Abstract
Objectives
Esophageal variceal bleeding is a severe complication of portal hypertension with significant morbidity and mortality. A substantial portion of cirrhotics fail to respond to conventional medical therapy and band ligation, necessitating alternative treatments including SEMS placement for acute refractory esophageal variceal bleeding. To perform a systematic review and structured meta-analysis of all eligible studies to evaluate the technically feasibility, safety, clinical efficacy, and survival advantage of SEMS for the treatment of acute esophageal variceal bleeding.
Methods
Searches of PubMed, EMBASE, Web of Science, and the Cochrane Library databases were performed through December 2015. Individual study proportions were transformed into a quantity using the Freeman – Tukey variant of the arcsine square root transformed proportion. Combined weighted proportions, and meta-regression were then determined.
Results
The search yielded 12 studies involving n=155 patients, included in our meta-analysis. The pooled clinical success rate in achieving hemostasis within 24 hours was 96% (95% CI, 0.90–1.00). Technical success for SEMS deployment endoscopically was achieved in 97% of patients (95% CI, 0.91–1.00). Total adverse events (including rebleeding after 48 hours, ulceration, and stent migration) were shown in 36% of patients after SEMS placement (95% CI, 0.23–0.50). The pooled 30 day and 60 day survival rate was 68% (95% CI, 0.56–0.80) and 64% (95% CI, 0.48–0.78), respectively.
Conclusion
This study demonstrated that esophageal SEMS placement is a technically feasible modality and highly efficacious in achieving hemostasis in acute esophageal variceal hemorrhage.
Keywords: Stents, SEMS, Esophageal varices, Cirrhosis, Bleeding
INTRODUCTION
Esophageal variceal bleeding accounts for 70% of all upper gastrointestinal bleeding episodes in patients with liver cirrhosis and is associated with significant morbidity and mortality.1 While short-term mortality has improved significantly from 42% in the 1980’s to today’s estimate of 15–20%, the number still remains unacceptably high.1–7 The current approach for acute esophageal variceal bleeding combines medical and endoscopic management including vasoactive drug therapy, endoscopic band ligation, and prophylactic antibiotic administration after initial hemodynamic resuscitation efforts.8–12 Endoscopic and pharmacologic treatment remains first-line therapy for active esophageal variceal hemorrhage; however, a large number of high-risk patients may not respond to traditional endoscopic or pharmacologic treatment. With 10–20% of cirrhotics refractory to medical and endoscopy therapy, alternative treatment options are necessary to curb the sizeable associated morbidity and morality.13
Traditional alternative therapies for patients with refractory esophageal variceal hemorrhage have included balloon tamponade and more recently, the use of transjugular intra-hepatic portosystemic shunt (TIPS).14,15 Both treatments are effective for achieving hemostasis in this patient population; however TIPS is associated with risk of worsening hepatic encephalopathy and balloon tamponade with significant risk of muscosal ischemia and aspiration.15–18 These potential adverse events, some of which are more common than others, are increasingly problematic for clinicians seeking an alternative means to achieve hemostasis. Additional barriers include availability of these modalities only at specialized centers where the use of TIPS may be more prominent. These limitations, coupled with the significant morbidity and mortality of variceal bleeding, necessitate more potential therapies available to clinicians, one with a high clinical efficacy and ease of implementation.
According to the Baveno V consensus conference, self-expanding metal stent (SEMS) deployment may be a option in cases of refractory esophageal variceal bleeding.19 While use of the device has been limited in real clinical practice within in the United States, SEMS placement has shown promising results to date.8 Current standard luminal stents are not amenable to achieving hemostasis in acute variceal hemorrhage; however, a specialized SEMS has been developed for this select patient population.20,21 This dedicated SEMS device is a removable, covered stent deployed endoscopically with the use of a guidewire. The early technical and clinical success shown in previous studies merit further consideration and advancement into mainstream clinical practice.
The primary aim of this study was to perform a systematic review and structured meta-analysis of all eligible studies to evaluate the technically feasibility, safety, clinical efficacy, and survival advantage of SEMS placement for the treatment of acute esophageal variceal bleeding.
