Abstract
Organophosphate (OP) chemicals include nerve agents and pesticides, and there is a growing concern of OP-based chemical attacks against civilians. Current antidotes are essential in limiting immediate mortality associated with OP exposure. However, further research is needed to identify molecular mechanisms underlying long-term neurological deficits following survival of OP toxicity in order to develop effective therapeutics. We have developed rat survival models of OP-induced status epilepticus (SE) that mimic chronic mortality and morbidity following OP intoxication. We have observed significant elevations in hippocampal calcium levels after OP SE that persisted for weeks following initial survival. Drugs inhibiting intracellular calcium–induced calcium release, such as dantrolene, levetiracetam, and carisbamate, lowered OP SE–mediated protracted calcium elevations. Given the critical role of calcium signaling in modulating behavior and cell death mechanisms, drugs targeted at preventing the development of the calcium plateau could enhance neuroprotection, help reduce morbidity, and improve outcomes following survival of OP SE.
Keywords: paraoxon, status epilepticus, cell death, calcium, dantrolene, carisbamate
The increasing risk for organophosphate exposure
Organophosphate (OP) chemicals include nerve agents such as sarin and pesticides such as parathion. These compounds are considered extremely lethal. The civilian population has been exposed to nerve agents under acts of war and terrorism. Recent examples include the reported 2015 sarin gas attack in Ghouta, Syria,1 the Tokyo subway sarin attack by the Aum Shinrikyo cult in 1995,2 and the 1988 Halabja chemical attack against Kurdish people in Iraq.3 OP-based pesticides have also been used against civilians during the Rhodesian War,4 and Indian children were accidentally exposed following consumption of pesticide-contaminated lunches.5 In addition, civilians are exposed to OPs intentionally via suicide attempts, occupationally, or due to industrial accidents. In fact, pesticide ingestion is one of the most common methods for committing suicide in developing nations.6–8 The military population has also been exposed to OP chemicals. Approximately 30% of the Gulf War veterans suffer from a cluster of symptoms commonly known as Gulf War Syndrome. Prolonged exposure to OP-based pesticides or exposure to sarin gas following demolition of chemical weapon stockpiles are among the possible causes of this syndrome.9–11 The ease of availability of pesticides make them attractive targets to weaponize and cause mass civilian causalities. Thus, there is a growing threat of OP toxicity in the current geopolitical environment. Research in this field has provided therapeutic antidotes that are critical in limiting immediate mortality associated with lethal OP intoxication.12 However, further research is needed to identify the molecular mechanisms underlying chronic mortality and morbidity in order to develop effective counteract therapeutics following OP exposure.13
Organophosphate poisoning: mechanisms, treatments, and challenges
Paraoxon (POX) is an active metabolite of parathion and is used in laboratory research to reliably model OP pesticide toxicity.14 Similarly, diisopropyl fluorophosphate (DFP) is used in civilian research as a nerve agent surrogate to model sarin exposure owing to the ease of handling associated with DFP.15–18 POX, DFP, and other OP chemicals are potent inhibitors of the enzyme acetylcholinesterase (AChE).19 Inhibition of AChE prevents breakdown of the neurotransmitter acetylcholine (ACh), and ACh levels rapidly build up at the synapses. Overstimulation of ACh receptors leads to the classical cholinergic crisis characterized by salivation, lacrimation, urination, and defecation. This is followed by respiratory depression and bradycardia. Nicotinic receptor stimulation causes muscle fasciculation. This is followed by tonic–clonic seizures and status epilepticus (SE), or prolonged seizure activity that continues unabated and can result in death if left untreated.20 SE activity is thought to involve recruitment of N-methyl-d-aspartate (NMDA) receptors following release of the excitatory neurotransmitter glutamate downstream of the ACh overstimulation.21–24 Current treatment strategies use atropine to control the cholinergic crisis, pralidoxime to reactivate AChE, and a benzodiazepine, such as diazepam or midazolam, to control seizures.25,26 While the current antidotes are critical in limiting immediate mortality associated with OP exposure, OP SE survivors are vulnerable to delayed mortality in the critical 2-week period after initial survival and the development of chronic neurological morbidities such as recurrent seizures, depression, and cognitive deficits.14,22,27–35 Thus, it is essential to develop valid animal models that mimic OP mortality and morbidity and to identify molecular mechanisms underlying long-term neurological deficits from survival of OP toxicity in order to develop effective counteract therapeutics.
