Abstract
One of the deleterious effects of acute nerve agent exposure is the induction of status epilepticus (SE). If SE is not controlled effectively, it causes extensive brain damage. Here, we review the neuropathology observed after nerve agent–induced SE, as well as the ensuing pathophysiological, neurological, and behavioral alterations, with an emphasis on their time course and longevity. Limbic structures are particularly vulnerable to damage by nerve agent exposure. The basolateral amygdala (BLA), which appears to be a key site for seizure initiation upon exposure, suffers severe neuronal loss; however, GABAergic BLA interneurons display a delayed death, perhaps providing a window of opportunity for rescuing intervention. The end result is a long-term reduction of GABAergic activity in the BLA, with a concomitant increase in spontaneous excitatory activity; such pathophysiological alterations are not observed in the CA1 hippocampal area, despite the extensive neuronal loss. Hyperexcitability in the BLA may be at least in part responsible for the development of recurrent seizures and increased anxiety, while hippocampal damage may underlie the long-term memory impairments. Effective control of SE after nerve agent exposure, such that brain damage is also minimized, is paramount for preventing lasting neurological and behavioral deficits.
Keywords: nerve agents, status epilepticus, seizures, basolateral amygdala, hippocampus, anxiety
Introduction
Nerve agents are organophosphorus compounds that inhibit acetylcholinesterase (AChE) via phosphorylation of a serine hydroxyl group at the esteratic site of the enzyme,1 resulting in the accumulation of acetylcholine in cholinergic synapses of the peripheral and the central nervous systems.2,3 One of the consequences of the excessive rise of acetylcholine in the brain is the induction of seizures, which rapidly progress to convulsive status epilepticus (SE). Unfortunately, surviving the exposure is unlikely to lead to recovery unless the nerve agent–induced SE is adequately controlled. This is because prolonged SE—of any etiology—can cause profound brain damage.4 The severity and brain localization of the neuropathological damage will determine to a great extent the severity and nature of the ensuing neurological and/or behavioral abnormalities.
In this review, we examine (1) which brain structures have been found to suffer the most severe neuropathology after nerve agent-induced SE, (2) what is known about differences in the susceptibility of principal neurons versus GABAergic interneurons to damage induced by nerve agent exposure, (3) pathophysiological alterations in affected brain regions, and (4) neurological and behavioral impairments. The time course and duration of these postexposure abnormalities are emphasized, so that it becomes clear whether they are transient or whether they persist for a long time, which would make the control of nerve agent–induced SE imperative not only in order to save the life of the individual, but also to prevent brain damage and its impact on quality of life.
Brain structures injured by nerve agent–induced SE
Assessment of brain damage by nerve agent–induced SE is performed primarily in animal models of exposure, using rodents in the majority of studies. The peripheral cholinergic crisis is typically treated with a muscarinic receptor antagonist and an oxime (which reactivates the inhibited AChE) administered before or soon after exposure to a nerve agent, and then the animal is allowed to experience SE for various durations, until SE is stopped by injection of an anticonvulsant or stops spontaneously. Soman has been the most commonly used nerve agent in these experiments, mainly because it is assumed that if an anticonvulsant treatment is effective against soman—an agent whose toxic effects are difficult to counteract—it should also be effective against other nerve agents. Although this is probably a reasonable assumption, there are not enough comparative data to confirm its validity. In general, there are differences between nerve agents in parameters such as LD50, dose of an anticonvulsant required to suppress seizures, or the degree to which they inhibit AChE in different brain regions and peripheral tissues,3 but there are no reports on qualitative differences in their toxic effects. In the studies reviewed below, when the findings are for exposure to one specific nerve agent, the type of agent is stated.
Brain pathology caused by nerve agent exposure is most commonly assessed by determining the extent of neurodegeneration in different brain regions, using methods such as Fluoro–Jade dyes, which selectively stain degenerating neurons,5,6 and/or by quantifying neuronal loss in brain regions of interest. The findings from these studies have suggested that the main cause of neuronal damage is the prolonged seizure activity, as degenerating neurons are found only in rats that develop seizures after exposure to soman,7–9 and neuropathology is dramatically reduced when seizures are adequately controlled,10–15 regardless of the type of nerve agent used.10 The mechanisms by which prolonged unremitting seizures can kill neurons involve excitotoxicity,16,17 oxidative stress,18,19 and inflammatory processes.20,21
The damage seen after nerve agent–induced SE is widespread and includes neocortical areas, the thalamus, and subcortical structures.7,22,23 Limbic structures, and particularly the amygdala and the hippocampus, but also the piriform and entorhinal cortices, suffer extensive damage.10–13,15,23,24 We have examined this in more detail in an attempt to delineate if there are certain regions of the amygdala and the hippocampus that are more susceptible than others to nerve agent–induced seizure damage and if, overall, the amygdala and the hippocampus are similarly susceptible. We found that the ventral hippocampus had significantly more neurodegeneration than the dorsal hippocampus, 1 day after soman-induced SE in rats.25 By 7 days postexposure, the ventral hippocampus still had more degenerating neurons, but the difference from the dorsal area was not statistically significant, probably due to the loss of many degenerating neurons during the 7-day period. The damage was present in the CA1, CA3, and hilar hippocampal subfields. We also found more severe neurodegeneration in the posteroventral regions of the lateral, basolateral, and medial amygdala nuclei compared to the anterodorsal regions of these nuclei. Overall, the extent of neurodegeneration in the whole amygdala was greater when compared with the whole hippocampus (both dorsal and ventral), but did not significantly differ from that in the ventral hippocampus.25
The basolateral nucleus of the amygdala (BLA) plays a central role in emotional behavior and, therefore, in the behavioral deficits that arise when this brain region is damaged. We have studied the time course of neuropathological alterations in the BLA after exposure of young-adult rats to soman, and have found that the number of degenerating neurons is the highest at 24 h postexposure and decreases over the course of 30 days.24 Neuronal loss, determined by performing unbiased design-based stereological counting of Nissl-stained neurons, is also already significant at 24 h postexposure, and there is no recovery at 14 days or 30 days postexposure,24 suggesting that neuronal regeneration that takes place in the amygdala after seizure-induced injury26 cannot compensate for the lost neurons, at least during this time period after the exposure.
