Abstract
Current oxime reactivators for organophosphate-inhibited cholinesterase (ChE) do not effectively cross the blood–brain barrier and therefore cannot restore brain ChE activity in vivo. Our laboratories have studied highly relevant sarin and VX surrogates, which differ from their respective nerve agents only in the leaving group and thereby leave ChE phosphylated with the same chemical moiety as sarin and VX. Our laboratories have invented novel substituted phenoxyalkyl pyridinium oximes (U.S. Patent 9,227,937 B2) that lead to reduced ChE inhibition in the brains of rats challenged with a high sublethal dosage of the sarin surrogate, whereas 2-PAM did not, using a paradigm designed to demonstrate brain penetration. In addition, these novel oximes also showed an attenuation of seizure-like behavior compared to rats treated with 2-PAM, giving additional evidence of the ability of these oximes to penetrate the blood–brain barrier. Further, some of these oximes provided 24-hour survival superior to 2-PAM and shortened the duration of seizure-like behavior when rats were challenged with lethal dosages of the sarin and VX surrogates, providing additional support for the concept of these life-saving oximes penetrating the brain.
Keywords: organophosphate, anticholinesterase, nerve agent surrogate, oxime, acetylcholinesterase reactivator
Introduction
The initial molecular lesion of the organophosphate (OP) anticholinesterases has been well known for many years. The OPs of concern are reactive and will persistently phosphylate the serine hydroxyl in the active site of serine esterases and serine proteases, with the most critical target being acetylcholinesterase (AChE) in synapses and neuromuscular junctions.1,2 This mechanism of toxicity has been employed in the development of some very potent acute neurotoxicants, the nerve agents and the OP insecticides. The nerve agents arose from the chemical technology of World War II, which was originally targeted to create better insecticides and which later migrated into the development of highly toxic OPs that could serve as chemical weapons.3–5 The persistent action of OPs occurs, in part, because the rate of spontaneous reactivation, through hydrolytic release of the phosphate moiety from the active site, is slow (taking hours to days). In addition a non-enzymatically mediated “aging” reaction occurs in which one of the alkyl groups dissociates from the remainder of the OP moiety, leaving the phosphylated enzyme charged and refractory to further spontaneous hydrolysis; no reactivation of the inhibited aged AChE occurs.6–9
Inhibition of critical AChE by OP anticholinesterases leads to the accumulation of the neurotransmitter acetylcholine in synapses and neuromuscular junctions, leading to hypercholinergic activity throughout the central and peripheral nervous systems.1 Lethal dose poisoning leads to death from respiratory failure, which results from both central and peripheral pathway hyperactivity.10 Combatting this hypercholinergic activity has involved the muscarinic cholinergic receptor antagonist atropine, a belladonna alkaloid that opposes the action of the excess acetylcholine at muscarinic synapses; atropine is highly effective, especially when administered repeatedly through the cholinergic crisis.11 In addition to atropine, oximes have also been employed as therapeutics.12,13 Oximes are potent nucleophiles and can attack the phosphylated serine, undergo a transphosphorylation reaction to an oxime–phosphate entity, and thereby restore the function of AChE.14–18 (However, it should be noted that the oxime–phosphates formed during the transphosphorylation are sometimes potent AChE inhibitors, so they could re-inhibit the reactivated AChE19). Oximes are effective AChE reactivators if they are present before the non-enzymatic aging process has occurred, but are not effective on inhibited, aged AChE.15
In addition to potential lethality, of great concern is also the fact that central AChE inhibition can lead to seizures from excess acetylcholine-mediated activation of excitotoxic glutamatergic pathways; prolonged seizures lead to brain damage.20 Because the best oxime reactivators, such as 2-PAM (pralidoxime; the current FDA-approved oxime reactivator in the United States),21 are pyridinium compounds, and therefore charged because of the presence of a quaternary ammonium ion, they cannot effectively cross the blood–brain barrier and act only in the peripheral nervous system.22–24 While peripheral AChE reactivation is helpful in maintaining respiration and other vital functions, current reactivators cannot restore AChE activity in the brain or have a direct positive impact on seizure attenuation.25 Consequently, an anticonvulsant, typically a benzodiazepine (e.g., diazepam), is usually also required once seizures initiate.26–28 Therefore a critical need for the therapeutic arsenal against OP anticholinesterases would be an oxime reactivator that can penetrate the blood–brain barrier and that can start to restore AChE activity and thus dampen the hypercholinergic activity, ideally before seizures are initiated or before seizures lead to brain damage.