Rat survival models of OP SE
Many OP studies in the literature have focused on the effects of low-dose, chronic OP exposure or the effects of OPs following in utero exposure.36–39 There are also studies reporting models of acute parathion40,41 and POX exposures.42–44 However, these models did not focus on evaluating long-term survival after lethal POX SE exposures. Development of OP SE models is also complicated by their variable pharmacokinetic and pharmacodynamics response, such as the challenges associated with parathion kinetics and differential metabolism.45–47 We wanted to further develop a reliable rat survival model for lethal OP exposure with SE that would replicate both the acute mortality and chronic morbidity associated with these agents. Such animal models could be very useful for studying the molecular mechanisms of OP toxicities and for screening medical countermeasures to improve survival following OP exposures.
To this end, we have developed two SE survival models of OP toxicity using lethal doses of POX 14 and DFP.16 The behavioral manifestations and electroencephalography (EEG) profiles for these OP SE models mimicked the signs and symptoms of acute OP intoxication. In this model, rats were exposed to a lethal dose (approximately twice the LD50) of an OP chemical (POX or DFP) and were treated with U.S. Food and Drug Administration–approved drugs to limit immediate mortality.26 Here, we will discuss the POX model of OP SE. One week before the SE experiments, rats were stereotaxically implanted with skull surface electrodes to record EEG. Briefly, 1 min after POX injection (2 mg/kg subcutaneous (SC)) animals received human-dose equivalents of 2-PAM (25 mg/kg intramuscular (IM) and atropine (0.5 mg/kg IM). Within 5–7 min following POX administration, rats displayed overt cholinergic symptoms and rapidly developed convulsions and SE-like activity. Onset of SE was determined by the presence of continuous class 4–5 level seizures using a modified Racine scale.48 One hour following onset of POX SE, animals were injected with midazolam (2 mg/kg IM) to terminate seizures. Surviving animals were then injected with saline (3cc/animal intraperitoneal (IP)), fed lactose milk as supportive care, and returned to their home cages. Surviving rats were housed individually in temperature- and light-controlled vivaria. All the rats were visually monitored once a week until their use in Ca2+ imaging or behavioral experiments. Chronic mortality (72-h and beyond) in these models of severe OP intoxication was 18–20%.14,16 These POX and DFP SE survival models manifested the same degree of delayed mortality and morbidity (see below) observed in the human OP-exposure condition.49–53
Development of Ca2+ plateau following survival from OP SE
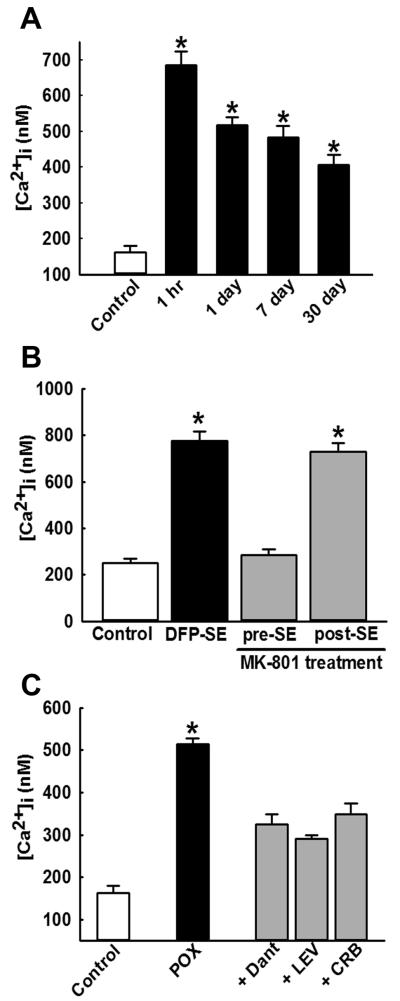
One of the important long-term molecular changes that occurs following the survival of SE induced by OPs or chemoconvulsants like pilocarpine is the development of sustained elevations in neuronal calcium levels ([Ca2+]i) known as the “Ca2+ plateau.”14,16,54–59 We have developed methodologies to acutely isolate hippocampal CA1 region neurons from brain slices using enzymatic and mechanical trituration. Neurons obtained using these methods show minimal signs of necrosis, exhibit normal electrophysiological membrane properties, and allow us to study Ca2+ dynamics in the absence of confounding factors such as glial response. Estimation of neuronal Ca2+ levels have revealed the development of a Ca2+ plateau wherein hippocampal neurons exhibit significantly elevated Ca2+ levels for weeks after the termination of POX SE14 (Fig. 1A). We have previously shown that, while the induction of Ca2+ plateau was dependent on the NMDA receptor during SE,16,54 the maintenance of the Ca2+ plateau for several weeks post-SE was independent of NMDA receptor activation and was mediated by persistent Ca2+ release from the endoplasmic reticulum through the mechanisms of Ca2+-induced Ca2+ release (CICR).56,57 Indeed, pretreatment with the NMDA antagonist MK-801 prevented the OP SE–induced elevations in hippocampal Ca2+ levels. However, application of MK-801 was not effective in lowering the elevated Ca2+ in hippocampal neurons isolated from rats 1 h after SE16 (Fig. 1B). On the other hand, treatments with dantrolene, levetiracetam, or carisbamate, inhibitors of the CICR mechanisms, were able to lower the elevated Ca2+ levels and abolish the Ca2+ plateau after SE14,57,60 (Fig. 1C). It is important to note that, while the NMDA receptor–mediated indiscriminant Ca2+ influx turns off after SE is terminated, a sustained Ca2+ release from the endoplasmic reticulum continues, owing to a long-lasting activation of molecular components involved in the CICR mechanisms. This is an important aspect of the long-lasting activation of the CICR system. Since Ca2+ ions act as major second messengers in multiple signaling cascades, the OP SE–induced prolonged elevations in [Ca2+]i can trigger neurodegenerative pathways and mediate pathological synaptic plasticity. These alterations in Ca2+ dynamics following OP toxicity could therefore underlie the associated neuronal injury, and together they may be responsible for the chronic neurological morbidities following OP SE survival14 (Fig. 4).
