Abstract
Exposure to the vesicating agents sulfur mustard (SM) and nitrogen mustard (NM) causes severe skin injury with delayed blistering. Depending upon the dose and time of their exposure, edema and erythema develop into blisters, ulceration, necrosis, desquamation, and pigmentation changes, which persist weeks and even years after exposure. Research advances have generated data that have started to explain the probable mechanism of action of vesicant-induced skin toxicity; however, despite these advances, effective and targeted therapies are still deficient. This review highlights studies on two SM analogs, chloroethyl ethyl sulfide (CEES) and NM, and CEES- and NM-induced skin injury mouse models that have substantially added to the knowledge on the complex pathways involved in mustard vesicating agent–induced skin injury. Furthermore, employing these mouse models, studies under the National Institutes of Health Countermeasures Against Chemical Threats program have identified the flavanone silibinin as a novel therapeutic intervention with the potential to be developed as an effective countermeasure against skin injury following exposure to mustard vesicating agents.
Keywords: sulfur mustard, nitrogen mustard, skin injury, silibinin
Introduction
Among the weapons of mass destruction (WMDs), chemical weapons are cost-effective and easy to synthesize. These have been commonly used in warfare as brutal agents to cause debilitating effects on humans.1,2 Chemical warfare agents (CWAs) have been in use since 1915, when chlorine was first used by Germans at Ypres. Subsequently, many toxic chemicals such as phosgene, sulfur mustard (SM), lewisite (L), hydrogen cyanide, and nerve agents like sarin have been used in various warfare conflicts, causing large numbers of casualties.2 Since its first use in World War I, the most widely used CWA with the highest military implication to date is the vesicating agent SM (2,2-dichloroethyl sulfide). This is observed in the recent Iran–Iraq war (1983–1988) and reports of its recent use by militant groups in the Middle East.3–5 Short- and long-term debilitating injuries from SM exposure occur mainly due to its absorption via ocular and dermal tissues, as well as lung inhalation, causing injury to these tissues as well as secondary toxic effects to the gastrointestinal, hematological, reproductive, nervous, and cardiac systems.6 In addition to SM, its analog and primary vesicating agent nitrogen mustard (NM; bis(2-chloroethyl) methylamine (HN2)) was developed as a CWA in the 1930s and 1940s by Germany and the United States and stockpiled by several countries during World War II.7,8 NM, though not used in warfare, is widely used as a chemotherapeutic agent. It also poses a similar threat to SM for use as a warfare or terrorist agent, since they both possess similar chemical and toxic properties.7–9 The mustard vesicating agents are alkylating agents with cytotoxic, mutagenic, and carcinogenic properties that upon exposure incur extensive damage and blistering to the most exposed skin tissue. The pathology of the mustard agent–induced acute and chronic effects, including skin inflammation, blistering, and cell death, has been studied in various animal models; however, the mechanisms underlying these consequences are complex and poorly defined. In recent years, several studies have shown that skin toxicity of mustard vesicants is mainly a consequence of their alkylating properties and/or thiol-depleting properties, which can cause macromolecular damage and the activation of several signaling pathways, mainly those related to DNA damage, oxidative stress, and inflammation.6,10–16 Based on some insights into the mechanism of action of vesicating agents so far, many approaches to treat skin injuries have been reported. These treatments include antioxidants and anti-inflammatory agents, nitric oxide synthase (iNOS) inhibitors, poly (ADP-ribose) polymerase (PARP) and matrix metalloprotease inhibitors, and regulators of DNA damage repair.17–24 However, regardless of extensive research attempts, a comprehensive understanding of the molecular mechanisms and signaling pathways involved in SM-induced skin injury in an efficient animal model is still lacking. This has been a major caveat in developing effective and targeted medical treatments to rescue vesicant-induced skin injuries, and, hence, an approved treatment against mustard vesicating agent–induced skin injury is not available. Based on the above background, our research efforts under the National Institutes of Health Countermeasures Against Chemical Threats (CounterACT) program were directed to establish useful vesicating mustard agent–induced skin injury models to elucidate the mechanism of vesicant-induced skin pathogenesis. Positive outcomes from our research efforts and efficacy studies employing the established skin injury models have identified novel mechanism-based therapies against vesicant-induced toxic effects, which are summarized in this article.