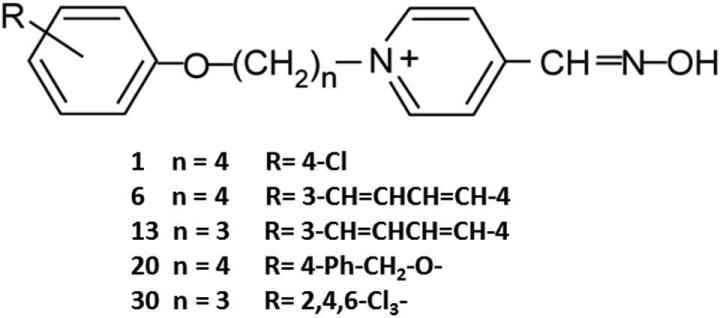
To address this critical need, our laboratories have invented a series of novel substituted phenoxyalkyl pyridinium oximes (U.S. Patent 9,227,937 B2) with the goal of identifying oximes that can penetrate the blood–brain barrier and reactivate phosphylated brain AChE in the intact animal.29 Figure 1 illustrates the structures of the five select novel oximes discussed here. The novel oximes in the entire series have alkyl chains of 3–5 carbons, with various substitutions on the phenoxy group. While these are pyridinium oximes, thereby containing a quaternary ammonium similar to other pyridinium oxime reactivators that do not cross the blood–brain barrier, our hypothesis was that the phenoxyalkyl moiety that increases the lipophilicity29 would tend to counterbalance the positive charge from the quaternary ammonium, allowing brain penetration.
Figure 1.
Structures of the novel oximes reported upon. Numbers in the left column represent the Mississippi State University numbers. n, the number of carbons in the alkyl chain; R, the substitution on the phenoxy ring.
Other laboratories have also investigated chemistries that might be effective reactivators that could penetrate the blood–brain barrier. The non-charged tertiary oximes monoisonitroacetone (MINA) and diacetylmonoxime (DAM) have been studied and have shown some efficacy against sarin, cyclosarin, and VX, with highest efficacy at low doses of nerve agent.30,31 Non-quaternary pyridine aldoximes, which were effective in vitro reactivators with tabun and VX, were not tested in vivo.14 Non-charged amidine–oxime reactivators showed efficacy in preventing lethality in mice treated with sarin and tabun surrogates when these oximes were administered 5 min after surrogate challenge.32 Ionizable hydroxyiminoacetamido amine reactivators have shown efficacy in promoting survival when administered as a pretreatment or a 1-min after-agent challenge.33
Our perspective has focused primarily on nerve agents that could affect not only soldiers in combat situations but also civilians who might be subject to terrorist attack, accidents, or acts by rogue nations. Our laboratories have been studying highly relevant nerve agent surrogates, in which the leaving group of the nerve agent has been substituted with another chemical group that makes these surrogates less toxic than their respective nerve agents but still potent anticholinesterases. The group phosphylating AChE is the same chemical moiety for both these surrogates and their respective nerve agents, making these surrogates highly relevant for the in vitro and in vivo study of reactivation potential of the novel oximes. The synthesis of the surrogates for sarin and VX has been described, and the surrogates have been chemically characterized and their biological potency described.34
