Abstract
Context
Although the liver is the primary target organ in acetaminophen (APAP) toxicity, other organs are affected. Previous data suggested chronic APAP abuse can be ototoxic and the mechanism involves APAP-induced oxidative stress and reactive metabolite (NAPQI)-induced endoplasmic reticulum stress. However, the effect of a single acute overdose on hearing has not been tested.
Objectives
To determine if a single acute APAP overdose causes hearing damage, and to explore possible mechanisms of APAP ototoxicity.
Materials and Methods
Male C57BL/6J mice were treated with a single human-relevant overdose of APAP (300 mg APAP per kg bodyweight). Blood, liver and cochleae were harvested at 0, 2, 6 and 24h post-APAP. In some mice, auditory brainstem responses (ABRs) to a range of frequencies were measured at 24h. The furosemide plus kanamycin (FS/K) model of drug ototoxicity was used as a positive control for hearing loss. NAPQI formation after APAP was assessed by measuring glutathione (GSH) depletion and covalent protein binding, and oxidative stress was assessed by measuring glutathione disulfide (GSSG).
Results
There was no evidence of reactive metabolite formation or hearing loss after a single overdose of APAP at a clinically relevant dose. However, there was a transient increase in oxidative stress.
Discussion
Although a single acute overdose was not ototoxic, there was evidence of oxidative stress which may support a role for oxidative stress in hearing loss due to chronic APAP abuse.
Conclusion
A single human-relevant acute overdose of acetaminophen (APAP) causes transient oxidative stress in cochleae but not hearing loss.
Keywords: Ototoxicity, Hepatotoxicity, Protein binding, Reactive oxygen species (ROS)
INTRODUCTION
Acetaminophen (APAP; paracetamol) overdose is the most common cause of acute liver failure in the U.S. (Lee, 2008). The mechanism of APAP hepatotoxicity involves conversion to the reactive intermediate N-acetyl-p-benzoquinone imine (NAPQI), catalyzed by cytochrome P450 enzymes (McGill and Jaeschke, 2013). NAPQI reacts with free sulfhydryl groups. As a result, it binds to glutathione (GSH) and to cysteine residues on proteins. Protein binding leads to mitochondrial dysfunction and oxidative stress (Meyers et al., 1988; Tirmenstein and Nelson, 1989; Jaeschke, 1990; Jaeschke et al., 2012). The oxidative stress activates the c-Jun N-terminal kinases (JNK) 1/2 that can then translocate into mitochondria and enhance the oxidative stress (Gunawan et al., 2006; Hanawa et al., 2008; Saito et al., 2010; Du et al., 2015). Mitochondrial damage causes release of endonucleases that cleave nuclear DNA (Bajt et al., 2006; Jaeschke et al., 2012). The result in both mice and humans is oncotic hepatocyte necrosis (Gujral et al., 2002; McGill et al., 2012; Antoine et al., 2012).
Although the liver is the primary site of APAP toxicity, other tissues are affected. There have been many reports of deafness caused by chronic APAP/opioid combination abuse (Friedman et al., 2000; Oh et al., 2000; Ho et al., 2007). However, it is not clear which of the two agents is responsible. It was recently shown that prolonged exposure to APAP can cause ototoxicity in vitro (Yorgason et al., 2010; Kalinec et al., 2014). Based on this, it seems likely that APAP is the ototoxic drug in APAP/opioid combinations. However, it is not known if a single acute overdose of APAP can affect hearing. Although such an effect has not been reported in the clinic, it may be missed in some cases. Overdose patients often have altered mental status and are incapable of communication. Also, the focus for clinicians is on treating liver injury and encephalopathy. We hypothesized that a single acute human-relevant APAP overdose can cause hearing loss in mice. Furthermore, because the proposed mechanisms for APAP ototoxicity from earlier work were APAP-induced oxidative stress and NAPQI-induced endoplasmic reticulum (ER) stress (Yorgason et al., 2010; Kalinec et al., 2014), we assessed oxidative stress and NAPQI formation. We used APAP toxicity in the liver as a comparison because the time course is well characterized (McGill et al., 2013). Furosemide plus kanamycin (FS/K) treatment was used as a positive control for hearing loss.
METHODS
Animals
Male C57BL/6J mice, 8–9 weeks age, were used. Although these mice develop age-related hearing problems, our age range is before the typical onset (Keithley et al., 2004). Animals were housed in a climate-controlled environment with 12 h light/dark cycle and ad libitum access to food and water. Mice in the APAP groups were deprived of food overnight before experiments to reduce variation caused by nutrition-dependent differences in drug metabolism, then injected i.p. or treated p.o. (by gavage) with 300 mg APAP per kg of bodyweight (mg/kg) dissolved in PBS. Animals were quickly sacrificed under anesthesia rapidly induced by isoflurane at 0, 2, 6 or 24 h post-APAP. For the 24 h group, food was returned at 6 h post-APAP. Blood was collected from the caudal vena cava and livers and cochleae were harvested. One piece of liver from each animal was fixed in phosphate-buffered formalin and embedded in paraffin for histology, while remaining liver and whole cochleae were frozen. Mice in the FS/K groups had access to food and water throughout the experiments and were injected s.c. with 1,000 mg/kg kanamycin in PBS followed 0.5 h later by i.p. injection of 400 mg/kg FS in PBS (pH 9.0). Separate experiments with different numbers of mice were performed for the biochemical studies and the ABR measurements. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kansas Medical Center.
