ratios in each bone were calculated at each time point tested. The results demonstrated that the KO/WT ratios of nasal bone and palatal length were significantly decreased (~20% decrease at 1 week, ~35% at 3 weeks, and ~30–35% at 6 weeks for both bones) over the postnatal development period, indicating that the growth in these bones was severely affected (Fig. 5B). On the other hand, the KO/WT ratios of mandibular bone were markedly increased during the development, indicating there may be a compensatory mechanism in this bone development (Fig. 5C). There were no significant changes of KO/WT ratios in other bones (Fig. 5D).
Figure 5. Growth impairment of specific craniofacial bones in Evc2 KO mice.
(A) The profile of averaged linear bone measurements. The average of all the linear bone measurements in each genotype was calculated at each time point by the total of linear measurements of all the craniofacial bones divided by the total number of bones and displayed as (average skull linear measurements). The profile of averaged measurement values during postnatal development in KO was similar to that in WT and Het, while the value in KO was lowered by 28.5% at 1 week, 28.0% at 3 weeks, and 22.3% at 6 weeks compared to WT, and lowered by 27.8% at 1 week, 29.4% at 3 weeks and by 27.6% at 6 weeks comparing to Het. The values in WT and Het were essentially same. (B, C, D) Values of linear measurements of each individual bone in KO at each time point were normalized to those in WT and are shown as (KO/WT ratio, %). (B) The KO/WT ratios of nasal (N-A) and palatal (Mu-Bu) bones were markedly decreased at 3 and 6 weeks. (C) Although mandibular bone growth was lowered at 1 week, the KO/WT ratio of this bone was significantly improved at 3 weeks and further increased at 6 weeks. (D) The change of KO/WT ratios of other craniofacial bones (E-Pr, E-Bu, Po-A, So-E, Po-E, E-Mu, Ml-Bl) was within ~20% of the range. N-A; nasal bone length, Mu-Bu; palatal length, Mn-Id; mandibular corpus length, Ml-Bl; mandibular lingual alveolar bone.
Discussion
In this study, we have characterized and analyzed the craniofacial morphology in a mouse model of EvC syndrome, Evc2/Lbn KO mice. Our data indicated that specific craniofacial bone structures in Evc2 KO mice were affected in the areas of nasal bone, total skull, cranial base, cranial vault, premaxilla, maxilla and palate showing the significant reduction in their size. On the other hand, mandible exhibited a different growth pattern; i.e. significantly smaller at 1 and 3 weeks, however, there was no statistical difference at 6 weeks (Figs. 2 and 5). Expectedly the expression pattern of Evc2 is strongly associated with these affected craniofacial bone areas including Meckel’s cartilage (MKB, YMo; unpublished data). However, it is not fully clear at this point as to why there was an increase of mandible length postnatally in KO. Unlike other bones in the craniofacial area, the body of mandible is initially formed from condensing mesenchyme through intramembranous ossification where Meckel’s cartilage serves as a template (Ramaesh and Bard, 2003). Subsequently mandible increases its size through endochondral ossification of condyle (Shen and Darendeliler, 2005). Based on the high levels of Evc2 expression in the Meckel’s cartilage, it is possible to speculate that impact of loss of Evc2 is more prominent during early stage of mandibular growth than postnatal growth when the mandible condylar growth mainly takes place (Shen and Darendeliler, 2005). It has been previously reported in some EvC cases that the zone of proliferative cartilage was affected (Smith and Hand, 1958) and chondrosternal epiphyseal plates showed delayed maturation (Peraita-Ezcurra et al., 2012). This observation was further supported by the characterization of bcd cattle (with Evc2/Lbn mutation), i.e. the long bones of the affected animals have insufficient endochondral ossification with irregularly arranged chondrocytes and partial disappearance of the epiphyseal growth plates (Takeda et al., 2002). These findings suggest that the craniofacial abnormalities by Evc2 deficiency may be caused by abnormal chondrocyte function including Meckel’s cartilage. Further studies are clearly warranted to investigate the chondrogenesis in craniofacial bone structures such as skull base.
In support with our findings, the previous cephalometric analysis reported that an EvC patient had the downward growth of mandible and open bite (Waldrigues et al., 1977). Another EvC case showed that the patient had a small size of maxilla and cranial base and that the analysis indicated retruded maxilla and steep and diverge mandible with the ramus rotated counter-clockwise. Although the angular relation of nasal bone was not described in the EvC patient, it was mentioned that the patient had depressed base of the nose (Susami et al., 1999). Consistently, angular measurements in KO where nasal bone, premaxilla and mandible were involved showed similar bone relations to these clinical cases. To the best of our knowledge the current study was the first to demonstrate that Evc2 deficiency causes the craniofacial defects likely seen in EvC patients.