Figure 1.
Development of Ca2+ plateau and its mechanism following OP-induced SE. (A) Hippocampal CA1 [Ca2+]i from age-matched control (white bar) and POX-exposed rats were isolated 1 h and 1, 7, and 30 days after SE (black bars). [Ca2+]i in POX-SE rats was significantly higher than control values at all the time points and did not return to baseline, even at 30 days post-SE (Ca2+ plateau). (B) Hippocampal CA1 [Ca2+]i from control rats (white bar), DFP-treated rats (black bar), and DFP + MK-801–treated rats (grey bars) were isolated 1 -h after SE. MK-801 pretreatment prevented the elevations in [Ca2+]i that occur following DFP-induced SE. However, MK-801 treatment 1 h after DFP-induced SE did not significantly affect DFP-SE induced [Ca2+]i elevations. (C)Hippocampal CA1 [Ca2+]i from control rats (white bar), POX-treated rats (black bars), and POX + drugs rats (grey bars) were isolated 48 h after SE. [Ca2+]i in neurons isolated from POX-SE rats treated with either dantrolene (DANT), levetiracetam (LEV), or carisbamate (CRB) were significantly lower than POX SE rats (no drugs) values at the respective time point. All data represented as mean ± SEM. *P < 0.05 Data in 1A and 1C reproduced from Ref. 14, with permission.
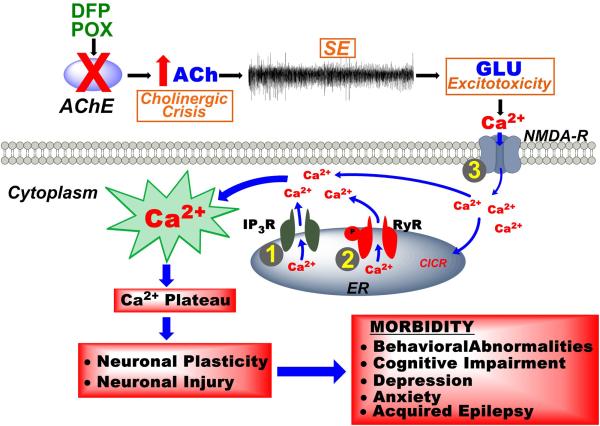
Figure 4.
Development of the calcium plateau following OP-induced SE and possible targets for countermeasures therapy. OP chemicals such as DFP or POX inhibit the enzyme acetylcholinesterase (AChE), initially producing a cholinergic crisis that propagates into self-sustaining SE and ultimately leads to glutamate excitotoxicity. Downstream activation of NMDA receptors leads to massive influx of Ca2+ ions into the postsynaptic neurons. Activation of Ca2+-induced Ca2+-release (CICR) mechanisms leads to release of Ca2+ into the cytoplasm from the endoplasmic reticulum (ER) via the ryanodine receptor (RyR) and the inositol–trisphosphate receptor (IP3R). While NMDA activation is required for genesis of the Ca2+ plateau, the maintenance is dependent on sustained Ca2+ release via CICR mechanisms. After SE is terminated, NMDA activation is shut off, but the Ca2+ release from ER continues, due to long-lasting activation of the CICR mechanisms. The Ca2+ plateau triggers neurodegenerative pathways leading to neuronal injury and activates nuclear signaling that can lead to the neuronal plasticity that underlies chronic morbidities characterized by the development of acquired epilepsy, memory deficits, and psychiatric impairments. Inhibiting the critical targets (1, 2, or 3) in the Ca2+ plateau cascade with pharmacological agents (dantrolene, levetiracetam, or ketamine) can exert neuroprotective effects and can decrease or prevent the development of the chronic neurological morbidities associated with OP SE survival.
