Abstract
Though valuation processes are fundamental to survival of the human species, hedonic dysregulation is at the root of an array of clinical disorders, including addiction, stress, and chronic pain, as evidenced by the allostatic shift in the relative salience of natural reward to drug reward observed among persons with severe substance use disorders. To address this crucial clinical issue, novel interventions are needed to restore hedonic regulatory processes gone awry in persons exhibiting addictive behaviors. This article describes a theoretical rationale and empirical evidence for the effects of one such new intervention, mindfulness-oriented recovery enhancement (MORE), on top-down and bottom-up mechanisms implicated in cognitive control and hedonic regulation. MORE is innovative and distinct from extant mindfulness-based interventions in that in unites traditional mindfulness meditation with reappraisal and savoring strategies designed to reverse the downward shift in salience of natural reward relative to drug reward, representing a crucial tipping point to disrupt the progression of addiction, which no other behavioral intervention has been designed to do. Though additional studies are needed, clinical and biobehavioral data from several completed and ongoing trials suggest that MORE may exert salutary effects on addictive behaviors and the neurobiological processes that underpin them.
Keywords: mindfulness, reappraisal, savoring, allostasis, reward, addiction
Introduction
In the past decade, the nascent field of contemplative science has struggled with the notion of value, wrestling to reconcile the traditional Buddhist prohibition against attachment with the Western traditions of hedonic and eudaimonic well-being. Studies suggest that mindfulness techniques may reduce evaluative processing in the brain.1–3 Yet, it would be premature to conclude that mindfulness and valuation are necessarily opposing cognitive processes and an oversimplification to assume that all forms of evaluative processing are sources of suffering. To the contrary, human values can provide a deep source of meaning. At the same time, valuation processes can become pathological, and may require treatments to augment hedonic regulation. It is with these considerations that mindfulness-oriented recovery enhancement (MORE)4 was developed as a distinct, novel mindfulness-based intervention (MBI) to help people recover from the clutches of addiction.
Hedonic valuation is fundamental to flexible adaption of an organism to its changing environmental context. The perceived reward and punishment value of environmental stimuli, encoded in dopaminergic and opioidergic activation of mesolimbic brain circuits, elicits approach-oriented (e.g., consummatory, affiliative) or avoidance-oriented (e.g., flight, defensive) behavioral repertoires, which preserve the survival of the individual and the species. While pleasure represents the subjective hedonic value of rewarding stimuli, pain represents the suffering (hedonic) and avoidance (motivational) responses evoked by a painful experience; preclinical and clinical studies suggest that these two opposing phenomena operate via an overlapping set of neural systems and neurochemical processes5 that instantiate a “common currency” of emotion in the brain.6 In other words, pleasure (and its relative absence) can be viewed as the common currency by which the central nervous system values and prioritizes various homeostatic goals, thereby allowing for selection among competing motives to organize and drive behavior.7,8
Despite phylogenetic conservation of neural mechanisms for hedonic valuation in the mammalian brain, the capacity to flexibly attribute value to stimulus contexts has evolved to be shaped by higher-order cognitive processes, including attentional control, beliefs, metacognition, and meaning making itself. That is, while all animals experience the desire to seek valued stimuli and to avoid painful stimuli, some philosophers propose that only humans have the desire to evaluate their own desires,9 and a parsimonious read of the data suggests that this is indeed the case.10 This apparently essential human attribute allows for a radical reframing of the meaning of stimuli, manifest in both daily life and in more extreme instances, such as grueling athletic feats of endurance, where the experience of pain can be transformed into pleasure, as well as in experiences of heartbreak, when a lover who was once coveted becomes abhorrent. Thus, hedonic experience can be regulated, both consciously and unconsciously, to modulate cognition, affect, and behavior, serving to mobilize resources and facilitate goal attainment even under conditions of heightened stress.
Though humans have the capacity to re-learn the hedonic value of stimuli, these same reward-learning processes can become dysregulated. Indeed, certain classes of rewarding stimuli that may not have been readily present in the natural environments of our mammalian ancestors are now ubiquitous and have the capacity to powerfully shape valuation processes. In that regard, the processing of natural rewards may be usurped by psychoactive substances that capitalize on the mesolimbic dopamine system to drive addictive behavior.11 This hijacking of brain reward systems occurs when the motivation to obtain natural rewards is re-organized around seeking drug-induced reward and the desire to alleviate dysphoria induced by withdrawal and aversive experiences (e.g., stress and pain).12,13 Drug-induced restructuring of reward learning is thought to result from neuroplastic modifications to brain circuits subserving stress (e.g., extended amygdala) and reward (e.g., ventral striatum), such that individuals with substance use disorders may become increasingly insensitive to natural reward from healthful and socially affiliative stimuli, while becoming increasingly sensitized to stress and dependent on drugs to preserve a dwindling sense of well-being.14 Ultimately, this allostatic process fuels the downward shift in the hedonic set point that undergirds addiction.
At the same time, disruptions in hedonic experience are not the sole cause of addictive behavior. Indeed, dual-process models posit that addiction results from dysregulation of bottom-up neural circuitry that codes for the salience of reward-related stimuli as well as impaired top-down frontal-executive brain circuitry that subserves cognitive-control processes, including proactive regulation of attention and emotion. Impairments in frontal-executive function prevent effective inhibitory control of overlearned, highly stereotyped, cognitive and behavioral repertoires for drug seeking and consumption, also known as automatic drug use action schemas, 15,16 which may be encoded via habit circuitry centered on the striatum.17,18 These schemas are elicited by drug-related interoceptive and exteroceptive cues, for which attention may become biased due to dopamine-driven incentive salience conferred by prior drug use episodes.19 When the prefrontal–striatal feedback loop is disrupted, the ability to regulate addictive automaticity is compromised. Stress, negative affect, and pain provide additional interoceptive input to elicit drug use schemas and further dysregulate prefrontal control over automaticity,20–23 amplifying addictive behavior.24 Neural systems underpinning these aversive states partially overlap and interact with those that underlie hedonic valuation of natural and drug rewards.13 The relative imbalance of hedonic and aversive states and dysregulation in the cortico–limbic–striatal networks that instantiate them results in an overall reward-processing deficit that may propel the downward spiral of behavioral escalation toward compulsive drug seeking.
