Abstract
Aims
To examine the safety and effectiveness of buprenorphine+naloxone sublingual tablets (BUP, as Suboxone®) provided after administration of extended-release injectable naltrexone (XR-NTX, as Vivitrol®) to reduce cocaine use in participants who met DSM-IV criteria for cocaine dependence and past or current opioid dependence or abuse.
Methods
This multi-centered, double-blind, placebo-controlled study, conducted under the auspices of the National Drug Abuse Treatment Clinical Trials Network, randomly assigned 302 participants at sites in California, Oregon, Washington, Colorado, Texas, Georgia, Ohio, New York, and Washington D.C., USA to 1 of 3 conditions provided with XR-NTX: 4mg/day BUP (BUP4, n=100), 16mg/day BUP (BUP16, n=100), or no buprenorphine (placebo; PLB, n=102). Participants received pharmacotherapy for 8 weeks, with 3 clinic visits per week. Cognitive Behavioral Therapy was provided weekly. Follow-up assessments occurred at 1 and 3 months post-intervention. The planned primary outcome was urine drug screen (UDS)-corrected, self-reported cocaine use during the last 4 weeks of treatment. Planned secondary analyses assessed cocaine use by UDS, medication adherence, retention, and adverse events.
Results
No group differences were found between groups for the primary outcome (BUP4 vs. PLB, p=0.262; BUP16 vs PLB, p=0.185). Longitudinal analysis of UDS data during the evaluation period using generalized linear mixed equations found a statistically significant difference between BUP16 and PLB (p=0.022, OR=1.71) but not for BUP4 (p=0.105, OR=1.05). No secondary outcome differences across groups were found for adherence, retention, or adverse events.
Conclusions
Buprenorphine+naloxone, used in combination with naltrexone, may be associated with reductions in cocaine use among people who meet DSM-IV criteria for cocaine dependence and past or current opioid dependence or abuse.
Introduction
Pharmacotherapy may be important to engage and retain individuals in treatment and reduce cocaine use. No medications are currently available specifically to treat cocaine use disorder, although extensive research testing new medications is ongoing. One promising medication is buprenorphine, a partial mu-opioid agonist and kappa-opioid antagonist. Interest in buprenorphine as a potential pharmacotherapy for cocaine use disorder elicited extensive research over the past 25 years, producing a range of findings on its efficacy in both clinical and preclinical work (1–13). Importantly, prior research has targeted primary opioid misusers who were also cocaine users. Montoya and colleagues (14) studied buprenorphine in participants who misused opioids and cocaine, and reported that 16mg daily sublingual buprenorphine is well tolerated and effective in reducing concomitant opioid and cocaine use. The therapeutic effect of buprenorphine on cocaine use appears to be independent of its effect on opioid use (9,15).
Concurrent administration of buprenorphine with an opioid antagonist such as naltrexone may minimize the risk of eliciting opioid craving or iatrogenic opioid dependence in cocaine-dependent individuals. Research by Rothman (15) and Gerra (9) documented buprenorphine effects on cocaine use when administered in combination with naltrexone. A recent review (17) noted that buprenorphine may be effective in reducing cocaine use in the presence of naltrexone and that the addition of naltrexone should alleviate concerns regarding physical dependence. A recent pre-clinical study demonstrated that the combination of buprenorphine and naltrexone reduced cocaine self-administration in rodents (13). Thus, the mechanism of this effect may be related to kappa antagonism (18), which may ameliorate dysphoria associated with cocaine withdrawal or other non-mu receptor effects/activity of buprenorphine.
Findings from the Gerra study (9) showed that a combination of 4mg sublingual buprenorphine with 50mg oral naltrexone led to significant reductions in cocaine use and was safe and well tolerated. Negligible pupillary changes suggested significant mu blocking effects from the combination, mitigating the risks of ongoing opioid misuse or dependence. Existing research and clinical observations provide a rationale to examine the therapeutic potential of buprenorphine for reducing cocaine use using concurrent administration of naltrexone to safeguard against the risk of inducing opioid-seeking behavior.
The current study investigated the safety and efficacy of sublingual buprenorphine+naloxone provided in combination with naltrexone for the treatment of cocaine dependence in individuals with a history of opioid abuse or dependence. The primary objective was to evaluate whether buprenorphine+naloxone administered with extended-release naltrexone would reduce the number of cocaine use days over the 30-day evaluation period compared to placebo. Secondary outcomes included: (1) safety of this medication combination; (2) cocaine use only using biologic measurement during the evaluation and follow-up periods; (3) abstinence in participants with high level of cocaine use at baseline; (4) compare treatment arms with respect to treatment adherence, compliance and retention; and (5) both opioid use and craving.
Methods
Design
This multi-centered double-blind, placebo-controlled study, conducted under the auspices of the National Drug Abuse Treatment Clinical Trials Network (CTN), provided extended-release, injectable naltrexone (Vivitrol®, XR-NTX) to participants and then randomly assigned them to one of three conditions for 8 weeks: buprenorphine+naloxone (Suboxone®, BUP) 4mg/day BUP (BUP4), 16mg/day BUP (BUP16), or placebo (PLB). Thrice-weekly clinic visits included observed dosing, provision of take-home medication, urine drug screens (UDS), and other assessments. Weekly individual Cognitive Behavioral Therapy (CBT) sessions were offered to all participants. Follow-up assessments occurred at 1 and 3 months post-intervention.
The study was conducted at 11 sites (in California, Oregon, Washington, Colorado, Texas, Georgia, Ohio, New York, and Washington D.C.) between September 2011 and March 2013. All sites obtained local institutional review board (IRB) approval prior to study initiation. The study was registered on ClinicalTrials.gov (identifier: NCT01402492). Details of study methods, design, and eligibility criteria have been published elsewhere (19).