Neuronal injury following OP SE
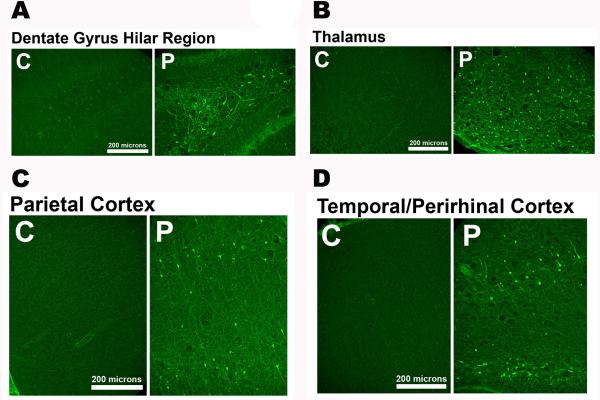
Neuronal loss in several brain regions has been observed following SE,54,55 OP SE,14,15 and exposure to other chemical threat agents.61 We have observed widespread neuronal loss induced by POX SE, as assessed using the Fluoro Jade (FJC) labeling technique.14,62 FJC+ neurons were observed within the hippocampus, parietal cortex, and in both the amygdala and thalamic nuclear regions of POX SE rats (Fig. 2). Damages to these critical brain areas have been implicated in memory impairment, depression, anxiety, epilepsy, and other neurological morbidities.27,63,64
Figure 2.
Neuronal injury following POX-induced SE. Representative photomicrographs of Fluoro-Jade C (FJC) staining in the dentate gyrus–hilus region, parietal cortex, amygdala, and thalamus 2 days after POX SE. Scale bars, 200 μm. Data adapted from Ref. 14.
Chronic morbidity following survival from OP SE
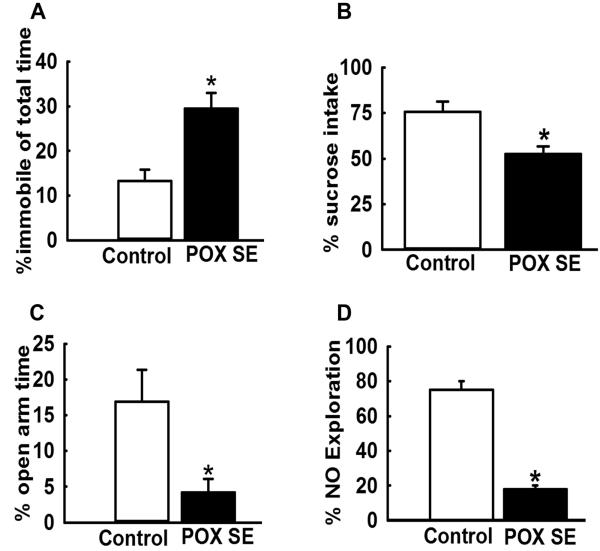
We have also analyzed OP SE survivors in these animal models for the development of neurological morbidities (Fig. 3). We have observed symptoms of chronic depression and memory impairments in these OP-exposed rats.62,65 OP SE survivors displayed increased immobility in the forced swim test, indicative of despair; reduced sucrose consumption in the sucrose preference test, indicative of anhedonia; and spend less time in the open arm of elevated plus maze, indicative of high anxiety.62,65 Despair, anhedonia, and anxiety are symptoms of depression. In addition, these rats performed poorly in the novel object recognition task, indicative of memory impairment.62,65 Survival from OP SE was also associated with significant neuronal damage throughout the limbic system, particularly in the hippocampus.14,16 These models provide a reproducible method of mimicking human survival of OP toxicity. In addition to lethal OP intoxication, chronic low-dose OP exposures have also been implicated in long-term neurological morbidities. For example, agricultural pesticide applicators and Gulf War veterans suspected of chronic OP exposure exhibit chronic neurological morbidities, such as depression and cognitive impairments.33,66,67
Figure 3.
Chronic behavioral morbidities following POX-induced SE. Approximately 3 months following POX SE, surviving rats were tested on various behavioral assays to assess symptoms of depression and memory impairments. (A) Increased immobility time in POX SE rats during the forced swim test, indicative of behavioral despair. (B) Decreased sucrose consumption in POX SE rats on the sucrose preference test, indicative of anhedonia (inability to feel pleasure). (C) Enhanced anxiety in POX SE rats as characterized by significantly less time spent in the open arm of the elevated plus maze. (D) Impaired recognition memory in POX SE rats on the novel object recognition test, as rays displayed significantly less time spent exploring the novel object. All data expressed as mean ± SEM, *P < 0.05, t-test, n = 8 rats. Data adapted from Ref. 65.