METHODS
Literature Search
A comprehensive search of the literature was performed to identify articles that examined the use of the SEMS device for the treatment of acute refractory esophageal variceal bleeding. Systematic searches of PubMed, EMBASE, Web of Science, and the Cochrane Library databases were performed through December 2015 using the following search terms “self-expanding metal stents”, “refractory esophageal varices”, “SEMS and esophageal variceal bleeding ”, “metal stents and varices”, and “stents and varices”. All relevant articles irrespective of language, year of publication, type of publication, or publication status were included. The titles and abstracts of all potentially relevant studies were screened for eligibility. The reference lists of studies of interest were then manually reviewed for additional articles by cross checking bibliographies. Two reviewers (TRM and BN) independently screened the titles and abstracts of all the articles according to predefined inclusion and exclusion criteria. Any differences were resolved by mutual agreement. In the case of studies with incomplete information, contact was attempted with the principal authors to obtain additional data.
Study Selection Criteria
Only studies investigating the use of SEMS placement for acute esophageal variceal bleeding were included. Stent types included: the Choo stent, Ella-Boubela-Danis stent, and the SX-Ella Danis stent (Ella-CS, Hradec Kralove, Czech Republic). Studies involving alternative endoscopic or interventional radiologic modalities were not included. Only human subject studies were included in the analysis. A particular study was excluded if deemed to have insufficient data, as well as review articles, editorials, and correspondence letters that did not report their own data. Due to the limited data available, case series and reported studies with fewer than 5 patients were excluded.
Outcome Measures
The primary outcome measurement in this study was treatment efficacy or clinical success - defined as cessation of bleeding to achieve hemostasis within 24 hours of SEMS placement. Secondary outcomes included technical success rate, complication rate and type of complication (rebleeding, ulceration, or stent migration), all cause 30 day survival rate, and 60 day survival rate. Additional information analyzed included average age of SEMS recipients, etiology of liver disease, severity of liver disease as measured by Model for End-Stage Liver Disease (MELD) or Child-Pugh Score, duration of follow-up period, previous treatments for which the patient was refractory, and definitive therapy after SEMS placement.
Statistical Analysis
This meta-analysis was performed by calculating pooled proportions. After appropriate studies were identified through systematic review, the individual study proportion was transformed into a quantity using the Freeman – Tukey variant of the arcsine square root transformed proportion. Then the pooled proportion was calculated as the back transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model and DerSimonian – Laird weights for the random effects model.14,22,23 Since this was a cumulative meta-analysis, publication bias was not assessed. Combined weighted proportions, and meta-regression were determined by use of the Stata 13.0 software package (Stata Corp LP, College Station, TX).
RESULTS
Characteristics of Included Studies
The initial search terms identified 415 studies after duplicate articles were removed. Of these, 63 articles were retrieved. A PRISMA flow chart of the literature search and selection process is shown in Figure 1. Final literature search and selection fulfilling the inclusion and exclusion criteria yielded 12 studies (n = 155 patients) using SEMS treatment for acute refractory esophageal variceal bleeding.21,24–34 One randomized, controlled trial was identified as a comparative study to balloon tamponade.30 The remaining studies were small sample-sized retrospective studies (mostly case series and reports). Patient characteristics of all included studies are summarized and highlighted in Table 1.
Figure 1.
PRISMA Flow Chart of Literature Search Results
Table 1.