Clinical biochemistry
Alanine aminotransferase (ALT) was measured using a kit from Pointe Scientific (Canton, MI). Briefly, ALT catalyzes the reaction of L-alanine and α-ketoglutarate to form pyruvate and L-glutamate. The pyruvate product can then be converted to L-lactate by lactate dehydrogenase, consuming NADH in the process. Because ALT catalyzes the rate-limiting step, the loss of light absorbance at 340 nm due to NADH consumption can be graphed over time to provide a slope that can then be used to calculate the activity of ALT. Both the intraday and interday coefficients of variation (%CV) are less than 5% for this assay over a wide range of ALT activities.
GSH measurement
GSH and glutathione disulfide (GSSG) were measured in liver and whole cochleae homogenate using a modified Tietze assay, as previously described in detail (McGill and Jaeschke, 2015). Briefly, the the total glutathione (GSH+GSSG) was measured with a cycling method using glutathione reductase (GR). In the assay, GSH reduces dithionitrobenzoate to a chromogenic product. The product absorbs light at 412 nm. Oxidized glutathione (GSSG) is recycled by (GR) so that it can continue to participate in the reaction until a pre-specified endpoint is reached. Accumulation of the chromogenic product was measured at 412 nm at 10 min and compared to a standard curve to calculate GSH concentration. GSSG was measured in the same way, except that GSH was masked by reaction with N-ethylmaleimide before the start of the cycling reaction with GR. In this way, only the GSSG is measured. The intra- and inter-day %CV values for this reaction are 3% and 11%, respectively (McGill and Jaeschke, 2015).
APAP-protein binding measurement
Protein-derived APAP-cysteine (APAP-cys) adducts were measured in liver and whole cochleae homogenate using high-performance liquid chromatography with electrochemical detection (HPLC-ECD), as described (Muldrew et al., 2002; McGill et al., 2013). Briefly, liver or cochleae tissue was homogenized in 10 mM sodium-acetate buffer (pH 6.5) to liberate proteins. The homogenates were then centrifuged at 12,000g for 5 min to pellet the cell and tissue debris. The supernatant containing free proteins from the tissue was then filtered through Bio-Spin 6 Tris columns (Bio-Rad, Hercules, CA) to remove low molecular weight (<6 kD) contaminants. The proteins in the filtrate were then digested with Type XIV protease mixture from Streptomyces griseus (Sigma, St. Louis, MO) to liberate the amino acids from the proteins, including the APAP-cys adduct. The proteases were then precipitated by 1:1 dilution of the samples with 40% trichloroacetic acid, followed by centrifugation at 12,000g for 5 min to pellet the protein. The supernatant was then subjected to HPLC-ECD to measure the protein-derived APAP-cys adduct. Validation of the entire method, including HPLC-ECD detection, has been described in detail (Muldrew et al., 2002). %CVs for this method are consistently ≤14%.
Auditory brainstem response measurement
Auditory brainstem response (ABR) thresholds were measured approximately 24 h prior to and following treatment with either APAP or FS/K. Each mouse was anesthetized with 1.5% isoflurane for the duration of ABR testing. Needle electrodes were inserted at the base of each pinna and the vertex of the scalp. Threshold measurements were made using the Intelligent Hearing Systems Smart EP program (IHS, Miami, FL). A series of tone bursts (500 μs) were presented to the left ear canal through a probe connected to a high frequency transducer. A range of frequencies (2, 4, 8, 16, and 32 kHz) were tested at various intensities, and threshold was defined as the lowest intensity at which an evoked potential could be reliably observed in two or more repetitions. ABRs were measured in 7 FS/K-treated mice to confirm that we could accurately detect hearing with minimal variation and in 4 APAP-treated mice. For the APAP-treated animals, the ABR testing was performed on a group of 24 h animals that was different from the group used to measure our biochemical parameters in order to avoid measuring effects that result from the prolonged anesthesia required for the ABR testing. However, parameters of liver injury were still measured in these animals to ensure that the APAP was absorbed and metabolized properly and has biological effects (plasma ALT: 3,010 ± 167 U/L).
Statistics
Normality was assessed using the Shapiro-Wilk test. For normal data, significance between two groups was assessed using Student’s t-test and significance across three or more groups was assessed using one-way analysis of variance (ANOVA) with Student-Newman-Keul’s post-hoc test. For non-normal data, significance was assessed using either Student’s t-test or ANOVA on ranks with post-hoc Dunn’s multiple comparisons. p < 0.05 was considered significant.