Clinicopathological and molecular toxicity of mustard vesicating agents
Toxic consequences, pathogenesis, and skin injury model
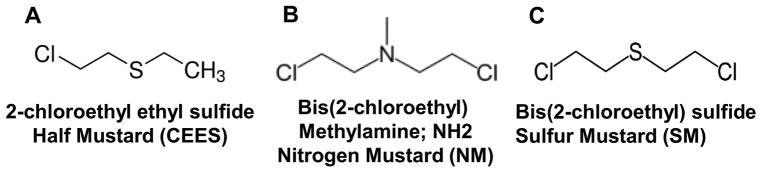
SM is an oily liquid that can be aerosolized or dispersed by explosive blasts, and exposure in humans can lead to its 20% absorption by skin tissue, causing acute and chronic lesions.3,6,25 Depending on the dose, temperature, and moisture conditions during exposure, the severity, time of cutaneous lesion appearance, and symptoms might differ.6,26 Skin exposure to mustard agents, depending on the dose and duration of exposure (threshold dose 10–20 μg/cm2 of liquid SM and 50–300 mg/min/m3 vapor to higher dose 40–100 μg/cm2 of liquid SM and 1000–2000 mg/min/m3 vapor), is associated with edema, erythema and discomfort, severe inflammation, apoptotic cell death, and epidermal–dermal separation leading to painful skin lesions, including blistering, followed by ulceration, desquamation, and necrosis.1,3–5,10,27 The late effects of SM reported from the exposed Iranian veterans show altered pigmentation, presence of cherry angiomas, eczema, hypertrophy, and dry and sensitive skin.3,6,28 Histopathology of skin lesions reveals that SM targets the basal epidermal keratinocytes in the skin tissue and causes cell death. This is followed by protease digestion of anchoring filaments at epidermal–dermal junctions, leading to basement membrane degeneration and triggering a skin blistering response via epidermal–dermal separation.27,29–31 In addition, dermal necrosis, with an increase in inflammatory cells, vascular dilation, and extravagated erythrocytes, is also observed.32,33 Since in vivo animal models that parallel the human toxic cutaneous pathophysiological effects of SM are critical to accelerate the development of effective targeted therapies, there have been extensive research efforts to develop useful, efficient, and cost-effective animal models. Various animal models, including mice, mouse ear, rabbits, weanling pig, and hairless guinea pigs, have been employed to mimic the histopathological consequences and to understand the underlying mechanisms of SM-induced skin lesions in humans.10,22,29,34–37 These models have been useful; however, owing to the requirement of containment facilities, it is not feasible to use SM to conduct comprehensive toxicity and mechanistic studies or routine efficacy studies to screen therapeutic agents. Therefore, its analog 2-chloroethyl ethyl sulfide (CEES) has been widely used as an experimental alternative for SM-related skin studies13–15,33,38–41 (Fig. 1). We have established a useful SKH-1 hairless mouse skin injury model with CEES, studied clinicohistopathological changes, and conducted beneficial efficacy studies employing this model.33,41,42 Employing CEES, we have also established mouse and human skin epidermal cells as useful skin injury models and conducted efficacy studies in these cells.15 CEES, a monofunctional alkylating agent, exhibits a toxicity profile similar to bifunctional mustard agents. The DNA damage produced by CEES is also reported to be due to its direct alkylating effects and increased reactive oxygen species (ROS) production.13,43 However, CEES does not form cross-links with DNA and hence is less toxic than SM. Nonetheless, NM, which is a bifunctional alkylating agent and SM analog, forms adducts and crosslinks with DNA, RNA, and proteins, similar to SM.6,44,45 Since both NM and SM are reported to exhibit similar mechanisms and skin toxic effects owing to their bifunctional alkylating properties, NM, which is commercially available, can be employed to simulate the toxicity of SM7,46,47 (Fig. 1). Recently, NM has been used as a surrogate to mimic SM injury in animal models, including mice and guinea pigs, to establish injury parameters and to screen promising therapies to counter NM- and SM-induced skin injuries.8,9,21 Nevertheless, due to a dearth of applicable quantifiable biomarkers, further comprehensive studies in our lab have developed a practical and cost-effective murine skin injury model and established quantitative end points with more applicable vesicant NM.48,49 NM application on to the dorsal skin of SKH-1 hairless mice caused clinically relevant cutaneous lesions, including edema, erythema, microblister formation, altered pigmentation, wounding, and xerosis.48 These NM-induced clinical sequelae of skin lesions are found to be associated with epidermal thickness, epidermal–dermal separation, epidermal cell death, epidermal denudation and ulceration, scab formation, parakeratosis, hyperkeratosis, and acanthosis.49 NM exposure in these mice also resulted in dermal necrosis and edema; increase in inflammatory cells, macrophages, and mast cells; red blood cell extravasation; and increased myeloperoxidase (MPO) activity related to neutrophil infiltration.49,50 Additionally, the clinical, histopathological, and immunohistochemical evaluation of the skin pathologic lesions inflicted by NM in SKH-1 hairless and C57BL/6 mice show that these lesions and their progression are comparable to those reported from SM exposure in human and other animal models, including hairless mice.36,49–51 Since hairless mouse is a superior murine model to study skin lesions and has been reported as a reliable model for investigating SM-induced skin toxicity,36,51,52 our completed studies in this mouse model with NM provide a valuable tool to further investigate mechanisms mediating vesicant-induced skin toxicity and conduct accelerated efficacy studies to identify and optimize effective targeted therapies.