Neuropathology data also exist at time intervals longer than 1 month after nerve agent intoxication. Ninety days after exposure of adult mice to soman, the number of neurons in the amygdala and the hippocampus is still significantly lower compared to controls.26 In postnatal day 21 rats that are exposed to soman, there are no degenerating neurons after soman exposure, yet both the amygdala and the hippocampus are significantly smaller compared to control animals at 30 and 90 days after the exposure;27 thus, despite the fact neurons in the developing brain are resistant to seizure-induced degeneration and death,28 other abnormalities such as seizure-induced metabolic changes29 may affect dendritic arborizations and overall synaptic organization, producing amygdala and hippocampal atrophy in the long term. Neuropathology that includes hippocampal atrophy has also been reported after exposure of adult rats to soman or sarin, and it is still present and progressively deteriorating at 90 days after exposure,22,23 while long-lasting atrophy of the whole brain has been found in guinea pigs after soman exposure.30
Atrophy in a number of brain regions has also been observed in victims of the terrorist attack in the Tokyo subway, in 1995, when the nerve agent sarin was released. Five to six years after the attack, victims presented with significant decrease in gray matter volume in the right insular cortex, the right temporal cortex, and the left hippocampus,31 as well as reduced volume of the anterior cingulate cortex32 and the amygdala.33 Information is not provided regarding the occurrence and duration of seizures in these human studies, but it is stated that the subjects “were treated in the emergency room for acute sarin intoxication,” which probably implies presence of seizures.
Susceptibility of principal neurons versus GABAergic interneurons
As discussed above, many brain regions are injured by nerve agent–induced SE, but the amygdala and the hippocampus have been studied more extensively because they are consistently found to suffer severe damage; another reason is probably that these two structures play a central—and, for the most part, well-delineated—role in memory and affective functions, which allows for more direct correlations of their pathology and pathophysiology with the resulting behavioral deficits. Knowing the extent of neuronal loss in these regions can help explain pathophysiological alterations and behavioral abnormalities, but also knowing the relative loss of excitatory neurons versus inhibitory interneurons can help explain alterations in the basal activity level and excitability of these neuronal networks, parameters that can profoundly affect their function. It is important, therefore, to review the existing information regarding the loss of principal/glutamatergic neurons versus the loss of GABAergic interneurons after nerve agent_induced SE; the data available have been obtained in the BLA, following exposure to soman.
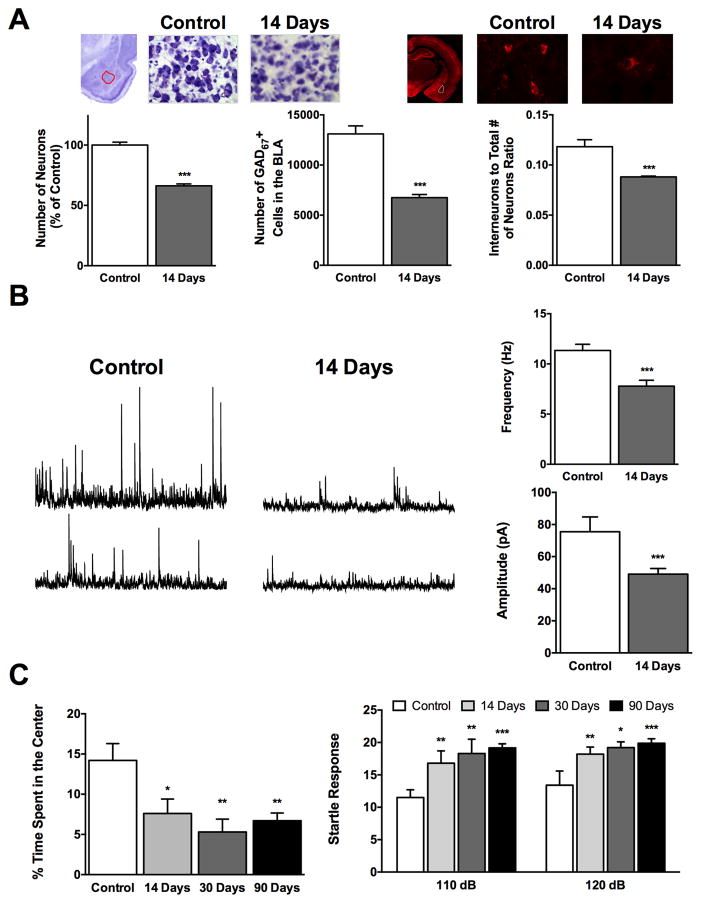
Despite the extensive neuronal loss in the rat BLA at 24 h after soman exposure,11,12,15,24 immunohistochemistry for GAD-67 has revealed that the number of GABAergic interneurons is not reduced at this time point.11,24 In contrast, 7 days later there is a 46% decrease in the interneuron population, with the ratio of interneurons to the total number of neurons being significantly smaller compared to control rats at 14 and 30 days after the exposure24 (Fig. 1A). Thus, there is loss of both principal cells and interneurons in the BLA after nerve agent–induced SE, but the interneurons follow a slow time course of death. Eventually, the BLA remains not only damaged with a reduced overall neuronal population, but also with a greater deficiency in inhibitory interneurons. Therefore, the absence of interneuron loss at 24 h after exposure cannot be interpreted to imply that these cells are more resistant to SE induced by nerve agents, only that their mechanism of death is slower. Since a significant number of principal cells have already been lost at 24 h postexposure, 11,12,15,24 interneurons lose some of their inputs and targets, which may contribute to their gradual death. However, regardless of the possible role that this may play in the delayed death of GABAergic interneurons, it is well known that the time course of neuronal death can vary widely. The same neuronal population can undergo either an immediate, necrotic death or a slow apoptotic death after excitotoxic damage; necrosis is associated with immediate loss of mitochondrial function, while apoptosis ensues in neurons that recover their mitochondrial function and energy levels, yet the excitotoxic insult has activated an endogenous cell death program.34 Principal BLA cells can undergo immediate, necrotic death (cell loss by 24 h after exposure) or slow, apoptotic-like death (since neurodegeneration continues for days after the initial 24 hours postexposure); regenerative processes may be overlapping,26,35 as suggested by the observation that the percentage of lost neurons does not change significantly up to 30 days postexposure, despite the ongoing degeneration.24 GABAergic BLA interneurons, on the other hand, seem to be less susceptible to necrosis and more susceptible to apoptosis, for reasons that remain to be determined.