Participants
Eligible participants were treatment-seeking adults age 18–65 with a DSM-IV diagnosis of cocaine dependence with past-year opioid dependence or abuse, or past-year opioid use with a lifetime history of opioid dependence. Participants were required to be in good general health with no known sensitivities to study medications and no serious medical or psychiatric conditions. Exclusion criteria included recent or ongoing use of medications which could interact adversely with study medications, or a current pattern of alcohol, benzodiazepine, or other sedative-hypnotic use which would preclude safe participation. Eligibility criteria were included to ensure abstinence from opioids and avoid the risk of prolonged XR-NTX-precipitated opioid withdrawal. Participants were excluded if they reported recent opioid use, had a UDS positive for opioids, had methadone maintenance treatment within 15 days of consent, buprenorphine maintenance treatment within 30 days of consent, required opioid analgesics, or had a body habitus that precluded the XR-NTX intramuscular gluteal injection. Participants received study medication and counseling at no cost and were compensated with up to $765 in gift cards or cash for completion of study components including screening, injections, clinic visits, and follow-up assessments.
Medications
Alkermes Inc. (Waltham, MA, USA) provided XR-NTX in single use kits for intramuscular gluteal injection (380mg) at the beginning of weeks 1 and 5. The combination sublingual tablet formulation of 4:1 buprenorphine:naloxone (BUP) was used in two doses: 2mg:0.5mg buprenorphine:naloxone, and 8mg:2mg buprenorphine:naloxone. Placebo (PLB) was prepared to look identical to BUP. Both BUP and PLB were provided by Reckitt Benckiser Pharmaceuticals (Hull, England).
BUP kits were assigned to participants such that each received 2 large (8mg BUP or placebo) and 2 small (2mg BUP or placebo) tablets daily: BUP4 received 2 active small and 2 placebo large tablets; BUP16 received 2 placebo small and 2 active large tablets; and PLB received 2 placebo small and 2 placebo large tablets. Induction onto sublingual medication occurred over the first two days and tapering off medication occurred over the last two days of the medication phase, using half the full medication dose (16mg group received 8mg/daily; 4mg group received 2mg/day). Sublingual medication dosage could be halved one time and maintained for the duration of the study to alleviate study drug-related adverse effects.
Procedures
Interested individuals were pre-screened and scheduled for an informed consent interview if found preliminarily eligible. Screening was completed within 30 days and included a naloxone challenge (0.8mg naloxone by injection) and tolerance of 50mg oral naltrexone to ensure opioid free status prior to XR-NTX injection. The challenge was conducted only after self-report of opioid abstinence for at least 7 days and provision of an opioid-negative UDS.
Random assignment was on a 1:1:1 ratio, stratified by site and opioid use level, conducted in a centralized process through the CTN Data and Statistics Center. High opioid use was operationally defined as: a) ever injected an opioid; or b) ≥2 years of regular opioid use; or c) opioid use on ≥20 days in the prior month. Low opioid use was defined as not meeting high opioid use criteria. Assignments were blinded to site staff and participants.
After XR-NTX injection and randomization, participants were provided with their first dose of sublingual medication. All in-clinic dosing was observed whereby study medical staff dispensed medication, observed dosing, and checked participants’ mouths to be sure the medication was completely dissolved. Participants were scheduled to attend clinic thrice weekly for 8 weeks. Follow-up assessments were conducted at 1 and 3 months post-intervention.
Weekly CBT during the 8-week treatment phase used a CBT treatment manual developed for this study. Individual therapy sessions were approximately 45 minutes and used a worksheet presenting a concept or brief exercise explaining one or more CBT principles.
Measures
Screening assessments included measures to confirm safety, eligibility, and detoxification status. Baseline assessments were completed during screening and included drug use, health information, dependence diagnoses, psychological status, and craving. Assessments during the medication phase were completed at each clinic visit to assess drug use, safety, and other related variables. The Addiction Severity Index (ASI;20) collected information on demographics, drug/alcohol use, medical/psychiatric health status, employment/support status, legal status, and family and social relationships. The Visual Analog Scale (VAS), a self-administered craving scale, required the participant to mark a line to correspond to his/her level of craving for cocaine or opioids. The Time-Line Follow Back (TLFB) (21) used a calendar to prompt participants for retrospective estimates of daily drug use over a specified period of time. Treatment-emergent adverse events (AEs) were defined as occurring on/after the day of the first XR-NTX injection. Dosing adherence was defined as percentage of number of expected pills taken, which also reflects a possible one-time clinical reduction in dose.
Qualitative UDS were conducted at every clinic visit with QuickTox® Drug Screen Dipcards, a CLIA-waived FDA-cleared device, to test for cocaine, amphetamines, methamphetamine, opiates, oxycodone, barbiturates, benzodiazepines, marijuana, methadone, and methylenedioxymethamphetamine (MDMA, Ecstasy). A commercially available adulterant test strip was used as an additional validity check. Prior to the naloxone challenge, a more sensitive opiate test with a 300ng/mL cut-off was used. On-site testing of buprenorphine was not conducted after randomization in order to maintain the study blind. Study logs documented dosing, CBT attendance, and the termination of medication and study participation. An ancillary study collected an additional blood sample for genetic analyses.
Sample Size
Sample size was computed from a simulation study that computed parameters from cocaine treatment literature, and use a beta-binomial model assuming a mean of 9.31 days (standard deviation 9.08) of cocaine use in the evaluation period for the placebo arm and various rates of underreporting use (0%, 20% and 40%). It was assumed that 5% of participants would be lost to follow-up before the evaluation period, and 18% of those remaining would drop out during the evaluation period. Based on these specifications, 300 participants randomized 1:1:1 would yield over 85% power to detect a difference of 3.16 days if underreporting occurred ≤20% of the time, and 78% if the rate was 40%.
Outcome Measures and Analyses
Primary outcome
The primary outcome measure was UDS-corrected number of self-reported cocaine use days during the evaluation period (last 4 weeks of treatment phase, days 25–54) comparing BUP groups to PLB group. An algorithm (ELCON;22) corrected for discordant results between negative self-reports of cocaine use (TLFB) and positive UDS. When a window was found in which self-report was negative but UDS was positive, a correction was made to change last self-report day in the window from negative to positive. Positive self-reports discordant with negative UDS were left unchanged. Analyses compared outcomes of each BUP condition to the placebo condition using a significance level of 0.025 to adjust for the two comparisons. A Wilcoxon rank-sum test (23) was utilized because it was anticipated that the distribution of the number of days of use would be highly non-normal. The null hypothesis for each test was the BUP would be associated with less or the same amount of cocaine use necessitating one-sided tests. SAS and STATA were used for all analyses.