Insofar as addiction involves dysfunction in controlled and automatic processes implicated in self-regulation and hedonic experience, it may be tractable to mental training programs that simultaneously target top-down and bottom-up mechanisms in the risk chain linking stress to addiction (Fig. 1). In that regard, MORE unites complementary aspects of mindfulness training, third-wave cognitive behavioral therapy (CBT), and principles from positive psychology into an integrative, dual-process intervention designed to target this multivariate risk chain. Though MORE has a range of targets (Fig. 2), the remainder of this paper focuses on the effects of MORE on shifting the relative salience of drug and natural reward as a key mechanism of action, presenting theoretical and empirical support for this claim. Because MORE differs from other MBIs largely on account of this hedonic mechanism, it deserves a more extensive discussion. Like other MBIs, MORE provides training in mindfulness meditation techniques (e.g., mindful breathing, body scan) that integrate focused attention and open monitoring styles of meditation.25 MORE may be innovative and unique in that in unites traditional mindfulness meditation with strategies designed to reverse the downward shift in salience of natural reward relative to drug reward, representing a crucial tipping point to disrupt the progression of addiction, which no other behavioral intervention has been designed to do. These unique strategies, integrating mindfulness with reappraisal and savoring techniques to restructure reward processes, are detailed in the next section.
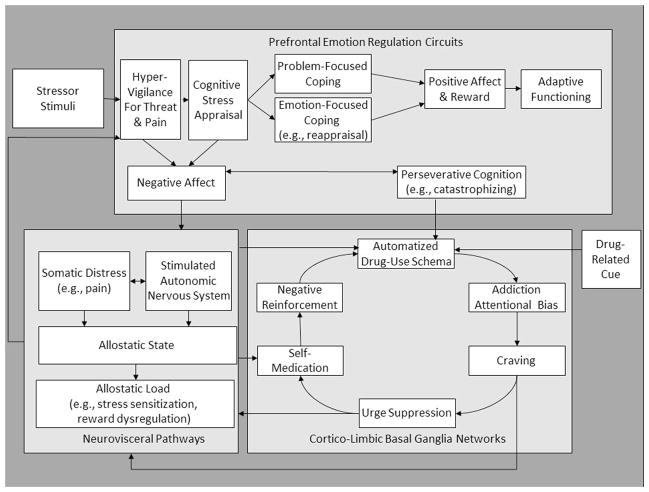
Figure 1.
A simplified schema of the risk chain linking stress to addictive behavior. In brief, stress involves a process of cognitive appraisal, where the individual consciously and unconsciously evaluates the stressor and its significance to the self.58 If the stressor is deemed to be a threat, autonomic and neuroendocrine systems adapt to the perturbations incurred by the stressor, that, when prolonged, result in allostatic load,59 including dysregulation of the extended amygdala, resulting in increased sensitization to stress and pain, and decreased sensitivity to natural reward, as discussed earlier. Yet, the stress process also involves coping. If the individual deems that the stressor is resolvable, s/he may engage in problem-focused coping; otherwise, s/he may engage in emotion-focused coping efforts, such as reappraisal.55 However, if the individual is unable to engage either of these forms of coping, s/he may fall into a cycle of perseverative cognition, which amplifies negative affect and stress physiology.60 When an individual with a history of using substances to cope is under such heightened conditions of distress and is presented with a drug-related cue, this activates drug-use action schema, biasing attention toward drug-relevant stimuli and amplifying craving.16 In the case of an individual who is attempting to abstain from or moderate drug use, s/he may try to suppress cravings, which paradoxically increases sympathetic nervous system activation and exhausts self-control resources.61 Thus, the individual surrenders to the urge and engages in substance use, which strengthens the addictive habit through negative reinforcement conditioning.62 Note that, for visual parsimony, important and complex recursive relationships among the presented constructs are omitted. For instance, drug cues can elicit increases in negative affect under many conditions,23 and stressor stimuli can serve as drug-related cues to directly activate drug-use action schema.16
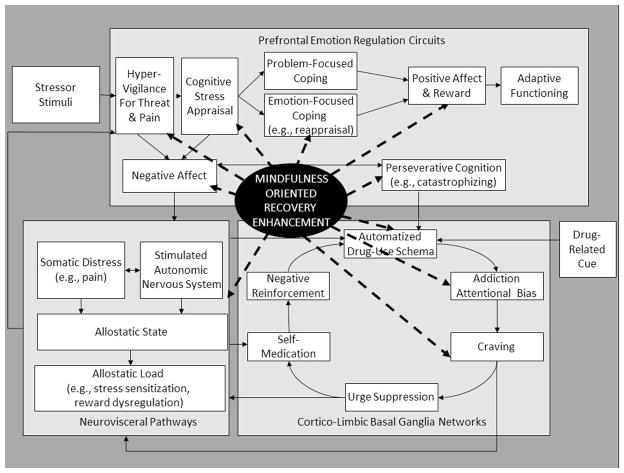
Figure 2.
MORE targets the risk chain linking stress to addictive behavior at a number of points. These points are enumerated below not in terms of priority, but rather in the temporal order that stress is thought to precipitate addictive responding. First, MORE aims to clarify cognitive appraisal processes, enabling the individual to achieve a more functional evaluation of the stressor context and his or her ability to cope with it. Second, MORE aims to decrease attentional hypervigilance toward threat and distressing somatic sensations, reducing exteroceptive and interoceptive input that might otherwise fuel the stress–addiction cycle. Third, MORE aims to increase regulation of negative emotions and maladaptive cognitions by strengthening emotion-focused coping processes, such as reappraisal. Fourth, MORE aims to directly stimulate natural-reward processing through cognitive training in savoring pleasant daily experiences. Fifth, MORE aims to disrupt perseverative cognition through mindful decentering from negative automatic thoughts. Sixth, MORE aims to strengthen top-down cognitive control over bottom-up drug-use action schemas via informal mindfulness practices designed to amplify awareness of automaticity. Seventh, MORE aims to decrease addiction attentional bias by strengthening attentional re-orienting capacity via mindful breathing techniques. Eighth, MORE aims to increase interoceptive awareness of craving and, consequently, to increase the ability to regulate craving, by deconstructing the craving experience into its constituent sensations, thoughts, emotions, and memories, and then countering them through metacognitive contemplation of the reasons to remain abstinent. Ninth, MORE aims to provide an effective alternative to suppression of unpleasant cognitions and drug cravings through mindful exposure and acceptance. And lastly, by promoting the ability to flexibly deploy the parasympathetic nervous system to balance acute and chronic sympathetic activation, MORE aims to downregulate the physiological stress reaction itself.
