Abstract
Introduction
Nerve regeneration across nerve constructs, such as acellular nerve allografts (ANAs), is inferior to nerve auto/isografts especially in the case of long defect lengths. Vascularization may contribute to poor regeneration. The time course of vascular perfusion within long grafts and constructs was tracked to determine vascularization.
Methods
Male Lewis rat sciatic nerves were transected and repaired with 6 cm isografts or ANAs. At variable days following grafting, animals were perfused with Evans Blue albumin, and grafts were evaluated for vascular perfusion by a blinded observer.
Results
Vascularization at the mid-graft was re-established within 3–4 days in 6 cm isografts, while it was established after 10 days in 6 cm ANAs.
Conclusion
Vascular perfusion is reestablished over a shorter time course in long isografts when compared to long ANAs.
Clinical Relevance
The differences in vascularization of long ANAs compared to auto/isografts suggest regenerative outcomes across ANAs could be impacted by vascularization rates.
Keywords: Autograft, acellular nerve allograft, hypoxia, ischemia, peripheral nerve, vascular perfusion
Introduction
Peripheral nerve injuries can result in a zone of injury that disrupts the continuity of nerve and causes a gap between the proximal and distal nerve ends. Surgically, direct reconstruction of these nerve ends without a “bridge” is rarely possible, and many alternatives, including autografts and constructs, have been used to “bridge the gap”.1,2 The period following nerve reconstruction subjects the bridge material to ischemic conditions, which potentially alter the regenerative environment and outcome across the bridge or graft. Our previous studies demonstrated that long grafts resulted in poor axonal regeneration and functional recovery compared to short grafts concurrent with an accumulation of cellular senescence.3 As tissue subjected to prolonged ischemia develops numerous pathological processes, including cell damage,4–11 an explanation for the poor regenerative outcomes and/or accumulation of senescence could be due to poor vascularization leading to ischemia and pathological consequences in long nerve grafts and constructs.
The primary method of revascularization of conventional nerve auto/isografts is longitudinal inosculation, or spontaneous end-to-end recoupling of existing vasculature.12,13 For constructs such as acellular nerve allografts (ANAs), vascularization depends on a new vascular network being established, as the processing to remove cells eliminates native endothelial cells.14 In order to further understand the regenerative outcomes associated with long nerve grafts, we investigated the primary differences in vascularization between long (6cm) auto/isografts versus ANAs. The purpose of this study was to determine whether long nerve grafts reestablished vascular perfusion and the period of time to establish vascularization.
Materials and Methods
Experimental design and animals
Twenty-one adult male Lewis rats (200–250 grams; Charles River Lab, Wilmington, MA) were recipients of either 60mm sciatic nerve isografts or ANAs following sciatic nerve transection. ANAs are nerve allografts processed with detergents to remove cells. An additional 6 adult male Lewis rats served as donors for the isograft group, while 5 adult Sprague-Dawley rats served as ANA donors. Rodents were housed in a central animal care facility. Food and water were provided ad libitum. Animals were monitored for appropriate postsurgical recovery and weight gain. All study procedures were approved by our institutional animal studies committee.
Two experimental animal groups were studied. The first group had 6 cm sciatic nerve isografts implanted, while the second group had 6 cm ANAs implanted. Animals were perfused at a designated endpoint. Animals with isografts were sacrificed at 3, 4, 5, and 10 days for analysis with fluorescent microscopy for vascular reperfusion (n=3/endpoint). Animals implanted with a 60mm ANA were sacrificed at 5, 10, and 20 days (n=3/endpoint) for similar analysis.