Figure 1.
Mustard vesicating agents (A) chloroethyl ethyl sulfide (CEES, (B) bis(2-chloroethyl) methylamine or nitrogen mustard (NH2, (NM)), and (C) bis(2-chloroethyl) sulfide (sulfur mustard, (SM)
Molecular mechanisms of mustard toxicity
Mustard vesicating agents induce cytotoxicity mainly owing to their alkylating activity, which is fast, irreversible, and generally goes undetected. This is primarily due to the fact that SM is difficult to smell, and skin lesions appear several hours after its exposure.6,26 Mustard agents can lose a chloride ion and undergo nucleophilic substitution to form a highly reactive cyclic sulfonium/aziridinium ion that can further react with the nucleophilic sites to form adducts or crosslinks.6,44 The potential targets of mustard agents are cellular macromolecules, including DNA, RNA, and proteins. However, DNA modification causing cytotoxicity is a major resultant effect, primarily due to the formation of interstrand crosslinks leading to cell cycle arrest and inhibition of DNA synthesis and cell replication.44 SM can also cause depletion of cellular thiols, primarily glutathione (GSH), and antioxidant enzymes, which can result in the accumulation of ROS and lipid peroxidation.6,22,53 In addition, nitric oxide, reported to be involved in SM-induced toxicity, could react with ROS to form toxic peroxynitrite, which plays a further role in enhancing oxidative stress.19 These molecular events could further lead to oxidative stress and macromolecular damage, which activate signaling pathways and cause gene expression, culminating in the vesicant-associated toxic responses.10,11,22 Moreover, DNA damage is reported as the key consequence following vesicant exposure and is also associated with enhanced production of inflammatory cytokines and chemokines and increased oxidative stress, as well as nitrosative stress.6,10,22,31,34,35,54 These responses could stimulate injury and accelerate inflammatory responses by activating the transcription factors leading to proinflammatory gene expression.10,55 Hence, it is critical to understand the complex network of signaling pathways involved in vesicant-induced DNA damage and inflammation and the role of oxidative stress in these toxic consequences in a relevant animal model. It has been reported that DNA damage and epidermal cell death involve the initiation of p53-related signaling and DNA damage repair pathways, caspases, poly (ADP-ribose) polymerase (PARP) activation, and the calmodulin pathway.5,22,24,33,43,56 In addition, vesicant exposure can cause the activation of mitogen-activated protein kinases (MAPKs) and transcription factors like AP-1 and NF-κB.5,31 Activation of these pathways can induce proinflammatory mediators like cyclooxygenase 2 (COX2) and matrix metalloproteinase (MMP) family proteases, which could be significant players in vesicant-mediated inflammation and vesication.10,11
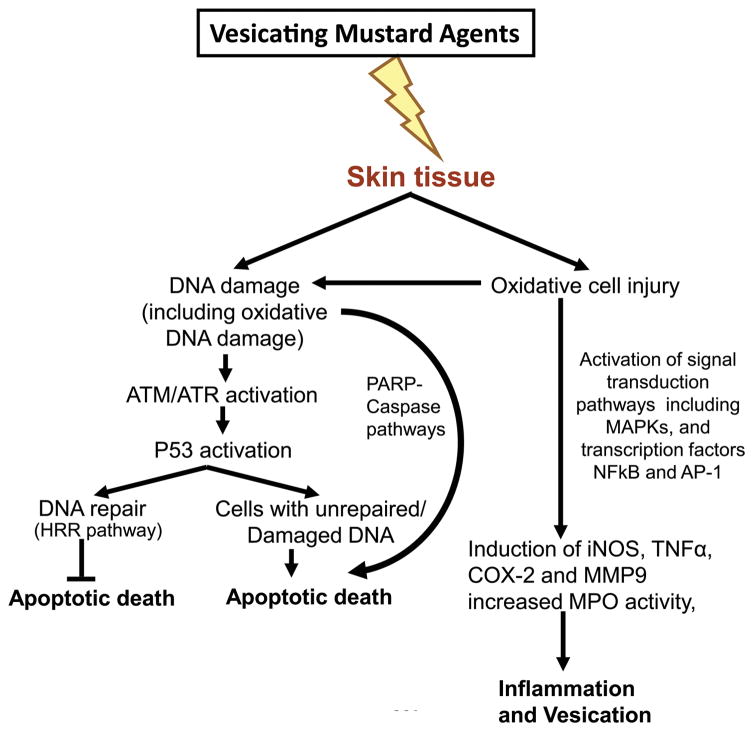
Using the NM-induced SKH-1 hairless mouse skin injury model, we further elucidated the molecular mechanisms and their interconnection with the progression of clinical and histopathological sequelae of skin injuries that were comparable to those reported in humans and animal models with SM.48,49,57 Studies in this animal model demonstrated that topical exposure to NM causes oxidative stress (increased lipid peroxidation and protein and DNA oxidation), DNA damage (H2A.X and p53 phosphorylation) and activation of the MAPK (ERK1/2, JNK1/2, and p38) and Akt pathways, which activate transcription factor AP1 and induce the expression of proinflammatory and proteolytic mediators (COX-2 and inducible nitric oxide (iNOS), MMP-9) and cytokine tumor necrosis factor alpha (TNFα)).57 These mechanistic findings parallel our earlier reported study in the CEES-induced SKH-1 hairless mouse model and further validated the molecular consequences of vesicant-induced skin injury14 (Fig. 2). In addition, the findings in skin epidermal cells and fibroblasts further suggest a DNA-damaging effect of CEES and the activation of ATM/ATR cell cycle checkpoint signaling, as well as caspase–PARP pathways, leading to cell cycle arrest and apoptosis/necrosis in these cells15 (Fig. 2).