Transforming addiction through the synergy of mindfulness, reappraisal, and savoring
If neural reward circuits provide a basic logic for goal selection,26 sustained training in selective attention to natural rewards over drug rewards may provide the learning signal needed to reverse addiction and restore default reward-processing parameters back toward valuation of intrinsically proregulatory objects and behaviors. This therapeutic process involves evocation of a self-reflexive, metacognitive awareness that transforms emotion via recoding of experience—a global workspace of consciousness in which schemas that encode the value of stimuli can be revised.27
With this overarching intention, MORE aims to modify associative learning mechanisms hijacked during the allostatic process of addiction by strengthening top-down proactive cognitive-control functions to restructure bottom-up reward learning from valuation of drug rewards to valuation of natural rewards.a This restructuring of reward processing may arise from restoration of the feedback loop between frontoparietal structures essential to metacognition and attention and limbic–striatal circuitry crucial to learning and motivation (Fig. 3).28 In this respect, MORE may represent a significant advance in the application of contemplative science to clinical intervention. While many existing MBIs use mindfulness to disrupt patterns of maladaptive associative learning, they eschew an explicit focus on promoting evaluative processing and therefore do not directly or intentionally engage in restructuring of learned responses. In contrast, MORE directly aims to restructure drug cue reactivity by enhancing natural reward responsiveness, using mindfulness to facilitate top-down, intentional governance of first- and second-order valuation processes.29 For addiction, which is primarily a disorder of salience dysregulation and learning processes gone awry, restructuring reward learning through reevaluation (second-order valuation) may be essential. In effect, the recovering addict must relearn on both conscious and unconscious levels what is, and is not, important in life, reevaluating the meaning of conditioned stimuli and responses that have become automatized over repeated cycles of positive and negative reinforcement by pharmacological agents of often great potency. For instance, is a drug craving to be slavishly obeyed, even in the face of adverse consequences to self and others? Is the love of one’s family more important than the fleeting pleasure of a drug high? To accomplish this reevaluation, what is needed is to suspend the initial habitual appraisal, broaden awareness to allow for novel information processing, and thereby increase sensitivity to the aversive contingencies of addiction and to the potential rewards of recovery.
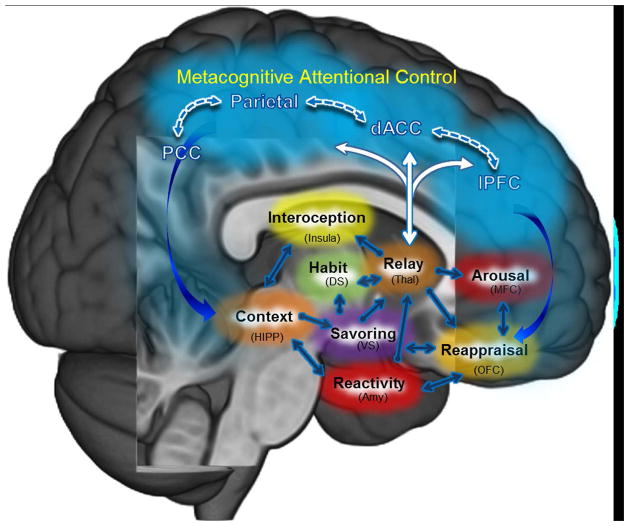
Figure 3.
Brain networks hypothesized to instantiate mindfulness-centered regulation of addictive behavior. The central tenet of this model28 posits that MORE and other mindfulness-based interventions may remediate the dysregulated habit behaviors, craving, and negative affect undergirding addiction by way of strengthening functional connectivity (1) within a metacognitive attentional control network (dlPFC, dACC, parietal cortex) and (2) between the metacognitive attentional control network and brain circuits subserving automaticity, memory consolidation, interoceptive awareness, and hedonic valuation processes. The model proposes that mindfulness training boosts connectivity between prefrontal, cingulate, and parietal nodes of an executive regulatory circuit to provide feedback to the striatum and medial temporal lobe on the reward value of various courses of action and their alignment with one’s overall goal orientation. Enhanced dynamic communication within this mindfulness-centered regulatory network may allow individuals to gain awareness of automatized behavioral habits, restructure conditioned associations, and maintain an optimal hedonic tone to support adaptive functioning. Abbreviations: dlPFC, dorsolateral prefrontal cortex; dACC, dorsal anterior cingulate cortex; PCC, posterior cingulate cortex; DS, dorsal striatum; VS, ventral striatum; Thal, thalamus; HIPP, hippocampus; Amy, amygdala; OFC, orbitofrontal cortex; MFC, medial prefrontal cortex.
New theory suggests that mindfulness meditation is well-suited to facilitate this reevaluation process. According to the mindfulness-to-meaning theory,30 mindfulness meditation can be used to disengage from automatic schema into a metacognitive state of awareness in which attention expands to encompass previously unattended data from which new cognitive structures can be constructed through a process of memory reconsolidation. Applying this theory to addiction,31 mindfulness may be used to interrupt drug-use action schema, which can then be consciously reconfigured in working memory to subserve adaptive goal pursuit (i.e., abstinence). Insofar as mindfulness allows access to an expanded set of information from which semantic and conditioned associations may be restructured,32 it can be used to enhance interoceptive awareness of the salience of various courses of action and experiences, thereby optimizing prediction error signaling in the brain to increase goodness-of-fit between behavior and goal states.33 This reconfiguration of salience attribution flexibly retunes attention onto novel targets, affording an enriched experience of their reward value in relation to their larger context as hedonic experience becomes infused with eudaimonic meaning. Through this process, mindfulness connects previously conditioned stimuli with new eudaimonic meanings, not by rejecting difficult life experiences and hedonics, but instead by situating adversity and hedonics into a novel context, allowing for an adaptive response rather than one dictated by past conditioning. In this way, the mindfulness-to-meaning theory proposes that mindfulness provides a means of restructuring reward learning.