Conclusions
Ca2+ ions are second messenger molecules in various signaling cascades that modulate behavior, memory, and cell death.55,56,68–72 The development of THE Ca2+ plateau is therefore a critical substrate for inducing neuronal damage and triggering many of the long-term plasticity changes following OP SE–induced brain injury.14,16,55,56 Given the role of CICR mechanisms in the maintenance of the Ca2+ plateau, drugs targeting the molecular components of this signaling mechanism could prove to be effective agents in extending neuroprotection following survival from OP SE. We have demonstrated neuroprotective and antiepileptogenic effects of dantrolene57 and carisbamate73 in an in vitro model of SE-induced acquired epilepsy. The ability of dantrolene, levetiracetam, and carisbamate to reduce or abolish the Ca2+ plateau could make them attractive neuroprotective adjuvant treatments following OP SE. These agents could also prove beneficial in reducing the chronic neurological morbidities observed in OP SE survivors. We are actively exploring these possibilities in our laboratories (Fig. 4).
Despite advances in developing more effective agents for controlling the cholinergic crisis associated with OP SE, there is a pressing need to develop treatments that prevent or reduce the high mortality and chronic morbidity associated with OP SE. This is an important area of research that has direct translational implications for clinical treatment.74,75 Development of animal models of OP SE are critical to identifying the molecular mechanisms underlying symptoms of OP toxicity. This knowledge can provide molecular targets that can be used to develop effective therapies for the treatment of OP SE (Fig. 4). This research indicated that agents that inhibit CICR and can reduce or prevent the Ca2+ plateau may be an innovative area for development of medical countermeasures that can lower mortality and morbidity following SE and OP SE.
Acknowledgments
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke Grant No. U01NS058213-10 to R.J.D. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. This work was also supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Gulf War Illness Research Program under Award No. (W81XWH-14-1-0478) to L.S.D. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Portions of this work were presented at the 9th Annual NIH Countermeasures against Chemical Threats (CounterACT) Network Research Symposium.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
References
- 1.Sellstrom A, Cairns S, Barbeschi M. Report of the United Nations Mission to Investigate Allegations of the Use of Chemical Weapons in the Syrian Arab Republic on the alleged use of chemical weapons in the Ghouta area of Damascus on 21 August 2013. https://disarmament-library.un.org/UNODA/Library.nsf/780cfafd472b047785257b1000501037/e4d4477c9b67de9085257bf800694bd2/$FILE/A%2067%20997-S%202013%20553.pdf.
- 2.Hood E. The Tokyo attacks in retrospect: sarin leads to memory loss. Environ Health Perspect. 2001;109:A542. doi: 10.1289/ehp.109-a542a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dingeman A, Jupa R. Chemical warfare in the Iran-Iraq conflict. Strategy and Tactics Magazine. 1987;113:51–52. [Google Scholar]
- 4.Moorcraft P, McLaughlin P. The Rhodesian War: A Military History. Pen & Sword; Yorkshire: 2008. [Google Scholar]
- 5.Than K. [Accessed May 6, 2016];Organophosphates: A Common But Deadly Pesticide. National Geographic. 2013 http://news.nationalgeographic.com/news/2013/07/130718-organophosphates-pesticides-indian-food-poisoning/ [Google Scholar]
- 6.Ajdacic-Gross V, Weiss MG, Ring M, et al. Methods of suicide: international suicide patterns derived from the WHO mortality database. Bulletin of the World Health Organization. 2008;86:726–732. doi: 10.2471/BLT.07.043489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konradsen F. Acute pesticide poisoning--a global public health problem. Danish medical bulletin. 2007;54:58–59. [PubMed] [Google Scholar]
- 8.Blakey DH, Lafontaine M, Lavigne J, et al. A screening tool to prioritize public health risk associated with accidental or deliberate release of chemicals into the atmosphere. BMC public health. 2013;13:253. doi: 10.1186/1471-2458-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haley RW, Tuite JJ. Epidemiologic evidence of health effects from long-distance transit of chemical weapons fallout from bombing early in the 1991 Persian Gulf War. Neuroepidemiology. 2013;40:178–189. doi: 10.1159/000345124. [DOI] [PubMed] [Google Scholar]
- 10.Special Assistant for Gulf War Illnesses [Accessed May 6, 2016];Environmental Exposure Report - Pesticides. 2001 http://www.gulflink.osd.mil.