In vitro studies
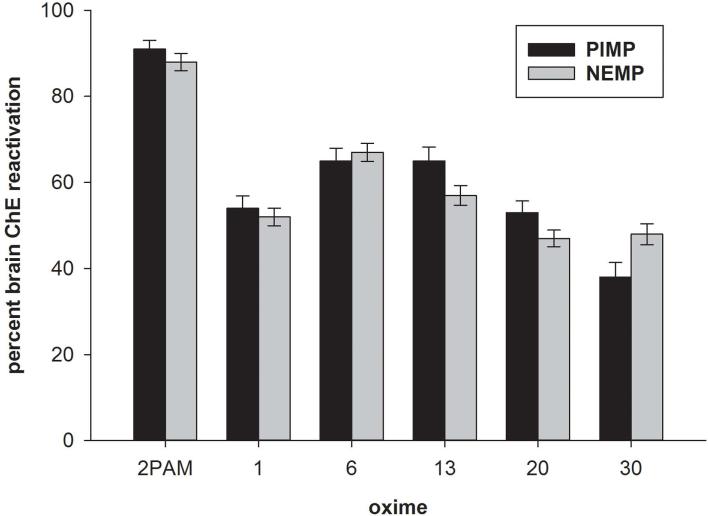
In order to determine whether the novel oximes possessed the ability to reactivate OP-phosphylated cholinesterase (ChE), in vitro assays were conducted with rat brain homogenates in which ChE had been inhibited by a sarin or a VX surrogate that had been synthesized in our laboratories. The sarin surrogate used, phthalimidyl isopropyl methylphosphonate (PIMP), is relatively unstable in aqueous solution and degrades within about 15 min, assuring little opportunity for surrogate reinhibition of reactivated ChE in the assay. The VX surrogate was nitrophenyl ethyl methylphosphonate (NEMP), which is stable in aqueous solution. We have reported the in vitro results with 35 of the novel oximes using a standard screening protocol of rat brain as a ChE source, which has been inhibited with either PIMP or NEMP at a concentration to yield about 80% inhibition (175 nM and 56 nM, respectively), followed by a 15-min reactivation period with a screening concentration (100 μM) of oxime. From these in vitro studies, reactivation was observed from 21–78% with our full panel of 35 novel oximes, compared to 2-PAM at 88–91%. The in vitro reactivation of the five novel oximes brought to in vivo testing (as described below) showed about 40–65% reactivation. Thus, all the novel oximes had some potential for reactivation, although with lesser potency than 2-PAM, but some of them approached the efficacy of 2-PAM,29 and all oximes displayed a similar reactivation potency with both surrogates (Fig. 2).
Figure 2.
Percent cholinesterase (ChE) reactivation in vitro in rat brain homogenates following inhibition with either phthalimidyl isopropyl methylphosphonate (PIMP; sarin surrogate) or nitrophenyl ethyl methylphosphonate (NEMP; VX surrogate) followed by incubation with 100 μM 2-PAM or one of five novel oximes; cholinesterase was measured spectrophotometrically. Method described and data originally published in Ref. 29.
In vivo screening studies with a high sublethal surrogate challenge
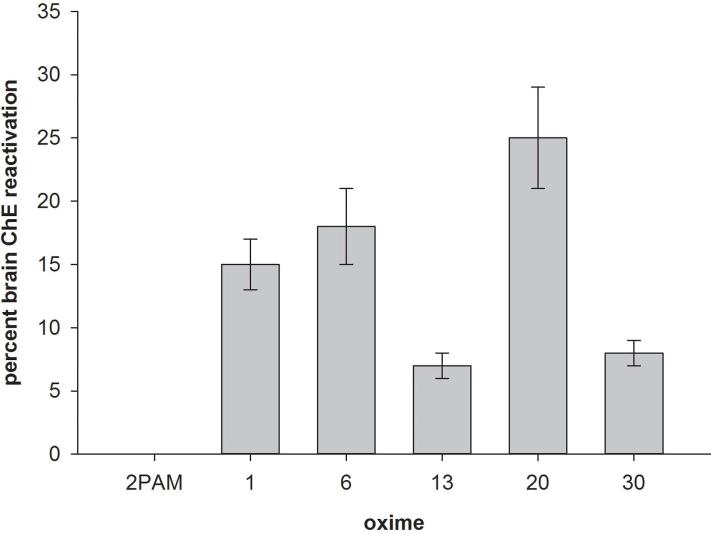
The more effective novel oximes as well as 2-PAM were then screened in an in vivo paradigm designed to indicate whether a novel oxime had the ability to cross the blood–brain barrier. A high sublethal intraperitoneal dosage (0.325 mg/kg) of the sarin surrogate nitrophenyl isopropyl methylphosphonate in DMSO vehicle (NIMP; stable in aqueous solution, so suitable for in vivo testing) was established, which led to about 80% peak brain ChE inhibition; this NIMP dosage did not require atropine or any other type of therapy to assure survival. This level of NIMP challenge resulted in strong signs of cholinergic