Secondary outcomes
The safety of the combination of buprenorphine+naloxone and extended-release naltrexone was evaluated by considering the number and proportion of participants in each condition experiencing at least one adverse event (AE). Cocaine use (via UDS) at the two follow-up visits was assessed via logistic regression adjusting for the baseline cocaine UDS result. Additional efficacy analyses included abstinence analysis based solely on urine testing and evaluation of abstinence in participants with a high level of use at baseline. The likelihood of having a cocaine-negative UDS during the evaluation period was analyzed longitudinally with generalized linear mixed models (GLMM;24–25) adjusting for baseline cocaine UDS results. All testing was one-sided.
Other analyses of urine testing involved a logistic regression of the odds of all UDS during the evaluation period being cocaine negative. A similar analysis was also performed with respect to have ≥75% of one’s UDS being cocaine-negative. Although most participants provided 12 UDS during the evaluation period (3 per week for 4 weeks), up to 15 UDS could be collected during this period due to scheduling of clinic visits. Lastly, participants with a high level of cocaine use at baseline were examined, where high use was defined as 20 or more days of use (reflecting 1/3 of use days in 30) in the 30 prior to completion of the baseline ASI assessment. Of interest was whether the change in the number of days of cocaine use by week 8, collected via the ASI, was associated with treatment arm. An ANOVA model was utilized due to normality of this outcome measure.
Retention measures including early terminations, missed visits, and visits attended were compared across groups, with time to dropout analyzed using Cox Proportional Hazards survival analysis (26). Dosing adherence is a measure of the number of 2mg and 8mg pills taken divided by the number of 2mg and 8mg pills expected to be taken. The mean number of 4mg doses and 16mg doses taken, and the proportion of doses taken over doses expected to be taken during the medication phase (accounting for dose reductions) are considered. Also analyzed are the number of expected and attended CBT sessions, the percentage of sessions attended, and the mean number of sessions attended per participant by treatment arm. An additional measure for assessing buprenorphine dosing adherence used blood samples collected at weeks 5 and 8. Buprenorphine and norbuprenorphine serum levels were deemed detectable if the serum level was ≥ 0.0500 ng/mL (lower limit of quantitation). For analysis, only participants with detectable blood levels who were on study medication and provided blood samples within 7 days of the end of medication were considered
The last pre-specified secondary outcome was an analysis of opioid use and craving to evaluate whether XR-NTX served to mitigate the development of physiologic dependence on buprenorphine. Opioid use was measured via UDS and self-report (via ASI) during the evaluation period and at follow-up. The self-reported opioid use outcome and the VAS craving score outcome measures were compared at baseline, week 8 and at follow-up.
Missing data
For the primary outcome, missing UDS were imputed as positive but missing TLFB in participants who reach the evaluation period (n=2) was pro-rated to the full 30-day interval. For the UDS analysis using generalized linear mixed model, missing urine results were not imputed as cocaine-positive since the model can get estimates that account for the uncertainty of the missing data by widening the confidence interval (26). Only participants with measurements at the appropriate visits were considered for the ASI analysis, post-intervention abstinence as well as the two opioid outcomes.
Results
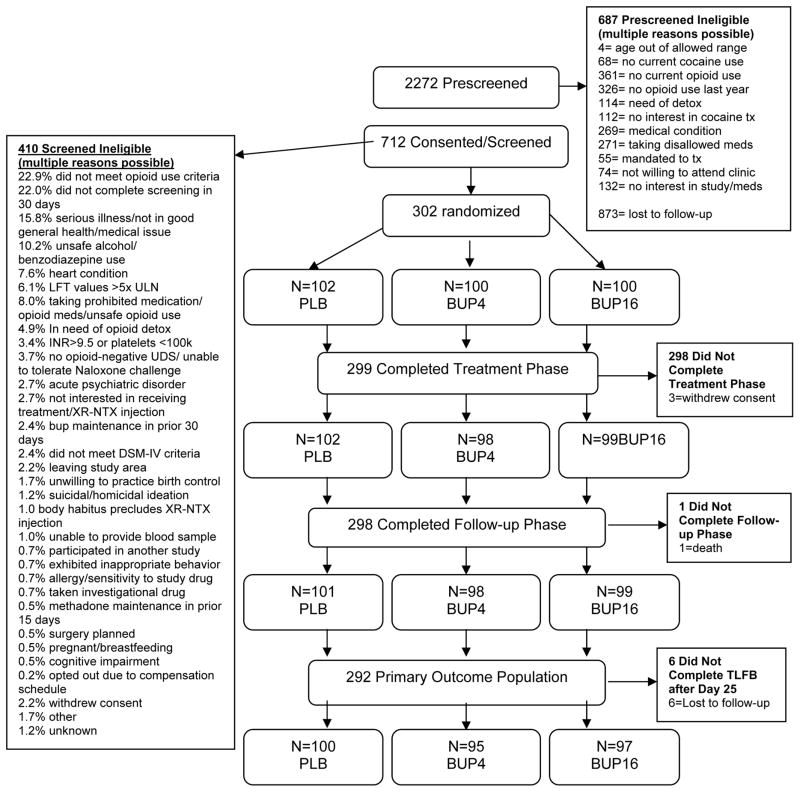
As seen in Figure 1, a total of 712 individuals consented to participate in the study and 302 were randomized to study condition (BUP4=100, BUP16=100, PLB=102). Table 1 describes participant baseline characteristics.
Figure 1.
CONSORT Diagram.
Table 1.