Surgical procedures and nerve grafting
All surgical procedures were performed aseptically using an operating microscope. Animals were anesthetized by subcutaneous administration of a mixture of 75 mg/kg ketamine hydrochloride (Fort Dodge Animal Health, Fort Dodge, IA) and 0.5 mg/kg dexmedetomidine hydrochloride (Orion Corporation, Espoo, Finland). Rats underwent a gluteal muscle splitting incision to expose the sciatic nerve and its trifurcation. Donor nerves were harvested as described previously,3 where nerves for ANAs were transferred to aseptic tubes to undergo detergent processing as previously described,3,15,16 while the others were immediately used as fresh nerve isografts. Donor animals were then euthanized. Isografts and ANAs were grafted using previously published methods.3 In all recipient animals, muscle and skin were re-approximated, anesthesia was reversed, and the animals were recovered on a warming pad and monitored for postoperative complications. Postoperative pain was managed using buprenorphine (0.05 mg/kg) every 8 to 12 hours as needed.
Vascularization assays
Animals were re-anesthetized and given an intravenous injection of 3mL of 5% weight/volume Evans Blue bovine albumin (EBA) [1% weight/volume Evans Blue (Sigma, St. Louis, MO) with 5% weight/volume bovine albumin], which was administered 20 minutes prior to sacrifice into the femoral vein. The nerve, including graft, was harvested and placed in 4% paraformaldehyde followed by 30% sucrose solution for cryoprotection. One hundred (100) μm thick longitudinal frozen sections were cut from the fixed specimens and examined using a fluorescent microscope (BX51, Olympus, Center Valley, PA) by a blinded operator. The graft was examined for blood vessel perfusion, noted by prominent fluorescent streaks (>20 μm long) in longitudinal sections. The integrity of the blood-nerve barrier was assessed by leakage of EBA as indicated by strong background fluorescence. Standard immunohistochemistry (IHC) was performed for CD31 (Abcam, Cambridge, MA; endothelial cells).
Results
Successful inosculation and vascular perfusion was demonstrated in a portion of the 6cm isograft group at the mid-graft by 3 days (Figure 1). Consistent vascularization of isografts was apparent by 4 days, as all animals had numerous perfused vessels. Leakage through the blood-nerve barrier was evident at 3 days; however, this leakage was qualitatively absent by 5 days. Previously, short (1cm) isografts established vascular perfusion by 2–3 days.12,13 As vascular perfusion was noted within 6cm isografts at 3–4 days, vascularization is presumably slower due to the increased graft length.
Figure 1. Vascularization micrographs and quantification of time to mid-graft perfusion in long nerve grafts.
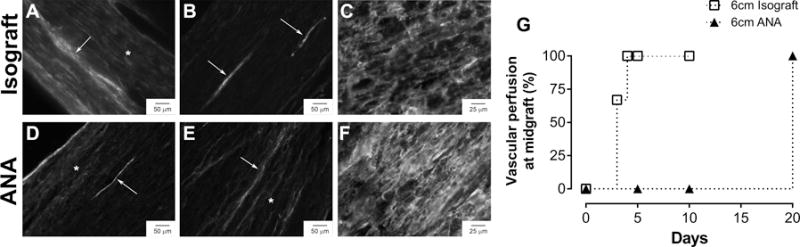
Photomicrographs of longitudinal nerve graft sections after perfusion with EBA and CD31 staining (A–F). Vascular perfusion into the middle of the isograft began by 3 days (A) and was well-established with minimal leakage of EBA by 5 days (B, G). Vascular perfusion was only present in the proximal and distal graft regions of long ANAs at 10 days (D) and completed by 20 days (E, G). Both grafts demonstrated the presence of endothelial cells (CD31) at 5 days regardless of perfusion status (C, F). Arrows indicate perfused, patent vessels, while asterisks indicate leakage of EBA.