Baseline Characteristics of Included Studies Assessing SEMS in Esophageal Varices
| Author | Year | No of Pts |
Gender (M/F) |
Mean Age in Yrs (Range) |
Type of Liver Disease |
Child Pugh Score |
MELD (mean) |
Grade of varices |
Previous Therapies |
Follow- up Period (day) |
Stent Type |
Length of Stent Placement days (range) |
Success of Stent Extractiona (%) |
Adverse Events (Total %) |
Definitive Treatment After Stent |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hubmann et al | 2006 | 20 | 18/2 | 52 (27–87) | 12 EtOH; 3 Viral; 0 NASH; 5 Other | CPA 0; CPB 8; CPC 12 | - | - | 5 Sclerotherpy; 18 EBL; 6 Balloon Tamponade | 60 | 2 Choo; 3 Ella-Boubella; 15 SX-Ella | 6 (2–14) | 100% | 1 ulceration; 5 stent migration (30%) | 1 Conservative; 5 TIPS; 3 Transplant |
| Zehetner et al | 2008 | 34 | 33/1 | 56 (32–91) | 26 EtOH; 4 Viral; 0 NASH; 4 Other | CPA 0; CPB 13; CPC 21 | - | - | 7 Sclerotherpy; 21 EBL; 0 Balloon Tamponade | - | 34 SX-Ella | 5 (1–14) | 100% | 1 ulceration; 7 stent migration (23.5%) | 2 TIPS; 8 Transplant |
| Hogan et al | 2009 | 10 | - | - | - | CPA 0; CPB 1; CPC 9 | - | - | - | - | 10 SX-Ella | 10 (4–14) | 100% | None (0%) | - |
| Wright et al | 2010 | 10 | 9/1 | 49.4 | 6 EtOH 2 Viral; 0 NASH; 2 Other | - | - | - | 0 Sclerotherpy; 5 EBL; 1 Balloon Tamponade | 42 | 10 SX-Ella | 9 (6–14) | 100% | 3 rebleeding; 1 ulceration (40%) | - |
| Dechene et al | 2012 | 8 | 6/2 | 63 | 3 EtOH; 3 Viral; 2 NASH; 0 Other | CPA 0; CPB 0; CPC 8 | - | - | 2 Sclerotherpy; 8 EBL; 2 Balloon Tamponade | - | 8 SX-Ella | 11 (4–17) | 100% | 3 rebleeding (37.5%) | 6 Conservative; 1 TIPS; 1 Transplant |
| Holster et al | 2013 | 5 | 3/2 | 58 (48–78) | 3 EtOH; 0 Viral; 0 NASH; 2 Other | CPA 0; CPB 1; CPC 1 | - | - | 0 Sclerotherpy; 5 EBL; 0 Balloon Tamponade | 180 | 5 SX-Ella | 52.4 (6–214) | 100% | 1 stent migration (20%) | 1 TIPS; 1 Transplant |
| Fierz et al | 2013 | 7 | 5/2 | 56 (41–68) | 5 EtOH; 2 Viral; 0 NASH; 0 Other | CPA 0; CPB 2; CPC 5 | - | - | 2 Sclerotherpy; 6 EBL; 0 Balloon Tamponade | 42 | 7 SX-Ella | 12 hours to 5 days | 100% | 2 stent migration (28.6%) | 3 TIPS |
| Zakaria et al | 2013 | 16 | 14/2 | 57 | 0 EtOH; 16 Viral; 0 NASH; 0 Other | CPA 2; CPB 8; CPC 6 | - | 5 with grade I or II; 11 with III or IV | - | - | 16 SX-Ella | 2 to 4 days | 100% | 2 rebleeding; 1 ulceration; 6 stent migration (56.3%) | - |
| Bremholm et al | 2013 | 9 | 3/6 | 55.2 (45–73) | 6 EtOH; 1 Viral; 0 NASH; 2 Other | - | - | - | - | - | 9 SX-Ella | 9.7 (4–22) | 100% | 2 rebleeding (22.2%) | - |
| Mishin et al | 2013 | 12 | 8/4 | 46.92 (24–62) | - | - | - | - | - | - | 12 SX-Ella | - | - | 1 rebleeding (8.3%) | - |
| Muller et al | 2015 | 11 | 8/3 | 64.2 (43–79) | 9 EtOH; 1 Viral; 0 NASH; 1 Other | CPA 1; CPB 6; CPC 3 | - | 1 with grade I; 2 with grade II; 6 with grade III; 2 with grade IV | - | 42 | 11 SX-Ella | 12.1 (5–24) | 100% | 2 ulceration; 7 stent migration (81.8%) | 2 TIPS; 1 Transplant |
| Escorsell | 2015 | 13 | 13/0 | 69 (40–81) | 8 EtOH; 3 Viral; 0 NASH; 2 Other | CPA 3; CPB+C 10 | 16.5 | 3 small; 10 large | 3 Sclerotherpy; 8 EBL; 0 Balloon Tamponade | 42 | 13 SX-Ella | 2–14 days | 100% | 7 stent migration (53.8%) | 5 Conservative; 4 TIPS |
Successful extraction was defined as technical ability to remove stent after placement by PEX-Ella extractor (Ella-CS) – percent calculated from only surviving patients
Types of Liver Disease: EtOH = alcoholic, Viral = hepatitis B or hepatitis C, NASH = non-alcoholic steatohepatitis
Child Pugh Score: CPA = Child Pugh A, CPB = Child Pugh B, CPC = Child Pugh C
Previous Therapies: EBL = Endoscopic band ligation
Definitive Treatment After Stent: TIPS = transjugular intrahepatic portosystemic shunt
Clinical Success
Treatment success, defined as absence of bleeding within 24 hours of SEMS placement was overwhelming positive amongst all included studies. The pooled clinical success rate was 96% (95% CI, 0.90 – 1.00) and reported in all 12 studies. Five studies reported ineffective control of bleeding with inability to achieve hemostasis within 24 hours – lowest clinical success rate of 78% by Hogan et al and Wright and colleagues.25,28,30,33,34 Figure 2 illustrates the Forest plot of clinical success. Relative risk (i.e. 95% CI) are reported for clinical success and other variables in the Supplemental Table 1.