Figure 1.
Status epilepticus (SE) induced by exposure to soman causes severe neuronal loss in the basolateral amygdala (BLA) that leads to long-term increases in the excitability of the BLA network and increased anxiety-like behavior. (A) Fourteen days after exposure of rats to soman (1.2 × LD50), there is a significant reduction in the total number of neurons—as determined by unbiased design-based stereological counting in Nissl-stained sections—and GABAergic interneurons in the BLA, with a decrease in the ratio of GABAergic to the total number of neurons in comparison to control rats. Photomicrographs in the top show Nissl-stained (left) and GAD-67 labeled (right) sections from the BLA of control rats and soman-exposed rats, 14 days after the exposure. Bar graphs show the group data on neuronal loss (data are from Ref. 24). (B) The frequency and amplitude of spontaneous IPSCs are significantly reduced 14 days after soman-induced SE; representative traces are shown on the left and group data on the right (from Ref. 41). (C) Anxiety-like behavior is increased after soman exposure, an impairment that persists at 90 days postexposure. The time spent in the center of the open field is shown on the left, and the amplitude of the startle responses to acoustic stimuli on the right (from Refs. 14 and 24).
The delayed death of BLA interneurons raises the question of whether the mechanisms by which they die allow for interventions that will reverse the process and lead to their recovery. These mechanisms need to be delineated, and may include processes already known to be associated with apoptosis, such as (1) excitotoxicity via activation of extrasynaptic NMDA receptors;36 (2) facilitation of NMDA receptor activity, in some neurons, by upregulation of D-serine, a positive NMDA receptor modulator;37 (3) prolonged elevations of intracellular calcium due to calcium-induced calcium release from intracellular stores;16 and (4) alterations in the expression and activity of cell-death regulatory proteins.38 It has been argued that direct actions of the nerve agent, independent of seizures, may also contribute to delayed neuronal death.39
Pathophysiological alterations in the amygdala and hippocampus after nerve agent–induced SE
The neuronal injury and loss that follow SE induced by nerve agent exposure can be expected to produce pathophysiological alterations in the affected regions, which in turn will affect their function. However, there is very little information available on such alterations, and what is available is limited to the amygdala and, to a lesser extent, the hippocampus. We will review it here, as these structures are central to both epileptogenesis and anxiety-related disorders or cognitive deficits that commonly develop after nerve agent exposure (see next section).
We have examined the impact of the neuronal loss on the physiology of the BLA, in in vitro brain slices containing the amygdala. The field potentials elicited in the BLA by stimulation of the external capsule had much smaller amplitudes in the soman-exposed rats compared to control rats, requiring high stimulus intensities to evoke a response.24 This weakening of evoked, glutamatergic field currents (for the receptors mediating BLA field potentials see Ref. 40), which was probably due to damage and loss of glutamatergic neurons, had recovered only partially at 30 days postexposure.24 Despite the low amplitude of the BLA field potentials after soman-induced SE, their duration was prolonged,14,24 which, along with an increased paired-pulse ratio,14,24 suggest reduced evoked inhibition; the prolonged duration of the field potentials and the increased paired-pulse ratio were still present at 30 days postexposure.14,24
In addition to the evoked inhibition, spontaneous inhibitory activity is also reduced after soman-induced SE, as reflected in the decreased frequency and amplitude of spontaneous inhibitory postsynaptic currents (IPSCs) recorded from BLA principal neurons (Fig. 1B), along with decreased frequency of miniature IPSCs.41 These changes in spontaneous GABAergic synaptic transmission are already present at 24 h postexposure,41 when the number of GABAergic interneurons has not yet been significantly reduced,11,24 suggesting that dysfunction of interneurons has already commenced at this time point. Fourteen days later, the spontaneous release of GABA is still significantly reduced, with no alterations in the amplitude of miniature IPSCs,41 reflecting the loss of GABAergic interneurons and suggesting possible dysfunction in presynaptic GABA release, with no postsynaptic alterations in GABAA receptor–mediated synaptic transmission. Neurotransmitter systems modulating GABAergic transmission in the BLA may also be altered. For example, in control rats, activation of α7 nicotinic receptors produces a transient but dramatic increase in the frequency and amplitude of spontaneous IPSCs recorded from principal BLA cells,42 but this effect is significantly less pronounced at 24 h and 14 days after soman-induced SE;41 to what extent this is due to the reduced number of GABAergic interneurons, desensitization of α7 nicotinic receptors after the prolonged inhibition of AChE following exposure to soman,24 or downregulation of these receptors remains to be clarified. The net result of the disproportional loss of BLA interneurons over the loss of principal neurons and the decrease in spontaneous GABAergic activity is an increase of spontaneous EPSCs in the BLA network.41 In this pathophysiological environment, synaptic plasticity in the BLA is also impaired, albeit transiently.14,24
In the hippocampus, decreased frequency of spontaneous EPSCs recorded from CA1 pyramidal neurons has been observed at 6–9 days after soman exposure,43 which probably implies reduction in the glutamatergic inputs to these neurons because of neuronal degeneration and loss in the CA1 and CA3 hippocampal subfields.11–13,15 During this time (6–9 days after exposure), the frequency of spontaneous IPSCs in CA1 pyramidal neurons has returned to control levels, after a transient reduction observed at 24 h after exposure, while their amplitude is larger than that in control rats.44 Thus, while in the BLA, hyperexcitability prevails, in the CA1 hippocampal area, inhibitory activity may be increased. It should be noted that, in contrast to the observations in the BLA, in the CA1 hippocampal area there is no significant loss of GABAergic interneurons at 7 days after soman-induced SE, despite the extensive reduction in the total number of neurons,11 an observation that seems consistent with the lack of reduction in the frequency of spontaneous IPSCs at this time point.44 In the next sections, we will examine if such alterations in the excitability of the BLA and CA1 circuitries are compatible with the observed neurological and behavioral derangements.