RESULTS
Time course of acetaminophen-induced liver injury
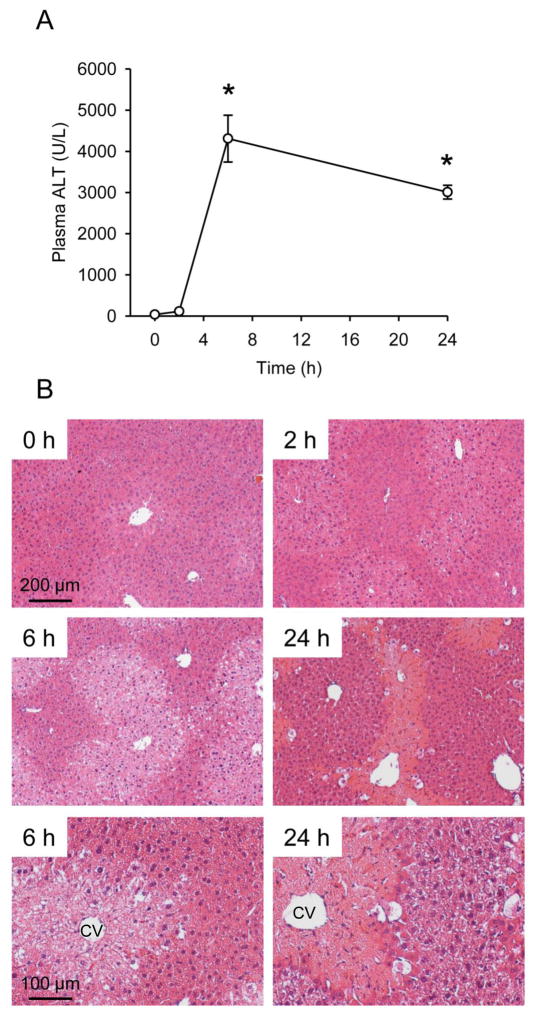
To characterize the timing of APAP toxicity in mice, animals were treated once with 300 mg/kg and sacrificed at 0, 2, 6 and 24 h post-APAP. This dose was chosen because the extent of liver injury that results is similar to what is seen in patients after a single acute APAP overdose, without lethality (McGill et al., 2012). Liver injury was assessed by measuring plasma ALT (a common clinical marker of liver cell death) and by histology. APAP caused a significant increase in ALT by 6 h, which decreased by 24 h (Fig. 1A). Similarly, evidence of tissue necrosis (i.e. hepatocyte swelling, nuclear disintegration, loss of basophilic staining) was visible around central veins in liver sections by 6 h (Fig. 1B). Consistent with earlier findings (McGill et al., 2013), these data show APAP rapidly induces liver injury within hours of overdose in mice, and the injury begins to resolve before 24 h.
Figure 1. Time course of acetaminophen-induced liver injury.
Mice were treated with 300 mg/kg acetaminophen (APAP). Plasma and livers were collected at the indicated time points. (A) Plasma alanine aminotransferase (ALT) activity. (B) H&E-stained liver sections at 100x (0 – 24 h) or 200x (6 and 24 h). 100 and 200 μm bars are included for scale. CV, central vein. Data are expressed as mean ± SEM for n = 3–4. *p < 0.05 vs. 0 h.
Auditory brainstem response after treatment with acetaminophen or furosemide/kanamycin
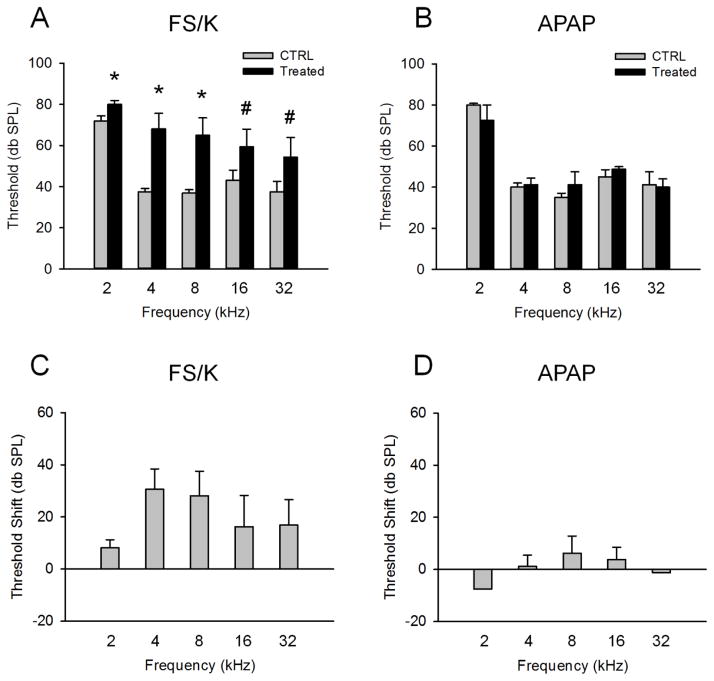
To determine whether or not overdose of APAP causes hearing loss, we measured ABRs in mice 24 h post-treatment. The FS/K model of drug ototoxicity was used as positive control to confirm that we can accurately detect hearing loss. These animals received 1,000 mg/kg kanamycin followed by 400 mg/kg FS 0.5 h later. This treatment has previously been shown to cause ototoxicity (Kraft et al., 2013). Furthermore, there is evidence that this is a clinically relevant model, as there are reports of hearing loss after combined treatment with aminoglycoside antibiotics and furosemide (Bates et al., 2002). FS/K increased ABR threshold at multiple frequencies at 24 h (Fig. 2A,C). However, no change in ABR threshold was observed at the same time point after APAP (Fig. 2B,D), despite the fact that this is well after the onset of liver injury and even the beginning of injury resolution in the liver. If APAP causes hair cell death, which is generally irreversible, we would expect it to have developed by this time point. These data show that a single acute clinically-relevant overdose of APAP does not result in significant hearing loss at 24 h after treatment.