No vascular perfusion was evident within ANAs at any graft location at 5 days; however, IHC revealed the presence of endothelial cells (CD31) across the entire graft (Figure 1). Vascular perfusion became evident in the proximal and distal ends of the ANAs at 10 days, and complete perfusion was established by 20 days. Leakage through the blood-nerve barrier was still partially apparent at 20 days. These results demonstrate long ANAs require greater time for vascularization compared to long isografts. This vascularization period for long ANAs is considerably greater than reported vascularization periods for other short (<3cm) tissue-engineered constructs, which typically vascularize within 5–7 days.17
Discussion
Overall, these data demonstrate that long auto/isografts and ANAs do contain blood vessels and vascularize following implantation. The time to vascularization increases in long nerve grafts, which is most prominently demonstrated in ANAs. As regenerative outcomes across long isografts are superior to long ANAs,3 these results suggest time to vascularization is an additional factor that influences regenerative outcomes across long nerve grafts. Tissues subjected to prolonged ischemia develop numerous pathological processes, including cell damage, oxidative stress, and induction of cellular senescence.4–11 Therefore, future studies considering the cause of poor regenerative outcomes across long nerve grafts will need to consider the period of ischemic insult and ischemic regions which develop within grafts and constructs for their potential impact on nerve regeneration.
Acknowledgments
Grant support: This work was supported in part by National Institutes of Neurological Disorders and Stroke of the National Institutes of Health under award number R56 NS33406 and R01 NS086773. Salary support was provided in part by the Barnes-Jewish Hospital Foundation for Matthew Wood. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Washington University.
Abbreviations
- ANA
Acellular nerve allograft
- EBA
Evans blue albumin
- CD31
Cluster of differentiation marker 31
- IHC
Immunohistochemistry
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- 1.Mackinnon SE, Dellon AL. Surgery of the Peripheral Nerve. Thieme Medical Publishers; 1988. [Google Scholar]
- 2.Mackinnon SE. Nerve Surgery. Thieme; 2015. [Google Scholar]
- 3.Saheb-Al-Zamani M, Yan Y, Farber SJ, Hunter DA, Newton P, Wood MD, Stewart SA, Johnson PJ, Mackinnon SE. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Experimental neurology. 2013;247:165–177. doi: 10.1016/j.expneurol.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. International review of cell and molecular biology. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111(18):2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 6.Welford SM, Giaccia AJ. Hypoxia and senescence: the impact of oxygenation on tumor suppression. Molecular cancer research : MCR. 2011;9(5):538–544. doi: 10.1158/1541-7786.MCR-11-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer cell. 2008;14(6):458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. The EMBO journal. 2012;31(5):1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen QM, Prowse KR, Tu VC, Purdom S, Linskens MH. Uncoupling the senescent phenotype from telomere shortening in hydrogen peroxide-treated fibroblasts. Experimental cell research. 2001;265(2):294–303. doi: 10.1006/excr.2001.5182. [DOI] [PubMed] [Google Scholar]
- 10.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature cell biology. 2003;5(8):741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. The international journal of biochemistry & cell biology. 2005;37(5):961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Best TJ, Mackinnon SE, Evans PJ, Hunter D, Midha R. Peripheral nerve revascularization: histomorphometric study of small- and large-caliber grafts. Journal of reconstructive microsurgery. 1999;15(3):183–190. doi: 10.1055/s-2007-1000090. [DOI] [PubMed] [Google Scholar]
- 13.Best TJ, Mackinnon SE, Midha R, Hunter DA, Evans PJ. Revascularization of peripheral nerve autografts and allografts. Plastic and reconstructive surgery. 1999;104(1):152–160. [PubMed] [Google Scholar]
- 14.Laschke MW, Menger MD. Vascularization in tissue engineering: angiogenesis versus inosculation. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 2012;48(2):85–92. doi: 10.1159/000336876. [DOI] [PubMed] [Google Scholar]
- 15.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10(9–10):1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 16.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10(11–12):1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 17.Fansa H, Schneider W, Keilhoff G. Revascularization of tissue-engineered nerve grafts and invasion of macrophages. Tissue Eng. 2001;7(5):519–524. doi: 10.1089/107632701753213147. [DOI] [PubMed] [Google Scholar]