Figure 2.
Schematic of important proposed pathways of mustard vesicating agent–induced skin injury based on CEES- and NM-induced in vitro and SKH-1 hairless mouse skin injury models.
Results from additional studies on pathways in SKH-1 hairless mice also show that p53 paucity caused a delay in NM-induced early apoptosis and inflammatory response, suggesting a crucial role of p53-related pathways in mustard vesicating agent–induced skin injury. Also, an increase in the homologous recombinant repair (HRR) pathway following NM exposure in mice and similar results in skin keratinocytes suggest that HRR is the key pathway involved in the repair of NM-induced DNA damage.13,57 Using Mpo knockout mice, our studies show that MPO, which produces microbicidal oxidants and is indicative of neutrophil infiltration, plays a significant role in NM-induced skin injuries.58
Outcomes from our studies suggest that, to rescue vesicant-caused skin injuries, antioxidants and pleiotropic agents (or cocktails) are needed that could target multiple pathways induced in the skin from mustard vesicant exposure.
Potential effective therapies
Treatment strategies
Mustard vesicating agents can cause debilitating skin injuries that are acute and chronic, which can take several months to heal, causing long-term complications. However, there are no approved and effective antidotes to ameliorate these effects and no standardized protocol for casualty management is available. The current therapeutic options, including topical corticosteroids, oral antihistamines, and emollient formulations, are mostly employed and focus on the management of skin symptoms that can improve life quality.6 Therapies used for SM-exposed soldiers in Iran and Germany included draining of blisters, washing of affected skin areas with Ringer’s lactate solution, and application of paraffin gauze or silver dressing (Actisorb), dexpathenol, flumentasone (Locacorten), a clioquinol–triamcinolone mixture, and pyoktanin solution.45 In addition to the reduction of injury symptoms, approaches directed towards accelerated wound healing therapies are important to reduce the long-term care and related consequences. Several treatment adjuncts have been tested in a pig skin injury model to accelerate wound healing, which include Flexan foam adhesive dressing, DuoDERM signal hydrocolloid dressing, and Amino-Plex spray with enhanced oxygen.59 Based on the known mechanisms of action of mustard vesicants, the major current treatment approaches being tested are ROS scavengers, anti-inflammatory agents, polymerase inhibitors, calcium modulators, MMP inhibitors, and regulators of DNA damage repair.18,20,21,23 The reported molecular events in mustard vesicating agent–induced skin injury largely involve oxidative stress and possible nitrosative stress that can lead to lipid peroxidation, protein oxidation, and DNA damage, which cause cell death and inflammation.19 Consistent with this, the agents that possess antioxidant and nitric oxide synthase inhibitor properties, like N-acetyl cysteine, superoxide dismutase, glutathione, melatonin, trolox, quercetin, and catalytic antioxidant metalloproteinases have displayed varying extents of protection against SM-induced cutaneous effects.19,22,53,60,61 Additionally, owing to the fact that mustard vesicants can activate multiple signaling pathways and involve direct DNA damage and inflammatory response, pleiotropic agents or a combination of agents that can target various pathways are likely to be more effective against vesicant-related skin injury. On this basis, the therapeutic efficacy of a small molecule natural flavanone silibinin (3R)-3,5,7-trihydroxy-2-[(2R,3R)-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)-2,3-dihydrobenzo[b][1,4]dioxin-6-yl]chroman-4-one) has been our major focus in ameliorating CEES- and NM-induced skin injury in established in vitro skin cells and SKH-1 hairless mouse skin injury models (Fig. 3).