Figure 2.
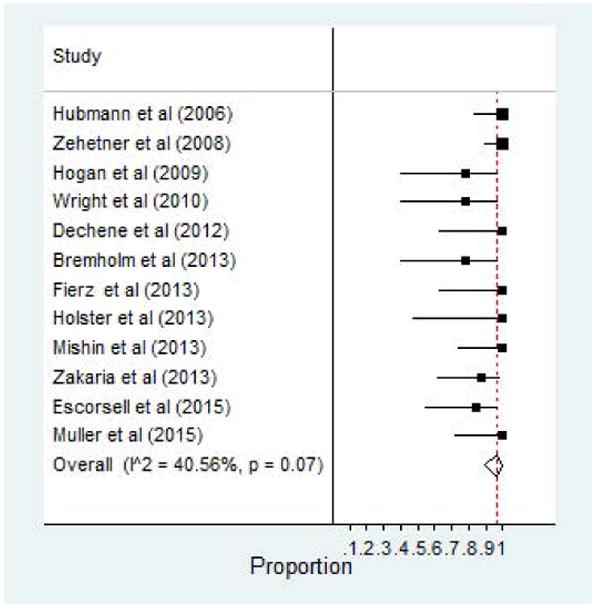
Clinical Success of SEMS for Esophageal Varices
Technical Success and Feasibility
SEMS deployment endoscopically with the use of a guidewire was reported in all 12 studies. Technical success was achieved in 97% of patients (95% CI, 0.91–1.00). Forest plot of technical success is demonstrated in Figure 3. Wright et al reported a technical failure due to malfunction of gastric balloon deflation while other authors reported various failures involving manipulation of the guidewire, stent migration, and other failed delivery methods.25 Although, technical difficulty and placement failure was reported, the vast majority of SEMS devices were placed without issue.
Figure 3.
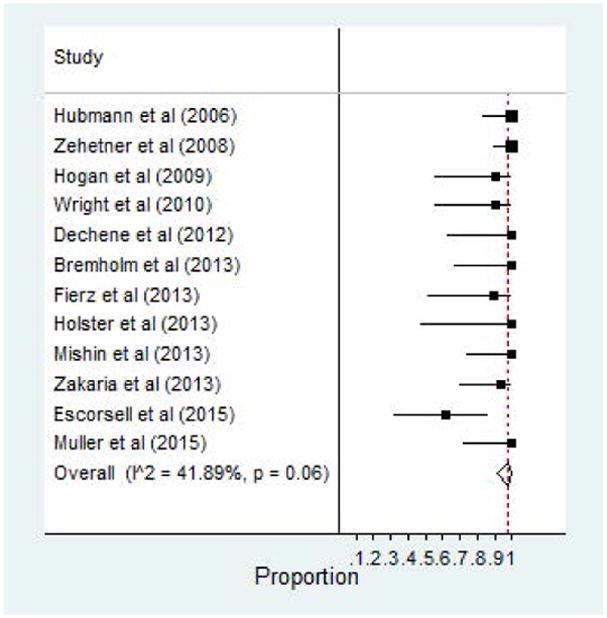
Technical Success of SEMS for Esophageal Varices
Adverse Events
Complications related to SEMS placement were also reported in all 12 studies. Total adverse events (including rebleeding after 48 hours, ulceration, and stent migration) were shown in 36% of patients after SEMS placement (95% CI, 0.23–0.50). Stent migration was by far the most common complication reported – Table 1. The second most common adverse event reported was ulceration, followed by rebleeding after initial hemostasis was achieved. One study, by Hogan and others, reported no complications among a cohort of 10 patients.33 Forest plot of total adverse events is shown in Figure 4.