Baseline Participant Characteristics by Treatment Arm*
| PLB (N=102) | BUP4 (N=100) | BUP16 (N=100) | |
|---|---|---|---|
| Demographic Characteristics | |||
| Gender; Male, % (n) | 77.5% (79) | 80.0% (80) | 78.0% (78) |
| Mean Age (SD) | 46.5 (7.78) | 46.8 (8.00) | 45.8 (10.08) |
| Race/Ethnicity, % (n) | |||
| American Indian or Alaska Native | 2.0% (2) | 2.0% (2) | 0% |
| Asian | 0% | 1.0% (1) | 0% |
| African American | 63.7% (65) | 58.0% (58) | 68.0% (68) |
| White | 26.5% (27) | 26.0% (26) | 25.0% (25) |
| Other | 2.0% (2) | 8.0% (8) | 2.0% (2) |
| Multiracial | 5.9% (6) | 5.0% (5) | 5.0% (5) |
| Hispanic | 10.8% (11) | 12.0% (12) | 8.0% (8) |
| Mean Years of Education (SD) | 13.1 (1.77) | 13.2 (1.84) | 12.8 (1.97) |
| % Employed (full or part time) | 42.2% (43) | 44.0% (44) | 49.0% (49) |
| Mean arrests (SD) | 8.8 (14.9) | 6.4 (10.6) | 7.7 (11.7) |
| Drug Use Characteristics | |||
| Mean Days of Cocaine Use in Last 30 Days (SD) | 10.8 (9.5) | 9.1 (8.4) | 9.2 (8.2) |
| Mean Lifetime Years of Cocaine Use (SD) | 18.1 (9.5) | 18.7 (10.0) | 17.7 (9.8) |
| % Cocaine-positive UDS | 57.8% | 51.0% | 64.0% |
| % DSM-IV Opioid Dependence Diagnosis | 72.5% | 64.0% | 70.0% |
| % DSM-IV Opioid Abuse Diagnosis | 27.5% | 22.0% | 23.0% |
| % Opioid Dependence in Full or Partial Remission | 0% | 14.0% | 7.0% |
| % High Opioid Use** | 63.7% | 66.0% | 74.0% |
| % DSM-IV Alcohol Dependence/Abuse Diagnosis | 69.6% | 82.0% | 74.0% |
| % DSM-IV Cannabis Dependence/Abuse Diagnosis | 47.1% | 49.0% | 40.0% |
| IV drug use | 35.3% | 35.0% | 28.0% |
No significant differences by treatment arm for any variable.
High opioid users were determined at baseline using the following criteria: a) ever injected an opioid; b) ≥2 years of regular opioid use; or c) opioid use on ≥20 days in the prior month. Participants who did not meet any of these criteria were considered Low opioid users. These were operationally defined and not clinically-derived definitions.
Cocaine Use
Cocaine use results are shown in Table 2. The results of primary outcome analyses showed no differences in cocaine use during the evaluation period from PLB for BUP4 (p=0.262) or BUP16 (p=0.185). Ten participants dropped out before initiating the evaluation period, yielding an effective sample size for the primary outcome of 292 (PLB=100, BUP4=95, BUP16=97). Analysis of concordance between cocaine use measures (negative self-report and positive UDS) shows that 32% of positive UDS correspond to negative self-report; when missing UDS are imputed as positive, the rate increases to 42%. Rates of missing UDS data were 16.1% for PLB, 16.1% for BUP4, and 16.4% for BUP16.
Table 2.
Cocaine Use during Evaluation Period (Days 25–54) and Follow-Up
| PLB | BUP4 | BUP4 vs. PLB | BUP16 | BUP16 vs. PLB | |
|---|---|---|---|---|---|
| PRIMARY OUTCOME | |||||
| Number of days of cocaine use during the evaluation period | |||||
| N | 102 | 100 | 100 | ||
| Mean (SD) | 8.2 (6.84) | 7.8 (7.23) | 7.9 (7.72) | ||
| P-value (one-sided) | - | 0.262 | 0.185 | ||
| Rank-biserial correlation | −0.09 | −0.08 | |||
| SECONDARY OUTCOMES | |||||
| Longitudinal analysis of UDS-based abstinence during the evaluation period | |||||
| N | 100 | 95 | 97 | ||
| Proportion UDS abstinent | 45.8% | 50.4% | 50.9% | ||
| Odds Ratio (95% one-sided CI) | 1.09 (0.75, ∞) | 1.71 (1.19, ∞) | |||
| P-value (one-sided) | 0.105 | 0.022 | |||
| UDS 100% abstinent during evaluation period | |||||
| N | 100 | 95 | 97 | ||
| Proportion 100% abstinent | 16.0% | 17.9% | 18.6% | ||
| Odds Ratio (95% one-sided CI) | 1.14 (0.61, ∞) | 1.19 (0.64, ∞) | |||
| P-value (one-sided) | 0.362 | 0.318 | |||
| UDS ≥75% abstinent during evaluation period | |||||
| N | 100 | 95 | 97 | ||
| Proportion ≥75% abstinent | 28.0% | 33.7% | 41.2% | ||
| Odds Ratio (95% one-sided CI) | 1.31 (0.78, ∞) | 1.80 (1.10, ∞) | |||
| P-value (one-sided) | 0.410 | 0.008 | |||
| UDS-based abstinence at follow-up visits | |||||
| One month follow-up | |||||
| N | 97 | 91 | 93 | ||
| Proportion abstinent | 48.5% | 49.5% | 45.2% | ||
| Odds Ratio (95% one-sided CI) | 1.04 (0.64, ∞) | 0.88 (0.54, ∞) | |||
| P-value (one-sided) | 0.446 | 0.325 | |||
| Three month follow-up | |||||
| N | 93 | 86 | 88 | ||
| Proportion abstinent | 50.5% | 52.3% | 54.6% | ||
| Odds Ratio (95% one-sided CI) | 1.07 (0.66, ∞) | 1.17 (0.72, ∞) | |||
| P-value (one-sided) | 0.406 | 0.295 | |||
| Higher use cocaine subsample analysis of the number of days of cocaine use from ASI | |||||
| N | 24 | 12 | 14 | ||
| Mean (SD) change (baseline - week 8) | 7.7 (8.1) | 18.8 (7.7) | 13.2 (10.0) | ||
| Proportion of variance due to BUP | 45.9% | 9.7% | |||
| P-value (one-sided) | <0.001 | 0.035 | |||
Longitudinal analysis of UDS during the evaluation period found a statistically significant difference between BUP16 and PLB (p=0.022) but no difference for BUP4 (p=0.105). Cocaine use at the follow-up time points did not differ across groups.