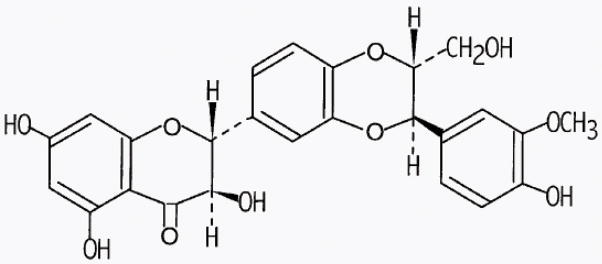
Figure 3.
Chemical structure of the flavanone silibinin.
Silibinin (C25H22O10; Fig. 2) is a nontoxic naturally occurring flavanone isolated mainly from the seeds of Milk Thistle (Silybum marianum (L.); Family Asteraceae). It has been widely consumed as a traditional medicine for years to heal liver disorders, hemorrhage, and bronchitis and as an antidote against poisoning by death cap mushroom.62 It is not yet approved for medical use and is commercially available as a dietary supplement.63 Apart from its anticancer efficacy against various epithelial cancers, its strong efficacy against skin cancer and chemical- and radiation-induced DNA damage and cell death, is evident from published reports.62,64 There is an abundance of reported data showing broad-spectrum efficacy and strong antioxidant, anti-inflammatory, anticancer and cancer-chemopreventive properties of silibinin, which is in clinical trials for some cancers.62,65,66 It has been reported that silibinin targets numerous signaling pathways, including oxidative stress and inflammation, which parallel those triggered following vesicant exposure.62,67,68 Hence, we evaluated the efficacy of silibinin against CEES-induced skin injury biomarkers reported in our earlier studies in skin epidermal cells, fibroblasts, and SKH-1 hairless mouse skin.15,42 The outcomes from these studies suggested a strong pleiotropic efficacy of silibinin (0.5 or 1 mg/mouse topical application and 10 μM silibinin 30 min after CEES exposure) in attenuating CEES-induced skin toxicity by targeting multiple signaling pathways, DNA damage, inflammation, vesication, and oxidative stress in the skin cells and mouse skin tissue, suggesting its strong potential as novel treatment for skin injuries by vesicants (Table 1). Silibinin is also reported to be effective in reversing SM-induced toxicity in skin epidermal cells.69 On the basis of these findings, mainly in the CEES-induced mouse skin injury model, silibinin efficacy was further assessed in bifunctional alkylating mustard NM-induced SKH-1 hairless mouse model. Results from this study also displayed a strong therapeutic effect of silibinin (1 or 2 mg/mouse applied on the exposed dorsal skin 30 min after NM exposure) in improving NM-induced skin lesions.70 These outcomes were similar to those observed in CEES-induced skin injury model, including DNA damage, apoptotic cell death, microvesication, inflammation, and histopathological damage, such as epidermal thickness, epidermal–dermal separation, parakeratosis, and epidermal denudation, similar to those reported following SM exposure70 (Table 1).
Table 1.
Effect of silibinin on CEES- or NM-induced injury end points in skin epidermal cells, skin fibroblasts, or SKH-1 hairless mice
| Injury end point | Model and vesicant | Effect of silibinin |
|---|---|---|
| Cell viability | Skin epidermal cells and fibroblast models with CEES | Decreases the CEES-induced Increase42 |
| Apoptotic cell death | Skin epidermal cells and fibroblast models with CEES SKH-1 hairless mouse skin injury model with CEES and NM |
Decreases the CEES- and NM- induced increase42,70 |
| Cellular and mitochondrial oxidative stress | Skin epidermal cells and fibroblast models with CEES | Decreases the CEES-induced increase42 |
| Skin epidermal thickness, denuding, or other changes | SKH-1 hairless mouse skin injury model with CEES and NM | Decreases the CEES- and NM- induced increase42,70 |
| Microvesication | SKH-1 hairless mouse skin injury model with NM | Decrease in NM-induced increase70 |
| Myeloperoxidase activity | SKH-1 hairless mouse skin injury model with CEES and NM | Decreases the CEES- and NM- induced increase42,70 |
| DNA damage (H2A.X or p53 phosphorylation) | Skin epidermal cells and fibroblast models with CEES SKH-1 hairless mouse skin injury model with CEES and NM |
Decreases the CEES- and NM- induced increase42,70 |
| Inflammatory and proteolytic mediators (COX-2, iNOS, MMP9) | SKH-1 hairless mouse skin injury model with CEES and NM | Decreases the CEES- and NM- induced increase42,70 |
| Transcription factors AP-1 and NF-κB | SKH-1 hairless mouse skin injury model with CEES | Decreases the CEES-induced increase42 |
| Lipid peroxidation, protein oxidation, and oxidative DNA damage | SKH-1 hairless mouse skin injury model with CEES and NM | Decreases the CEES- and NM- induced increase42,70 |
On the basis of the above results, silibinin is being further tested and optimized in the form of a topically applicable humanized formulation against SM-induced skin injury in the SKH-1 hairless mouse model. Since the skin of hairless guinea pigs is reported to be morphologically similar to human skin, consisting of multi-cell layered epidermis,26 efficacy studies using silibinin formulation with optimal treatment regimen in SM-induced skin injury in hairless mice will next be performed in this animal model. The outcomes from these studies could allow further optimization and development and lead to approval of medical use of silibinin as an effective novel therapeutic agent against vesicant-induced skin injury. On the basis of clinical studies in humans for prostate cancer, silibinin is found to be safe for oral human use, and it is further being clinically tested for its topical use to prevent radiation dermatitis.71
Conclusions and future directions
The recent developments in the Middle Eastern countries where the alleged use of CWAs has been reported in addition to a surge of terrorist organizations increased the probability of an attack with SM, which could have a deleterious impact on the military and civilian population there and in other parts of the world. Since there is no approved countermeasure available for human use in case of a mass casualty scenario, development of silibinin for topical application could be a promising approach, and is under investigation in other animal skin injury models with SM. The development of a useful and cost-effective skin injury model with NM for laboratory use has assisted in the identification and optimization of silibinin as an effective pleiotropic therapy against CEES- and NM-induced skin toxicity. However, since there are complex and multiple mechanisms involved in mustard vesicant–induced skin toxicity, development of new antioxidants, combinations of drugs that can reduce injury and accelerate wound healing, and use of cutting-edge technologies that can reveal more precise molecular targets and improve delivery will be useful to implement.