Figure 4.
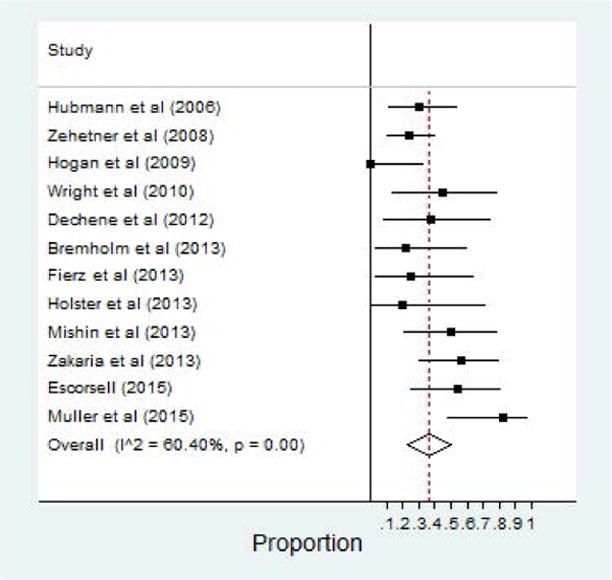
Adverse Events of SEMS for Esophageal Varices
Mortality and Survival Data
Data on all cause mortality was also abstracted from the included studies. A total of 8 of the 12 studies reported a 30 day mortality rate.21,24,25,27,30–33 Three of these included studies assessed 6 week survival rate.25,30,32 Of those 8 studies, the pooled survival rate was 68% (95% CI, 0.56–0.80). The most common etiology for death was due to progressive hepatic damage and multi-organ failure. Eight studies in all also reported survival rates at 60 days post-SEMS placement as well 64% (95% CI, 0.48–0.78).21,24–27,29,31,32 Forest plot of 30 day survival as well as 60 day survival are highlighted in Figure 5 and 6, respectively.
Figure 5.
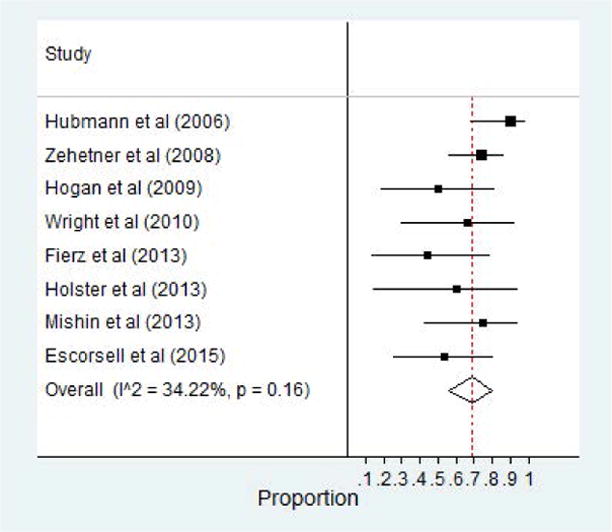
30 Day Survival Rate of Patients with SEMS for Esophageal Varices
Figure 6.