In keeping with this theoretical framework, in MORE, patients are first taught mindful breathing and body scan meditations to build attentional stability, set-shifting capacity, and increased metacognitive awareness. As the patient develops greater cognitive control, mindfulness skills are used to synergize more advanced contemplative techniques not found in other MBIs that are designed to restructure valuation processes underpinning addiction. For instance, in MORE, patients are taught to actively contemplate the consequences of indulging in and abstaining from the addictive behavior. During this process, mindfulness is used to first suspend extant drug-use action schema and then to stabilize attention on affectively laden mental simulations of potential future consequences. When mind wandering occurs, the patient reorients attention back to the mental simulation of these consequences, elaborating on them and gradually building a wider network of associations until they become infused with meaning. This technique is part of a broader family of mindful reappraisal approaches taught in MORE, which have parallels in the Tibetan mind-training practices of duk ngal lam du drub pa30 (“transforming adversity into the path of enlightenment”), which use mindfulness to potentiate cognitive reappraisal34 of maladaptive thoughts contributing to negative emotions and addictive behaviors. MORE aims to increase psychological flexibility by explicitly teaching mindfulness skills in tandem with cognitive restructuring techniques. Patients are taught to disengage from negative appraisals and restructure them until they abate, and adaptive appraisals are constructed to promote adaptive coping and a sense of eudaimonic meaning.
In complementary fashion, MORE provides training in mindful savoring skills, in which mindfulness meditation is used to intentionally orient and sustain attention on the sensory features (i.e., visual, auditory, olfactory, gustatory, or tactile) of natural rewards while metacognitively reflecting on any positive emotions or higher-order meaning arising in response to the rewarding event, without clinging. This latter point is crucial: MORE does not promote attachment to positive experience, but rather aims to foster a deep appreciation of moment-by-moment positive experience, no matter how fleeting. To learn savoring, patients are first instructed to focus mindful attention on a bouquet of flowers, attending to and appreciating their pleasant colors, textures, and scents, as well as the touch of the petals against their skin. During this process, participants are instructed to adopt a metacognitive awareness of their experience, and to attend to and absorb any positive emotions arising from their encounter with the flowers, as well as any cognitive or affective dimensions of meaning emerging from the experience. This kind of savoring involves not only attending to the most perceptually salient features of an object or event, but also becoming conscious of its more subtle features and affective impressions, broadening and deepening the array of sensations and experiences to be derived from the savored experience.35,36 This savoring technique has parallels in meditative practices from the Tibetan Bön five-elements tradition,37 which were held to produce sensations of contentment and bliss, as well as rebalance hedonic tone in the face of stressors and perturbations from the socioenvironment. Patients are instructed to practice mindful savoring with naturally rewarding objects and events in their everyday lives—for example, to savor the beauty of a prismatic sunset, the satisfaction from a job well done, the gentle touch of a loved one’s hand, or the sibilant trill of a bird in a tree.
These mindful reappraisal and mindful savoring techniques represent complex sequences of emotion regulatory strategies, involving iterative admixtures of attentional orienting, appraisal, valuation, acceptance, cognitive broadening, and reappraisal. By integrating traditional mindfulness meditation with reappraisal and savoring, MORE aims to enhance emotion regulation flexibility, providing a range of strategies to be adaptively deployed, depending on the need or context (as an example, see Figure 4 for a flow of intervention components, targets, and mechanisms in an adaptation of MORE for opioid misuse among chronic pain patients). For instance, when addiction attentional bias is to be disrupted, mindful breathing can be engaged; when negative affect is to be downregulated, mindful reappraisal can be employed; and when positive affect is to be upregulated, mindful savoring can be utilized. When these strategies are looped recursively within and across emotion regulatory episodes, reentrant processing may be fostered, broadening contextual awareness and building novel meanings. This therapeutic approach is innovative, in that extant MBIs ostensibly use mindfulness to disrupt elaborative processing rather than to guide and potentiate it.b At the same time, the MORE approach has similarities with classical contemplative practices focused on developing insight, in which the practitioner is instructed to devote mindful attention to consciously contemplate various precepts, philosophical realizations, or koans. Indeed, I (and other scholars38) argue that one original intention of mindfulness was to stabilize attention in service of focusing on salutary appraisals, that is, “right views” of that most fundamental of human addictions—the addiction to the concept of an unchanging and permanent Self. In Buddhist traditions, mindfulness was not an end in itself, but rather a skillful means of gaining insight into the interdependent and impermanent nature of reality. In this sense, MORE is fully consistent with the tradition of utilizing mindfulness to achieve clear comprehension (samprajanya) and wisdom (prajna).c Yet, rather than foist specific views or values upon clients, MORE aims to help individuals construct their own sense of personal meaning to fuel the recovery process.
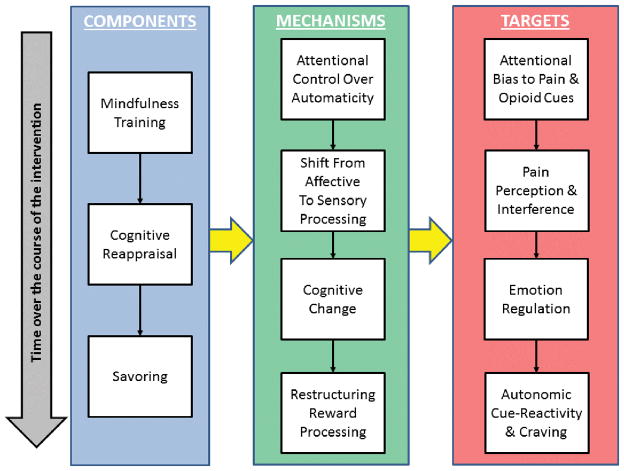
Figure 4.