Acknowledgments
This work was supported by the Countermeasures Against Chemical Threats (CounterACT) Program, Office of the Director National Institutes of Health (OD) and the National Institute of Environmental Health Sciences (NIEHS) (Grant Number U54 ES015678).
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
References
- 1.Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacological reviews. 1996;48:289–326. [PubMed] [Google Scholar]
- 2.Ganesan K, Raza SK, Vijayaraghavan R. Chemical warfare agents. Journal of pharmacy & bioallied sciences. 2010;2:166–178. doi: 10.4103/0975-7406.68498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balali-Mood M, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol. 2005;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 4.Ghabili K, et al. Mustard gas toxicity: the acute and chronic pathological effects. Journal of applied toxicology : JAT. 2010;30:627–643. doi: 10.1002/jat.1581. [DOI] [PubMed] [Google Scholar]
- 5.Ghabili K, et al. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Critical reviews in toxicology. 2011;41:384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- 6.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Sharma M, Vijayaraghavan R, Ganesan K. Comparison of toxicity of selected mustard agents by percutaneous and subcutaneous routes. Indian journal of experimental biology. 2008;46:822–830. [PubMed] [Google Scholar]
- 8.Wormser U, Brodsky B, Reich R. Topical treatment with povidone iodine reduces nitrogen mustard-induced skin collagenolytic activity. Archives of toxicology. 2002;76:119–121. doi: 10.1007/s00204-001-0307-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith KJ, et al. Histopathologic and immunohistochemical features in human skin after exposure to nitrogen and sulfur mustard. The American Journal of dermatopathology. 1998;20:22–28. doi: 10.1097/00000372-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Shakarjian MP, et al. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kehe K, et al. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Sabourin CL, et al. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. Journal of biochemical and molecular toxicology. 2002;16:263–272. doi: 10.1002/jbt.10050. [DOI] [PubMed] [Google Scholar]
- 13.Inturi S, et al. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free radical biology & medicine. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal A, et al. Sulfur mustard analog induces oxidative stress and activates signaling cascades in the skin of SKH-1 hairless mice. Free radical biology & medicine. 2009;47:1640–1651. doi: 10.1016/j.freeradbiomed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tewari-Singh N, et al. Biological and molecular mechanisms of sulfur mustard analogue-induced toxicity in JB6 and HaCaT cells: possible role of ataxia telangiectasia-mutated/ataxia telangiectasia-Rad3-related cell cycle checkpoint pathway. Chemical research in toxicology. 2010;23:1034–1044. doi: 10.1021/tx100038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain AK, et al. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicology letters. 2011;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElroy CS, Day BJ. Antioxidants as potential medical countermeasures for chemical warfare agents and toxic industrial chemicals. Biochemical pharmacology. 2015;100:1–11. doi: 10.1016/j.bcp.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiermann H, Worek F, Kehe K. Limitations and challenges in treatment of acute chemical warfare agent poisoning. Chemico-biological interactions. 2013;206:435–443. doi: 10.1016/j.cbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Laskin JD, et al. Oxidants and antioxidants in sulfur mustard-induced injury. Annals of the New York Academy of Sciences. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith WJ. Therapeutic options to treat sulfur mustard poisoning--the road ahead. Toxicology. 2009;263:70–73. doi: 10.1016/j.tox.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Anumolu SS, et al. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials. 2011;32:1204–1217. doi: 10.1016/j.biomaterials.2010.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paromov V, et al. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. Journal of burns and wounds. 2007;7:e7. [PMC free article] [PubMed] [Google Scholar]
- 23.Kehe K, et al. Sulfur mustard research-strategies for the development of improved medical therapy. Eplasty. 2008;8:e32. [PMC free article] [PubMed] [Google Scholar]
- 24.Korkmaz A, et al. Molecular targets against mustard toxicity: implication of cell surface receptors, peroxynitrite production, and PARP activation. Archives of toxicology. 2006;80:662–670. doi: 10.1007/s00204-006-0089-x. [DOI] [PubMed] [Google Scholar]