Figure 2. Auditory brainstem responses after acetaminophen treatment.
Mice were treated with 300 mg/kg acetaminophen (APAP) or with 1000 mg/kg kanamycin followed by 400 mg/kg furosemide 0.5 h later (FS/K). Auditory brainstem responses (ABRs) were measured at the indicated frequencies before (CTRL) and 24 h after treatment. (A) ABRs in FS/K-treated mice. (B) ABRs in APAP-treated mice. (C) Hearing threshold shifts in FS/K-treated mice. (D) Hearing threshold shifts in APAP-treated mice. Data are expressed as mean ± SEM. n = 4 for APAP. n =7 for FS/K. *p < 0.05 vs. CTRL. #p < 0.1 vs. CTRL.
Time courses of protein binding in the liver and cochleae after acetaminophen
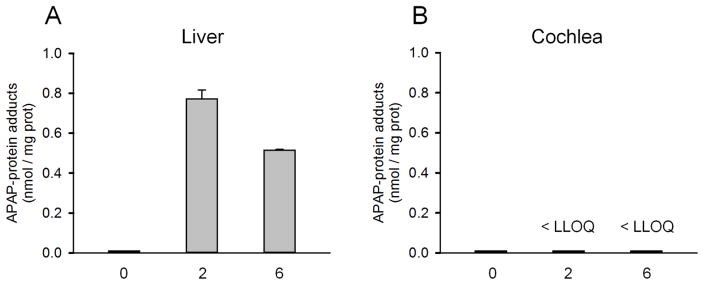
Although APAP overdose did not cause appreciable hearing loss at 24 h, it is still possible that the reactive metabolite of APAP forms in cochleae. To test this, we measured APAP-protein binding in cochleae and compared with the liver. Although NAPQI is the reactive metabolite that attacks sulfhydryl groups, the structure reverts to APAP upon binding leading to APAP-protein adducts. While we could measure APAP-protein adducts in the liver at 2 and 6 h post-treatment (Fig. 3A), we were unable to detect any protein binding in cochleae even at 6 h (Fig. 3B) – after the peak of binding in the liver (Fig. 3A) (McGill et al., 2013). These data suggest NAPQI does not form in cochleae after a clinically-relevant acute APAP overdose.
Figure 3. Protein binding after acetaminophen treatment.
Mice were treated with 300 mg/kg acetaminophen (APAP). Livers and cochleae were collected at the indicated time points. (A) APAP-protein adducts in liver tissue after APAP treatment. (B) APAP-protein adducts in cochlea tissue after APAP treatment. Data are expressed as mean ± SEM for n = 3–4.