Model of the therapeutic action of MORE as a treatment for prescription opioid misuse among chronic pain patients. This schema depicts the temporal flow of intervention components from top to bottom, beginning with mindfulness training, which, by virtue of its effects on increasing attentional control and metacognitive awareness, is thought to synergize later training in cognitive reappraisal and savoring skills. This treatment sequence is designed to capitalize on the synergy of these components to stimulate a cascade of therapeutic mechanisms, which build on one another and require increasing degrees of cognitive control (e.g., capacity for sustained attention, attentional re-orienting, inhibitory control, working memory, set shifting). These mechanisms include attentional regulation of automatic cognitive and behavioral habits; disengaging and shifting attention from emotional to sensory processing of pain and craving sensations (i.e., cultivating interoceptive awareness in the context of pain and craving); cognitive change via flexibly selecting among many possible meanings of adverse life circumstances; and restructuring the relative incentive salience of natural and drug-related rewards. These mechanisms are intended to produce stepwise change in a series of intervention targets that are addressed sequentially, as motivational ambivalence is resolved and participants become ready to alter their opioid use patterns. Note that this model is a simplification of a complex, recursive, and multivariate process. This conceptual framework posits numerous specific pathways from components to mechanisms to targets (i.e., the three components are hypothesized to produce differential effects on the various targets via different weightings of mechanistic influences), but, for visual parsimony, we have chosen to represent the causal flow in a simplified manner with yellow arrows.
In these ways, MORE uses mindfulness as a cognitive amplifier to enhance a domain-general cognitive resource for restructuring reward-learning processes, including boosting the flexibility of attention allocation to re-evaluate and align one’s behavior and goals. Once adaptive cognitive and behavioral sequences have been constructed and practiced, they may be evoked with less effort and top-down cognitive control. It remains an open question as to what dose of MORE is optimal to restructure valuation of drug reward to natural reward.
Clinical impact and biobehavioral mechanisms of MORE
Recent studies support these claims of clinical benefit and hypothesized mechanisms of action. Thus far, MORE has been tested in three completed randomized controlled trials (RCTs). In the first trial, MORE was tested as a treatment for alcohol-dependent adults (n = 53) in long-term residential treatment. The results indicated that, compared to a support-group control condition (n = 26), MORE (n = 27) was associated with greater reductions in perceived stress and thought suppression, as well as enhanced recovery of parasympathetically mediated heart rate variability (HRV) from stress and alcohol cue exposure at post-treatment.39 In a second trial, a modified version of MORE was tested as a treatment for prescription opioid misuse among chronic pain patients prescribed long-term opioid analgesics (n = 115). Compared with a support-group control condition (n = 58), participants in MORE (n = 57) evidenced greater reductions in pain, stress arousal, and opioid craving, and were more likely to no longer meet criteria for opioid use disorder at post-treatment.40 In a third pragmatic RCT (n = 180), participants with co-occurring substance dependence, traumatic stress, and psychiatric disorders were randomly assigned to participate in MORE (n = 64), CBT (n = 64), or treatment-as-usual in a therapeutic community (n = 52). MORE outperformed the CBT intervention with regard to reductions in drug craving, posttraumatic stress, and negative affect, and produced greater increases in positive affect than treatment as usual.41 In addition to these trials, a recent pilot study indicated that participation in MORE is associated with significant reductions in cigarette smoking among nicotine-dependent individuals.42,43 Ongoing research is examining the effects of MORE on behavioral addictions, including internet gaming disorder and overeating among obese cancer survivors.44
Subsequent mechanistic analyses indicated that participation in MORE was associated with reduced attentional bias for emotionally threatening cues,45 improved parasympathetically mediated HRV during attention to emotional information,46 and enhanced cardiac46 and electrocortical indices of natural reward processing (i.e., late positive potential (LPP).47 Further, MORE appears to exert addiction-specific effects, including decreasing the correlation strength between drug craving and addictive behavior,40 reducing drug cue reactivity,46 and modulating attentional bias for addiction-related stimuli.39,48 In support of the theoretical proposal articulated in the current paper, MORE’s effects on increasing autonomic and electroencephalographic responses to natural reward stimuli were associated with reductions in drug craving,46,47 suggesting that MORE may restructure reward processing. This hypothesis has garnered additional support from recent analyses indicating that MORE increases autonomic responsiveness to natural reward cues relative to drug cues, and that such increases in relative responsiveness of natural to drug-related reward significantly predict decreased substance misuse at follow-up.48 Taken together, MORE’s therapeutic effects on transdiagnostic mechanisms and addiction-specific targets suggest its potential promise as an intervention for addictive disorders and other comorbid conditions.
Preliminary neuroimaging evidence suggests that the synergy of mindfulness, reappraisal, and savoring skills training in MORE may treat dysfunction in prefrontal–striatal circuits integral to reward processing and addiction. In a pilot functional magnetic resonance imaging (fMRI) study of MORE as an intervention for nicotine addiction,42,43 smokers (n = 13) participated in either MORE or a time-control condition, and underwent two fMRI scans—one at baseline and one at week 8. During the fMRI protocol, smokers completed a cue-reactivity task, in which cigarette images were viewed, and an event-related positive emotion–regulation task, in which participants were asked to either view a positive image or upregulate positive affective responses to the image via savoring. On the cue-reactivity task, significant group x time effects were observed in the rostral anterior cingulate cortex (rACC) and ventral striatum, such that participants in the MORE intervention exhibited significant decreases in striatal and rACC responses to cigarette cues over time relative to those in the no-treatment control condition. In complementary fashion, during the positive emotion–regulation task, significant group x time effects were observed in the ventral striatum and rACC for savor trials relative to view trials, such that compared to the no-treatment control group, participation in MORE was associated with increased striatal and rACC responses during savoring. As additional evidence that MORE may restructure reward processing, increases in striatal and rACC savoring responses significantly predicted increases in positive affect and decreases in the number of cigarettes smoked over the 8-week study period. Moreover, significant group x time effects were observed for resting-state functional connectivity (rsFC), such that smokers participating in MORE evidenced significantly greater increases in rsFC between the rACC and the orbitofrontal cortex, which were also correlated with reduced smoking, improved positive affect, and enhanced ventral striatal activation during savoring. Study findings, while preliminary, suggest that participation in MORE is associated with a restructuring of frontostriatal circuitry functions implicated in hedonic regulation and addictive behavior.