- 25.Goverman J, et al. Sulfur mustard gas exposure: case report and review of the literature. Annals of burns and fire disasters. 2014;27:146–150. [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KJ, et al. Sulfur mustard: its continuing threat as a chemical warfare agent, the cutaneous lesions induced, progress in understanding its mechanism of action, its long-term health effects, and new developments for protection and therapy. J Am Acad Dermatol. 1995;32:765–776. doi: 10.1016/0190-9622(95)91457-9. [DOI] [PubMed] [Google Scholar]
- 27.Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic & clinical pharmacology & toxicology. 2006;99:273–282. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- 28.Balali-Mood M, et al. Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam Clin Pharmacol. 2005;19:713–721. doi: 10.1111/j.1472-8206.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 29.Greenberg S, et al. Characterization of the initial response of engineered human skin to sulfur mustard. Toxicological sciences : an official journal of the Society of Toxicology. 2006;90:549–557. doi: 10.1093/toxsci/kfi306. [DOI] [PubMed] [Google Scholar]
- 30.Hayden PJ, et al. Microvesicating Effects of Sulfur Mustard on an In Vitro Human Skin Model. Toxicology in vitro : an international journal published in association with BIBRA. 2009;23(7):1396–405. doi: 10.1016/j.tiv.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Graham JS, et al. Wound healing of cutaneous sulfur mustard injuries: strategies for the development of improved therapies. Journal of burns and wounds. 2005;4:e1. [PMC free article] [PubMed] [Google Scholar]
- 32.Smith KJ, et al. Histopathologic features seen in sulfur mustard induced cutaneous lesions in hairless guinea pigs. J Cutan Pathol. 1995;22:260–268. doi: 10.1111/j.1600-0560.1995.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 33.Tewari-Singh N, et al. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicological sciences : an official journal of the Society of Toxicology. 2009;108:194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodsky B, et al. Early effects of iodine on DNA synthesis in sulfur mustard-induced skin lesions. Archives of toxicology. 2006;80:212–216. doi: 10.1007/s00204-005-0032-6. [DOI] [PubMed] [Google Scholar]
- 35.Smith KJ, et al. Histopathologic features seen with different animal models following cutaneous sulfur mustard exposure. Journal of dermatological science. 1997;14:126–135. doi: 10.1016/s0923-1811(96)00560-9. [DOI] [PubMed] [Google Scholar]
- 36.Joseph LB, et al. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Experimental and molecular pathology. 2011;91:515–527. doi: 10.1016/j.yexmp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benson JM, et al. Time course of lesion development in the hairless guinea-pig model of sulfur mustard-induced dermal injury. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:348–357. doi: 10.1111/j.1524-475X.2011.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, et al. Protection by antioxidants against toxicity and apoptosis induced by the sulphur mustard analog 2-chloroethylethyl sulphide (CEES) in Jurkat T cells and normal human lymphocytes. British journal of pharmacology. 2004;141:795–802. doi: 10.1038/sj.bjp.0705591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jowsey PA, Williams FM, Blain PG. DNA damage, signalling and repair after exposure of cells to the sulphur mustard analogue 2-chloroethyl ethyl sulphide. Toxicology. 2009;257:105–112. doi: 10.1016/j.tox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, et al. Effect of carbachol on phospholipase C-mediated phosphatidylinositol 4,5-bisphosphate hydrolysis, and its modulation by isoproterenol in rabbit corneal epithelial cells. Current eye research. 1995;14:563–571. doi: 10.3109/02713689508998403. [DOI] [PubMed] [Google Scholar]
- 41.Jain AK, et al. 2-Chloroethyl ethyl sulfide causes microvesication and inflammation-related histopathological changes in male hairless mouse skin. Toxicology. 2011;282:129–138. doi: 10.1016/j.tox.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tewari-Singh N, et al. Silibinin attenuates sulfur mustard analog-induced skin injury by targeting multiple pathways connecting oxidative stress and inflammation. PloS one. 2012;7:e46149. doi: 10.1371/journal.pone.0046149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. The Journal of pharmacology and experimental therapeutics. 2009;328:732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inturi S, et al. Activation of DNA damage repair pathways in response to nitrogen mustard-induced DNA damage and toxicity in skin keratinocytes. Mutation research. Fundamental and molecular mechanisms of mutagenesis. 2014;763–764C:53–63. doi: 10.1016/j.mrfmmm.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kehe K, et al. Acute effects of sulfur mustard injury--Munich experiences. Toxicology. 2009;263:3–8. doi: 10.1016/j.tox.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 46.Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clinical and experimental dermatology. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- 47.Batal M, et al. Temporal and spatial features of the formation of DNA adducts in sulfur mustard-exposed skin. Toxicology and applied pharmacology. 