- 11.Institute of Medicine: Board on the Health of Select Populations [Accessed May 6, 2016];Gulf War and Health: Treatment of Chronic Multisymptom Illness. 2013 http://www.iom.edu/Reports/2013/Gulf-War-and-HealthTreatment-for-Chronic-Multisymptom-Illness.aspx.
- 12.Broomfield CA, Kirby SD. Progress on the road to new nerve agent treatments. J Appl Toxicol. 2001;21(Suppl 1):S43–46. doi: 10.1002/jat.804. [DOI] [PubMed] [Google Scholar]
- 13.Jett DA. Neurotoxic pesticides and neurologic effects. Neurologic clinics. 2011;29:667–677. doi: 10.1016/j.ncl.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande LS, Carter DS, Phillips KF, et al. Development of status epilepticus, sustained calcium elevations and neuronal injury in a rat survival model of lethal paraoxon intoxication. Neurotoxicology. 2014;44C:17–26. doi: 10.1016/j.neuro.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Lein PJ, Liu C, et al. Spatiotemporal pattern of neuronal injury induced by DFP in rats: a model for delayed neuronal cell death following acute OP intoxication. Toxicol Appl Pharmacol. 2011;253:261–269. doi: 10.1016/j.taap.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande LS, Carter DS, Blair RE, et al. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol Sci. 2010;116:623–631. doi: 10.1093/toxsci/kfq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaja-Milatovic S, Gupta RC, Aschner M, et al. Protection of DFP-induced oxidative damage and neurodegeneration by antioxidants and NMDA receptor antagonist. Toxicol Appl Pharmacol. 2009;240:124–131. doi: 10.1016/j.taap.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pibiri F, Kozikowski AP, Pinna G, et al. The combination of huperzine A and imidazenil is an effective strategy to prevent diisopropyl fluorophosphate toxicity in mice. Proc Natl Acad Sci U S A. 2008;105:14169–14174. doi: 10.1073/pnas.0807172105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuovinen K. Organophosphate-induced convulsions and prevention of neuropathological damages. Toxicology. 2004;196:31–39. doi: 10.1016/j.tox.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 21.Mello LE, Cavalheiro EA, Tan AM, et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 22.Rice AC, Floyd CL, Lyeth BG, et al. Status epilepticus causes long-term NMDA receptor-dependent behavioral changes and cognitive deficits. Epilepsia. 1998;39:1148–1157. doi: 10.1111/j.1528-1157.1998.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 23.Costa MS, Rocha JB, Perosa SR, et al. Pilocarpine-induced status epilepticus increases glutamate release in rat hippocampal synaptosomes. Neurosci Lett. 2004;356:41–44. doi: 10.1016/j.neulet.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Tyler AL, Mahoney JM, Richard GR, et al. Functional network changes in hippocampal CA1 after status epilepticus predict spatial memory deficits in rats. J Neurosci. 2012;32:11365–11376. doi: 10.1523/JNEUROSCI.1516-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eddleston M, Buckley NA, Eyer P, et al. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chemical Hazards Emergency Medical Management [Accessed May 6, 2016];Nerve Agents - Emergency Department/Hospital Management. 2013 http://chemm.nlm.nih.gov/na_hospital_mmg.htm#top.