Glutathione depletion and oxidative stress in the liver and cochleae after acetaminophen
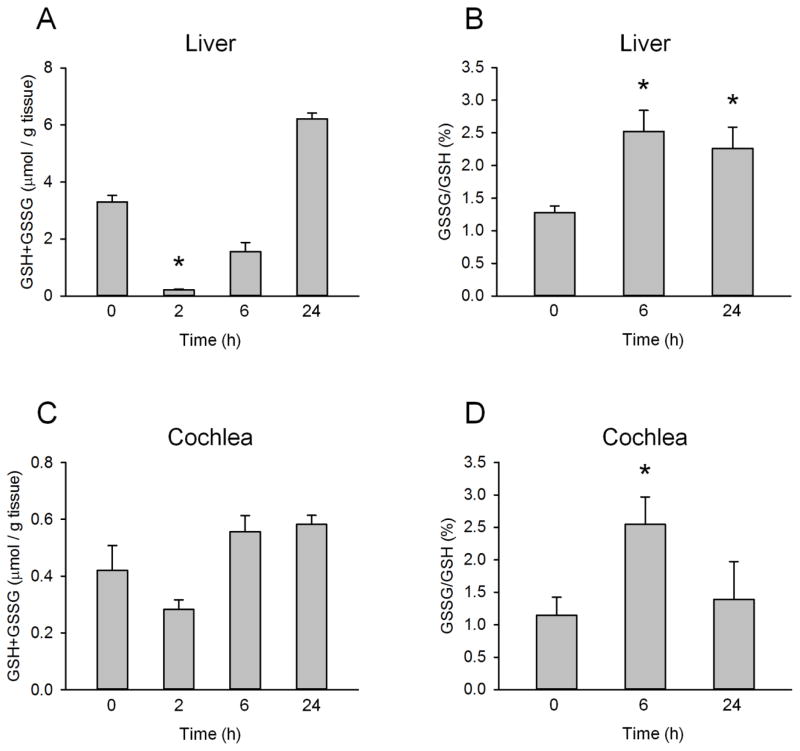
We next measured GSH in the liver and cochleae as an additional indicator of NAPQI formation because NAPQI is known to bind to and deplete GSH. As expected, extensive GSH depletion was observed in the liver as early as 2 h post-APAP (Fig. 4A). Importantly, hepatic GSH began to recover by 6 h (Fig. 4A), suggesting NAPQI forms early after APAP overdose and is completed before 6 h as APAP is rapidly eliminated. This is consistent with what we have previously shown (McGill et al., 2013) and with our protein binding data. However, GSH depletion was not observed in cochleae from the APAP-treated mice (Fig. 4B), supporting the idea that NAPQI formation does not occur in cochleae. Although there was a trend toward depletion that could have been suggestive of NAPQI formation, the results were not significant.
Figure 4. Glutathione and glutathione disulfide levels after acetaminophen treatment.
Mice were treated with 300 mg/kg acetaminophen (APAP). Livers and cochlea were collected at the indicated time points. (A) Total glutathione (GSH+GSSG) levels in the liver. (B) Percentage of glutathione in the oxidized form (GSSG) in the liver. (C) Total glutathione levels in cochleae. (D) Percentage of glutathione as GSSG in cochleae. Data are expressed as mean ± SEM for n = 4. *p < 0.05 vs. 0 h.
To test the possibility that acute APAP can cause oxidative stress in cochleae, we also measured the oxidized form of GSH, glutathione disulfide (GSSG). Interestingly, we observed significant increases in GSSG in both liver and cochleae at 6 h (Fig. 4B,D). However, while GSSG remained elevated in the liver to at least 24 h, the effect in cochleae was only detected at 6 h. These data suggest that some oxidative stress develops in cochleae at an early time point in response to APAP, despite the fact that our single acute overdose model does not cause appreciable hearing loss at 24 h.
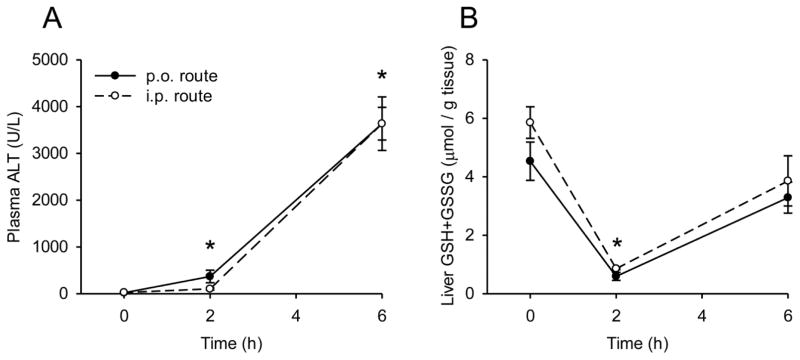
The effects of exposure route on metabolism and injury
The experiments described above were all performed using i.p. administration of APAP. However, the i.p. route is not clinically relevant. Most patients are exposed to APAP orally. To address this, we compared liver injury and GSH depletion between mice treated either i.p. or by oral gavage (p.o.) with 300 mg/kg APAP to determine if there are differences in the kinetics of metabolism and bioactivation or well-established biological effects. The time courses of liver GSH and plasma ALT levels were similar between the two groups of animals (Fig. 5). These data suggest that the i.p. and p.o. routes are likely equivalent for APAP metabolism and toxicity studies such as this.
Figure 5. Comparison of hepatic glutathione and plasma ALT levels after i.p. and p.o. acetaminophen treatment.
Mice were treated either i.p. or p.o. with 300 mg/kg acetaminophen (APAP). Plasma and livers were collected at the indicated time points. (A) Plasma alanine aminotransferase (ALT) activity. (B) Total liver glutathione (GSH+GSSG) levels. Data are expressed as mean ± SEM for n = 3. *p < 0.05 vs. 0 h.