Our results demonstrated that anterior cross bite may seem to be consistent phenotype in Evc2 KO mice at postnatal stages (Figs. 1B and 1C). At this point, it is not fully clear whether EvC patients have this particular occlusion phenotype, while many of previous literatures described that they have malocclusion (Varela and Ramos, 1996) or Susami et al. reported a female case of EvC showing an open bite and an angle class III molar relation (Susami et al., 1999). It is noteworthy to address that the anterior cross bite phenotype in Evc2 KO mice evidently affects the feeding behavior, which potentially contributes to the bone size difference as a secondary effect.
In conclusion, our data indicate that Evc2 is indispensable for preserving the normal craniofacial bone size as well as bone-to-bone relations during murine postnatal craniofacial bone development and lack of Evc2 affects these relations leading to specific bone growth impairment.
Acknowledgments
Grant sponsors: NIH/NIDCR; DE019527 (Y.Mo.), NIH/NIDCR; DE020843 (Y.Mi.).
Footnotes
Conflict of interest
All authors declare no conflicts of interest in this paper.
Literature cited
- Agerholm JS, Arnbjerg J, Andersen O. Familial chondrodysplasia in Holstein calves. J Vet Diagn Invest. 2004;16:293–298. doi: 10.1177/104063870401600406. [DOI] [PubMed] [Google Scholar]
- Caparros-Martin JA, Valencia M, Reytor E, Pacheco M, Fernandez M, Perez-Aytes A, Gean E, Lapunzina P, Peters H, Goodship JA, Ruiz-Perez VL. The ciliary Evc/Evc2 complex interacts with Smo and controls Hedgehog pathway activity in chondrocytes by regulating Sufu/Gli3 dissociation and Gli3 trafficking in primary cilia. Hum Mol Genet. 2013;22:124–139. doi: 10.1093/hmg/dds409. [DOI] [PubMed] [Google Scholar]
- Curry CJ, Hall BD. Polydactyly, conical teeth, nail dysplasia, and short limbs: a new autosomal dominant malformation syndrome. Birth Defects Orig Artic Ser. 1979;15:253–263. [PubMed] [Google Scholar]
- Ellis RW, van Creveld S. A Syndrome Characterized by Ectodermal Dysplasia, Polydactyly, Chondro-Dysplasia and Congenital Morbus Cordis: Report of Three Cases. Arch Dis Child. 1940;15:65–84. doi: 10.1136/adc.15.82.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom C, Linde A, Thilander B. Craniofacial morphology and growth in the rat. Cephalometric analysis of the effects of a low calcium and vitamin D-deficient diet. J Anat. 1982;134:299–314. [PMC free article] [PubMed] [Google Scholar]
- Galdzicka M, Patnala S, Hirshman MG, Cai JF, Nitowsky H, Egeland JA, Ginns EI. A new gene, EVC2, is mutated in Ellis-van Creveld syndrome. Mol Genet Metab. 2002;77:291–295. doi: 10.1016/s1096-7192(02)00178-6. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Setty S, Sivakumar A, Pai KM. Report of a new syndrome: focus on differential diagnosis and review of Ellis-van Creveld, Curry-Hall, acrofacial dysostosis, and orofacial digital syndromes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:670–676. doi: 10.1016/j.tripleo.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Goor D, Rotem Y, Friedman A, Neufeld HN. Ellis-van Creveld syndrome in identical twins. Br Heart J. 1965;27:797–804. doi: 10.1136/hrt.27.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanemann JA, de Carvalho BC, Franco EC. Oral manifestations in Ellis-van Creveld syndrome: report of a case and review of the literature. J Oral Maxillofac Surg. 2010;68:456–460. doi: 10.1016/j.joms.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Jayo MJ, Leipold HW, Dennis SM, Horton WH. Bovine dwarfism: clinical, biochemical, radiological and pathological aspects. Zentralbl Veterinarmed A. 1987;34:161–177. doi: 10.1111/j.1439-0442.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Jones TH, McClintock AE, Smith GF, Williams G. Achondroplasia in British Friesians. Vet Rec. 1978;102:404. doi: 10.1136/vr.102.18.404-a. [DOI] [PubMed] [Google Scholar]
- Julian LM, Tyler WS, Gregory PW. The current status of bovine dwarfism. J Am Vet Med Assoc. 1959;135:104–109. [PubMed] [Google Scholar]
