Abstract
Aging is a dealkylation reaction of organophosphorus (OP)-inhibited acetylcholinesterase (AChE). Despite many studies to date, aged AChE cannot be reactivated directly by traditional pyridinium oximes. This review summarizes strategies that are potentially valuable in the treatment against aging in OP poisoning. Among them, retardation of aging seeks to lower the rate of aging through the use of AChE effectors. These drugs should be administered before AChE is completely aged. For postaging treatment, realkylation of aged AChE by appropriate alkylators may pave the way for oxime treatment by neutralizing the oxyanion at the active site of aged AChE. The other two strategies, upregulation of AChE expression and introduction of exogenous AChE, cannot resurrect aged AChE but may compensate for lowered active AChE levels by in situ production or external introduction of active AChE. Upregulation of AChE expression can be triggered by some peptides. Sources of exogenous AChE can be whole blood or purified AChE, either from human or nonhuman species.
Keywords: acetylcholinesterase, aging, resurrection, realkylation, upregulation, exogenous
Introduction
Acetylcholinesterase (AChE) is a serine hydrolase found in brain synapses, neuromuscular junctions (NMJs), and erythrocytes. Using a catalytic triad of Glu–His–Ser, its role is to silence nerve impulses by selectively hydrolyzing acetylcholine, a neurotransmitter. Inhibition of AChE can lead to a cholinergic crisis, which is caused by the accumulation of acetylcholine at synapses and NMJs.1 Inhibition of AChE would result in the continuous firing of nerve impulses, thereby causing greatly increased parasympathetic actions and twitches of voluntary muscles, which may lead to death due to seizures or respiratory failure. Organophosphorus (OP) chemical nerve agents are a type of suicide AChE inhibitor. They act by covalently binding to the catalytic serine and blocking the active site (Fig. 1). OPs have been used as pesticides (e.g., malathion) and as chemical warfare agents (e.g., VX, sarin, soman, and tabun). Every year, OP-based pesticides kill approximately 200,000 people worldwide, especially in rural areas of developing countries.2 OP-based chemical warfare agents have been used in armed conflicts, such as the Iran–Iraq war3 and the recent Syrian civil war,4 as well as terrorist attacks, such as the Tokyo subway sarin attack.5 Clinically, OP poisoning can be treated by a combination of anticholinergic drugs (e.g., atropine) and oximes (e.g., 2-PAM). Atropine acts as an antagonist of acetylcholine receptors, while oximes (more likely, their deprotonated form, the oximates) nucleophilically substitute the phosphorylated serine into the active site of the enzyme to reactivate the OP-inhibited AChE.1
Figure 1.
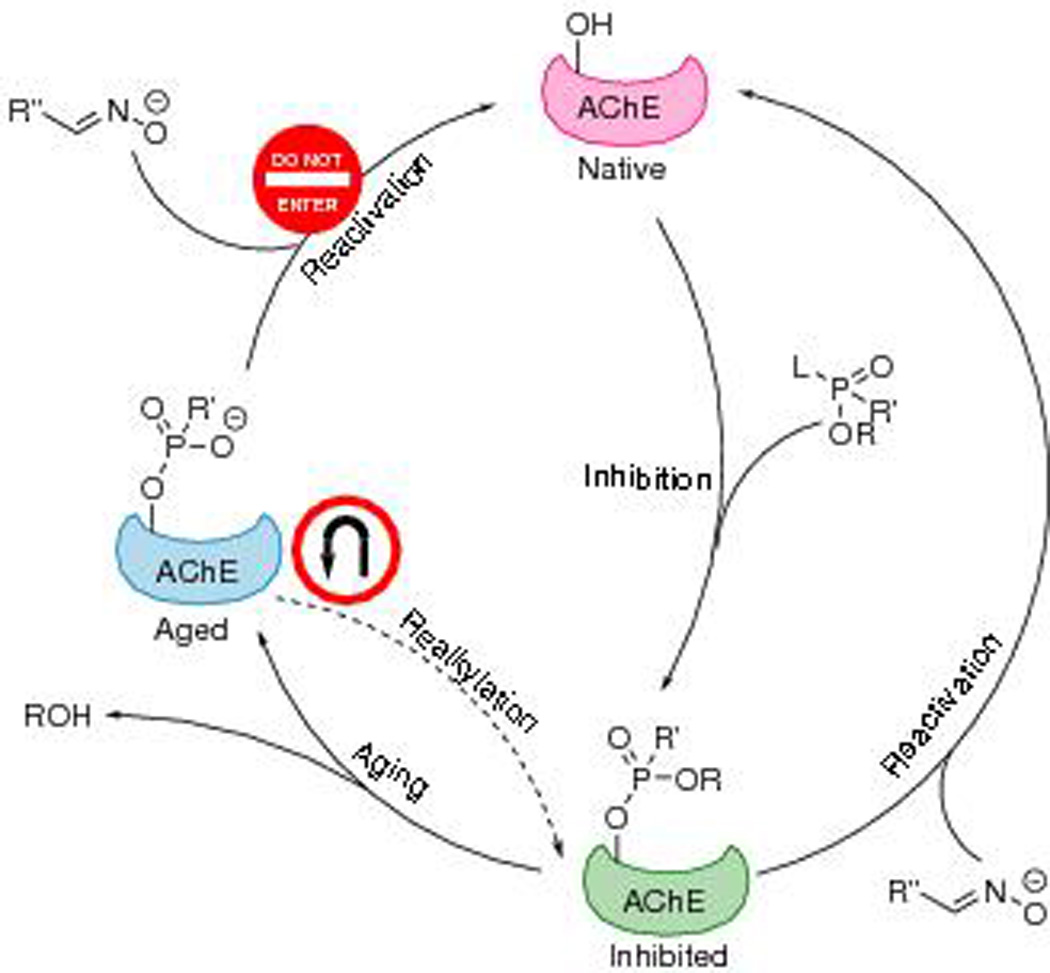
Scheme illustrating inhibition, reactivation, aging, and realkylation pathways of AChE. “L” stands for the leaving group that is lost in inhibition. Realkylation may bring the aged AChE back to the inhibited, but unaged, state and hence facilitate reactivation by oximes.
Though a variety of different oximes have been developed to treat OP poisoning, none of them are universally effective against all OPs.1 For instance, HI-6 is less efficient against tabun than against VX, while TMB-4, HLö-7, and LüH-6 show the opposite reaction selectivity.6,7 Even more problematic, a dealkylation process can follow inhibition, which is the loss of a second leaving group from OP, thereby producing an oxyanion on the phosphoryl group. This process is referred to as aging (or ageing in British English). To date, the aged form of AChE has been recalcitrant to reactivation by any oxime (Fig. 1). Electrostatic attraction between the oxyanion and the positively charged catalytic histidine stabilizes the structure of aged AChE, making it unresponsive to reactivation.8 Furthermore, electrostatic repulsion from the phosphoryl oxyanion orients the oximate away from the enzyme’s active site, precluding the nucleophilic attack.9 Victims exposed to fast-aging OPs, such as soman, which has an aging half-time (t1/2) as short as several minutes,10 have a minimal chance of survival. Since the discovery of aging in the 1950s,11 no clinical treatment has been fully developed to prevent aging or to recover the activity of aged AChE. Most research on OP poisoning focuses on treatments before aging. Despite the difficulty, researchers’ efforts in finding antiaging cures have continued. In this review, we summarize the methods and compounds that may be used as potential therapeutic pathways against the aging of AChE.