DISCUSSION
The liver is the primary target for toxicity after APAP overdose, but it has been known for decades that extrahepatic tissues can be affected (Boyer and Rouff, 1971; Placke et al., 1987). In fact, reports of APAP toxicity in other organ systems have proliferated in recent years, including claims that APAP causes lung injury, asthma and neurobehavioral disorders (Tiegs et al., 2014). While many of those claims are controversial, it is clear from clinical reports that chronic APAP/opioid abuse can cause profound hearing loss. However, it is not known which agent causes the ototoxicity (Friedman et al., 2000; Oh et al., 2000; Ho et al., 2007). Two recent studies showed that continuous exposure to APAP under in vitro conditions can damage auditory cells (Yorgason et al., 2010; Kalinec et al., 2014). The mechanism of ototoxicity was thought to be oxidative stress caused by the parent compound and endoplasmic reticulum stress caused by NAPQI (Kalinec et al., 2014). Altogether, it seems likely that APAP is responsible for the hearing loss that has been observed after chronic APAP/opioid abuse. It is known that repeated exposure to APAP can lead to resistance to the hepatotoxic effects of the drug if the dose is gradually increased (Shayiq et al., 1999) and this may explain why liver injury was not noted in any APAP/opioid abuse patients.
We wondered if any ototoxicity occurs after a single acute overdose of APAP in vivo. We chose a dose of APAP that causes liver injury similar to what is seen in humans and is not lethal. Higher doses can be lethal in fasted mice, but lethal APAP overdose is relatively rare in humans. National studies have reported <1% mortality (Altyar et al., 2015), although this percentage is much higher at referral centers that receive the most severe cases (McGill et al., 2012). Lethal doses also present experimental challenges. Overall, our data do not support the idea that a human-relevant single acute APAP overdose causes ototoxicity. However, the finding of oxidant stress without evidence of NAPQI is very surprising considering the critical role that protein binding is believed to play in the initiation of oxidative stress in the liver during hepatotoxicity (McGill and Jaeschke, 2013). Further research is needed to understand how APAP directly causes oxidative stress in cochleae.
We concluded that NAPQI formation did not occur in cochleae. NAPQI is an electrophilic metabolite of APAP that reacts with nucleophilic groups, particularly the sulfhydryl groups of cysteine. Cysteine is found in both GSH and proteins. Upon reaction, the structure of NAPQI reverts to APAP (Rosen et al., 1983), so adducts with protein-derived cysteine are referred to as APAP-cys adducts and adducts with GSH are referred to as APAP-GSH. APAP-GSH is not detected by either our GSH or APAP-cys adduct assay. As a result, reduced GSH levels in tissue reflect NAPQI formation, while our APAP-cys assay detects only protein-derived adducts (Muldrew et al., 2002). We assessed NAPQI formation indirectly using both of these distinct methods. Neither approach showed evidence that APAP is metabolized to NAPQI in cochleae.
We chose to measure glutathione oxidation as a marker of oxidative stress. Glutathione oxidation is well-known to occur in APAP hepatotoxicity, and is generally considered the best indicator of oxidative stress in models of APAP toxicity. Although lipid peroxidation (LPO) was once thought to be involved in the pathophysiology of APAP hepatotoxicity, it is no longer widely believed to play a role (Jaeschke et al., 2012). It has been shown that a greater than 10-fold increase in lipid peroxidation over controls is necessary to cause cell death in the liver (Mathews et al., 1994). However, the amount of lipid peroxidation that occurs in rodents after APAP overdose is quite low, usually <3-fold over controls and even unmeasurable in some studies (Knight et al., 2003). Past studies reporting lipid peroxidation-mediated liver injury after APAP overdose relied upon mice fed diets low in vitamin E and high in polyunsaturated fats, which potentiates LPO but is not clinically relevant (Jaeschke et al., 2012; Wendel and Feuerstein, 1981; Wendel et al., 1982). Thus, although some LPO may occur in APAP toxicity, it is no longer thought to be important. Another, more relevant form of oxidative stress than LPO is protein nitration caused by peroxynitrite that is produced by the reaction of nitric oxide with superoxide. Recent data show that protein nitration is important in the mechanisms of APAP-induced liver injury (Bajt et al., 2003; Cover et al., 2005; Hinson et al., 2004). It may be interesting to assess protein nitration in cochleae in future studies.
Two possible explanations for the lack of NAPQI are that APAP is extensively metabolized before it reaches cochleae, or that it does not cross the blood-labyrinth barrier (BLB). However, previous experiments have shown that APAP accumulates in multiple tissues in mice at levels similar to liver (Fischer et al., 1981). Furthermore, APAP is a small molecule and neutral at physiological pH, so it readily diffuses across membranes (McGill and Jaeschke, 2013). In fact, it is known to cross the blood-brain barrier (Fischer et al., 1981; Fukuda et al., 2005). Thus, it is unlikely that either of these mechanisms can explain our results. A better explanation may be a lack of phase I drug-metabolizing enzymes (DMEs) in cochleae. For example, gentamicin, which is an aminoglycoside antibiotic similar to kanamycin, is not toxic to guinea pig hair cells in vitro, but becomes toxic after incubation with liver homogenate (Crann et al., 1992). This suggests cochlear cells do not express the DMEs necessary to form the reactive metabolite of gentamicin. However, this idea was challenged by the later finding that gentamicin is still ototoxic in vivo when directly administered into the ear (Husmann et al., 1998). Clearly, additional research is needed to characterize expression of DMEs in cochleae. Although kanamycin is not the same as gentamicin, they belong to the same class of drugs and are generally believed to have similar mechanisms of otoxicity.