toxicity, including a display of seizure-like behavior. Time of peak inhibition was 1 h after challenge with NIMP. The oxime, either 2-PAM or a novel oxime (bromide salt, in DMSO) was administered intramuscularly at a screening dosage of 0.1 mmol/kg at the time of peak brain ChE inhibition; therefore, with the surrogate in the process of being cleared, the opportunity for reinhibition of reactivated peripheral ChE would have been minimized or eliminated. Using this paradigm, any reduction in brain ChE inhibition should reflect reactivation by the oxime that has entered the brain. With this paradigm, five out of 11 novel oximes tested displayed a reduction of brain ChE inhibition of at least 5% at 30 min after oxime administration (with a reduction of 25% with the most efficacious oxime) (Fig. 3). 2-PAM yielded no reduction in brain ChE inhibition using this same paradigm. The more effective of the novel oximes also resulted in an attenuation of the seizure-like behavior compared to that observed with 2-PAM.29 Therefore the data obtained with the high sub-lethal challenge paradigm are consistent with the ability of the novel oximes to enter the brain and the inability of 2-PAM to do so.
Figure 3.
Percent cholinesterase (ChE) reactivation in vivo in rat brain homogenates following a high sublethal dosage intraperitoneal challenge with nitrophenyl isopropyl methylphosphonate (NIMP; sarin surrogate) followed by an intramuscular administration of 0.1 mmol/kg 2-PAM or one of five novel oximes; cholinesterase was measured spectrophotometrically. Reactivation was calculated through comparison of brain ChE inhibition in oxime-treated rats to non-oxime–treated rats. Method described and data originally published in Ref. 29.
In vivo lethal dosage challenge studies
Once the above screening for the ability to penetrate the brain identified several lead oximes, lethal dosage challenges were conducted to determine whether these oximes had the ability to be effective antidotes against life-threatening dosages of surrogates. An oxime that could save lives and also penetrate the brain could be an effective replacement for the current oximes that only act peripherally. While our perspective with our novel oximes has been brain-penetration ability, clearly it was important to know whether they were capable of saving lives from lethal dose exposures, especially since our oximes do not display as high in vitro efficacy as reactivators compared to 2-PAM. It was predicted that they would have some efficacy in providing survival and also that they might have a positive effect on seizure-like behavior cessation because of their ability to penetrate the blood–brain barrier and presumably provide a dampening effect on the hypercholinergic activity that results in seizures.