There was no significant difference in the proportion of participants achieving complete cocaine abstinence between PLB and BUP4 (p=0.362) or BUP16 (p=0.318). Comparing the number of participants with ≧75% cocaine-negative UDS during the evaluation period, a significantly higher percentage of the BUP16 group met this threshold after adjusting for baseline cocaine UDS than the PLB group (p=0.008). No significant difference was found between PLB and BUP4 (p=0.41).
The higher cocaine use groups provided with buprenorphine showed a significantly greater reduction in self-reported cocaine use days from baseline to week 8.
Dosing Adherence, Behavioral Treatment Compliance, and Retention
Table 3 summarizes dosing adherence, CBT compliance, and visit attendance (retention). The table indicates that approximately 87% of the participants received the second XR-NTX injection. Approximately 80% of participants at week 5 and 70% of participants at week 8 who were assigned to a BUP arm had a detectable buprenorphine blood level. No statistically significant differences were found for any of these variables by treatment arm.
Table 3.
Medication Adherence, Detectable Buprenorphine Blood Level, Behavioral Treatment Compliance, and Retention by Treatment Arm*
| PLB (N=102) | BUP4 (N=100) | BUP16 (N=100) | |
|---|---|---|---|
| Medication Adherence** | |||
| Mean Number of 2mg pills reported taken (SD) | 87.5 (31.81) | 83.5 (34.04) | 82.3 (32.58) |
| Mean Number of 8mg pills reported taken (SD) | 87.5 (31.81) | 83.5 (34.04) | 82.3 (32.58) |
| Mean Proportion Sublingual Medication Adherence (SD) | 91.3 (13.07) | 91.9 (11.17) | 87.4 (16.43) |
| Percentage Received XR-NTX Injection #2 | 89 (87.3%) | 86 (86.0%) | 88 (88.0%) |
| Detectable Buprenorphine Blood Level | |||
| Week 5 Buprenorphine Blood Level | 1/86 (1.2%) | 61/82 (74.4%) | 63/85 (74.1%) |
| Week 5 Norbuprenorphine Blood Level | 2/86 (2.3%) | 67/82 (81.7%) | 66/85 (77.7%) |
| Week 5 Buprenorphine/Norbuprenorphine Blood Level | 2/86 (2.3%) | 68/82 (82.9%) | 67/85 (78.8%) |
| Week 8 Buprenorphine Blood Level | 0/74 (0.0%) | 34/67 (50.8%) | 44/66 (66.7%) |
| Week 8 Norbuprenorphine Blood Level | 1/74 (1.3%) | 43/67 (64.2%) | 45/66 (68.2%) |
| Week 8 Buprenorphine/Norbuprenorphine Blood Level | 1/74 (1.3%) | 46/67 (68.7%) | 47/66 (71.2%) |
| Behavioral Treatment Compliance | |||
| Number of CBT Sessions Attended/Expected | 672/816 | 655/800 | 653/800 |
| Percent Expected CBT Sessions Attended | 82.4% | 81.9% | 81.6% |
| Mean CBT*** Sessions Attended per Participant (SD) | 6.6 (2.03) | 6.6 (2.17) | 6.5 (2.14) |
| Retention | |||
| Participants who provided self-report data during evaluation period (Weeks 5–8) | 100 (98%) | 95 (95%) | 97 (97%) |
| Mean Days to Last Dose (SD) | 48.9 (16.0) | 47.2 (17.7) | 48.4 (16.1) |
| Early Medication Terminations | 15.7% | 21.0% | 17.0% |
| Mean # Missed Visits per Participant | 3.2 | 3.5 | 3.7 |
| Attendance Weeks 1–4 | 89.6% | 87.4% | 86.9% |
| Attendance Weeks 5–8 | 84.9% | 81.8% | 82.0% |
| Attendance Month 1 follow-up visit | 95.1% | 91.0% | 93.0% |
| Attendance Month 3 follow-up visit | 91.2% | 87.0% | 91.0% |
No statistically significant difference for any variable presented in Table 3;
no significant difference in buprenorphine blood level between the two groups who received buprenorphine.
CBT=Cognitive Behavioral Therapy
Presented in Table 3 are medication terminations, missed visits, and visits attended for the first and second halves of the study and for each follow-up visit by condition. No statistically significant difference in any measure of retention was found across treatment arms.
Adverse Events/Serious Adverse Events (SAEs)
Table 4 provides information about AEs/SAEs by medication condition. Although 63.2% of participants reported at least one AE over the study duration, 33.3% of AEs were not study-related. None of the 37 SAEs were deemed by the Medical Monitor to be study-related. There were no significant differences in numbers of AEs/SAEs by treatment arm. There were few withdrawal- or overdose-related AEs. Additionally, there were no significant AEs related to induction onto XR-NTX.
Table 4.