Retardation of aging
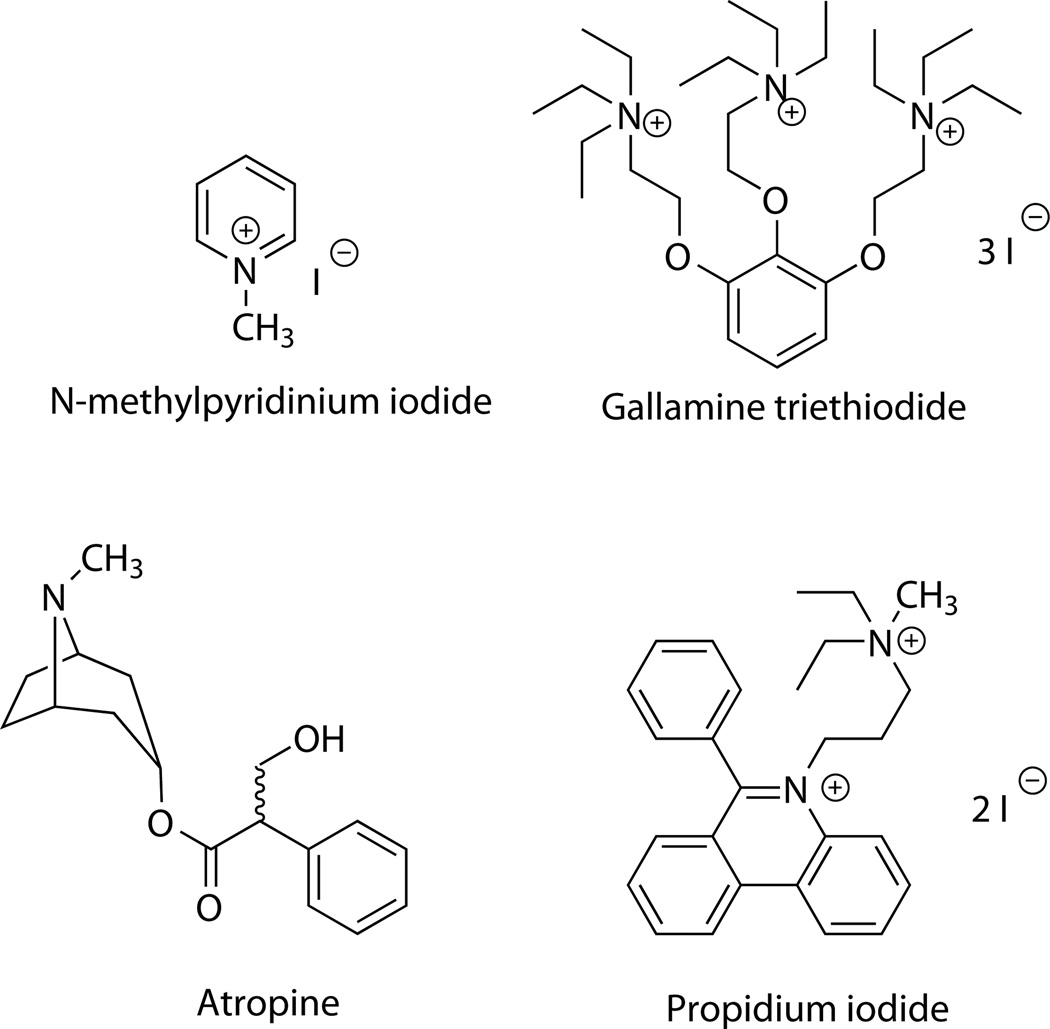
If aging can be considerably slowed down by any drug, there will be an opportunity to reactivate the inhibited AChE with oximes before the AChE becomes aged. Some effectors were proven capable of retarding aging. In 1966, Berry and Davies reported that the use of 2–40 mM N-methylpyridinium iodide (Fig. 2) lowered the aging rate of soman-inhibited AChE by 33–69% and cyclosarin-inhibited AChE by 52–100%.12 In the 1970s, Crone reported a study on the effect of six quaternary ammoniums on the aging kinetics of sarin.13 Among them, 0.1 mM of gallamine triethiodide (Fig. 2) slowed down aging by 77%. Sterri investigated several imidazole compounds for their activities to retard aging.14 Among them, 1 mM 2,4-dimethylimidazole lowered the aging rate of sarin-inhibited AChE by 70%. It is interesting that atropine (Fig. 2) also displays a retardation effect against aging. As reported by van Dongen et al. in 1987, this anticholinergic drug, which is widely used in the treatment of OP poisoning, slowed down the aging of sarin- and GS-inhibited rat and human AChE by 28–60% when applied at 1 mM.15 Nevertheless, the clinical dose of atropine is orders of magnitude lower than this level and consequently, will not exhibit any significant aging-retardation effect. The aforementioned gallamine triethiodide is also an anticholinergic drug.
Figure 2.
Examples of AChE effectors that retard aging.
Not all aging effectors slow down aging. Some can accelerate this process. Schoene compared a library of 27 aging effectors, of which most are pyridinium compounds, on their influence on the aging of sarin-inhibited AChE.16,17 At a concentration of up to 2 mM, most of the tested compounds exhibited retardation of aging, while a few, such as 1,1′-(oxy-bis(methylene))-bis-(4-((E)-(2-carbamoylhydrazono)methyl)pyridin-1-ium) and hydrazinecarboxamide, accelerated aging slightly.
How these effectors work is not fully understood. Some of them, such as gallamine triethiodide and propidium iodide, were reported as allosteric ligands of AChE and bind at a peripheral anionic site.18 Their concentration–effect correlations can be non-monotonic: as reported by Schoene, for example, the aging acceleration effect of semicarbazide increased with concentration, reaching a maximum at 1.25 mM, then decreased.17
Though many pyridinium compounds can slow down aging, and this structural moiety is present in many oxime drugs, these oximes may not be bifunctional against inhibition and aging. On the contrary, they may accelerate aging. For example, in the presence of 2 mM 1-heptyl-pyridinium iodide, the aging rate of tabun-inhibited bovine AChE was lowered by 56%; the complex of the same inhibited enzyme with 1-heptyl-2-hydroxyiminomethyl-pyridinium iodide displayed an aging rate coefficient that increased by 260%.19
None of the aforementioned effectors have been clinically utilized in order to slow down aging, presumably due to their low activities. Most of these effectors are effective only when applied on the mM scale, which is too high for clinical treatments and may exhibit toxicity. Propidium iodide (Fig. 2), however, can slow down the aging of soman-inhibited AChE by about twofold at 10 µM.20 Another limitation is that, once all AChE of the patient is aged, these drugs will no longer be effective, since they are intended to only retard aging rather than to resurrect the aged form of AChE.
Realkylation of aged AChE: make a U-turn
If the road is closed, why not turn back? A straightforward strategy against aging is to reverse this dealkylation reaction by realkylation of the oxyanion on the phosphoryl adduct (Fig. 1). In this way, the negative charge on the phosphoryl group will be neutralized after realkylation, and the inhibited enzyme can be reactivated by oximes again. Unlike aging retardation, which needs to be performed in time before all AChE gets aged, realkylation of aged AChE is a postaging approach. Several types of electrophilic alkylating agents, including sulfonates, haloketones, methoxypyridiniums, sulfoniums, and quinone methide precursors, have been proposed to be used for realkylation of aged AChE. A potential risk that should be taken into account with this approach is the possible off-target reactivities of the realkylators. The drug will exhibit toxicity if it undesirably alkylates irrelevant proteins, nucleic acids, or any residues other than the phosphorylated serine of the aged AChE.
Sulfonates
Sulfonates are known as good leaving groups in substitution reactions. In 1969, Blumbergs et al. reported the synthesis of a series of sulfonic esters and proposed to use them as aged AChE realkylators (Fig. S1A).21 Reactivity of one compound was estimated by alkylation of various small anions, including several phosphonates, halides, and some other inorganic anions (Fig. S1B), which was monitored by nuclear magnetic resonance (NMR) spectroscopy.22 However, none of these compounds were tested with aged AChE.