It should be noted that the research described herein is of a developmental nature, and that the clinical outcomes and mechanistic findings discussed above require replication in additional, well-controlled studies. Indeed, the MORE research program is in its inception. Though prior RCTs of MORE have employed active control conditions39,40 in an attempt to control for nonspecific therapeutic factors (e.g., social support, attention by a caring professional, expectation of benefit) that might otherwise confound evaluations of intervention efficacy, only recently has MORE begun to be compared to more robust, empirically supported therapies (e.g., CBT; see Ref. 49). Additional studies are needed to compare MORE to other robust treatments to provide a powerful test of the comparative effectiveness of this new intervention. Similarly, because MORE is a multimodal intervention that combines mindfulness training with reappraisal and savoring techniques, the differential efficacy of these intervention components is unknown. Dismantling trials50 and factorial experimental designs, such as the multiphase optimization strategy (MOST),51 are needed to identify the independent and interactive effects of these mindfulness, reappraisal, and savoring components, as well as their common and unique mechanisms. In that regard, it would be particularly informative to compare MORE with other MBIs (such as mindfulness-based stress reduction or mindfulness-based relapse prevention) to demonstrate the added value of restructuring hedonic processes via reappraisal and savoring, above and beyond the therapeutic effects of basic mindfulness practices.
Conclusion
Across these completed and ongoing studies, evidence is amassing that suggests that MORE, as an MBI, targets mechanisms of hedonic dysregulation in the treatment of addiction. To be clear, it is not yet known whether MORE modulates universal and/or unique mechanistic targets, because the range of treatment conditions to which this intervention has been compared has been limited to date. Further complicating this issue, few of the mechanistic targets (e.g., reward responsiveness, addiction attentional bias) studied in the MORE research program have been explored in studies of other behavioral treatments for addiction (e.g., CBT, motivational interviewing).
Although many more studies are needed, the models outlined here provide a future research agenda with a number of testable hypotheses. First, MORE should be tested in several full-scale RCTs involving active control conditions and sample sizes of several hundred participants to definitively establish the efficacy of the treatment for a range of addictive disorders. Second, MORE should be tested head-to-head against another MBI and/or empirically supported intervention for the treatment of addiction in one or more multisite comparative effectiveness trials, to determine whether MORE offers significant advantages over other evidence-based practices. Third, careful phenotypic characterization of clinical trial participants is required to identify treatment moderators and answer the question “for whom does MORE work best?” Fourth, future process research could use ecological momentary assessment and advanced statistical techniques, such as multivariate autoregressive latent trajectory modeling (for an example of this analytic approach used in the context of a clinical trial of mindfulness-based cognitive therapy, see Ref. 52), to elucidate dynamic change trajectories of participants in MORE via time-lagged, functional analyses of the impact of mindfulness, reappraisal, and savoring practice on symptoms. Fifth, as indicated above, dismantling and/or factorial studies, as well as additional biobehavioral measurement approaches, are needed to further parse the mechanisms of MORE. Specifically, fMRI and positron emission tomography (PET) studies could be used to identify the effects of MORE on neural mechanisms of cognitive control and hedonic regulation, while genome-wide transcriptional profiling could be used to explore the effects of MORE peripheral indices of gene expression relevant to the molecular mediators of addiction, stress, and pain.
A number of hypotheses have been generated from the new perspective emerging from the MORE research program, including the following testable predictions to be pursued in future research: (1) mindfulness strengthens top-down cognitive control; (2) enhancing attentional stability, set-shifting capacity, and metacognitive awareness via mindfulness meditation can facilitate higher-order valuation processes; (3) mindfulness facilitates the flexible reconfiguration of information within working memory; (4) mindfulness increases interoceptive awareness to optimize reward prediction error, and thereby improves decision making; (5) by inducing positive affective tone, mindfulness tunes attention toward positive information; (6) tuning attention toward positive information augments natural reward processing; (7) boosting natural-reward processing reduces the comparative salience of drug cues and thereby decreases addiction risk; and (8) such restructuring of reward processing is underpinned by changes in frontostriatal connectivity. These hypotheses, and the research questions surrounding them, will be answered in the coming years as my colleagues and I pursue increasingly sophisticated measurement protocols and research designs to reveal the clinical impact and biobehavioral mechanisms of MORE. As MORE is unpacked and further developed, pursuing these lines of research can ultimately enrich understanding of the ways in which mind training can modulate the pathophysiology of hedonic dysregulation. In so doing, this work may cut to the heart of issues as fundamental as embodied cognition and reciprocal causation at the mind–body interface.53
In the end, it would be unwise for contemplative science to dismiss evaluation and higher-order thought as incidental or anathema to well-being. To the contrary, the human species evolved advanced cognitive capacities for meaning making and thinking about thinking, enabling humans to radically restructure their values and goals in service of the survival and flourishing of the species. Thus, it is a fool’s errand to attempt to use mindfulness to anesthetize the very essence that makes us human. Rather, psychological flexibility is needed to adapt to the rigors of life. There is indeed a time to change one’s course of action by thinking, and a time to accept one’s experience by quietly observing and absorbing the world in all its beauty and wonder. May we, as scientists and clinicians, have the wisdom to know the difference.
Acknowledgments
E.L.G. was supported by Grant R34DA037005 from the National Institutes of Health (NIH) in preparing this manuscript. The conclusions in this article are those of the author and do not necessarily represent the official position of the NIH.
Footnotes
MORE aims to enhance processing of natural rewards, including non-harmful hedonic pleasures, health-promoting behaviors, aesthetic appreciation of natural beauty, prosocial engagement, and the sense of accomplishment, purpose, and eudaimonic meaning in life.