Experiments were set up to determine challenge dosages of NIMP and NEMP that would be lethal to all rats that received only atropine as therapy (essentially an LD99 level) and that would yield only a moderate level of survival with atropine and 2-PAM at 24 h.35 This experimental approach would allow a determination of improved efficacy for survival by our novel oximes if that were the case. Some of the conditions were changed from the earlier sublethal challenge studies to make these experiments more consistent with the lethality studies typically conducted for characterization of nerve agent effects and therapy. Our lead oximes were converted by ion exchange from their original bromide salt form to mesylate salts for better aqueous solubility. The procedure for novel oxime synthesis has been described.36
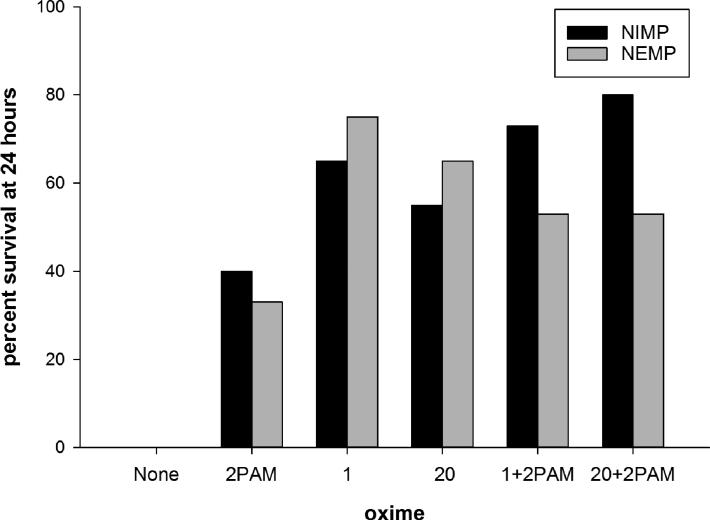
The surrogates were dissolved in Multisol and administered subcutaneously in the back of the neck. (Multisol, a biocompatible vehicle, consists of 48.5% water, 40% propylene glycol, 10% ethanol, and 1.5% benzyl alcohol.) The challenge dosages of NIMP and NEMP administered were 0.6 and 0.65 mg/kg, respectively. Atropine (free base in saline) was given intramuscularly in the thigh muscle at 0.65 mg/kg at 25–30 min after surrogate challenge, the time of onset of seizure-like behavior; this atropine dosage was in the range of atropine typically used by laboratories studying nerve agent therapy in laboratory animal models. Rats treated with oximes also received 2-PAM or novel oximes (in Multisol) intramuscularly at the time of seizure-like behavior onset. Oxime therapy dosage was 0.146 mmol/kg, which is the human equivalent dosage for three autoinjectors of 2-PAM. Novel oximes were tested alone and in combination with 2-PAM, at the same dosage of each oxime that was tested alone. Detailed observations of signs of toxicity including any recovery toward normal behavior were made for the first 8 h following surrogate treatment, and survivors at 24 h were humanely euthanized. Four novel oximes were tested, with oximes 1 and 20 showing better efficacy than 2-PAM, either alone (20 replications each) or in combination with 2-PAM (15 replications each). The other two oximes tested, oximes 6 and 13, were less effective than 2-PAM and their study was discontinued with fewer replications in order to reduce animal usage.35
While 2-PAM in the above-described paradigm yielded 40% survival with NIMP, oximes 1 and 20 alone yielded 65% and 55%, respectively, and oximes 1 and 20 in combination with 2-PAM yielded 73 and 80%, respectively (Fig. 4). While 2-PAM yielded 33% survival with NEMP, oximes 1 and 20 alone yielded 75 and 65%, respectively, and oximes 1 and 20 in combination with 2-PAM both yielded 53%. Therefore, both oxime 1 and oxime 20 performed better than 2-PAM and performed even more effectively in combination with 2-PAM with the sarin surrogate NIMP. While very good performance with oximes 1 and 20 compared to 2-PAM was observed with the VX surrogate NEMP, the combinations of oximes 1 and 20 with 2-PAM were somewhat worse than oximes 1 and 20 alone, although the combinations were still better than 2-PAM alone. The reason behind the differences in results with the two surrogates for the combinations of the novel oximes with 2-PAM is not known, although we speculate that the higher levels of oximes present in the combination therapies with NEMP might result from the formation of higher levels of toxic oxime phosphates, since some oxime phosphates are potent anticholinesterases.19
Figure 4.
Percent survival at 24 h of rats treated with a lethal level challenge of nitrophenyl isopropyl methylphosphonate (NIMP; sarin surrogate) or nitrophenyl ethyl methylphosphonate (NEMP; VX surrogate) plus 0.65 mg/kg atropine. At time of seizure-like behavior initiation (25–30 min), rats tested with oximes were administered intramuscularly 0.146 mmol/kg 2-PAM or novel oximes 1 or 20 alone or in combination with 2-PAM. There were 20 replications for oximes alone or 15 replications for oxime combinations. Method described and data originally published in Ref. 35.