Haloketones
Soon after, in 1970, Steinberg et al. tested a library of more than 20 alkylating agents, mainly haloketones, R-C(=O)CH2Br, against p-nitrophenyl methylphosphonate, which mimics the phosphorylated serine residue in the aged-AChE enzyme (Fig. S2).23 The halide ion is displaced by this model phosphonate compound during the reaction, leading to realkylation. Steinberg et al. used this methodology to evaluate the potential in vivo efficacy of haloketones with such an in vitro model phosphonate. Affected by the adjacent carbonyl, the halogen-bonded carbon of a haloketone is more reactive to nucleophiles than is an alkyl halide. Similar reactions between haloketones and phosphates have also been reported for other uses.24,25 In Steinberg’s work, seven compounds in the library were selected to be examined further with real AChE aged with32P-labeled soman. TMB-4 was chosen as the reactivating oxime. Removal of the radioactive label was monitored as an implication of reactivation of realkylated AChE. None of tested compounds, however, successfully removed the radioactive label from aged AChE. The authors did not confirm whether the resurrection was halted at the binding step, the realkylation step, or the subsequent oxime treatment.
Methoxypyridinium
Inspired by the interconversion between 2-methoxypyridine and N-methylpyridone, Quinn and coworkers proposed to alkylate aged AChE with a series of methoxypyridiniums in 2013 (Fig. 3).26 These positively charged compounds transfer methyl groups to nucleophiles, yielding corresponding neutral pyridones as the by-product. Alkylation reactivities of a series of compounds were tested with sodium methyl methylphosphonate, which mimics the phosphoryl group of aged AChE (as was done by Steinberg et al.23). Two reaction mechanisms were proposed: as Figure 3 illustrates, the methyl group can be either transferred directly to the phosphonate or via a pseudo-six-membered-ring transition state. In the same year, these researchers evaluated the IC50 of more than 30 compounds of this type against native human AChE in order to investigate their affinities to this enzyme.27 The values ranged from 50 nM to 131 µM. However, alkylation efficacies of these drug candidates with aged AChE were not reported in any peer-reviewed publications. An et al. investigated the realkylation of sarin-aged AChE by Quinn’s realkylator using quantum mechanical/molecular mechanical (QM/MM) calculations.28 An intense π–π interaction between the W86 residue and the drug was found to hinder the reaction.
Figure 3.
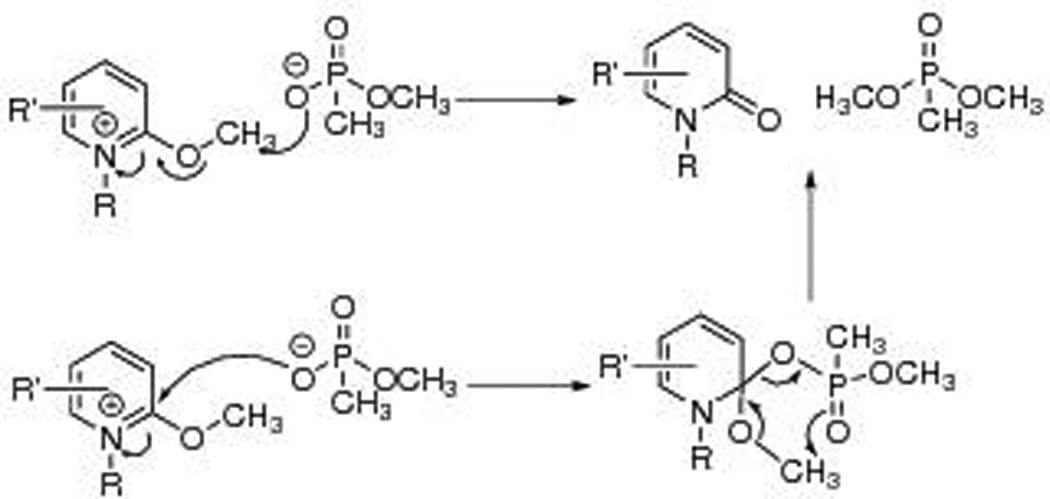
Quinn et al. proposed to alkylate aged AChE with methoxypyridiniums. Two possible mechanisms of methyl transfer from methoxypyridiniums to phosphonates were proposed. Adapted from Refs. 26 and 27.
Sulfoniums
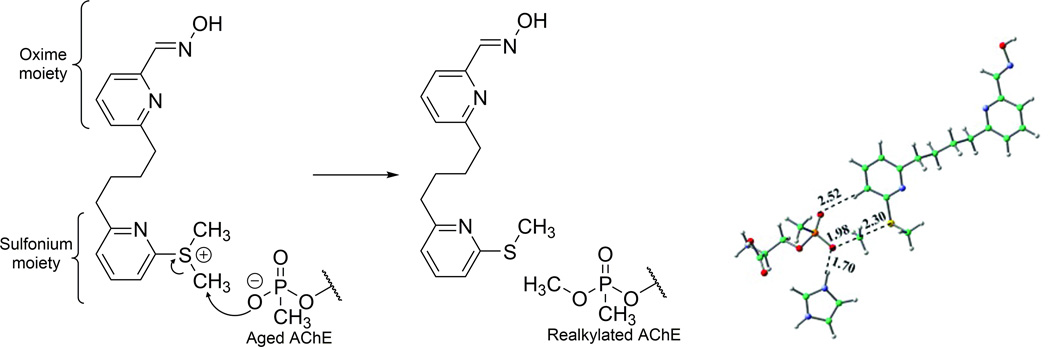
Tertiary sulfoniums can also act as alkylators. S-adenosyl methionine (SAM) is known as a cosubstrate in biological transmethylation. Inspired by biological sulfoniums, Chandar et al. designed several sulfoniums as realkylators of aged AChE, investigated them in silico, and compared them with N-methyl-2-methoxypyridium.29 Particularly, compound 3 in this publication was designed as a bifunctional drug candidate with a sulfonium moiety and an oxime moiety (Fig. 4). The activation energy of reaction, as well as the orientation of the molecule in the gorge of AChE, was investigated. The potential energy surfaces were calculated with density functional theory at the M05-2X/6-31G* level of theory and with consideration of implicit (aqueous) solvation. Steered molecular dynamics (SMD) with the GROMOS 96 force field were used to “pull” the compound out from the active site of aged AChE, and their studies indicate that the orientation of the drug flips as it enters or leaves the enzyme gorge, allowing both moieties to react with the active site. However, to our knowledge, these sulfoniums have not been experimentally synthesized or examined.
Figure 4.
Compound 3 proposed by Chandar et al., and its methyl transfer reaction with methylphosphonate-bound serine, catalyzed by histidine. Adapted from Ref. 29.
Quinone methide precursors
Quinone methides (QMs (Fig. S3A)) can be regarded as delocalization-stabilized carbocations, which are reactive to nucleophiles. For better stability, a QM can be synthesized and applied as its precursor (QMP (Fig. S3B)), which is a phenol with a leaving group on the benzylic carbon. QMPs can alkylate nucleophiles directly via SN2 substitution or via corresponding QMs as reactive intermediates (Fig. S3C). They have been reported as alkylators of proteins30–33 and nucleic acids.34–38 More interestingly, in 1999, Zhou et al. reported a QM capable of alkylating phosphodiesters.39 Bakke et al. also reported a QM that can alkylate dibutyl phosphate in 2005.40 These studies suggest the possibility to realkylate aged AChE using a QM or QMP, considering the structural similarity between small phosphates and the phosphylated serine of aged AChE. However, to date, no QM or QMP effective against aged AChE has been reported in any peer-reviewed publications.