It is dubious that evaluative processing can be completely suppressed or suspended for any extended period of time in the human brain. Complete suspension of evaluative processing is unlikely in light of the presence of primordial brain structures and functions devoted to discriminating appetitive and aversive stimuli, which have been evolutionarily conserved for millennia due to their inherent survival value. Moreover, due to the semantic-narrative orientation that is so fundamental to psychological development in Western society, practitioners will inevitably re-engage with cognitive appraisals of self and world following the acute phase of mindfulness practice. Thus, while existing MBIs, such as mindfulness-based stress reduction and mindfulness-based cognitive therapy, may explicitly eschew evaluation in an effort to champion nonjudgment as a guiding therapeutic principle, valuing nonjudgment is itself a form of valuation or prioritization of some values over other values. Thus, ostensibly non-evaluative, nonjudgmental approaches in these MBIs may actually promote implicit valuation of particular behaviors and attitudes (e.g., self-compassion, loving-kindness toward others). In MORE, the implicit is made explicit, as a matter of course.
At the deepest stage of mindfulness practice referenced in MORE (and other MBIs), the emptiness of the autobiographical self and the interdependent nature of reality may be realized, leading to a collapsing of the distinction between the subject who appraises and the object that is appraised, a nondual awareness that nullifies both aversion to the stressor as well as craving of one’s desires.54 If there is no distinction between self or other, and no goal to seek or obtain, stress cannot arise during the transactional process, since stress results from the primordial defense response, in which the self attempts to preserve its own survival.55 When there is no self to defend, how can there be stress? That said, the self is comprised of many processes and layers of consciousness (c.f., Damasio’s distinction between extended and core self56), not all of which are malignant defenses to the encounter with the world. To the contrary, many higher-order, autobiographical, self-related processes are crucial for successful adaptation, including the maximization of hedonic and eudaimonic well-being. Indeed, the attribution of semantic and episodic meaning to experience allows for negotiation of the sociocultural environment and is therefore essential to healthy psychological development in Western society.57 It is my contention that mindfulness practice may have enduring, eudaimonic effects when practitioners “get off the meditation cushion” and refocus on their autobiographical selves constructed from their individual memories and enriched by language. Thus, while some veins of contemporary scholarship claim that mindfulness invariably decreases self-referential, evaluative processing, my colleagues and I30,32 have argued that the practice of mindfulness augments flexible cognitive control and thereby facilitates appraisal and meaning-making processes as the culturally embedded, autobiographical self navigates through life’s challenges.
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc Cogn Affect Neurosci. 2007;2(4):313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor V, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Vago DR, Silbersweig DA. Self-awareness, self-regulation, and self-transcendence (S-ART): a framework for understanding the neurobiological mechanisms of mindfulness. [Accessed September 7, 2015];Front Hum Neurosci. 2012 :6. doi: 10.3389/fnhum.2012.00296. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3480633/ [DOI] [PMC free article] [PubMed]
- 4.Garland EL. Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain. Washington, D.C: NASW Press; 2013. [Google Scholar]
- 5.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 6.Cabanac M. The dialectics of pleasure. Pleas Brain. 2010:113–124. [Google Scholar]
- 7.Cabanac M. Sensory Pleasure. Q Rev Biol. 1979;54(1):1–29. doi: 10.1086/410981. [DOI] [PubMed] [Google Scholar]
- 8.McFarland DJ, Sibly RM. The behavioural final common path. Philos Trans R Soc B Biol Sci. 1975;270(907):265–293. doi: 10.1098/rstb.1975.0009. [DOI] [PubMed] [Google Scholar]
- 9.Frankfurt H. Freedom of the will and the concept of a person. J Philos. 1971;67:5–20. [Google Scholar]
- 10.Steklis HD, Lane RD. The Unique Human Capacity for Emotional Awareness: Psychological, Neuroanatomical, Comparative and Evolutionary Perspectives. In: Watanabe S, Kuczaj S, editors. Emotions of Animals and Humans. The Science of the Mind. Springer; Japan: 2012. [Accessed August 21, 2015]. pp. 165–205. http://link.springer.com/chapter/10.1007/978-4-431-54123-3_8. [Google Scholar]
- 11.Volkow ND, Fowler JS, Wang G-J. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Alcaro A, Panksepp J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 14.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 15.Pierce RC, Vanderschuren L. Kicking the habit: The neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- 17.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 19.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacol Berl. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 20.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dias-Ferreira E, Sousa JC, Melo I, et al. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 22.Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiffany ST. Substance Abuse and Emotion. Washington, DC: US: American Psychological Association; 2010. Drug craving and affect; pp. 83–108. [Google Scholar]
- 24.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shizgal P, Hyman SE. Principles of Neural Science. 5. New York: McGraw Hill; Motivational and addictive states. [Google Scholar]
- 27.Sundararajan L. Toward a Reflexive Positive Psychology Insights from the Chinese Buddhist Notion of Emptiness. Theory Psychol. 2008;18(5):655–674. [Google Scholar]
- 28.Garland E, Froeliger B, Howard M. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Name Front Psychiatry. 2013;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gross JJ. Emotion regulation: Current status and future prospects. Psychol Inq. 2015;26(1):1–26. [Google Scholar]