Long-term neurological effects of nerve agent exposure
Neurological evaluations have been performed in victims of the sarin attacks in Matsumoto in 1994 and Tokyo in 1995. Although, in these human studies, there is significant heterogeneity in the severity of the intoxication the subjects experienced and in their resilience to nerve agent toxicity (partly due to varying ages, gender, and possibly other factors), it is evident that abnormal electroencephalographic results suggestive of epileptiform activity are common and persistent for years after the incidents.45,46
Animal studies have shown that the neuronal damage produced by a prolonged SE—of any etiology—is often followed by epileptogenesis, leading to the appearance of recurrent seizures.47,48 Epileptogenesis also takes place after SE induced by nerve agents.22,49 A large percentage of rats who experience SE after soman exposure develop spontaneous recurrent seizures, which first appear 5–10 days after the exposure49 and are present throughout a 3-month postexposure observation period.22 More than one brain region and mechanism may be involved in the epileptogenesis that follows nerve agent–induced SE, but the significant loss of interneurons in the BLA and the resulting hyperexcitability is likely to play an important role. The BLA has been known to be a highly seizurogenic neuronal network, and also one from which seizures can readily propagate to other brain regions.50 Evidence suggests that the BLA may also be the site from where seizures propagate and progress to generalized SE after nerve agent exposure; the most important pieces of evidence supporting this notion are that (1) microinjection of nerve agents into different brain regions can elicit convulsions only when the nerve agent is injected into the BLA,51 and (2) generation of SE after soman exposure requires significant inhibition of AChE activity specifically in the BLA.8 With reduced background inhibition and a net increase in spontaneous excitatory activity,41 the BLA could be responsible for or contribute to triggering spontaneous seizures.
The hippocampus is also well known to be a seizurogenic structure, and therefore it may play an essential role in the development of recurrent seizures after nerve agent–induced SE. The ventral hippocampus may be more important in this regard, because it suffers more extensive neuronal degeneration than the dorsal part25 and may have a higher propensity than the dorsal hippocampus for generating epileptiform activity.52 However, as mentioned in the previous section, there is no significant loss of GABAergic interneurons in the CA1 hippocampal area at 7 days after soman-induced SE, despite the extensive reduction in the total number of neurons,11 and, at about the same time point, GABAergic activity is not reduced.44 It is not known if inhibitory neurons and/or their activity are reduced in the CA1 area at later postexposure time points, but mechanisms other than reduced GABAergic activity, such as aberrant reorganization of hippocampal neuronal circuitries53,54 could contribute to spontaneous recurrent seizures arising from the hippocampus.
Long-term cognitive deficits and behavioral disorders after nerve agent exposure
Cognitive deficits related to learning and memory have been observed after exposure to nerve agents. In rats, there is impaired learning ability in the Morris water maze at 2 months after soman-induced seizures.55 Filliat et al. studied mice for up to 90 days after soman-induced SE and found that impaired performance in the Morris water maze, which tests long-term spatial memory, and the T-maze, which tests short-term spatial memory, persisted at the 90-day postexposure time point.56 The severity of the memory impairment correlated with the severity of neuropathology in the CA1 hippocampal area, as well as with the severity of convulsive SE as deduced by the extent of weight loss.56 Similarly, Collombet et al. found impaired performance in the Morris water maze and T-maze at 30 days and 90 days after soman-induced SE, in mice.26 In the study by Filliat et al., some soman-exposed mice that had little or no neurodegeneration in the CA1 area displayed memory deficits, but only in the Morris water maze test (not in the T-maze) and only at the 90-day time point (not at 30 days postexposure), suggesting that even mild toxicity can produce delayed cognitive deficits. Delayed impairment (3 months after soman exposure) in the Morris water maze after relatively mild intoxication has also been reported in guinea pigs.57 There are also human data indicating disturbances in memory functions and other cognitive deficits, long after acute organophosphate intoxication. These data derive from accidental or occupational exposure or from studies on the victims of the sarin terrorist attacks in Japan, and seem to suggest that not only severe but even mild organophosphate intoxication, without convulsive SE, can produce cognitive disturbances (reviewed in Ref. 39).
Correlations of the impairments in spatial learning and memory with pathophysiological alterations in the hippocampus after nerve agent poisoning have not been made. However, it is well established that hippocampal memory formation is associated with and requires synaptic plasticity in the hippocampus. It remains to be determined whether the impaired spatial memory after nerve agent exposure is associated with reduced synaptic plasticity in the hippocampus, and if this correlates with neuronal damage, abnormal reorganization of hippocampal neuronal circuitries, and/or increased GABAergic inhibition.
Another behavioral abnormality that is consistently observed after nerve agent-induced SE is increased anxiety-like behavior. Seven to 9 days after soman-induced SE, rats display elevated acoustic startle responses.58 Studies at longer time intervals after soman-induced SE also indicate increased anxiety; thus, the amount of time the rats spend in the center of the open field is significantly reduced, while the amplitudes of the startle responses to acoustic stimuli are significantly increased, at 14 days,24 30 days,14,15,24 and 90 days14 after the exposure (Fig. 1C). Similarly, mice exhibit increased anxiety in the light/dark box and the elevated plus maze at 30 and 90 days after soman-induced SE.59
The animal data are in accord with findings from studies of the victims of the sarin terrorist attack in Japan, which have revealed that the main behavioral deficit present at 6–8 months after the attack60 and still persisting 5–7 years later46,61,62 is increased anxiety, with symptomatology consistent with posttraumatic stress disorder (PTSD). In addition, 5–6 years after the sarin Tokyo attack, victims with a history of PTSD had atrophied amygdala,33 a pathology that has been associated with anxiety disorders.63–65 The core feature, however, of anxiety disorders including PTSD in humans,66,67 and increased anxiety-like behavior in animals,68–70 is increased excitability of the amygdala, and the BLA in particular.66–70 Therefore, the GABAergic interneuronal loss,11,24 decreased GABAergic activity,41 and increased spontaneous excitatory activity in the BLA,41 as observed in animal studies, may explain the development of increased anxiety after nerve agent exposure (Fig. 1A–C). It should also be noted that the ventral hippocampus, which suffers severe damage after nerve agent–induced SE,25 plays an important role in regulating anxiety along with the BLA;71 therefore, the neuropathology of the ventral hippocampus may also play an important role in the anxiety-related behavioral derangements that develop after nerve agent exposure.