Upregulation of AChE expression
Another strategy for postaging treatment of OP poisoning is the upregulation of AChE expression. Rotundo reported expression of AChE in chicken muscle cells after OP poisoning.41 All cell-associated AChE was initially inhibited with diisopropyl fluorophosphate (DFP), a membrane-permeable OP. Following removal of unreacted OP, the cells were incubated in complete medium, and the AChE level was determined at various time intervals. An obvious increase of AChE level was seen within 2 h of observation. This phenomenon is attributed to the freshly produced active AChE from the cells rather than the spontaneous reactivation of inhibited AChE, considering the fast aging and slow spontaneous reactivation of DFP.42 The expression of native AChE may prove useful in postaging treatment of OP poisoning if it can be accelerated. In this case, newly synthesized and active AChE can replace any impaired form of AChE (either inhibited or aged), hence recovering the total AChE activity in the victim. In the meantime, however, aged AChE will not be resurrected.
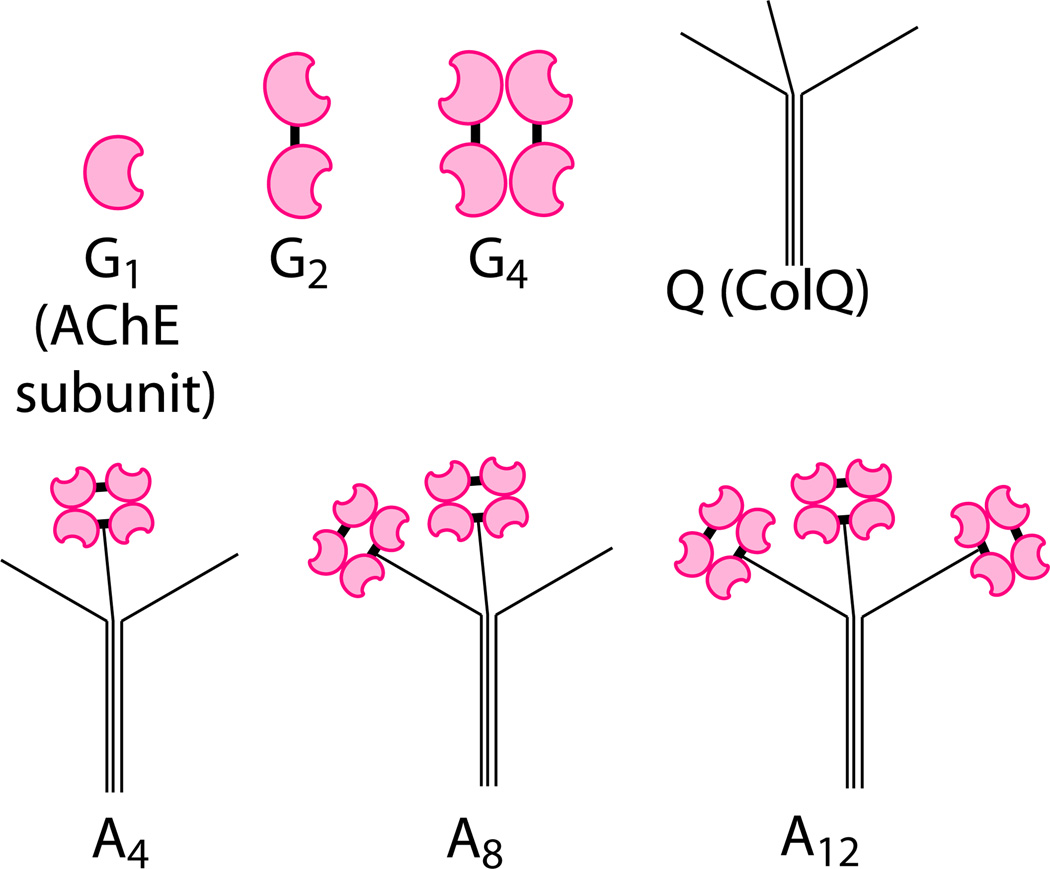
AChE at NMJs can be assembled into dimers (G2), tetramers (G4), and further hetero-oligomers (A4, A8, and A12, (i.e., ColQ-AChE)) with a collagen-like tail (Q, (i.e., ColQ)), as illustrated in Figure 5.43 The N-terminal domain of each strand of this triple-helix tail can be linked to an AChE tetramer. Most of the newly translated AChE before assembly is incompletely folded, hence catalytically inactive and rapidly degraded; indeed, only correctly folded and assembled AChE can transit the Golgi apparatus and be secreted or anchored to the cell membrane.41 Assembly with Q into oligomers could rescue AChE from degradation. A research report by Ruiz and Rotundo reveals that the overexpression and knockdown of Q are sufficient to up- and downregulate the total AChE activity in skeletal muscle, respectively.44
Figure 5.
Molecular forms of AChE and Q tail. Each AChE subunit, shown in magenta, possesses its own active site. Bold dashes in black represent disulfide bonds. Adapted from Ref. 43.
By means of mutation and truncation of Q, Bon et al. discovered that a proline-rich attachment domain (PRAD) in Q consisting of 17 amino acids (CCLLTPPPPPMFPPPFF), as well as 8-kDa polyproline, was capable of promoting G4 assembly and upregulating AChE expression.45 Specifically, in this study, polyproline was added to the culture medium as an exogenous synthetic peptide, rather than transfected and endogenously expressed. These facts reveal the possibility of applying synthetic PRAD or polyproline as a peptide drug against OP poisoning, even after aging occurs. Some mutants of PRAD, such as ESTP10, also contributed to G4 formation, but are less efficient than the wild-type sequence. Rotundo has patented using this type of peptide as a postaging treatment against OP poisoning, but the patent has yet to be granted.46
Exogenous AChE
The third strategy to recover the total AChE activity after OP poisoning or even aging is to introduce exogenous AChE to the victims. Like upregulation of AChE, this strategy cannot resurrect aged AChE. Instead, it compensates for lost AChE activity by adding some amount of active AChE.
Blood transfusion and human AChE
Whole blood is a common source of human AChE for research. Then why not use it for OP-poisoning treatment? Besides AChE, blood also contains butyrylcholinesterase (BChE), which can act as a bioscavenger of OPs.47 In 1976, Okonek et al. reported the change of total activity of AChE in the blood in parathion-poisoned rats upon blood-exchange transfusion. Indeed, the AChE level was recovered from ~10% to >50% after exchange transfusion of 6.7 mL of blood per 100 g body weight.48
In 2011, Ryniak et al. reported a clinical case, in which a patient, self-poisoned by parathion, was treated with a combination of conventional drugs and whole-blood transfusion.49 The blood AChE level of the patient dropped below detection limit within ~10 h, and multiorgan failure symptoms then emerged. After 5 days of treatment with obidoxime and anticholinergic drugs, the condition did not improve until the transfusion of 500 mL of whole blood. Six hours after the transfusion, AChE level was restored to 55%. The administration of drugs was continued. The patient was discharged from the hospital on the 16th day. Note that the aging half-time (t1/2) of paraoxon, which is the active metabolite of parathion, is 5.4–45.5 h.42 Most of the endogenous AChE of the patient had presumably been aged before the blood transfusion.
The limitations of this treatment include challenges in whole-blood storage, incompatibility between blood types (especially Rh− blood types), and risk of blood-transmitted diseases. Human AChE isolated from whole blood may be a better alternative in this respect. However, the supply of human AChE from blood still depends on whole-blood donation. Moreover, these treatments will be more costly than small-molecule realkylators of aged AChE.