- 30.Garland EL, Farb NAR, Goldin P, Fredrickson BL. Mindfulness broadens awareness and builds eudaimonic meaning: A process model of mindful positive emotion regulation. Psychol Inq. 2015;26(4):293–314. doi: 10.1080/1047840X.2015.1064294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McConnell P, Froeliger BE. Mindfulness, mechanisms and meaning: Perspectives from the cognitive neuroscience of addiction. Psychol Inq. doi: 10.1080/1047840X.2015.1076701. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garland EL, Farb NA, Goldin PR, Fredrickson BL. The Mindfulness-to-Meaning Theory: Extensions, Applications, and Challenges at the Attention–Appraisal–Emotion Interface. Psychol Inq. 2015;26(4):377–387. doi: 10.1080/1047840X.2015.1064294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci. 2015;16(7):419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahl CJ, Lutz A, Davidson RJ. Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends Cogn Sci. 2015;19(9):515–523. doi: 10.1016/j.tics.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant FB, Chadwick ED, Kluwe K. Understanding the processes that regulate positive emotional experience: Unsolved problems and future directions for theory and research on savoring. Int J Wellbeing. 2011;1:107–126. [Google Scholar]
- 36.Frijda NH, Sundararajan L. Emotion refinement: A theory inspired by Chinese poetics. Perspect Psychol Sci. 2007;2:227–241. doi: 10.1111/j.1745-6916.2007.00042.x. [DOI] [PubMed] [Google Scholar]
- 37.Rinpoche TW. Healing with Form, Energy, and Light: The Five Elements in Tibetan Shamanism, Tantra, and Dzogchen. Snow Lion Publications; 2002. [Accessed January 21, 2016]. https://books.google.com/books?hl=en&lr=&id=MVXyFEhgmQIC&oi=fnd&pg=PR13&dq=tibetan+bon+five+elements&ots=upZJhhgHca&sig=1B6FZjgrsuGhM23cur43YxShjZ0. [Google Scholar]
- 38.Dreyfus G. Is mindfulness present-centred and non-judgmental? A discussion of the cognitive dimensions of mindfulness. Contemp Buddhism. 2011;12(01):41–54. [Google Scholar]
- 39.Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results of a randomized controlled pilot trial. J Psychoactive Drugs. 2010;42(2):177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garland EL, Manusov EG, Froeliger B, Kelly A, Williams JM, Howard MO. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J Consult Clin Psychol. 2014;82(3):448. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garland EL, Roberts-Lewis A, Tronnier CD, Graves R, Kelly K. Mindfulness-Oriented Recovery Enhancement as a transdiagnostic treatment for co-occurring substance dependence, traumatic stress, and psychiatric disorders: Proximal outcomes from a pragmatic randomized trial. Manuscr Submitt Publ. 2015 doi: 10.1016/j.brat.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eichberg C, Mathew A, Baddeley J, et al. Mindfulness-Oriented Recovery Enhancement (MORE): A novel smoking-cessation treatment focused on cognitive reappraisal and the savoring of naturalistic rewards. Poster Present Front Neurosci Charlest SC. 2015 [Google Scholar]
- 43.Froeliger B, Mathew A, Eichberg C, et al. Restructuring reward mechanisms in nicotine addiction: a pilot fMRI study of Mindfulness Oriented Recover Enhancement in cigarette smokers. doi: 10.1155/2017/7018014. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garland EL, Beck A, Hansen P, et al. Integrated Mindfulness-Oriented Recovery Enhancement and physical health intervention for obese cancer survivors: Preliminary results from a pilot randomized controlled trial. Boston, MA: 2015. [Google Scholar]
- 45.Garland EL, Howard MO. Mindfulness-oriented recovery enhancement reduces pain attentional bias in chronic pain patients. Psychother Psychosom. 2013;82(5):311–318. doi: 10.1159/000348868. [DOI] [PubMed] [Google Scholar]
- 46.Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 2014;231(16):3229–3238. doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garland EL, Froeliger B, Howard MO. Neurophysiological evidence for remediation of reward processing deficits in chronic pain and opioid misuse following treatment with Mindfulness-Oriented Recovery Enhancement: exploratory ERP findings from a pilot RCT. J Behav Med. 2014;38(2):327–336. doi: 10.1007/s10865-014-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garland EL. Mindfulness-Oriented Recovery Enhancement modulates neurocognitive mechanisms and reward system function in addiction, stress, and pain. Sloan Kettering Memorial Hospital; New York, NY: 2015. [Google Scholar]
- 49.Garland EL, Roberts-Lewis A, Tronnier CD, Graves R, Kelley K. Mindfulness-Oriented Recovery Enhancement versus CBT for co-occurring substance dependence, traumatic stress, and psychiatric disorders: Proximal outcomes from a pragmatic randomized trial. Behav Res Ther. 2016;77:7–16. doi: 10.1016/j.brat.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garland EL. Dismantling mindfulness-based cognitive therapy for recurrent depression implicates lack of differential efficacy for mindfulness training. Evid Based Ment Health. 2014;17(3):94–94. doi: 10.1136/eb-2014-101856. [DOI] [PubMed] [Google Scholar]
- 51.Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30(1):65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- 52.Garland EL, Geschwind N, Peeters F, Wichers M. Mindfulness training promotes upward spirals of positive affect and cognition: multilevel and autoregressive latent trajectory modeling analyses. [Accessed July 1, 2015];Front Psychol. 2015 :6. doi: 10.3389/fpsyg.2015.00015. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313604/ [DOI] [PMC free article] [PubMed]
- 53.Maturana H, Varela F. The Tree of Knowledge: The Biological Roots of Human Understanding. Boston: Shambala; 1987. [Google Scholar]
- 54.Garland EL. The meaning of mindfulness: A second-order cybernetics of stress, metacognition, and coping. Complement Health Pract Rev. 2007;12:15–30. [Google Scholar]
- 55.Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- 56.Damasio AR. Investigating the biology of consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353(1377):1879–1882. doi: 10.1098/rstb.1998.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vygotsky LS. Mind in Society: The Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 58.Ellsworth PC, Scherer KR. Appraisal processes in emotion. In: Davidson RJ, editor. Handbook of Affective Sciences. New York: Oxford University Press; 2002. pp. 572–595. [Google Scholar]
- 59.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 60.Brosschot JF, Verkuil B, Thayer JF. Conscious and unconscious perseverative cognition: is a large part of prolonged physiological activity due to unconscious stress? J Psychosom Res. 2010;69:407–416. doi: 10.1016/j.jpsychores.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Moss AC, Erskine JAK, Albery IP, Allen JR, Georgiou GJ. To suppress, or not to suppress? That is repression: Controlling intrusive thoughts in addictive behaviour. Addict Behav. 2015;44:65–70. doi: 10.1016/j.addbeh.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 62.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]