2013;273:644–650. doi: 10.1016/j.taap.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Tewari-Singh N, et al. Clinically-relevant cutaneous lesions by nitrogen mustard: useful biomarkers of vesicants skin injury in SKH-1 hairless and C57BL/6 mice. PloS one. 2013;8:e67557. doi: 10.1371/journal.pone.0067557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tewari-Singh N, et al. Cutaneous injury-related structural changes and their progression following topical nitrogen mustard exposure in hairless and haired mice. PloS one. 2014;9:e85402. doi: 10.1371/journal.pone.0085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain AK, et al. Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie. 2014;66:129–138. doi: 10.1016/j.etp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mouret S, et al. Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice. Toxicology letters. 2014;232:68–78. doi: 10.1016/j.toxlet.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Ricketts KM, et al. Inflammatory cytokine response in sulfur mustard-exposed mouse skin. Journal of applied toxicology : JAT. 2000;20(Suppl 1):S73–76. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat685>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Tewari-Singh N, et al. Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. The Journal of pharmacology and experimental therapeutics. 2011;336:450–459. doi: 10.1124/jpet.110.173708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruff AL, Dillman JF. Signaling molecules in sulfur mustard-induced cutaneous injury. Eplasty. 2007;8:e2. [PMC free article] [PubMed] [Google Scholar]
- 55.Sabourin CL, Petrali JP, Casillas RP. Alterations in inflammatory cytokine gene expression in sulfur mustard-exposed mouse skin. Journal of biochemical and molecular toxicology. 2000;14:291–302. doi: 10.1002/1099-0461(2000)14:6<291::AID-JBT1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 56.Kehe K, et al. Inhibition of poly(ADP-ribose) polymerase (PARP) influences the mode of sulfur mustard (SM)-induced cell death in HaCaT cells. Archives of toxicology. 2008;82:461–470. doi: 10.1007/s00204-007-0265-7. [DOI] [PubMed] [Google Scholar]
- 57.Kumar D, et al. Nitrogen mustard exposure of murine skin induces DNA damage, oxidative stress and activation of MAPK/Akt-AP1 pathway leading to induction of inflammatory and proteolytic mediators. Toxicology letters. 2015;235:161–171. doi: 10.1016/j.toxlet.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain AK, et al. Myeloperoxidase deficiency attenuates nitrogen mustard-induced skin injuries. Toxicology. 2014;320:25–33. doi: 10.1016/j.tox.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graham JS, et al. Medical management of cutaneous sulfur mustard injuries. Toxicology. 2009;263:47–58. doi: 10.1016/j.tox.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 60.McElroy CS, Day BJ. Antioxidants as potential medical countermeasures for chemical warfare agents and toxic industrial chemicals. Biochemical pharmacology. 2016;100:1–11. doi: 10.1016/j.bcp.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tewari-Singh N, et al. Catalytic antioxidant AEOL 10150 treatment ameliorates sulfur mustard analog 2-chloroethyl ethyl sulfide-associated cutaneous toxic effects. Free radical biology & medicine. 2014;72:285–295. doi: 10.1016/j.freeradbiomed.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer metastasis reviews. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh RP, Agarwal R. Cosmeceuticals and silibinin. Clinics in dermatology. 2009;27:479–484. doi: 10.1016/j.clindermatol.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhanalakshmi S, et al. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25:1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 65.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 66.Deep G, et al. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 67.Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocrine-related cancer. 2006;13:751–778. doi: 10.1677/erc.1.01126. [DOI] [PubMed] [Google Scholar]
- 68.Gu M, et al. Differential effect of silibinin on E2F transcription factors and associated biological events in chronically UVB-exposed skin versus tumors in SKH-1 hairless mice. Molecular cancer therapeutics. 2006;5:2121–2129. doi: 10.1158/1535-7163.MCT-06-0052. [DOI] [PubMed] [Google Scholar]
- 69.Balszuweit F, et al. Silibinin as a potential therapeutic for sulfur mustard injuries. Chemico-biological interactions. 2013;206:496–504. doi: 10.1016/j.cbi.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 70.Jain AK, et al. Flavanone silibinin treatment attenuates nitrogen mustard-induced toxic effects in mouse skin. Toxicology and applied pharmacology. 2015;285:71–78. doi: 10.1016/j.taap.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flaig TW, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. The Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]