Production of recombinant human AChE from transgenic plants50,51 or mammal cell lines52,53 can eliminate the dependence on human blood. An Agrobacterium-mediated transient coexpression system in Nicotiana benthamiana plants reported by Rosenberg et al. exhibited high yield and promising stability.50 The p19 suppressor of gene silencing from tomato bushy stunt virus and PRAD (discussed above) were cotransfected along with the AChE gene in order to maximize the yield. Polyvinylpyrrolidone was added to prevent polyphenols in the extract from damaging AChE. Approximately 70 mg was harvested from ~ 200 g of leaves. The obtained AChE was stable in aqueous solutions for weeks at up to 37 °C. Production of recombinant AChE also allows some additional features that endogenous AChE lacks. Radić and coworkers reported a Y337A/F338A mutant of human AChE, which proved resistant against soman aging.53 Atsmon et al. reported a PEGylated recombinant human AChE with a long plasma half-life (26.7 h in humans).51 A further extended plasma half-life (> 60 h) was achieved with a chimeric protein generated by Mazor and coworkers, which combines human AChE and the Fc region of human IgG1.54
Nonhuman AChE
Livestock blood and brains are produced on a large scale as by-products in animal slaughter. Electric organs of the electric eel (Electrophorus electricus), electric ray (Torpedo californica), and other electric fishes are particularly rich in AChE. Nonhuman AChE can be harvested from these animals at a lower cost compared to human AChE. The retail prices of human erythrocyte AChE from Sigma-Aldrich Co. LLC is U.S. $467 every 50 units.55 By contrast, the price of electric eel AChE (type V-S) from the same provider is only $179.50 every 1000 units.56 None of these enzymes have been investigated as treatments of OP poisoning. They may, however, prove to be valuable candidates as exogenous AChE, considering their inexpensive production. Immunogenicity and allergenicity are possible risks that must be taken into account before these exogenous proteins are introduced to the human body.
Though plants and bacteria possess no nervous systems, some of them also naturally produce acetylcholine hydrolases. Some are related to environmental stimuli, such as heat stress57 and gravity,58 while some are produced for metabolism.59 In spite of their sizes, structures, physiological functions, and presumably catalysis mechanisms, which differ from those of animal AChE, they have also been referred to as “AChE” or “cholinesterase” by numerous researchers.
In the 1950s, Goldstein et al. reported the adaptive production of AChE from Pseudomonas fluorescens, a bacterium.60,61 Considerable production of this protein relies on induction by adding choline or acetylcholine to the medium. This enzyme is more active against propionylcholine than acetylcholine. Most of expressed AChE was intracellular rather than excreted. Fitch also investigated AChE from this bacterium, both in terms of its physiological role and its catalytic properties.59,62 Over the past 4 decades, researchers have been studying adaptive production of AChE from P. aeruginosa, another species in Pseudomonas genus.63–67 Sánchez et al. identified this enzyme as encoded by gene PA4921 in the SGNH hydrolase superfamily.63 The molecular weight is around 30 kDa. There are also publications on AChEs from plants, including eggplants, beans, and maize.68–76 These AChEs belong to the lipase GDSL (Gly–Asp–Ser–Leu) family.68
In 1991, Nieto et al. reported an AChE from Aeromonas hydrophila, which acts as a toxin to fish, the host of this bacterium.77 Unlike Pseudomonas AChE, this enzyme is excreted and smaller (~ 15 kDa). The specific activity was claimed to be approximately three times as high as that of electric eel AChE. This enzyme was also discovered in other species of the Aeromonas and Vibrio genera.78,79 It may be capable of crossing the blood–brain barrier (BBB) of fish to enter the central nervous system, as implied by an experiment published in 1993.80 The enzyme was injected intraperitoneally (into the body cavity) into rainbow trout. A dramatic increase (up to two orders of magnitude) of AChE activity in the brain was observed. A 45-kDa protein was identified as the proenzyme of this AChE, and displayed lower activity.81 Further proteomic and crystallographic investigations are needed to clarify its gene, structure, and catalytic mechanism. The high activity, small size, and good BBB permeability of Aeromonas AChE reveal the potential of this enzyme as a protein drug candidate to compensate for impaired AChE level due to OP poisoning. Furthermore, as an excreted protein, it can be economically and conveniently harvested from the culture medium without homogenizing the cells.
AChE–fasciculin-2 chimeric protein
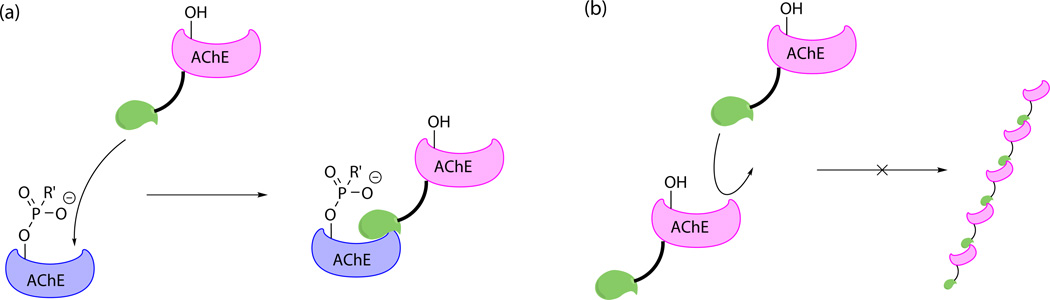
Fasciculin-2 (Fas2) is a toxin found in snake venom. It acts by noncovalently binding to a peripheral site of AChE near the catalytic gorge and allosterically changing the conformation of AChE.82 Rotundo developed and patented an AChE–Fas2 chimeric protein for treatment of OP poisoning (Fig. 6A).83 In this protein, a Fas2 domain is linked downstream of an AChE domain. The Fas2 domain guides and anchors the protein to NMJs and synapses by binding to endogenous AChE (either OP inhibited or not), while the AChE domain hydrolyzes acetylcholine. Compared to exogenous AChE without the Fas2 moiety, this chimeric protein is expected to be more enriched at NMJs and synapses, where it is more in demand. Immobilization may also slow down its metabolism. Microscope images visualized AChE–Fas2 bound to mouse NMJs after intravenous injection. Injection of 5 µg of AChE–Fas2 enhanced the survival rate of DF-treated (2 × LD50) mice from 17% to 92%. Karnovsky-Roots staining confirmed the recovered AChE activity at NMJs of surviving mice. Aggregation of this chimeric protein owing to head-to-tail binding is prevented by choosing a mutant AChE, which is still active but does not bind Fas2 (Fig. 6B).Rotundo et al. have not disclosed any information regarding the safety of this approach. But a single dose may be acceptable in terms of immunogenicity.
Figure 6.
(A) The Fas2 moiety (displayed in green) links the active AChE moiety (magenta) to the aged endogenous AChE (blue). (B) The AChE moiety is mutated to avoid binding to the Fas2 moiety and aggregation. Adapted from Ref. 83.
Conclusions
Aging is a major barrier in the treatment of OP poisoning. Aged AChE cannot be directly reactivated by oximes. Some effectors can slow down aging, allowing more time for conventional oxime therapy. High concentrations of the discovered effectors required for this aging retardation effect is limiting the application of this approach. Moreover, they must be administrated before the patient’s AChE is completely aged.
For resurrection of aged AChE, researchers have been developing and testing alkylators to bring the enzyme back to the inhibited state, which can then be reactivated by conventional oximes. Some of these compounds have exhibited encouraging reactivity with model phosphonates, which emulate aged AChE. But candidates capable of realkylating the aged form of AChE have not yet been discovered. This may be due to ineffective binding between these compounds and the catalytic gorge of AChE or unfavorable orientation at the active site. Computational chemistry may continue to guide the rational design of new alkylators in the future. Another possibility is that the realkylation itself was a success, but the subsequent oxime reactivation was not viable, perhaps due to an inappropriate choice of oxime or steric hindrance in the active site. Mass spectrometry may be employed to verify or to rule out this possibility. If the aged AChE is successfully realkylated, the corresponding mass shift can be observed in the appropriate peptide in the digest. Off-target reactivities of the realkylators should also be studied to prevent them from alkylating other residues of AChE or other proteins.