Treatment-Emergent Adverse Events and Serious Adverse Events by Treatment Arm
| PLB (N=102) | BUP4 (N=100) | BUP16 (N=100) | |
|---|---|---|---|
| ADVERSE EVENTS (AEs) | |||
| % (n) Participants with treatment-emergent AEs | 59.8% (61) | 66.0% (66) | 64.0% (64) |
| Number of treatment-emergent AEs | 145 | 192 | 209 |
| Severity of treatment-emergent AEs, % (n) | |||
| Grade 1 – Mild | 55.2% (80) | 53.6% (103) | 61.2% (128) |
| Grade 2 – Moderate | 32.4% (47) | 41.7% (80) | 34.9% (73) |
| Grade 3 – Severe | 12.4% (18) | 4.7% (9) | 3.8% (8) |
| Relationship of treatment-emergent AEs, % (n) | |||
| Not related | 39.3% (57) | 32.8% (63) | 29.7% (62) |
| Causal relationship to naltrexone only | 21.4% (31) | 23.4% (45) | 20.1% (42) |
| Causal relationship to buprenorphine/placebo only | 21.4% (31) | 21.9% (42) | 34.4% (72) |
| Causal relationship to buprenorphine/placebo and naltrexone | 17.9% (26) | 21.9% (42) | 15.8% (33) |
| Types of AEs deemed possibly/definitely-related to buprenorphine (n) | 57 | 84 | 105 |
| Abdominal cramps, upset stomach | 2 | 1 | 1 |
| Acid reflux | 1 | 0 | 0 |
| Anxiety, subjective anxiety | 0 | 0 | 4 |
| Brief psychotic episode | 0 | 1 | 0 |
| Change in smell, taste, bitter metallic taste | 0 | 1 | 2 |
| Sweats, cold sweats, night sweats | 1 | 2 | 4 |
| Constipation, hard stools | 9 | 9 | 12 |
| Decreased/loss of appetite | 1 | 3 | 2 |
| Decreased libido, sex drive | 2 | 1 | 0 |
| Depressed mood | 1 | 0 | 0 |
| Diarrhea | 2 | 0 | 4 |
| Disoriented, mental cloudiness | 0 | 0 | 2 |
| Dizziness, lightheaded | 5 | 9 | 10 |
| Drowsiness, sedation, sleepiness | 1 | 3 | 6 |
| Opiate/drug withdrawal symptoms | 2 | 2 | 1 |
| Dry mouth | 3 | 0 | 3 |
| Elevated liver function | 0 | 1 | 0 |
| Fatigue | 2 | 4 | 3 |
| Feeling high, euphoria | 0 | 1 | 1 |
| Gagging on medication | 0 | 0 | 1 |
| General body soreness, musculoskeletal pain/cramps | 2 | 1 | 0 |
| Headache | 1 | 1 | 3 |
| Hematemesis | 0 | 0 | 1 |
| Hot flashes | 0 | 1 | 1 |
| Increased opiate craving | 1 | 0 | 0 |
| Insomnia, interrupted sleep | 2 | 3 | 1 |
| Irritable mood | 0 | 2 | 0 |
| Itching | 0 | 0 | 3 |
| Leg restlessness | 0 | 1 | 0 |
| Loss of consciousness | 0 | 0 | 1 |
| Nausea | 13 | 24 | 23 |
| Nervousness, restlessness | 0 | 1 | 2 |
| Runny nose | 0 | 1 | 0 |
| Sensitive teeth | 1 | 0 | 0 |
| Sluggishness | 0 | 0 | 2 |
| Tearing | 0 | 1 | 0 |
| Tinnitus | 0 | 0 | 1 |
| Urinary retention | 0 | 0 | 1 |
| Vomiting | 5 | 10 | 10 |
| SERIOUS ADVERSE EVENTS (SAEs) | |||
| % (n) Participants with treatment-emergent SAEs | 11.8% (12) | 13.0% (13) | 8.0% (8) |
| Number of treatment-emergent SAEs | 14 | 14 | 9 |
| Relationship of treatment-emergent SAEs, % (n) | |||
| Not related as assessed by Medical Monitor | 100% (14) | 100% (14) | 100% (9) |
| Type of SAEs (n) | |||
| Death | 1 | 0 | 0 |
| Inpatient admission to hospital | 13 | 14 | 9 |
Opioid Use and Craving
The results of analyses of opioid use and craving are summarized in Table 5. Self-reported opioid use was significantly reduced for all groups from baseline to all time-points but there was no difference across treatment arms based on UDS. A greater percentage of the BUP16 group reported opioid use at the 1-month follow-up compared to the PLB group (p=0.046). Opioid craving was reduced for all groups from baseline to all time points. At the 1-month follow-up BUP16 participants reported greater craving for opioids than PLB group, and at the 3-month follow-up, both BUP arms reported greater craving than PLB (p=0.014, p=0.001, respectively).
Table 5.
UDS, Self-Reported Opioid Use and Craving of Randomized Participants by Treatment Arm
| PLB | BUP 4 | p-value PLB vs BUP4 | BUP 16 | p-value PLB vs BUP16 | |
|---|---|---|---|---|---|
| % Opioid-Negative UDS | |||||
| Baseline* | 100% | 100% | 100% | ||
| Evaluation Period | 99.5% | 99.0% | 0.161 | 98.2% | 0.110 |
| 1-Month Follow-up | 95.9% | 98.9% | 0.198 | 93.5% | 0.473 |
| 3-Month Follow-up | 95.7% | 95.4% | 0.910 | 95.4% | 0.936 |
| Mean Days of Self-Reported Opioid Use in Past 30 (SD)** | |||||
| Baseline | 2.0 (4.2) | 1.7 (3.9) | 0.295 | 2.2 (3.9) | 0.295 |
| Evaluation Period | 0.45 (3.09) | 0.09 (0.32) | 0.141 | 0.18 (0.92) | 0.826 |
| 1-Month Follow-up | 0.26 (1.59) | 0.19 (1.02) | 0.380 | 0.97 (3.81) | 0.046 |
| 3-Month Follow-up | 0.61 (3.08) | 0.59 (3.49) | 0.488 | 0.82 (3.81) | 0.339 |
| Mean VAS Opioid Craving Score (SD)*** | |||||
| Baseline | 22.2 (23.9) | 23.6 (22.9) | 0.334 | 25.4 (28.1) | 0.192 |
| Evaluation Period | 6.8 (14.1) | 7.3 (16.9) | 0.331 | 8.1 (17.8) | 0.161 |
| 1-Month Follow-up | 6.3 (14.1) | 5.5 (13.7) | 0.338 | 11.3 (21.9) | 0.032 |
| 3-Month Follow-up | 3.5 (8.1) | 7.9 (17.2) | 0.014 | 10.7 (20.7) | 0.001 |
Study design required an opioid-negative UDS at baseline to proceed to randomization
Significant reductions (p<0.001) in self-reported opioid use in past 30 days (ASI) from baseline to Week 8, baseline to 1-Month follow-up, and baseline to 3-Month follow-up for all treatment conditions.
Significant reductions (p<0.001) in opioid craving from baseline to all time points for all treatment conditions.