The ample evidence of the association between hyperexcitability of the amygdala and increased anxiety implies causality.72 In the case of the sarin attack victims who developed PTSD, no studies have been done to determine if their amygdala displays exaggerated responses to fearful stimuli, as typically seen in PTSD patients;66,67 however, there is evidence for pathology of the amygdala and other brain regions that are involved in the expression of anxiety.33 The prevailing assumption appears to be that PTSD in these sarin attack victims developed due to stress experienced during the terrorist attack and the consolidation of lasting fear memories. It is important, however, to ask whether the emotional stress experienced during the attack produced the pathological alterations that manifested behaviorally into PTSD, or if sarin itself and/or the seizures induced by exposure to sarin affected certain brain regions—most importantly the amygdala—producing (or contributing to) pathological and pathophysiological alterations that resulted in the development of PTSD. The animal data would suggest the latter. All rats who are exposed to a nerve agent and undergo cholinergic crisis will experience stress, but if neuropathology is minimized with administration of an anticonvulsant at 20 min14 or even 1 h15 after the exposure, the development of increased anxiety-like behavior is prevented;14,15 these findings suggest that it is the brain damage—induced by seizures in this case—more than the stress that produced increased anxiety. In support of this view, increased anxiety also develops after experimental brain injury, which is inflicted while the animal is anesthetized and probably does not experience any severe stress;73 importantly, the injury induces loss of GABAergic neurons and decreased spontaneous GABAergic activity in the BLA.73 It is possible, therefore, that, in addition to the fear-eliciting memories that contribute to PTSD symptomatology, any insult that will result in hyperexcitability of the amygdala has the potential of producing or contributing to the development of an anxiety disorder, including PTSD.72
Controlling nerve agent–induced SE prevents brain damage and the long-term behavioral sequelae
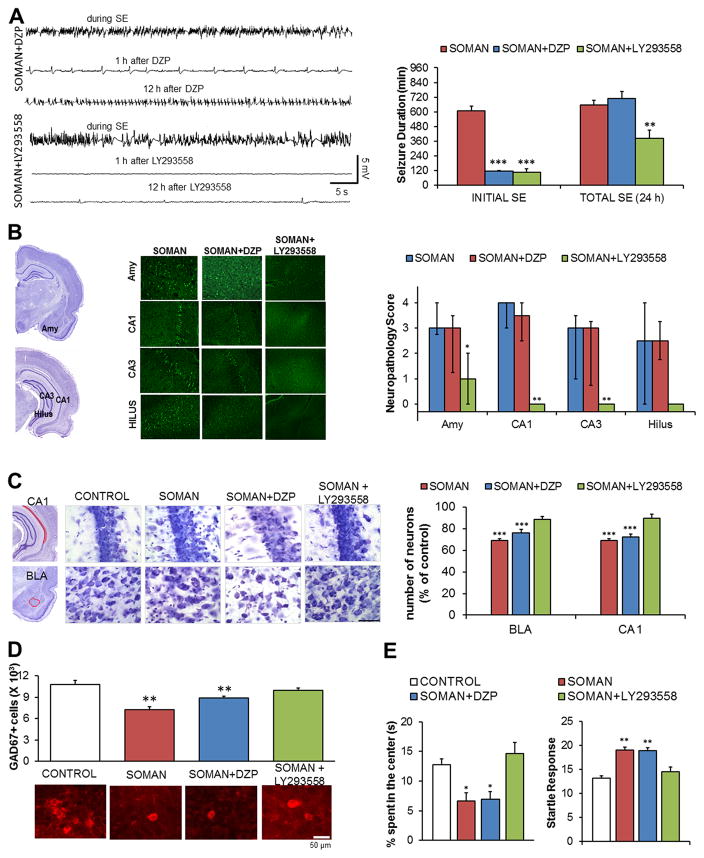
Although mild nerve agent intoxication may produce long-term functional deficits39 via mechanisms that are still unclear, there is a wealth of evidence to suggest that the SE induced by the exposure is the primary cause of the brain damage and the long-term neurological and behavioral sequelae.7–15 For this reason, the scientific community has been actively seeking to discover new drugs that will provide seizure control and neuroprotection in the event of an emergency involving the release of nerve agents. It is not the purpose of this review to describe these studies and compare the efficacies of different drugs and drug combinations that have been discovered. However, it is important to point out that pharmacologically terminating the initial SE will not necessarily prevent neuropathology and its consequences; seizures and SE can return and, therefore, they must be monitored for at least 24 h postexposure. We have observed the return of seizures after stopping the initial soman-induced SE by intramuscular injection of diazepam,15 which is the anticonvulsant currently approved by the U.S. Food and Drug Administration for the management of nerve agent–induced SE. Despite the cessation of the initial SE by diazepam, the total duration of SE within 24 h after exposure was not lower in the diazepam-treated group compared to the soman-exposed group that did not receive anticonvulsant treatment (Fig. 2A); it is probably for this reason that diazepam did not provide protection against neuronal degeneration (Fig. 2B), neuronal loss (Fig. 2C and D), and behavioral deficits15 (Fig. 2E). Additional injections of diazepam as seizures reappear may not only risk severe cardiorespiratory suppression, but may also have little effectiveness in suppressing seizures, due to downregulation and/or the many alterations that occur in the synaptic and extrasynaptic GABAA receptors in the acute phase, during and post-SE.74,75 Our studies have shown that anticonvulsant treatments are effective when they include antagonism of the glutamatergic system;11–15 glutamate receptors are not only not downregulated, but their number increases during excessive excitatory activity.75 The AMPA/GluK1 receptor antagonist LY293558 has been exceptionally effective in suppressing seizures11,13,14 (Fig. 2A), protecting against neurodegeneration (Fig. 2B) and neuronal loss (Fig. 2C,D), and preventing the development of long-term increased anxiety (Fig. 2E). It must be cautioned, however, that in these studies LY293558 has been compared with diazepam against only one nerve agent (soman); it remains to be determined whether similar results would be obtained with other nerve agents.