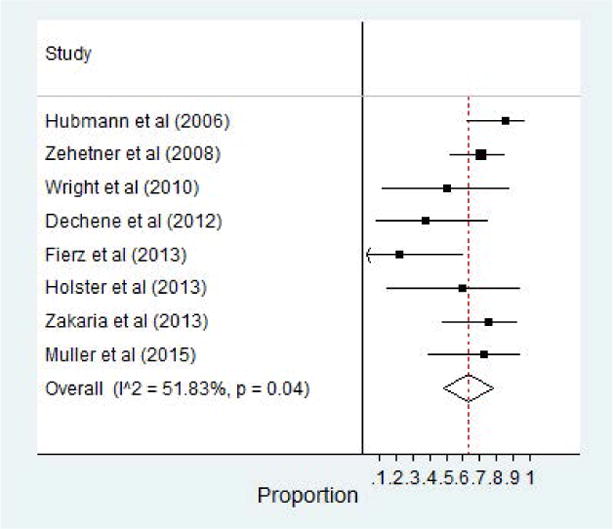
60 Day Survival Rate of Patients with SEMS for Esophageal Varices
DISCUSSION
This study demonstrated that esophageal SEMS placement is a technically feasible and effective (96% success rate) modality in achieving hemostasis in acute esophageal variceal hemorrhage. Stent placement is relatively safe with few complications. The high clinical success rate and minimal adverse event profile suggest that specialized SEMS placement appears to be a viable alternative for patients with esophageal variceal bleeding that fail traditional endoscopic or pharmacologic therapy.
With a substantial patient population that fails to respond to conventional medical therapy and band ligation, a variety of potential alternatives have been established. Current clinical practice in the United States relies upon balloon tamponade though recent data suggests TIPS may have an expanded role as both an early and rescues modality.14 A recent study by Escorsell and colleagues aimed to compare traditional balloon tamponade versus a SEMS device.30 This recent study was a randomized controlled trial in effort to better evaluate this clinical conundrum. Escorsell et al demonstrated that, SEMS placement was associated with a greater efficacy and less serious adverse events when compared to balloon tamponade. Additionally, the overwhelmingly positive technical success as demonstrated in this meta-analysis suggests an expanded or first-line role for SEMS placement in the emergent setting. Based upon these results, these authors favored the use of SEMS for patients with acute esophageal variceal hemorrhage uncontrolled with medical or traditional endoscopic treatment.
When compared to balloon tamponade, it appears the use of SEMS placement has a less severe side effect profile potentially making it a more attractive rescue modality. However, as compared to TIPS, both therapies require some degree of expertise that may not be readily available at non-tertiary centers. SEMS placement requires deployment over a guidewire under direct visualization with conscious sedation. Additionally, the SEMS is only a bridge therapy. A large percentage of patients included in the 12 studies ultimately received TIPS therapy at the time of SEMS removal. SEMS extraction also requires a PEX-Ella extractor (Ella-CS) for removal of the stent necessitating repeat endoscopy. While stent extraction was done with relative ease as reported in 11 of the 12 studies, the additional requirement of a specialized retraction device may limit an expanded use of this device. Additionally, it remains unclear whether stent retraction and the device associated may have resulted in cause for rebleeding or mucosal ulceration. Lastly, a major limitation of SEMS placement relates to timing of stent removal. As illustrated in Table 1, the timing of stent placement was heterogeneous and may impact technical success and retraction capability at inexperienced centers.
While this study suggests an expanded role for specialized SEMS placement, further consideration is warranted with regard to cost and availability. Additionally, clinical expertise and unfamiliarity among United States endoscopists may factor in to variable technical success and clinical success results. The cost of this modality as well as availability remains unclear as it relates to clinical practice in United States. Future studies are needed to truly evaluate the cost effectiveness of specialized SEMS placement for the management of refractory esophageal variceal bleeding.
Our results should be interpreted bearing in mind the following: First, this summative analysis was based purely on the published medical literature. We did not have access to individual patient data, which could have allowed us to perform more detailed analysis, especially on factors associated with response to SEMS and survival (i.e. acute liver failure and procedure related complications). Second, aside from the small, inherent heterogeneity limitations of meta-analyses that exist between studies, a major limitation of this study is the modality of choice. The initial study of SEMS use for variceal hemorrhage was performed by Hubmann and colleagues.21 It is important to note that, this early study did not exclusively use the SX-Ella Danis stent (Ella-CS, Hradec Kralove, Czech Republic), specialized designed to treat bleeding esophageal varices. Instead, the Choo stent and the Ella-Boubela-Danis stent were also deployed. All other studies exclusively utilized the SX-Ella Danis stent (Ella-CS, Hradec Kralove, Czech Republic). Also of note, all authors defined hemostasis within 24 hours in a heterogeneous manner, which may prevent the ability to generalize this data. Aside from this, the translation of these results clinically is difficult as the stent device is specifically designed for acute esophageal variceal hemorrhage and not the traditional luminal stent. Lastly, as this was a cumulative meta-analysis, publication bias was not assessed which resulted in an inability to exclude some bias in the results of this study.