The brain cholinesterase inhibition levels in the 24-h survivors with NIMP were slightly lower in the rats treated with the novel oximes either alone or in combination with 2-PAM (74–78%) compared to 2-PAM alone (85%), but there were no statistically significant differences. The brain cholinesterase inhibition levels in the survivors with NEMP were slightly lower in rats treated with the novel oximes alone (59–63%) compared to 2-PAM alone (66%), and again were not statistically significant. It is not surprising that the levels of brain ChE inhibition at 24 h in novel oxime–treated rats and 2-PAM–treated rats were not appreciably different, since the surrogates were at such high levels at these lethal-level challenges that they probably remained in circulation longer than the oximes did. In contrast to NIMP, rats treated with NEMP and the combinations of novel oximes and 2-PAM had slightly higher brain ChE inhibition than animals treated with either 2-PAM or the novel oximes alone. While no explanation is available at this time, these results are consistent with the lower survival of the combination-treated rats than the novel oximes alone, and could be the result of an oxime phosphate derivative that is a potent anticholinesterase. This potential explanation is under further study in our laboratories.35
Lastly, the animals in these lethal dosage studies were observed for cessation of seizure-like behavior. With both NIMP and NEMP, all 2-PAM treated animals surviving during the first 8 hours were still displaying seizure-like behavior until 8 h after the surrogate challenge. In contrast, all surviving animals treated with oxime 1 or 20, either alone or in combination with 2-PAM, showed cessation of seizure-like behavior by about 6 h. This reduction in time to cessation of seizure-like behavior is consistent with the action of ChE reactivation within the brain by our novel oximes (although these oximes would not be effective in a short enough time frame to replace an anticonvulsant).35
While we do not yet have definitive proof that our oximes penetrate the blood–brain barrier (such as from pharmacokinetic studies, which are planned), our experimental paradigm for the sublethal challenge provided convincing evidence that they are capable of entering the brain. In that paradigm, oximes were not administered until the time of peak brain ChE inhibition, indicating that insufficient surrogate was present in the blood to yield any further brain ChE inhibition. Therefore, oxime was administered at a time of ChE inhibition recovery. Any increase in brain ChE activity greater than observed in the non-oxime–treated controls (as occurred with the novel oximes discussed here) strongly suggests reactivator entry into the brain. In our studies, 2-PAM did not yield any increases in brain ChE activity over controls, consistent with the inability of 2-PAM to appreciably cross the blood–brain barrier. Peripheral ChE reactivation would not have led to increased brain ChE activity. Therefore convincing, although circumstantial, support for the brain penetrability of the novel oximes was obtained. While other reactivator chemistries may also penetrate the blood–brain barrier, most of the in vivo testing done with those were performed either prophylactically or very shortly after the challenge,30–33 making them potentially less useful for unanticipated mass casualties, such as might occur following a terrorist attack. Our novel oximes seem to be able to show efficacy with an appreciable delay following challenge.
Summary and conclusions
Using lethal-level challenges of surrogates for sarin and VX that are highly relevant for ChE reactivation studies, our laboratories have accumulated evidence that some novel substituted phenoxyalkyl pyridinium oximes invented at Mississippi State University can assure better 24-h survival than 2-PAM in laboratory rats. In addition, these novel oximes reduced the time until cessation of seizure-like behavior. Using high sublethal–level challenges of these surrogates, the novel oximes but not 2-PAM resulted in a reduction of brain ChE inhibition at 30 min using a paradigm that was designed to identify reactivators that could penetrate the blood–brain barrier; these oximes also attenuated seizure-like behavior in these rats. Thus, data have accumulated on these novel oximes that are consistent with the ability of these oximes to penetrate the blood–brain barrier and reactivate sarin and VX surrogate–treated laboratory rats. Our laboratories are continuing experiments characterizing the biological efficacy of these novel oximes.
Acknowledgments
This research was supported in part by the Defense Threat Reduction Agency (1.E0056-08-WR-C) through the Henry M. Jackson Foundation for the Advancement of Military Medicine, INC (0000169320); the funding source did not provide input into the study's design, conduct or interpretation. This research was also supported in part by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number U01NS083430. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors wish to thank Marilynn Alldread, Joshua Bennett, W. Shane Bennett, Erle Chenney, C. Andrew Leach, Royce Nichols, Ronald Pringle and Dr. Robert Wills for their assistance with technical procedures or data analysis.