Figure 2.
Effective control of SE is necessary for the prevention of neuropathology and behavioral deficits. The data presented here compare the efficacies of LY293558 (an AMPA/GluK1 receptor antagonist) and diazepam (DZP) against soman. (A) Both LY293558 and DZP, administered 1 h after exposure of rats to soman (1.4 × LD50), terminated the initial SE, but seizures returned in the DZP-treated rats; as a result, only LY293558 reduced the total duration of SE within 24 h after the exposure. Electroencephalographic traces are shown on the left and group data on the right (from Refs. 11 and 15). (B) Neuronal degeneration is prevented by LY293558, but not by DZP treatment. Data shown were obtained 7 days after the exposure. The panoramic Nissl-stained sections (left) show the areas where neurodegeneration was evaluated, representative photomicrographs of Fluoro–Jade C–stained sections from the amygdala (Amy) and the hippocampus are shown in the middle, and group data are shown on the right (from Refs. 11 and 15). (C) Neuronal loss in the BLA and the CA1 hippocampal area is prevented by LY293558, but not by DZP treatment. Data were obtained 7 days after the exposure. The panoramic Nissl-stained sections (left) show the areas where neuronal loss was assessed. The Nissl-stained sections in the middle (total magnification 630×; scale bar, 50 μm) are representative photomicrographs from the four groups. The bar graph (right) shows group data (from Refs. 11 and 15). (D) Loss of GABAergic interneurons in the BLA is prevented by LY293558, but not by DZP treatment. Data were obtained 7 days after the exposure. The bar graph shows group data; representative photomicrographs from the corresponding groups are shown below the graph (from Refs. 11 and 15).(E) Increased anxiety-like behavior, 30 days after soman exposure, was prevented by LY293558, but not by DZP treatment. Left graph: time spent in the center of the open field. Right graph: amplitude of the startle responses to 120-dB acoustic stimulus (from Refs. 14 and 15).
Conclusions
The studies reviewed above indicate that the neuropathological, pathophysiological, neurological, and behavioral alterations that are caused by acute exposure to nerve agents are very long lasting. In addition, the limited data available from humans are consistent with the observations in animal models; this is very important considering that it is not ethical and feasible to use humans in studies of nerve agent exposure. Effective control of SE is of paramount importance, not only in order to prevent death, but also to prevent brain damage. Therefore, new anticonvulsant treatments aiming at counteracting nerve agent–induced SE must aim not only at saving the lives of the exposed individuals, but also at providing sufficient neuroprotection to prevent the development of long-term neurological and behavioral impairments.
Acknowledgments
Our research has been supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant Number 5U01NS058162-07].
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Sirin GS, Zhou Y, Lior-Hoffmann L, et al. Aging mechanism of soman inhibited acetylcholinesterase. J Phys Chem B. 2012;116:12199–12207. doi: 10.1021/jp307790v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajgar J, Fusek J, Kassa J, et al. An attempt to assess functionally minimal acetylcholinesterase activity necessary for survival of rats intoxicated with nerve agents. Chem Biol Interact. 2008;175:281–285. doi: 10.1016/j.cbi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Shih TM, Kan RK, McDonough JH. In vivo cholinesterase inhibitory specificity of organophosphorus nerve agents. Chem Biol Interact. 2005;157–158:293–303. doi: 10.1016/j.cbi.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 4.Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000;41(Suppl 2):S23–30. doi: 10.1111/j.1528-1157.2000.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmued LC, Stowers CC, Scallet AC, et al. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 6.Eyüpoglu IY, Savaskan NE, Bräuer AU, et al. Identification of neuronal cell death in a model of degeneration in the hippocampus. Brain Res Brain Res Protoc. 2003;11:1–8. doi: 10.1016/s1385-299x(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 7.Myhrer T, Andersen JM, Nguyen NH, et al. Soman-induced convulsions in rats terminated with pharmacological agents after 45 min: neuropathology and cognitive performance. Neurotoxicology. 2005;26:39–48. doi: 10.1016/j.neuro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, et al. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology. 2013;38:84–90. doi: 10.1016/j.neuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Filliat P, Baubichon D, Burckhart MF, et al. Memory impairment after soman intoxication in rat: correlation with central neuropathology. Improvement with anticholinergic and antiglutamatergic therapeutics. Neurotoxicology. 1999;20:535–549. [PubMed] [Google Scholar]
- 10.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo TH, Qashu F, Apland JP, et al. The GluK1 (GluR5) kainate/AMPA receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther. 2011;336:303–312. doi: 10.1124/jpet.110.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiredo TH, Aroniadou-Anderjaska V, Qashu F, et al. Neuroprotective efficacy of caramiphen against soman and mechanisms of its action. Br J Pharmacol. 2011;164:1495–1505. doi: 10.1111/j.1476-5381.2011.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, et al. Efficacy of the GluK1/AMPA receptor antagonist LY293558 against seizures and neuropathology in a soman-exposure model without pretreatment and its pharmacokinetics after intramuscular administration. J Pharmacol Exp Ther. 2012;344:133–140. doi: 10.1124/jpet.112.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prager EM, Figueiredo TH, Long RP, 2nd, et al. LY293558 prevents soman-induced pathophysiological alterations in the basolateral amygdala and the development of anxiety. Neuropharmacology. 2014;89C:11–18. doi: 10.1016/j.neuropharm.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, et al. The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: Comparison with UBP302. J Pharmacol Exp Ther. 2014;351:359–372. doi: 10.1124/jpet.114.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande LS, Carter DS, Phillips KF, et al. Development of status epilepticus, sustained calcium elevations and neuronal injury in a rat survival model of lethal paraoxon intoxication. Neurotoxicology. 2014;44:17–26. doi: 10.1016/j.neuro.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujikawa DG. The role of excitotoxic programmed necrosis in acute brain injury. Comput Struct Biotechnol J. 2015;13:212–221. doi: 10.1016/j.csbj.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cock HR. The role of mitochondria and oxidative stress in neuronal damage after brief and prolonged seizures. Prog Brain Res. 2002;135:187–196. doi: 10.1016/S0079-6123(02)35018-0. [DOI] [PubMed] [Google Scholar]