To our knowledge, this is the first ever pooled analysis on SEMS placement in patients with acute esophageal variceal hemorrhage. By combining data from all available studies, we were able to report the best evidence on the effectiveness of SEMS in management of acute esophageal variceal hemorrhage. This systematic review and meta-analysis suggests an expanded use of specialized SEMS placement for the treatment of acute esophageal variceal bleeding. The technical success of SEMS placement was overwhelming positive and achieved hemostasis is a vast majority of patients with minimal SEMS-related complications. While this should be directly evaluated with comparative studies for true performance measures, we suggest that this specialized SEMS should be considered relatively early in patients with acute esophageal variceal hemorrhage who fail primary pharmacologic and endoscopic treatment. However, caution should be exercised in patients with progressive hepatic damage and multi-organ failure.
Supplementary Material
Acknowledgments
Financial Support: Supported by NIH 5 T32 DK 7356-37 (BN)
The authors have no acknowledgements.
Footnotes
Author Contributions – Study concept and design: TRM, BN; Acquisition and analysis of data: BN; Interpretation of data: TRM, BN; Initial draft: TRM; Critical revision of manuscript: BN. All authors approved the final draft submitted.
Potential Conflicts of Interest: The authors have no potential conflicts of interest to report.
CONFLICT OF INTERESTS
The authors have no potential conflicts of interest to report. B.N. was supported by grants from NIH 5 T32 DK 7356-37.
References
- 1.D’Amico G, De Franchis R Cooperative Study G. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 2.North Italian Endoscopic Club for the S, Treatment of Esophageal V. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983–9. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 3.Augustin S, Altamirano J, Gonzalez A, et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol. 2011;106:1787–95. doi: 10.1038/ajg.2011.173. [DOI] [PubMed] [Google Scholar]
- 4.Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol. 2009;7:1347–54. doi: 10.1016/j.cgh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652–9. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- 6.Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003;98:653–9. doi: 10.1111/j.1572-0241.2003.07294.x. [DOI] [PubMed] [Google Scholar]
- 7.Jamal MM, Samarasena JB, Hashemzadeh M. Decreasing in-hospital mortality for oesophageal variceal hemorrhage in the USA. Eur J Gastroenterol Hepatol. 2008;20:947–55. doi: 10.1097/MEG.0b013e32830280c7. [DOI] [PubMed] [Google Scholar]
- 8.de Franchis R, Baveno VF. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–8. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD Practice Guidelines Committee of American Association for Study of Liver D, Practice Parameters Committee of American College of G. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JH, Shergill AK, Acosta RD, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221–7. doi: 10.1016/j.gie.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Runyon BA, Committee APG. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 12.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–82. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Pagan JC, Reverter E, Abraldes JG, Bosch J. Acute variceal bleeding. Seminars in respiratory and critical care medicine. 2012;33:46–54. doi: 10.1055/s-0032-1301734. [DOI] [PubMed] [Google Scholar]
- 14.Njei B, Laine L. Early Use of TIPS and Outcomes Patients with Cirrhosis Acute Esophageal Variceal Bleeding: Analysis the U.S. Nationwide Inpatient Sample (NIS) Database, 2000–2010. ACG Annual Scientific Meeting 2018. Am J Gastroenterol. 2015 Oct;11:1. [Google Scholar]
- 15.Panes J, Teres J, Bosch J, Rodes J. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig Dis Sci. 1988;33:454–9. doi: 10.1007/BF01536031. [DOI] [PubMed] [Google Scholar]