- 27.de Araujo Furtado M, Rossetti F, Chanda S, et al. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology. 2012;33:1476–1490. doi: 10.1016/j.neuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Helmstaedter C. Cognitive outcome of status epilepticus in adults. Epilepsia. 2007;48(Suppl 8):85–90. doi: 10.1111/j.1528-1167.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 29.Neligan A, Shorvon SD. Prognostic factors, morbidity and mortality in tonic-clonic status epilepticus: a review. Epilepsy Res. 2011;93:1–10. doi: 10.1016/j.eplepsyres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowsky K, Arzimanoglou A. Outcome and prognosis of status epilepticus in children. Seminars in pediatric neurology. 2010;17:195–200. doi: 10.1016/j.spen.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Read MI, Andreianova AA, Harrison JC, et al. Cardiac electrographic and morphological changes following status epilepticus: effect of clonidine. Seizure. 2014;23:55–61. doi: 10.1016/j.seizure.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Rod CS. Status epilepticus in the developing brain: Long-term effects seen in humans. Epilepsia. 2009;50:32–33. doi: 10.1111/j.1528-1167.2009.02374.x. [DOI] [PubMed] [Google Scholar]
- 33.Phillips KF, Deshpande LS. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology. 2016;52:127–133. doi: 10.1016/j.neuro.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock L, Keifer M, Daniell WE, et al. Chronic central nervous system effects of acute organophosphate pesticide intoxication. The Lancet. 1991;338:223–227. doi: 10.1016/0140-6736(91)90356-t. [DOI] [PubMed] [Google Scholar]
- 35.Savage EP, Keefe TJ, Mounce LM, et al. Chronic neurological sequelae of acute organophosphate pesticide poisoning. Arch Environ Health. 1988;43:38–45. doi: 10.1080/00039896.1988.9934372. [DOI] [PubMed] [Google Scholar]
- 36.Levin ED, Timofeeva OA, Yang L, et al. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res. 2010;208:319–327. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauh VA, Perera FP, Horton MK, et al. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci U S A. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruckart PZ, Kakolewski K, Bove FJ, et al. Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environ Health Perspect. 2004;112:46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivens IA, Schmuck G, Machemer L. Learning and memory of rats after long-term administration of low doses of parathion. Toxicol Sci. 1998;46:101–111. doi: 10.1006/toxs.1998.2508. [DOI] [PubMed] [Google Scholar]
- 40.Dunn C, Bird SB, Gaspari R. Intralipid fat emulsion decreases respiratory failure in a rat model of parathion exposure. Acad Emerg Med. 2012;19:504–509. doi: 10.1111/j.1553-2712.2012.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gresham C, Rosenbaum C, Gaspari RJ, et al. Kinetics and efficacy of an organophosphorus hydrolase in a rodent model of methyl-parathion poisoning. Acad Emerg Med. 2010;17:736–740. doi: 10.1111/j.1553-2712.2010.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albuquerque EX, Pereira EF, Aracava Y, et al. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc Natl Acad Sci U S A. 2006;103:13220–13225. doi: 10.1073/pnas.0605370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrikovics I, Papahadjopoulos D, Hong K, et al. Comparing therapeutic and prophylactic protection against the lethal effect of paraoxon. Toxicol Sci. 2004;77:258–262. doi: 10.1093/toxsci/kfg185. [DOI] [PubMed] [Google Scholar]
- 44.Todorovic MS, Cowan ML, Balint CA, et al. Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res. 2012;101:268–276. doi: 10.1016/j.eplepsyres.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellison CA, Tian Y, Knaak JB, et al. Human hepatic cytochrome P450-specific metabolism of the organophosphorus pesticides methyl parathion and diazinon. Drug Metab Dispos. 2012;40:1–5. doi: 10.1124/dmd.111.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eyer F, Meischner V, Kiderlen D, et al. Human parathion poisoning. A toxicokinetic analysis. Toxicol Rev. 2003;22:143–163. doi: 10.2165/00139709-200322030-00003. [DOI] [PubMed] [Google Scholar]
- 47.Jan YH, Richardson JR, Baker AA, et al. Vitamin K3 (menadione) redox cycling inhibits cytochrome P450-mediated metabolism and inhibits parathion intoxication. Toxicol Appl Pharmacol. 2015;288:114–120. doi: 10.1016/j.taap.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 49.Steenland K, Jenkins B, Ames RG, et al. Chronic neurological sequelae to organophosphate pesticide poisoning. Am J Public Health. 1994;84:731–736. doi: 10.2105/ajph.84.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamashita M, Yamashita M, Tanaka J, et al. Human mortality in organophosphate poisonings. Veterinary and human toxicology. 1997;39:84–85. [PubMed] [Google Scholar]
- 51.Karki P, Ansari JA, Bhandary S, et al. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J. 2004;45:385–389. [PubMed] [Google Scholar]
- 52.Munidasa UA, Gawarammana IB, Kularatne SA, et al. Survival pattern in patients with acute organophosphate poisoning receiving intensive care. Journal of toxicology. Clinical toxicology. 2004;42:343–347. doi: 10.1081/clt-120039539. [DOI] [PubMed] [Google Scholar]