References
- 1.Ecobichon DJ. Toxic effects of pesticides. In: Klaassen CD, editor. Casarett and Doull's Toxicology. McGraw-Hill; New York: 2001. pp. 763–810. [Google Scholar]
- 2.Taylor P, Radic Z, Hosca NA, et al. Structural basis for the specificity of cholinesterase catalysis and inhibition. Toxicol. Lett. 1995;82-83:453–458. doi: 10.1016/0378-4274(95)03575-3. [DOI] [PubMed] [Google Scholar]
- 3.Tucker JB. War of Nerves: Chemical Warfare from World War I to Al-Qaeda. Pantheon Books; New York, NY: 2007. [Google Scholar]
- 4.Johnson NH, Larson JC, Meek EC. Historical perspectives of chemical warfare agents. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. Elsevier; London, U.K.: 2015. pp. 7–16. [Google Scholar]
- 5.Smart JK. History of chemical and biological warfare: an American perspective. In: Sidell FR, Takafuji ET, Franz DR, editors. Medical Aspects of Chemical and Biological Warfare, Textbook of Military Medicine. Office of the Surgeon General, Bordon Institute; Washington, D.C.: 1997. pp. 9–86. [Google Scholar]
- 6.Aldridge WN, Reiner E. Enzyme inhibitors as substrates—Interactions of esterases with esters of organophosphorus and carbamic acids. North-Holland Publishing Company; Amsterdam, London: 1972. [Google Scholar]
- 7.Worek F, Diepold C, Eyer P. Dimethylphophoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch. Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- 8.Shafferman A, Ordentlich A, Barak D, et al. Aging of phosphylated human acetylcholinesterase: catalytic processes mediated by aromatic and polar residues of the active centre. Biochem. J. 1996;318:833–840. doi: 10.1042/bj3180833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worek F, Thiermann H, Szinicz L, et al. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem. Pharmacol. 2004;68:2237–2248. doi: 10.1016/j.bcp.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 10.Marrs TC, Maynard RL, Sidell FR. In: Chemical Warfare Agents: Toxicology and Treatment. Marrs TC, editor. John Wiley & Sons; Wiltshire: 2007. [Google Scholar]
- 11.Brown JH, Taylor P. Muscarinic receptor agonists and antagonists. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th Ed. McGraw-Hill; New York, NY: 2001. pp. 155–173. [Google Scholar]
- 12.Gerald DR., Sr. Organophosphate poisoning. Emerg. Med. Serv. 2002;31:64–69. [PubMed] [Google Scholar]
- 13.Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J. Physiol. Paris. 1998;92:375–378. doi: 10.1016/S0928-4257(99)80008-4. [DOI] [PubMed] [Google Scholar]
- 14.Mercey G, Verdelet T, Renou J, et al. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res. 2012;45:756–766. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- 15.Worek F, Thiermann H. The value of novel oximes for treatment of poisoning by organophosphorus compounds. Pharmacol. Ther. 2013;139:249–259. doi: 10.1016/j.pharmthera.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J. Toxicol. Clin. Toxicol. 2002;40:803–816. doi: 10.1081/clt-120015840. [DOI] [PubMed] [Google Scholar]
- 17.Worek F, Szinicz L, Eyer P, et al. Evaluation of oxime efficacy in nerve agent poisoning: development of a kinetic-based dynamic model. Toxicol. Appl. Pharmacol. 2005;209:193–202. doi: 10.1016/j.taap.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Shih TM, Skovira JW, O'Donnell JC, et al. In vivo reactivation by oximes of inhibited blood, brain and peripheral tissue cholinesterase activity following exposure to nerve agents in guinea pigs. Chem. Biol. Interact. 2010;187:207–214. doi: 10.1016/j.cbi.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Herkenhoff S, Szinicz L, Rastogi VK, et al. Effect of organophosphorus hydrolyzing enzymes on obidoxime induced reactivation of organophosphate-inhibited human acetylcholinesterase. Arch Toxicol. 2004;78:338–343. doi: 10.1007/s00204-004-0547-2. [DOI] [PubMed] [Google Scholar]