Discussion
No treatment differences were found between the buprenorphine and placebo groups during the evaluation period (days 25–54) when self-reported cocaine use and UDS were combined in the primary outcome analysis. Analyses of UDS-based abstinence, however, showed a significant reduction in cocaine use between PLB and BUP16. The difference in these findings may be due to discordance between negative self-report and cocaine-positive UDS data. Post-hoc analyses of higher cocaine use participants (≥20 days of cocaine use in the 30 days before baseline) also found significant differences in self-reported cocaine use between the PLB and both BUP4 and BUP16 groups. These results are consistent with findings from prior clinical trials evaluating the effects of buprenorphine+naltrexone on cocaine use and deserve further replication and confirmation. Whereas Gerra (9) demonstrated reductions in cocaine use in individuals with opioid dependence treated with the buprenorphine/naltrexone combination, the present study examined primarily a cocaine-dependent population with varying degrees of opioid use.
Of interest are findings regarding medication adherence. About 87% of participants received the second XR-NTX injection, and self-reported sublingual medication adherence was about 90% across all treatment arms. About 80% of BUP4 and BUP16 participants who were still on medication and provided a blood test at week 5 had a detectable buprenorphine/norbuprenorphine blood plasma level; at week 8 the rate was about 70%. A rate less than 100% calls into question whether participants actually took sublingual study drug as reported. It is possible that diversion of buprenorphine diversion occurred, but findings may reflect the inclusion of participants who did not take study medication. Future analyses will investigate outcomes for participants with and without detectable buprenorphine blood levels.
These findings support the overall safety of the medication combination. No significant differences in AEs were found between conditions, and rates of SAEs were low (10.9% total) with none related to study medication, indicating that buprenorphine in combination with naltrexone is generally safe and well-tolerated. The process of induction onto naltrexone, including the naloxone challenge used to confirm an opioid-negative status, was also well tolerated without significant incidence of precipitated withdrawal or AEs (27). Importantly, there were no cases of opioid overdose during the trial, suggesting that any attempts to override mu opioid receptor blockade by naltrexone were unsuccessful and that participants did not use excess opioids upon removal of mu receptor blockade after medication dosing. Self-reported adherence to dosing and clinic attendance were higher than 80%, suggesting general acceptability and feasibility of administering this combination.
There was no evidence of escalation of opioid use or craving during the medication phase, suggesting that naltrexone effectively blocked the mu opioid effects of buprenorphine when used in this study, which approximated real-world treatment settings. Although craving decreased from baseline through all time points for all groups, the BUP16 group reported a greater craving score at the 1- and 3-month follow-ups compared to PLB, and BUP4 reported greater craving than PLB at the 3-month follow-up. It is unclear why the BUP groups reported greater craving for opioids at the follow-up time points than the placebo group, given that craving scores in both BUP groups were significantly lower at follow-up than at baseline, and scores at the follow-up time points were not significantly higher than scores obtained at the end of the active medication period. Similarly, though a greater proportion of the BUP16 group reported opioid use at 1-month follow-up compared to the PLB group, self-reported use was significantly lower at all time points compared to baseline across groups, and no difference in opioid UDS was found at any time point. Overall, findings suggest that administration of BUP in the presence of XR-NTX was not associated with escalation of opioid use or craving during either active medication or follow-up periods. Recently published results of effects of buprenorphine and naltrexone on cocaine use in rodents demonstrate reductions in cocaine use with minimum risk of producing opioid dependence (13). These animal data provide additional evidence that concurrent administration of these medications may thwart opioid dependence.
Strengths of this study include retention and medication adherence with incorporation of observed medication dosing to ensure adequate exposure to study medication. Also, participation of 11 study sites broadens generalizability of findings. Because this study took place in multiple community treatment programs across the country, results demonstrate feasibility of implementing and treating patients with medication combinations not previously adopted in such settings. Nevertheless, there are limitations to what can be learned from this first trial examining combination of buprenorphine in presence of long-acting naltrexone.
A notable limitation is the inability to generalize results to opioid-naïve cocaine users given the population recruited, in which a past or current opioid use disorder diagnosis was required. Given that findings showed no study-induced escalation of opioid use during the medication phase, a future study could include participants with no opioid use disorder history; however, future studies should continue to monitor closely for evidence of iatrogenic initiation, exacerbation, or reinstatement of opioid use disorder. This study also did not control for the potential independent effect of naltrexone on outcomes (as all participants received naltrexone); future studies could evaluate the differential effects of naltrexone on cocaine use, administered alone or in combination with buprenorphine. Finally, analyses presented in this paper have not adjusted for multiple tests.
Conclusion
Although the primary outcome analysis did not detect significant differences in cocaine use between treatment groups, some UDS analyses found that participants randomized to higher dose (16 mg/day) of buprenorphine provided significantly more cocaine-negative urine samples, compared to participants randomized to placebo. Furthermore, the medication combination used in this study appeared to be safe with little risk of inducing iatrogenic opioid dependence. The combination of naltrexone and buprenorphine deserves further confirmatory study as pharmacotherapy for cocaine use disorder.
Acknowledgments
Support provided through the National Institute on Drug Abuse (DA13045; DA13035; DA13046, DA015815, HHSN271200900034C/N01DA-9-2217, HHSN271201200017C/N01DA-12-2229, HHSN271201400028C/N01DA-14-2237); Reckitt Benckiser Pharmaceuticals, Alkermes Pharmaceuticals.
We are grateful for the dedication of staff at each participating Community-based Treatment Program (CTP) and Regional Research and Training Center (RRTC): Albert Einstein College of Medicine (Bronx, NY) and Bellevue Hospital Center (New York, NY) in the Greater New York Node; Atlanta VA Medical Center (Atlanta, GA) in the Southern Consortium Node; Addiction Research & Treatment Services (Denver, CO) and CODA Inc. (Portland, OR) in the Western States Node; Howard University (Washington, DC) in the Mid-Atlantic Node; Maryhaven (Columbus, OH) in the Ohio Valley Node; Recovery Centers of King County (Seattle, WA) in the Pacific Northwest Node; South Texas Veterans Health Care System (San Antonio, TX) in the Texas Node; and BAART Programs (San Francisco, CA) and UCLA Integrated Substance Abuse Programs (Los Angeles, CA) in the Pacific Region Node. We would also like to thank our partners at the Center for the Clinical Trials Network at the National Institute on Drug Abuse, the Clinical Coordinating Center and Data and the Statistics Center at the Emmes Corporation, Reckitt-Benckiser Pharmaceuticals, and Alkermes Pharmaceuticals.