One issue with the present study is that we measured hearing at a single time point. We chose the 24 h time point in part because evidence of ototoxicity was observed under in vitro conditions by 24 h (Yorgason et al., 2010; Kalinec et al., 2014). It is possible that ototoxicity develops later after APAP. However, the latter seems unlikely because the NAPQI formation, oxidative stress, and clearance of APAP are complete in mice long before 24 h (McGill et al., 2013), so any damage would be expected to develop by this time point, as in the liver. Furthermore, although hearing loss can develop days after treatment with kanamycin, for example, that delay is due to slow release of the drug from cochlear fluid resulting in prolonged exposure. As stated, APAP crosses membranes, so that would not be expected to occur. Finally, if any hearing loss occurred before 24 h, it should still be detectable at 24 h because, unlike liver cells, cochlear cells have very limited capacity to regenerate. Thus, hearing loss is a permanent effect.
It may be important to note that the kinetics of APAP metabolism in humans and human hepatocytes differ from mice and mouse hepatocytes (McGill et al., 2011). Metabolism and formation of NAPQI appears to happen much faster in mice (McGill et al., 2011; McGill et al., 2013). This does not seem to be a result of differences in absorption, as APAP is rapidly absorbed in both species. As mentioned, it is a small molecule that lacks charge at physiological pH and can therefore cross lipid membranes. In fact, APAP is used to study gastric emptying in humans because it is so rapidly absorbed (Heading et al., 1973; Nimmo et al., 1973). We have demonstrated that it is also rapidly absorbed in mice after enteral administration, based on the observation that GSH depletion is complete as early as 2 h after administration similar to i.p. treatment. However, there is evidence that NAPQI formation occurs over a longer period of time in human hepatocytes (McGill et al., 2011). Thus, APAP toxicity in humans is delayed compared with mice likely because of differences in the kinetics of NAPQI formation. Despite this, it is clear that NAPQI formation and protein binding occur in humans and human hepatocytes, just as in mice (Davern et al., 2006; McGill et al., 2011). Furthermore, there is strong evidence that the mechanisms of APAP hepatotoxicity are the same in mice and humans (McGill et al., 2012; Antoine et al., 2012).
CONCLUSIONS
This is the first study to test the effect of a single human-relevant acute APAP overdose on cochleae. We did not observe ototoxicity. Moreover, we were unable to detect evidence of NAPQI formation. However, there was transient oxidative stress at 6 h. If ototoxicity occurs in humans after a single acute overdose, then the mechanism of injury differs from the mechanism of hepatotoxicity which requires NAPQI. The likely mechanism would be APAP-induced oxidative stress. This may also be true in patients with hearing loss due to chronic APAP/opioid abuse. Interventions designed to scavenge reactive oxygen species could be useful in those cases.
Acknowledgments
This work was supported in part by National Institutes of Health grants R01 DK102142 (H.J.), P20 GM103549 (H.J.), T32 ES007079 (M.R.M.), and T32 HD057850 (S.K.). Additional support came from the Gunner Proud Scholars Award for Otolaryngology from the University of Kansas Medical Center (S.K.) and a Biomedical Research Training Program grant from the University of Kansas Medical Center (S.K.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
References
- Altyar A, Kordi L, Skrepnek G. Clinical and economic characteristics of emergency department visits due to acetaminophen toxicity in the USA. BMJ Open. 2015;5:e007368. doi: 10.1136/bmjopen-2014-007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–225. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bates DE, Beaumont SJ, Baylis BW. Ototoxicity induced by gentamicin and furosemide. Ann Pharmacother. 2002;36:446–451. doi: 10.1345/aph.1A216. [DOI] [PubMed] [Google Scholar]
- Boyer TD, Rouff SL. Acetaminophen-induced hepatic necrosis and renal failure. JAMA. 1971;218:440–441. [PubMed] [Google Scholar]
- Crann SA, Huang MY, McLaren JD, Schacht J. Formation of a toxic metabolite from gentamicin by a hepatic cytosolic fraction. Biochem Pharmacol. 1992;43:1835–9. doi: 10.1016/0006-2952(92)90718-x. [DOI] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Du K, Xie Y, McGill MR, Jaeschke H. Pathophysiological significance of c-Jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2015 doi: 10.1517/17425255.2015.1071353. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LJ, Green MD, Harman AW. Levels of acetaminophen and its metabolites in mouse tissues after a toxic dose. J Pharmacol Exp Ther. 1981;219:281–286. [PubMed] [Google Scholar]