- 53.Burns CJ, McIntosh LJ, Mink PJ, et al. Pesticide exposure and neurodevelopmental outcomes: review of the epidemiologic and animal studies. J Toxicol Environ Health B Crit Rev. 2013;16:127–283. doi: 10.1080/10937404.2013.783383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raza M, Blair RE, Sombati S, et al. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc Natl Acad Sci U S A. 2004;101:17522–17527. doi: 10.1073/pnas.0408155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeLorenzo RJ, Sun DA, Deshpande LS. Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintainance of epilepsy. Pharmacol Ther. 2005;105:229–266. doi: 10.1016/j.pharmthera.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagarkatti N, Deshpande LS, DeLorenzo RJ. Development of the calcium plateau following status epilepticus: role of calcium in epileptogenesis. Expert Rev Neurother. 2009;9:813–824. doi: 10.1586/ern.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagarkatti N, Deshpande LS, Carter DS, et al. Dantrolene inhibits the calcium plateau and prevents the development of spontaneous recurrent epileptiform discharges following in vitro status epilepticus. Eur J Neurosci. 2010;32:80–88. doi: 10.1111/j.1460-9568.2010.07262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Filbert M, Levine E, Ballough G. Neuroprotection for nerve agent-induced brain damage by blocking delayed calcium overload: a review. J Med CBR Def. 2005;3:1–21. [Google Scholar]
- 59.Persiyantseva NA, Birikh KR, Dvoretskova EA, et al. Role of protein kinase C in Ca(2+) homeostasis disorders in cultured rat neurons during hyperstimulation of glutamate receptors. Bull Exp Biol Med. 2008;145:595–599. doi: 10.1007/s10517-008-0159-6. [DOI] [PubMed] [Google Scholar]
- 60.Nagarkatti N, Deshpande LS, DeLorenzo RJ. Levetiracetam inhibits both ryanodine and IP3 receptor activated calcium induced calcium release in hippocampal neurons in culture. Neurosci Lett. 2008;436:289–293. doi: 10.1016/j.neulet.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zolkowska D, Banks CN, Dhir A, et al. Characterization of seizures induced by acute and repeated exposure to tetramethylenedisulfotetramine. J Pharmacol Exp Ther. 2012;341:435–446. doi: 10.1124/jpet.111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips KF, Deshpande LS, Huang B, et al. Behavioral depression and memory impairment following organophosphate diisopropyl fluorophosphate induced status epilepticus in rats. Presented at American Epilepsy Society; Philadelphia, PA. 2015. [Google Scholar]
- 63.Battaglia FP, Benchenane K, Sirota A, et al. The hippocampus: hub of brain network communication for memory. Trends Cogn Sci. 2011;15:310–318. doi: 10.1016/j.tics.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- 65.Deshpande LS, Phillips K, Huang B, et al. Chronic behavioral and cognitive deficits in a rat survival model of paraoxon toxicity. Neurotoxicology. 2014;44:352–357. doi: 10.1016/j.neuro.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brimfield AA. Chemicals of military deployments: revisiting Gulf War Syndrome in light of new information. Prog Mol Biol Transl Sci. 2012;112:209–230. doi: 10.1016/B978-0-12-415813-9.00007-6. [DOI] [PubMed] [Google Scholar]
- 67.Odegard TN, Cooper CM, Farris EA, et al. Memory impairment exhibited by veterans with Gulf War Illness. Neurocase. 2013;19:316–327. doi: 10.1080/13554794.2012.667126. [DOI] [PubMed] [Google Scholar]
- 68.Deshpande LS, Limbrick DD, Jr., Sombati S, et al. Activation of a novel injury-induced calcium-permeable channel that plays a key role in causing extended neuronal depolarization and initiating neuronal death in excitotoxic neuronal injury. J Pharmacol Exp Ther. 2007;322:443–452. doi: 10.1124/jpet.107.123182. [DOI] [PubMed] [Google Scholar]
- 69.Deshpande LS, Sun DA, Sombati S, et al. Alterations In Neuronal Calcium Levels Are Associated With Cognitive Deficits After Traumatic Brain Injury. Neurosci Lett. 2008;441:115–119. doi: 10.1016/j.neulet.2008.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. doi: 10.1007/978-3-7091-0932-8_17. [DOI] [PubMed] [Google Scholar]
- 71.Baker KD, Edwards TM, Rickard NS. The role of intracellular calcium stores in synaptic plasticity and memory consolidation. Neurosci Biobehav Rev. 2013;37:1211–1239. doi: 10.1016/j.neubiorev.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Chadwick W, Mitchell N, Martin B, et al. Therapeutic targeting of the endoplasmic reticulum in Alzheimer's disease. Curr Alzheimer Res. 2013;9:110–119. doi: 10.2174/156720512799015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deshpande LS, Nagarkatti N, Ziobro JM, et al. Carisbamate prevents the development and expression of spontaneous recurrent epileptiform discharges and is neuroprotective in cultured hippocampal neurons. Epilepsia. 2008;49:1795–1802. doi: 10.1111/j.1528-1167.2008.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jett DA. Finding new cures for neurological disorders: a possible fringe benefit of biodefense research? Science translational medicine. 2010;2:23ps12. doi: 10.1126/scitranslmed.3000752. [DOI] [PubMed] [Google Scholar]
- 75.Jett DA, Yeung DT. The CounterACT Research Network: basic mechanisms and practical applications. Proceedings of the American Thoracic Society. 2010;7:254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]