- 20.McDonough JH, Jr., Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci. Biobehav. Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 21.Wilson IB, Ginsburg B. A powerful reactivator of alkylphosphate-inhibited acetylcholinesterase. Biochim. Biophys. Acta. 1955;18:168–170. doi: 10.1016/0006-3002(55)90040-8. [DOI] [PubMed] [Google Scholar]
- 22.Clement JG. Efficacy of pro-PAM (N-methyl-1,6-dihydropyridine-2-carbaldoxime hydrochloride) as a prophylaxis against organophosphate poisoning. Toxicol. Appl. Pharmacol. 1979;47:305–311. doi: 10.1016/0041-008x(79)90325-9. [DOI] [PubMed] [Google Scholar]
- 23.Sakurada K, Matsubara K, Shimizu K, et al. Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochem. Res. 2003;28:1401–1407. doi: 10.1023/a:1024960819430. [DOI] [PubMed] [Google Scholar]
- 24.Kenley RA, Howd RA, Uyeno ET. Effects of PAM, proPAM, and DFP on behavior, thermoregulation, and brain AChE in rats. Pharmacol. Biochem. Behav. 1982;17:1001–1008. doi: 10.1016/0091-3057(82)90485-3. [DOI] [PubMed] [Google Scholar]
- 25.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol. Appl. Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 26.McDonough JH, Jr., Jaax NK, Crowley RA, et al. Atropine and/or diazepam therapy protects against soman-induced neural and cardiac pathology. Fundam. Appl. Toxicol. 1989;13:256–276. doi: 10.1016/0272-0590(89)90262-5. [DOI] [PubMed] [Google Scholar]
- 27.McDonough JH, Jr., Zoeffel LD, McMonagle J, et al. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 28.Gilat E, Kadar T, Levy A, et al. Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol. Appl. Pharmacol. 2005;209:74–85. doi: 10.1016/j.taap.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Chambers JE, Chambers HW, Meek EC, et al. Testing of novel brain-penetrating oxime reactivators of acetylcholinesterase inhibited by nerve agent surrogates. Chem. Biol. Interact. 2013;203:135–138. doi: 10.1016/j.cbi.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Skovira JW, O'Donnell JC, Koplovitz I, et al. Reactivation of brain acetylcholinesterase by monoisonitrosoacetone increases the therapeutic efficacy against nerve agents in guinea pigs. Chem. Biol. Interact. 2010;187:318–324. doi: 10.1016/j.cbi.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Shih TM, Skovira JW, O'Donnell JC, et al. Treatment with tertiary oximes prevents seizures and improves survival following sarin intoxication. J. Mol. Neurosci. 2010;40:63–69. doi: 10.1007/s12031-009-9259-7. [DOI] [PubMed] [Google Scholar]
- 32.Kalisiak J, Ralph EC, Cashman JR. Nonquaternary reactivators for organophosphate-inhibited cholinesterases. J. Med. Chem. 2012;55:465–474. doi: 10.1021/jm201364d. [DOI] [PubMed] [Google Scholar]
- 33.Radic Z, Sit RK, Kovarik Z, et al. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J. Biol. Chem. 2012;287:19337. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meek E, Chambers H, Coban A, et al. Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol. Sci. 2012;126:525–533. doi: 10.1093/toxsci/kfs013. [DOI] [PubMed] [Google Scholar]
- 35.Chambers JE, Meek EC, Bennett JP, et al. Novel substituted phenoxyalkyl pyridinium oximes enhance survival and attenuate seizure-like behavior of rats receiving lethal levels of nerve agent surrogates. Toxicology. 2016;339:51–57. doi: 10.1016/j.tox.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meek EC, Chambers HW, Pringle RB, et al. The effect of PON1 enhancers on reducing acetylcholinesterase inhibition following organophosphate anticholinesterase exposure in rats. Tox. Sci. 2015;336:79–83. doi: 10.1016/j.tox.2015.08.002. [DOI] [PubMed] [Google Scholar]