- 19.Puttachary S, Sharma S, Stark S, et al. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res Int. 2015;2015:745613. doi: 10.1155/2015/745613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legido A, Katsetos CD. Experimental studies in epilepsy: immunologic and inflammatory mechanisms. Semin Pediatr Neurol. 2014;21:197–206. doi: 10.1016/j.spen.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Spradling KD, Lumley LA, Robison CL, et al. Transcriptional responses of the nerve agent-sensitive brain regions amygdala, hippocampus, piriform cortex, septum, and thalamus following exposure to the organophosphonate anticholinesterase sarin. J Neuroinflamm. 2011;8:2–21. doi: 10.1186/1742-2094-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadar T, Cohen G, Sahar R, et al. Long-term study of brain lesions following soman, in comparison to DFP and metrazol poisoning. Hum Exp Toxicol. 1992;11:517–523. doi: 10.1177/096032719201100613. [DOI] [PubMed] [Google Scholar]
- 23.Kadar T, Shapira S, Cohen G, et al. Sarin-induced neuropathology in rats. Hum Exp Toxicol. 1995;14:252–259. doi: 10.1177/096032719501400304. [DOI] [PubMed] [Google Scholar]
- 24.Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, et al. The recovery of acetylcholinesterase activity and the progression of neuropathological and pathophysiological alterations in the rat basolateral amygdala after soman-induced status epilepticus: relation to anxiety-like behavior. Neuropharmacology. 2014;81:64–74. doi: 10.1016/j.neuropharm.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apland JP, Figueiredo TH, Qashu F, et al. Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology. 2010;31:485–492. doi: 10.1016/j.neuro.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collombet JM, Béracochéa D, Liscia P, et al. Long-term effects of cytokine treatment on cognitive behavioral recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res. 2011;221:261–70. doi: 10.1016/j.bbr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Miller SL, Aroniadou-Anderjaska V, Figueiredo TH, et al. A rat model of nerve agent exposure applicable to the pediatric population: The anticonvulsant efficacies of atropine and GluK1 antagonists. Toxicol Appl Pharmacol. 2015;284:204–216. doi: 10.1016/j.taap.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes GL, Ben-Ari Y. Seizures in the developing brain: perhaps not so benign after all. Neuron. 1998;21:1231–1234. doi: 10.1016/s0896-6273(00)80642-x. [DOI] [PubMed] [Google Scholar]
- 29.Nehlig A, Pereira de Vasconcelos A. The model of pentylenetetrazol-induced status epilepticus in the immature rat: short- and long-term effects. Epilepsy Res. 1996;26:93–103. doi: 10.1016/s0920-1211(96)00045-9. [DOI] [PubMed] [Google Scholar]
- 30.Gullapalli RP, Aracava YY, Zhuo J, et al. Magnetic resonance imaging reveals that galantamine prevents structural brain damage induced by an acute exposure of guinea pigs to soman. Neurotoxicology. 2010;31:67–76. doi: 10.1016/j.neuro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Yamasue H, Abe O, Kasai K, et al. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]
- 32.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers MA, Yamasue H, Abe O, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res. 2009;174:210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 35.Ohira K. Injury-induced neurogenesis in the mammalian forebrain. Cell Mol Life Sci. 2011;68:1645–1656. doi: 10.1007/s00018-010-0552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brassai A, Suvanjeiev RG, Bán EG, et al. Role of synaptic and nonsynaptic glutamate receptors in ischaemia induced neurotoxicity. Brain Res Bull. 2015;112:1–6. doi: 10.1016/j.brainresbull.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Liu YH, Wang L, Wei LC, et al. Up-regulation of D-serine might induce GABAergic neuronal degeneration in the cerebral cortex and hippocampus in the mouse pilocarpine model of epilepsy. Neurochem Res. 2009;34:1209–1218. doi: 10.1007/s11064-008-9897-0. [DOI] [PubMed] [Google Scholar]
- 38.Bengzon J, Mohapel P, Ekdahl CT, et al. Neuronal apoptosis after brief and prolonged seizures. Prog Brain Res. 2002;135:111–119. doi: 10.1016/S0079-6123(02)35011-8. [DOI] [PubMed] [Google Scholar]
- 39.Pereira EF, Aracava Y, DeTolla LJ, Jr, et al. Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J Pharmacol Exp Ther. 2014;350:313–321. doi: 10.1124/jpet.114.214932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aroniadou-Anderjaska V, Post RM, Rogawski MA, et al. Input-specific LTP and depotentiation in the basolateral amygdala. NeuroReport. 2001;12:635–640. doi: 10.1097/00001756-200103050-00041. [DOI] [PubMed] [Google Scholar]
- 41.Prager EM, V, Pidoplichko I, Aroniadou-Anderjaska V, et al. Pathophysiological mechanisms underlying increased anxiety after soman exposure: Reduced GABAergic inhibition in the basolateral amygdala. Neurotoxicology. 2014;44:335–343. doi: 10.1016/j.neuro.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pidoplichko VI, Prager EM, Aroniadou-Anderjaska V, et al. Alpha7-containing nicotinic acetylcholine receptors on interneurons of the basolateral amygdala and their role in the regulation of the network excitability. J Neurophysiol. 2013;110:2358–2369. doi: 10.1152/jn.01030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexandrova EA, Alkondon M, Aracava Y, et al. Galantamine prevents long-lasting suppression of excitatory synaptic transmission in CA1 pyramidal neurons of soman-challenged guinea pigs. Neurotoxicology. 2014;44:270–278. doi: 10.1016/j.neuro.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexandrova EA, Aracava Y, Pereira EF, et al. Pretreatment of guinea pigs with galantamine prevents immediate and delayed effects of soman on inhibitory synaptic transmission in the hippocampus. J Pharmacol Exp Ther. 2010;334:1051–1058. doi: 10.1124/jpet.110.167700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sekijima Y, Morita H, Yanagisawa N. Follow-up of sarin poisoning in Matsumoto. Ann Intern Med. 1997;127:1042. doi: 10.7326/0003-4819-127-11-199712010-00028. [DOI] [PubMed] [Google Scholar]