- Friedman RA, House JW, Luxford WM, Gherini S, Mills D. Profound hearing loss associated with hydrocodone/acetaminophen abuse. Am J Otol. 2000;21:188–191. doi: 10.1016/s0196-0709(00)80007-1. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kitaichi K, Abe F, Fujimoto Y, Takagi K, Takagi K, Morishima T, Hasegawa T. Altered brain penetration of diclofenac and mefenamic acid, but not acetaminophen, in Shiga-like toxin II-treated mice. J Pharmacol Sci. 2005;97:525–532. doi: 10.1254/jphs.fp0040752. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heading RC, Nimmo J, Prescott LF, Tothill P. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol. 1973;47:415–421. doi: 10.1111/j.1476-5381.1973.tb08339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T, Vrabec JT, Burton AW. Hydrocodone use and sensorineural hearing loss. Pain Physician. 2007;10:467–472. [PubMed] [Google Scholar]
- Husmann KR, Morgan AS, Girod DA, Durham D. Round window administration of gentamicin: a new method for the study of ototoxicity of cochlear hair cells. Hear Res. 1998;125:109–19. doi: 10.1016/s0378-5955(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Thein P, Parsa A, Yorgason J, Luxford W, Urrutia R, Kalinec F. Acetaminophen and NAPQI are toxic to auditory cells via oxidative and endoplasmic reticulum stress-dependent pathways. Hear Res. 2014;313:26–37. doi: 10.1016/j.heares.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and ahl locus in mice. Hear Res. 2004;188:21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Fariss MW, Farhood A, Jaeschke H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol Sci. 2003;76:229–236. doi: 10.1093/toxsci/kfg220. [DOI] [PubMed] [Google Scholar]
- Kraft S, Hsu C, Brough DE, Staecker H. Atoh1 induces auditory hair cell recovery in mice after ototoxic injury. Laryngoscope. 2013;123:992–999. doi: 10.1002/lary.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142–152. doi: 10.1055/s-2008-1073114. [DOI] [PubMed] [Google Scholar]
- Mathews WR, Guido DM, Fisher MA, Jaeschke H. Lipid peroxidation as molecular mechanism of liver cell injury during reperfusion after ischemia. Free Radic Biol Med. 1994;16:763–770. doi: 10.1016/0891-5849(94)90191-0. [DOI] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. A direct comparison of methods used to measure oxidized glutathione in biological samples: 2-vinylpyridine and N-ethylmaleimide. Toxicol Mech Methods. 2015 doi: 10.3109/15376516.2015.1094844. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Lebofsky M, Norris HR, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose-response, mechanisms and clinical implications. Toxicol Appl Pharmacol. 2013;269:240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nimmo J, Heading RC, Tothill P, Prescott LF. Pharmacological modification of gastric emptying: effects of propantheline and metoclopromide on paracetamol absorption. Br Med J. 1973;1:587–589. doi: 10.1136/bmj.1.5853.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh AK, Ishiyama A, Baloh RW. Deafness associated with abuse of hydrocodone/acetaminophen. Neurology. 2000;54:2345. doi: 10.1212/wnl.54.12.2345. [DOI] [PubMed] [Google Scholar]
- Placke ME, Wyand DS, Cohen SD. Extrahepatic lesions induced by acetaminophen in the mouse. Toxicol Pathol. 1987;15:381–387. doi: 10.1177/019262338701500401. [DOI] [PubMed] [Google Scholar]
- Rosen GM, Rauckman EJ, Ellington SP, Dahlin DC, Christie JL, Nelson SD. Reduction and glutathione conjugation reactions of N-acetyl-p-benzoquinone imine and two dimethylated analogues. Mol Pharmacol. 1984;25:151–157. [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayiq RM, Roberts DW, Rothstein K, Snawder JE, Benson W, Ma X, Black M. Repeat exposure to incremental doses of acetaminophen provides protection against acetaminophen-induced lethality in mice: an explanation for high acetaminophen dosage in humans without hepatic injury. Hepatology. 1999;29:451–463. doi: 10.1002/hep.510290241. [DOI] [PubMed] [Google Scholar]
- Tiegs G, Karimi K, Brune K, Arck P. New problems arising from old drugs: second-generation effects of acetaminophen. Expert Rev Clin Pharmacol. 2014;7:655–662. doi: 10.1586/17512433.2014.944502. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxicity regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]
- Wendel A, Feuerstein S. Drug-induced lipid peroxidation in mice – I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981;30:2513–2520. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- Wendel A, Jaeschke H, Gloger M. Drug-induced lipid peroxidation in mice – II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol. 1982;31:3601–3605. doi: 10.1016/0006-2952(82)90582-2. [DOI] [PubMed] [Google Scholar]
- Yorgason JG, Kalinec GM, Luxford WM, Warren FM, Kalinec F. Acetaminophen ototoxicity after acetaminophen/hydrocodone abuse: evidence from two parallel in vitro mouse models. Otolaryngol Head Neck Surg. 2010;142:814–819. doi: 10.1016/j.otohns.2010.01.010. [DOI] [PubMed] [Google Scholar]