Alternatively, newly produced active AChE can be used to restore the damaged AChE activity in the bodies of victims. Some peptides that can upregulate the expression of AChE, such as PRAD and polyproline, have been discovered. To replace aged AChE, an exogenous source of active AChE, such as whole blood, recombinant human AChE, and bacterial AChE, can also be used. Fas2–AChE chimeric proteins were also proposed for this purpose. The Fas2 moiety can anchor the protein to the NMJ and synapses, where endogenous native AChE is naturally located.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the National Institutes of Health CounterACT program (1U01–NS087983). Computational support has been generously provided by the Ohio Supercomputer Center.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard P. Reactivators of Acetylcholinesterase Inhibited by Organophosphorus Nerve Agents. Accounts Chem. Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- 2.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macilwain C. Study proves Iraq used nerve gas. Nature. 1993;363:3. doi: 10.1038/363003b0. [DOI] [PubMed] [Google Scholar]
- 4.United Nations. Report on the Alleged Use of Chemical Weapons in the Ghouta Area of Damascus on 21 August, 2013. [Accessed August 17, 2015];2013 Sep 13; 2013. http://www.un.org/disarmament/content/slideshow/Secretary_General_Report_of_CW_Investigation.pdf. [Google Scholar]
- 5.Tu AT. Basic Information on Nerve Gas and the Use of Sarin by Aum Shinrikyo. J Mass Spectrom. Soc. Jpn. 1996;44:293–320. [Google Scholar]
- 6.Worek F, Aurbek N, Koller M, Becker C, Eyer P, Thiermann H. Kinetic analysis of reactivation and aging of human acetylcholinesterase inhibited by different phosphoramidates. Biochem. Pharmacol. 2007;73:1807–1817. doi: 10.1016/j.bcp.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Carletti E, Aurbek N, Gillon E, Loiodice M, Nicolet Y, Fontecilla-Camps J, Masson P, Thiermann H, Nachon F, Worek F. Structure-activity analysis of aging and reactivation of human butyrylcholinesterase inhibited by analogues of tabun. Biochem. J. 2009;421:97–106. doi: 10.1042/BJ20090091. [DOI] [PubMed] [Google Scholar]
- 8.Carletti E, Colletier J, Dupeux F, Trovaslet M, Masson P, Nachon F. Structural evidence that human acetylcholinesterase inhibited by tabun ages through O-dealkylation. J Med. Chem. 2010;53:4002–4008. doi: 10.1021/jm901853b. [DOI] [PubMed] [Google Scholar]
- 9.Sanson B, Nachon F, Colletier J, Froment M, Toker L, Greenblatt HM, Sussman JL, Ashani Y, Masson P, Silman I, Weik M. Crystallographic Snapshots of Nonaged and Aged Conjugates of Soman with Acetylcholinesterase, and of a Ternary Complex of the Aged Conjugate with Pralidoxime. J Med. Chem. 2009;52:7593–7603. doi: 10.1021/jm900433t. [DOI] [PubMed] [Google Scholar]
- 10.Shafferman A, Ordentlich A, Barak D, Stein D, Ariel N, Velan B. Aging of phosphylated human acetylcholinesterase: catalytic processes mediated by aromatic and polar residues of the active centre. Biochem. J. 1996;318:833–840. doi: 10.1042/bj3180833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobbiger F. Effect of nicotinhydroxamic acid methiodide on human plasma cholinesterase inhibited by organophosphates containing a dialkylphosphato group. Brit. J. Pharmacol. 1955;10:356–362. doi: 10.1111/j.1476-5381.1955.tb00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry WK, Davies DR. Factors influencing the rate of 'aging' of a series of alkyl methylphosphonyl-acetylcholinesterases. Biochem. J. 1966;100:572–576. doi: 10.1042/bj1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crone HD. Can allosteric effectors of acetylcholinesterase control the rate of ageing of the phosphonylated enzyme? Biochem. Pharmacol. 1974;23:460–463. doi: 10.1016/0006-2952(74)90439-0. [DOI] [PubMed] [Google Scholar]
- 14.Sterri SH. Effect of imidazoles and pH on aging of phosphylated acetylcholinesterase. Biochem. Pharmacol. 1977;26:656–658. doi: 10.1016/0006-2952(77)90044-2. [DOI] [PubMed] [Google Scholar]
- 15.Van Dongen CJ, Elskamp RM, De Jong LPA. Influence of atropine upon reactivation and ageing of rat and human erythrocyte acetylcholinesterase inhibited by soman. Biochem. Pharmacol. 1987;36:1167–1169. doi: 10.1016/0006-2952(87)90428-x. [DOI] [PubMed] [Google Scholar]
- 16.Schoene K. Aging of soman-inhibited acetylcholinesterase: Inhibitors and accelerators. Biochim. Biophys. Acta. 1978;525:468–471. doi: 10.1016/0005-2744(78)90243-7. [DOI] [PubMed] [Google Scholar]
- 17.Schoene K, Steinhanses J, Wertmann A. Aging of soman-inhibited acetylcholinesterase. pH-rate profiles and temperature dependence in absence and in presence of effectors. Biochim. Biophys. Acta. 1980;616:384–388. doi: 10.1016/0005-2744(80)90156-4. [DOI] [PubMed] [Google Scholar]
- 18.Bourne Y, Taylor P, Radić Z, Marchot P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003;22:1–12. doi: 10.1093/emboj/cdg005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong LPA, Wolring GZ. Effect of 1-(AR)alkyl-2-hydroxyiminomethyl-pyridinium salts on reactivation and aging of acetyl-cholinesterase inhibited by ethyl dimethylphosphoramidocyanidate (tabun) Biochem. Pharmacol. 1978;27:2229–2235. doi: 10.1016/0006-2952(78)90082-5. [DOI] [PubMed] [Google Scholar]
- 20.Puu G, Artursson E, Bucht G. Reactivation of nerve agent inhibited human acetylcholinesterases by HI-6 and obidoxime. Biochem. Pharmacol. 1986;35:1505–1510. doi: 10.1016/0006-2952(86)90116-4. [DOI] [PubMed] [Google Scholar]
- 21.Blumbergs P, Ash AB, Daniher FA, Stevens CL, Michel HO, Hackley BE, Epstein J. Alkylating agents containing a quaternary nitrogen group. J Org. Chem. 1969;34:4065–4070. [Google Scholar]
- 22.Ash AB, Blumbergs P, Stevens CL, Michel HO, Hackley BE, Epstein J. Relative nucleophilicity. Methylation of anions in aqueous media. J Org. Chem. 1969;34:4070–4072. [Google Scholar]
- 23.Steinberg GM, Lieske CN, Boldt R, Goan JC, Podall HE. Model studies for the reactivation of aged phosphonylated acetylcholinesterase. Use of alkylating agents containing nucleophilic groups. J Med. Chem. 1970;13:435–446. doi: 10.1021/jm00297a024. [DOI] [PubMed] [Google Scholar]
- 24.Sachnov SJ, Schulz PS, Wasserscheid P. A convenient method to access long-chain and functionalised mixed methylphosphonate esters and their application in the synthesis of ionic liquids. Chem. Commun. 2011;47:11234–11236. doi: 10.1039/c1cc14490a. [DOI] [PubMed] [Google Scholar]
- 25.Park C, Givens RS. New Photoactivated Protecting Groups. 6. p-Hydroxyphenacyl: A Phototrigger for Chemical and Biochemical Probes. J Am. Chem. Soc. 1997;119:2453–2463. [Google Scholar]