- 46.Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Lothman EW, Bertram EH., 3rd Epileptogenic effects of status epilepticus. Epilepsia. 1993;34(Suppl 1):S59–70. doi: 10.1111/j.1528-1157.1993.tb05907.x. [DOI] [PubMed] [Google Scholar]
- 48.Dudek FE, Hellier JL, Williams PA, et al. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog Brain Res. 2002;135:53–65. doi: 10.1016/S0079-6123(02)35007-6. [DOI] [PubMed] [Google Scholar]
- 49.de Araujo Furtado M, Rossetti F, Chanda S, et al. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology. 2012;33:1476–1490. doi: 10.1016/j.neuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Aroniadou-Anderjaska V, Fritsch B, Qashu F, et al. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008;78:102–116. doi: 10.1016/j.eplepsyres.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDonough JH, Jr, McLeod CG, Jr, Nipwoda MT. Direct microinjection of soman or VX into the amygdala produces repetitive limbic convulsions and neuropathology. Brain Res. 1987;435:123–137. doi: 10.1016/0006-8993(87)91593-9. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert M, Racine RJ, Smith GK. Epileptiform burst responses in ventral vs dorsal hippocampal slices. Brain Res. 1985;361:389–391. doi: 10.1016/0006-8993(85)91309-5. [DOI] [PubMed] [Google Scholar]
- 53.Mello LE, Cavalheiro EA, Tan AM, et al. Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia. 1993;34:985–995. doi: 10.1111/j.1528-1157.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 54.Dudek FE, Obenaus A, Schweitzer JS, et al. Functional significance of hippocampal plasticity in epileptic brain: electrophysiological changes of the dentate granule cells associated with mossy fiber sprouting. Hippocampus. 1994;4:259–265. doi: 10.1002/hipo.450040306. [DOI] [PubMed] [Google Scholar]
- 55.Joosen MJ, Jousma E, van den Boom TM, et al. Long-term cognitive deficits accompanied by reduced neurogenesis after soman poisoning. NeuroToxicology. 2009;30:72–80. doi: 10.1016/j.neuro.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 56.Filliat P, Coubard S, Pierard C, et al. Long-term behavioral consequences of soman poisoning in mice. Neurotoxicology. 2007;28:508–519. doi: 10.1016/j.neuro.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Mamczarz J, Kulkarni GS, Pereira EFR, et al. Galantamine counteracts development of learning impairment in guinea pigs exposed to the organophosphorus poison soman: clinical significance. Neurotoxicology. 2011;32:785–798. doi: 10.1016/j.neuro.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langston JL, Wright LK, Connis N, et al. Characterizing the behavioral effects of nerve agent-induced seizure activity in rats: increased startle reactivity and perseverative behavior. Pharmacol Biochem Behav. 2012;100:382–391. doi: 10.1016/j.pbb.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Coubard S, Beracochea D, Collombet JM, et al. Long-term consequences of soman poisoning in mice: part 2. Emotional behavior. Behav Brain Res. 2008;191:95–103. doi: 10.1016/j.bbr.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 60.Murata K, Araki S, Yokoyama K, et al. Asymptomatic sequelae to acute sarin poisoning in the central and autonomic nervous system 6 months after the Tokyo subway attack. J Neurol. 1997;244:601–606. doi: 10.1007/s004150050153. [DOI] [PubMed] [Google Scholar]
- 61.Ohtani T, Iwanami A, Kasai K, et al. Post-traumatic stress disorder symptoms in victims of Tokyo subway attack: a 5-year follow-up study. Psychiatry Clin Neurosci. 2004;58:624–629. doi: 10.1111/j.1440-1819.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 62.Hoffman A, Eisenkraft A, Finkelstein A, et al. A decade after the Tokyo sarin attack: a review of neurological follow-up of the victims. Mil Med. 2007;172:607–610. doi: 10.7205/milmed.172.6.607. [DOI] [PubMed] [Google Scholar]
- 63.Irle E, Ruhleder M, Lange C, et al. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J Psychiatry Neurosci. 2010;35:126–131. doi: 10.1503/jpn.090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage. 2003;19:80–90. doi: 10.1016/s1053-8119(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 65.Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–966. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 66.Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 67.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aroniadou-Anderjaska V, V, Pidoplichko I, Figueiredo TH, et al. Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GluK1 receptors. Neuroscience. 2012;27:157–169. doi: 10.1016/j.neuroscience.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pidoplichko VI, Aroniadou-Anderjaska V, Prager EM, et al. ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety. J Neurosci. 2014;34:3130–3141. doi: 10.1523/JNEUROSCI.4009-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baculis BC, Diaz MR, Valenzuela CF. Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacol Biochem Behav. 2015;137:78–85. doi: 10.1016/j.pbb.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Felix-Ortiz AC, Beyeler A, Seo C, et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aroniadou-Anderjaska V. Anxiety and amygdalar hyperexcitability: The chicken or the egg? Ann Depress Anxiety. 2015;2:1038. [Google Scholar]
- 73.Almeida-Suhett CP, Prager EM, Pidoplichko VI, et al. Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PLoS One. 2014;9:e102627. doi: 10.1371/journal.pone.0102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci. 2008;28:2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Naylor DE. Glutamate and GABA in the balance: convergent pathways sustain seizures during status epilepticus. Epilepsia. 2010;51(Suppl 3):106–109. doi: 10.1111/j.1528-1167.2010.02622.x. [DOI] [PubMed] [Google Scholar]