- 16.Rossle M. TIPS: 25 years later. J Hepatol. 2013;59:1081–93. doi: 10.1016/j.jhep.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Chong CF. Esophageal rupture due to Sengstaken-Blakemore tube misplacement. World J Gastroenterol. 2005;11:6563–5. doi: 10.3748/wjg.v11.i41.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avgerinos A, Klonis C, Rekoumis G, Gouma P, Papadimitriou N, Raptis S. A prospective randomized trial comparing somatostatin, balloon tamponade and the combination of both methods in the management of acute variceal haemorrhage. J Hepatol. 1991;13:78–83. doi: 10.1016/0168-8278(91)90867-b. [DOI] [PubMed] [Google Scholar]
- 19.Laine L, Abid S, Albillos A, et al. Treatment of acute bleeding. In: de Franchis, editor. Portal Hypertension V: Proceedings of the Fifth Baveno International Consensus Workshop. Blackwell Publishing Ltd; Oxford, UK: 2011. pp. 103–115. [Google Scholar]
- 20.Benko L, Danis J, Czompo M, et al. DSC examination of the esophagus after two different self-expanding stents implantation. J Therm Anal Calorim. 2006;83:715–20. [Google Scholar]
- 21.Hubmann R, Bodlaj G, Czompo M, et al. The use of self-expanding metal stents to treat acute esophageal variceal bleeding. Endoscopy. 2006;38:896–901. doi: 10.1055/s-2006-944662. [DOI] [PubMed] [Google Scholar]
- 22.Stuart A, Ord JK. Kendall’s Advanced Theory of Statistics. 6. London: Edward Arnold; 1994. [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Zehetner J, Shamiyeh A, Wayand W, Hubmann R. Results of a new method to stop acute bleeding from esophageal varices: implantation of a self-expanding stent. Surg Endosc. 2008;22:2149–52. doi: 10.1007/s00464-008-0009-7. [DOI] [PubMed] [Google Scholar]
- 25.Wright G, Lewis H, Hogan B, Burroughs A, Patch D, O’Beirne J. A self-expanding metal stent for complicated variceal hemorrhage: experience at a single center. Gastrointest Endosc. 2010;71:71–8. doi: 10.1016/j.gie.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Dechene A, El Fouly AH, Bechmann LP, et al. Acute management of refractory variceal bleeding in liver cirrhosis by self-expanding metal stents. Digestion. 2012;85:185–91. doi: 10.1159/000335081. [DOI] [PubMed] [Google Scholar]
- 27.Holster IL, Kuipers EJ, van Buuren HR, Spaander MC, Tjwa ET. Self-expandable metal stents as definitive treatment for esophageal variceal bleeding. Endoscopy. 2013;45:485–8. doi: 10.1055/s-0032-1326227. [DOI] [PubMed] [Google Scholar]
- 28.Zakaria MS, Hamza IM, Mohey MA, Hubamnn RG. The first Egyptian experience using new self-expandable metal stents in acute esophageal variceal bleeding: pilot study. Saudi J Gastroenterol. 2013;19:177–81. doi: 10.4103/1319-3767.114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller M, Seufferlein T, Perkhofer L, Wagner M, Kleger A. Self-Expandable Metal Stents for Persisting Esophageal Variceal Bleeding after Band Ligation or Injection-Therapy: A Retrospective Study. PloS one. 2015;10:e0126525. doi: 10.1371/journal.pone.0126525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escorsell A, Pavel O, Cardenas A, et al. Esophageal balloon tamponade Vs esophageal stent in controlling acute refractory variceal bleeding: A multicenter RCT. Hepatology. 2015 doi: 10.1002/hep.28360. [DOI] [PubMed] [Google Scholar]
- 31.Mishin I, Zastavnitsky G, Ghidirim G, Bunic G. Self-expanding metal stents: a new hemostasis method for bleeding esophageal varices. Hepatol Int. 2013;7(Suppl I):S540. [Google Scholar]
- 32.Fierz FC, Kistler W, Stenz V, Gubler C. Treatment of esophageal variceal hemorrhage with self-expanding metal stents as a rescue maneuver in a swiss multicentric cohort. Case Rep Gastroenterol. 2013;7:97–105. doi: 10.1159/000350192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan B, Patch D, Burroughs A, O’Beirne J. Use of the SX-Ella Self-Expanding Mesh Metal Stent in the Management of Complex Variceal Haemorrhage: Initial Experience in a Single Centre. J Hepatol. 2009:S86–S87. [Google Scholar]
- 34.Bremholm L, Bendtsen F, Hansen EF. Self-expanding Metal Stents for Acute Treatment of Bleeding Oesophageal Varices. 17th UEGW/WCOG; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.