Footnotes
Authors’ Ethical Statement and Declaration of Interest
Walter Ling has served as a consultant for Reckitt Benckiser Pharmaceuticals. Andrew Saxon has served as a speaker for Reckitt Benckiser Pharmaceuticals, and as a member of a scientific advisory board for Alkermes, Inc. John Rotrosen is Lead Investigator of a NIDA CTN study (CTN-0051) for which Reckitt-Benckiser has donated Suboxone. No other financial or other possible conflicts of interest exist for authors. Alkermes Inc. (Waltham, MA, USA) provided XR-NTX. Both BUP and PLB were provided by Reckitt Benckiser Pharmaceuticals (Hull, England).
Clinical Trials Registration and Hypothesis Registration: NCT01402492
References
- 1.Brown EE, Finlay JM, Wong JT, Damsma G, Fibiger HC. Behavioral and neurochemical interactions between cocaine and buprenorphine: Implications for the pharmacotherapy of cocaine abuse. J Pharmacol Exp Ther. 1991;256:119–25. [PubMed] [Google Scholar]
- 2.Kamien JHB, Spealman RD. Modulation of the discriminative-stimulus effects of cocaine by buprenorphine. Behav Pharmacol. 1991;2:517–20. [PubMed] [Google Scholar]
- 3.Mello N, Lukas S, Mendelson J, Drieze J. Naltrexone–buprenorphine interactions: Effects on cocaine self-administration. Neuropsychopharmacology. 1993;9:211–24. doi: 10.1038/npp.1993.57. [DOI] [PubMed] [Google Scholar]
- 4.Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine suppresses cocaine self-administration by rhesus monkeys. Science. 1989;245:859–62. doi: 10.1126/science.2772637. [DOI] [PubMed] [Google Scholar]
- 5.Misra AL, Pontani RB, Vadlamani NL. Blockade of tolerance to morphine analgesia by cocaine. Pain. 1989;38:77–84. doi: 10.1016/0304-3959(89)90076-6. [DOI] [PubMed] [Google Scholar]
- 6.Spealman RD, Bergman J. Modulation of the discriminative stimulus effects of cocaine by mu and kappa opioids. J Pharmacol Exp Ther. 1992;261:607–15. [PubMed] [Google Scholar]
- 7.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine versus methadone maintenance for concurrent opiate dependence and cocaine abuse. Arch Gen Psychiatry. 1994;54:713–20. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 8.Amass L, Bickel WK, Higgins ST, Badger GJ. Alternate-day dosing during buprenorphine treatment of opioid dependence. Life Sci. 1994;54:1215–28. doi: 10.1016/0024-3205(94)00848-5. [DOI] [PubMed] [Google Scholar]
- 9.Gerra G, Fantoma A, Zaimovic A. Naltrexone and buprenorphine combination in the treatment of opioid dependence. J Psychopharmacol. 2006;20:806–14. doi: 10.1177/0269881106060835. [DOI] [PubMed] [Google Scholar]
- 10.Ling W, Amass L, Shoptaw S, et al. Buprenorphine Study Protocol Group. A multi-center randomized trial of buprenorphine-naloxone versus clonidine for opioid detoxification: Findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100:1090–100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schottenfeld RS, Chawarski MC, Pakes JR, Patalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry. 2005;162:340–9. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- 12.Woody GE, Poole S, Subramaniam G. Extended vs. short-term buprenorphine-naloxone for treatment of opioid addicted youth: A randomized trial. JAMA. 2008;300:2002–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee S, Vendruscolo LF, Misra KK, Schlosburg JE, Koob GF. A combination of buprenorphine and naltrexone blocks compulsive cocaine intake in rodents without producing dependence. Sci Transl Med. 2012;4:110. doi: 10.1126/scitranslmed.3003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya I, Gorelick DA, Preston KL, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmaco Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothman RB, Gorelick DA, Heishman SJ, et al. An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. J Substa Abuse Treat. 2000;18:277–81. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 16.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–92. [PubMed] [Google Scholar]
- 17.McCann DJ. Potential of buprenorphine/naltrexone in treating polydrug addiction and co-occurring psychiatric disorders. Clin Pharmaco Ther. 2008;83:627–630. doi: 10.1038/sj.clpt.6100503. [DOI] [PubMed] [Google Scholar]
- 18.Romero DV, Partilla JS, Zheng QX, et al. Opioid peptide receptor studies. Buprenorphine is a potent and selective m/k antagonist in the [35S]-GTP-g-S functional binding assay. Synapse. 1999;34:83–94. doi: 10.1002/(SICI)1098-2396(199911)34:2<83::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Mooney LJ, Nielsen S, Saxon A, Hillhouse M, Thomas C, Hasson A, Stablein D, McCormack J, Lindblad R, Ling W. Cocaine use reduction with buprenorphine (CURB): rationale, design, and methodology. Contemp Clin Trials. 2013;34:196–204. doi: 10.1016/j.cct.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 21.Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. The Human Press Inc; 1992. [Google Scholar]
- 22.Oden NL, VanVeldhuisen PC, Wakim PG, Trivedi MH, Somoza E, Lewis D. Power of automated algorithms for combining time-line follow-back and urine drug screening test results in stimulant-abuse clinical trials. Am J Drug Alcohol Abuse. 2011;37:350–7. doi: 10.3109/00952990.2011.601777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Elteren PH. On the combination of independent two-sample tests of Wilcoxon. Bulletin of the International Statistical Institute. 1960;37:351–61. [Google Scholar]
- 24.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. Journal of the American Statistical Association. 1993;88:9–25. [Google Scholar]
- 25.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2. John Wiley & Sons; 2011. [Google Scholar]
- 26.Cox DR. Regression models and life tables (with discussion) J R Stat Soc. 1972;B34:187–220. [Google Scholar]
- 27.Lindblad R, McCormack J, Saxon A, Hillhouse MP, Thomas C, Hasson A, Mooney L, VanVeldhuisen P, Ling W. Induction onto extended-release naltrexone in 302 cocaine-dependent opioid users. Presented at the 2013 meeting of the College on Problems of Drug Dependence; 2013; June, San Diego, California. [Google Scholar]