- Kurian K, Shanmugam S, Harsh Vardah T, Gupta S. Chondroectodermal dysplasia (Ellis van Creveld syndrome): a report of three cases with review of literature. Indian J Dent Res. 2007;18:31–34. doi: 10.4103/0970-9290.30920. [DOI] [PubMed] [Google Scholar]
- Peraita-Ezcurra M, Martinez-Garcia M, Ruiz-Perez VL, Sanchez-Gutierrez ME, Fenollar-Cortes M, Velez-Monsalve C, Ramos-Corrales C, Pastor I, Santonja C, Trujillo-Tiebas MJ. Ellis-van Creveld syndrome in a fetus with rhizomelia and polydactyly. Report of a case diagnosed by genetic analysis, and correlation with pathological andradiologic findings. Gene. 2012;499:223–225. doi: 10.1016/j.gene.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Bard JB. The growth and morphogenesis of the early mouse mandible: a quantitative analysis. J Anat. 2003;203:213–222. doi: 10.1046/j.1469-7580.2003.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Blair HJ, Rodriguez-Andres ME, Blanco MJ, Wilson A, Liu YN, Miles C, Peters H, Goodship JA. Evc is a positive mediator of Ihh-regulated bone growth that localises at the base of chondrocyte cilia. Development. 2007;134:2903–2912. doi: 10.1242/dev.007542. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Ide SE, Strom TM, Lorenz B, Wilson D, Woods K, King L, Francomano C, Freisinger P, Spranger S, Marino B, Dallapiccola B, Wright M, Meitinger T, Polymeropoulos MH, Goodship J. Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nat Genet. 2000;24:283–286. doi: 10.1038/73508. [DOI] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Tompson SW, Blair HJ, Espinoza-Valdez C, Lapunzina P, Silva EO, Hamel B, Gibbs JL, Young ID, Wright MJ, Goodship JA. Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. Am J Hum Genet. 2003;72:728–732. doi: 10.1086/368063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage---a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–699. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- Smith HL, Hand AM. Chondroectodermal dysplasia (Ellis-van Creveld syndrome); report of two cases. Pediatrics. 1958;21:298–307. [PubMed] [Google Scholar]
- Susami T, Kuroda T, Yoshimasu H, Suzuki R. Ellis-van Creveld syndrome: craniofacial morphology and multidisciplinary treatment. Cleft Palate Craniofac J. 1999;36:345–352. doi: 10.1597/1545-1569_1999_036_0345_evcscm_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Takeda H, Takami M, Oguni T, Tsuji T, Yoneda K, Sato H, Ihara N, Itoh T, Kata SR, Mishina Y, Womack JE, Moritomo Y, Sugimoto Y, Kunieda T. Positional cloning of the gene LIMBIN responsible for bovine chondrodysplastic dwarfism. Proc Natl Acad Sci U S A. 2002;99:10549–10554. doi: 10.1073/pnas.152337899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M, Ramos C. Chondroectodermal dysplasia (Ellis-van Creveld syndrome): a case report. Eur J Orthod. 1996;18:313–318. doi: 10.1093/ejo/18.4.313. [DOI] [PubMed] [Google Scholar]
- Waldrigues A, Grohmann LC, Takahashi T, Reis HM. Ellis-van Creveld syndrome. An inbred kindred with five cases. Rev Bras Pesqui Med Biol. 1977;10:193–198. [PubMed] [Google Scholar]
- Weyers H. [A correlated abnormality of the mandible and extremities (dysostosis acrofacialis)] Fortschr Geb Rontgenstr. 1952;77:562–567. [PubMed] [Google Scholar]
- Ye X, Song G, Fan M, Shi L, Jabs EW, Huang S, Guo R, Bian Z. A novel heterozygous deletion in the EVC2 gene causes Weyers acrofacial dysostosis. Hum Genet. 2006;119:199–205. doi: 10.1007/s00439-005-0129-2. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Moritomo Y, Takami M, Hirata S, Kikukawa Y, Kunieda T. Localization of a locus responsible for the bovine chondrodysplastic dwarfism (bcd) on chromosome 6. Mamm Genome. 1999;10:597–600. doi: 10.1007/s003359901052. [DOI] [PubMed] [Google Scholar]
- Zhang H, Takeda H, Tsuji T, Kamiya N, Rajderkar S, Louie K, Collier C, Scott G, Ray M, Mochida Y, Kaartinen V, Kunieda T, Mishina Y. Generation of Evc2/Limbin global and conditional KO mice and its roles during mineralized tissue formation. Genesis. 2015;53:612–626. doi: 10.1002/dvg.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]