- 26.Topczewski JJ, Quinn DM. Kinetic assessment of N-methyl-2-methoxypyridinium species as phosphonate anion methylating agents. Org. Lett. 2013;15:1084–1087. doi: 10.1021/ol400054m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topczewski JJ, Lodge AM, Yasapala SN, Payne MK, Keshavarzi PM, Quinn DM. Reversible inhibition of human acetylcholinesterase by methoxypyridinium species. Bioorg. Med. Chem. Lett. 2013;23:5786–5789. doi: 10.1016/j.bmcl.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 28.An Y, Zhu Y, Yao Y, Liu J. Is it possible to reverse aged acetylcholinesterase inhibited by organophosphorus compounds? Insight from the theoretical study. Phys. Chem. Chem. Phys. 2016;18:9838–9846. doi: 10.1039/c5cp07991h. [DOI] [PubMed] [Google Scholar]
- 29.Chandar NB, Lo R, Ganguly B. Quantum chemical and steered molecular dynamics studies for one pot solution to reactivate aged acetylcholinesterase with alkylator oxime. Chem-Biol. Interact. 2014;223:58–68. doi: 10.1016/j.cbi.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Thompson DC, Perera K, London R. Spontaneous hydrolysis of 4-trifluoromethylphenol to a quinone methide and subsequent protein alkylation. Chem-Biol. Interact. 2000;126:1–14. doi: 10.1016/s0009-2797(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 31.Bolton JL, Turnipseed SB, Thompson JA. Influence of quinone methide reactivity on the alkylation of thiol and amino groups in proteins: studies utilizing amino acid and peptide models. Chem-Biol. Interact. 1997;107:185–200. doi: 10.1016/s0009-2797(97)00079-3. [DOI] [PubMed] [Google Scholar]
- 32.McCracken PG, Bolton JL, Thatcher GRJ. Covalent modification of proteins and peptides by the quinone methide from 2-tert-butyl-4,6-dimethylphenol: Selectivity and reactivity with respect to competitive hydration. J Org. Chem. 1997;62:1820–1825. [Google Scholar]
- 33.Reboud-Ravaux M, Wakselman M. Quinone methides and aza-quinone methides as latent alkylating species in the design of mechanism-based inhibitors of serine proteases and beta-lactamases. Wiley Ser. React. Intermed. Chem. Biol. 2009;1:357–383. [Google Scholar]
- 34.Liu Y, Rokita SE. Inducible Alkylation of DNA by a Quinone Methide-Peptide Nucleic Acid Conjugate. Biochem. 2012;51:1020–1027. doi: 10.1021/bi201492b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Q, Qu Y, Mangrum JB, Wang X. DNA alkylation with N-methylquinolinium quinone methide to N2-dG adducts resulting in extensive stops in primer extension with DNA polymerases and subsequent suppression of GFP expression in A549 cells. Chem. Res. Toxicol. 2011;24:402–411. doi: 10.1021/tx100351c. [DOI] [PubMed] [Google Scholar]
- 36.Lewis MA, Graff Yoerg D, Bolton JL, Thompson JA. Alkylation of 2'-deoxynucleosides and DNA by quinone methides derived from 2,6-di-tert-butyl-4-methylphenol. Chem. Res. Toxicol. 1996;9:1368–1374. doi: 10.1021/tx960115+. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Song Y, Zhang L, He H, Zhou X. Quinone methide derivatives: important intermediates to DNA alkylating and DNA cross-linking actions. Curr. Med. Chem. 2005;12:2893–2913. doi: 10.2174/092986705774454724. [DOI] [PubMed] [Google Scholar]
- 38.Dufrasne F, Gelbcke M, Neve J, Kiss R, Kraus J. Quinone methides and their prodrugs: a subtle equilibrium between cancer promotion, prevention, and cure. Curr. Med. Chem. 2011;18:3995–4011. doi: 10.2174/092986711796957301. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q, Turnbull KD. Phosphodiester alkylation with a quinone methide. J Org. Chem. 1999;64:2847–2851. doi: 10.1021/jo9823745. [DOI] [PubMed] [Google Scholar]
- 40.Bakke BA, McIntosh MC, Turnbull KD. Improved alkylation and product stability in phosphotriester formation through quinone methide reactions with dialkyl phosphates. J Org. Chem. 2005;70:4338–4345. doi: 10.1021/jo050050s. [DOI] [PubMed] [Google Scholar]
- 41.Rotundo RL. Biogenesis of acetylcholinesterase molecular forms in muscle. Evidence for a rapidly turning over, catalytically inactive precursor pool. J Biol. Chem. 1988;263:19398–19406. [PubMed] [Google Scholar]
- 42.Worek F, Thiermann H, Szinicz L, Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem. Pharmacol. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 43.Massoulie J, Bon S. The molecular forms of cholinesterase and acetylcholinesterase in vertebrates. Annu. Rev. Neurosci. 1982;5:57–106. doi: 10.1146/annurev.ne.05.030182.000421. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz CA, Rotundo RL. Dissociation of transcription, translation, and assembly of collagen-tailed acetylcholinesterase in skeletal muscle. J Biol. Chem. 2009;284:21488–21495. doi: 10.1074/jbc.M109.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bon S, Coussen F, Massoulié J. Quaternary associations of acetylcholinesterase: II. The polyproline attachment domain of the collagen tail. J Biol. Chem. 1997;272:3016–3021. doi: 10.1074/jbc.272.5.3016. [DOI] [PubMed] [Google Scholar]
- 46.Rotundo RL, inventors; University Of Miami (FL, USA), assignee 12/514,384. US patent. 2007 Aug 14; 2007.
- 47.Huang Y, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Côté M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemée N, Wilgus H, Bégin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Karatzas CN, Langermann S. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proceedings of the National Academy of Sciences. 2007;104:13603–13608. doi: 10.1073/pnas.0702756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okonek S, Boelcke G, Hollmann H. Therapeutic properties of haemodialysis and blood exchange transfusion in organophosphate poisoning. Eur. J. Intensive Care Med. 1976;2:13–18. doi: 10.1007/BF00571891. [DOI] [PubMed] [Google Scholar]
- 49.Ryniak S, Harbut P, Goździk W, Sokołowski J, Paciorek P, Hałas J. Whole blood transfusion in the treatment of an acute organophosphorus poisoning-a case report. Med. Sci. Monit. 2011;17:109–111. doi: 10.12659/MSM.881922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg YJ, Walker J, Jiang X, Donahue S, Robosky J, Sack M, Lees J, Urban L. A highly stable minimally processed plant-derived recombinant acetylcholinesterase for nerve agent detection in adverse conditions. Sci. Rep. 2015;5:13247. doi: 10.1038/srep13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atsmon J, Brill-Almon E, Nadri-Shay C, Chertkoff R, Alon S, Shaikevich D, Volokhov I, Haim KY, Bartfeld D, Shulman A, Ruderfer I, Ben-Moshe T, Shilovitzky O, Soreq H, Shaaltiel Y. Preclinical and first-in-human evaluation of PRX-105, a PEGylated, plant-derived, recombinant human acetylcholinesterase-R. Toxicol. Appl. Pharm. 2015;287:202–209. doi: 10.1016/j.taap.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 52.D. Ruark C, Chapleau RR, Mahle DA, Gearhart JM. Organophosphorus Inhibition and Characterization of Recombinant Guinea Pig Acetylcholinesterase. Protein Peptide Lett. 2015;22:862–868. doi: 10.2174/0929866522666150728114754. [DOI] [PubMed] [Google Scholar]
- 53.Kovarik Z, Macek Hrvat N, Katalinic M, Sit RK, Paradyse A, Zunec S, Musilek K, Fokin VV, Taylor P, Radic Z. Catalytic soman scavenging by Y337A/F338A acetylcholinesterase mutant assisted with novel site-directed aldoximes. Chem. Res. Toxicol. 2015;28:1036–1044. doi: 10.1021/acs.chemrestox.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noy-Porat T, Cohen O, Ehrlich S, Epstein E, Alcalay R, Mazor O. Acetylcholinesterase-Fc Fusion Protein (AChE-Fc): A Novel Potential Organophosphate Bioscavenger with Extended Plasma Half-Life. Bioconjugate Chem. 2015;26:1753–1758. doi: 10.1021/acs.bioconjchem.5b00305. [DOI] [PubMed] [Google Scholar]
- 55.Sigma-Aldrich Co. LLC. Acetylcholinesterase from Human Erythrocytes. [Accessed April 11, 2016];2016 Apr 11; 2016. http://www.sigmaaldrich.com/catalog/product/sigma/c0663?lang=en®ion=US. [Google Scholar]
- 56.Sigma-Aldrich Co. LLC. Acetylcholinesterase from Electrophorus electricus (Electric Eel) [Accessed April 11, 2016];2016 Apr 11; 2016. http://www.sigmaaldrich.com/catalog/product/sigma/c2888?lang=en®ion=US. [Google Scholar]
- 57.Momonoki YS, Momonoki T. Changes in acetylcholine-hydrolyzing activity in heat-stressed plant cultivars. Jpn J. Crop Sci. 1993;62:438–446. [Google Scholar]
- 58.Momonoki YS, Hineno C, Noguchi K. Acetylcholine as a signaling system to environmental stimuli in plants: III. Asymmetric solute distribution controlled by ACh in gravistimulated maize seedlings. Plant Prod. Sci. 1998;1:83–88. doi: 10.1626/pps.1.83. [DOI] [PubMed] [Google Scholar]
- 59.Fitch WM. Studies on a cholinesterase of Pseudomonas fluorescens. I. Enzyme induction and the metabolism of acetylcholine. Biochem. 1963;2:1217–1221. doi: 10.1021/bi00906a007. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein DB. Induction of cholinesterase biosynthesis in Pseudomonas fluorescens. J Bacteriol. 1959;78:695–702. doi: 10.1128/jb.78.5.695-702.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldstein DB, Goldstein A. An adaptive bacterial cholinesterase from a Pseudomonas species. J Gen. Microbiol. 1953;8:8–17. doi: 10.1099/00221287-8-1-8. [DOI] [PubMed] [Google Scholar]
- 62.Fitch WM. Studies on a cholinesterase of Pseudomonas fluorescens. II. Purification and properties. Biochem. 1963;2:1221–1227. doi: 10.1021/bi00906a008. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez DG, Otero LH, Hernández CM, Serra AL, Encarnación S, Domenech CE, Lisa ÁT. A Pseudomonas aeruginosa PAO1 acetylcholinesterase is encoded by the PA4921 gene and belongs to the SGNH hydrolase family. Microbiol. Res. 2012;167:317–325. doi: 10.1016/j.micres.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Lucchesi G, Lisa T, Casale C, Domenech C. Carnitine resembles choline in the induction of cholinesterase, acid phosphatase, and phospholipase C and in its action as an osmoprotectant in Pseudomonas aeruginosa. Curr. Microbiol. 1995;30:55–60. doi: 10.1007/BF00294525. [DOI] [PubMed] [Google Scholar]
- 65.Lisa TA, Garrido MN, Domenech CE. Induction of acid phosphatase and cholinesterase activities in Pseudomonas aeruginosa and their in-vitro control by choline, acetylcholine and betaine. Mol. Cell. Biochem. 1983;50:149–155. doi: 10.1007/BF00285640. [DOI] [PubMed] [Google Scholar]
- 66.Garber N, Nachshon I. Localization of cholinesterase in Pseudomonas aeruginosa strain K. J Gen. Microbiol. 1980;117:279–283. doi: 10.1099/00221287-117-1-279. [DOI] [PubMed] [Google Scholar]
- 67.Tani Y, Nagasawa T, Sugisaki H, Ogata K. Purification and characterization of the cholinesterase of Pseudomonas aeruginosa A-16. Agric. Biol. Chem. 1975;39:1287–1294. [Google Scholar]
- 68.Sagane Y, Nakagawa T, Yamamoto K, Michikawa S, Oguri S, Momonoki YS. Molecular characterization of maize acetylcholinesterase. A novel enzyme family in the plant kingdom. Plant Physiol. 2005;138:1359–1371. doi: 10.1104/pp.105.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Momonoki YS, Momonoki T. Changes in acetylcholine levels following leaf wilting and leaf recovery by heat stress in plant cultivars. Jpn J. Crop Sci. 1991;60:283–290. [Google Scholar]
- 70.Fluck RA, Jaffe MJ. Cholinesterases from plant tissues VI. Preliminary characterization of enzymes from Solanum melongena L. and Zea mays L. Biochim. Biophys. Acta. 1975;410:130–134. doi: 10.1016/0005-2744(75)90213-2. [DOI] [PubMed] [Google Scholar]
- 71.Fluck RA, Jaffe MJ. Cholinesterases from plant tissue: V. Cholinesterase is not pectin esterase. Plant Physiol. 1974;54:797–798. doi: 10.1104/pp.54.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fluck RA, Jaffe MJ. Cholinesterases from Plant Tissues: III. Distribution and Subcellular Localization in Phaseolus aureus Roxb. Plant Physiol. 1974;53:752–758. doi: 10.1104/pp.53.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartmann E, Kilbinger H. Occurrence of light-dependent acetylcholine concentrations in higher plants. Experientia. 1974;30:1387–1388. doi: 10.1007/BF01919649. [DOI] [PubMed] [Google Scholar]
- 74.Riov J, Jaffe MJ. Cholinesterases from plant tissues. II. Inhibition of bean cholinesterase by 2-isopropyl-4-dimethylamino-5-methylphenyl-1-piperidine carboxylate methyl chloride (AMO-1618) Plant Physiol. 1973;52:233–235. doi: 10.1104/pp.52.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riov J, Jaffe MJ. Cholinesterases from plant tissues: I. Purification and characterization of a cholinesterase from mung bean roots. Plant Physiol. 1973;51:520–528. doi: 10.1104/pp.51.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Evans ML. Promotion of cell elongation in Avena coleoptiles by acetylcholine. Plant Physiol. 1972;50:414–416. doi: 10.1104/pp.50.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieto TP, Santos Y, Rodríguez LA, Ellis AE. An extracellular acetylcholinesterase produced by Aeromonas hydrophila is a major lethal toxin for fish. Microb. Pathogenesis. 1991;11:101–110. doi: 10.1016/0882-4010(91)90003-s. [DOI] [PubMed] [Google Scholar]
- 78.Rodríguez LA, Fernández AIG, Nieto TP. Production of the lethal acetylcholinesterase toxin by different Aeromonas hydrophila strains. J Fish Dis. 1993;16:73–78. [Google Scholar]
- 79.Pérez MJ, Rodríguez LA, Nieto TP. The acetylcholinesterase ichthyotoxin is a common component in the extracellular products of Vibrionaceae strains. J Appl. Microbiol. 1998;84:47–52. doi: 10.1046/j.1365-2672.1997.00311.x. [DOI] [PubMed] [Google Scholar]
- 80.Rodríguez LA, Ellis AE, Nieto TP. Effects of the acetylcholinesterase toxin of Aeromonas hydrophila on the central nervous system of fish. Microb. Pathogenesis. 1993;14:411–415. doi: 10.1006/mpat.1993.1040. [DOI] [PubMed] [Google Scholar]
- 81.Pérez MJ, Rodríguez LA, Fernández-Briera A, Nieto TP. A 45-kDa acetylcholinesterase protoxin of Aeromonas hydrophila: purification and immunogenicity in fish. Fems Microbiol. Lett. 2002;211:23–27. doi: 10.1111/j.1574-6968.2002.tb11198.x. [DOI] [PubMed] [Google Scholar]
- 82.Bourne Y, Taylor P, Marchot P. Acetylcholinesterase inhibition by fasciculin: Crystal structure of the complex. Cell. 83:503–512. doi: 10.1016/0092-8674(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 83.Rotundo RL, inventors; University of Miami (FL, USA), assignee WO2014059403. US patent. 2013 Oct 14; 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.