Abstract
We used magnetoencephalography to examine lateralization and binaural interaction of the middle-latency and late-brainstem components of the auditory evoked response (the MLR and SN10, respectively). Click stimuli were presented either monaurally, or binaurally with left- or right-leading interaural time differences (ITDs). While early MLR components, including the N19 and P30, were larger for monaural stimuli presented contralaterally (by approximately 30 and 36 % in the left and right hemispheres, respectively), later components, including the N40 and P50, were larger ipsilaterally. In contrast, MLRs elicited by binaural clicks with left- or right-leading ITDs did not differ. Depending on filter settings, weak binaural interaction could be observed as early as the P13 but was clearly much larger for later components, beginning at the P30, indicating some degree of binaural linearity up to early stages of cortical processing. The SN10, an obscure late-brainstem component, was observed consistently in individuals and showed linear binaural additivity. The results indicate that while the MLR is lateralized in response to monaural stimuli—and not ITDs—this lateralization reverses from primarily contralateral to primarily ipsilateral as early as 40 ms post stimulus and is never as large as that seen with fMRI.
Keywords: middle-latency response, lateralization, binaural interaction, interaural time difference, auditory brainstem response, SN10
Introduction
The auditory middle-latency response (MLR), with vertex-positive deflections at 13, 30, and 50 ms (P13, P30, P50) and vertex-negative deflections at 19 and 40 ms (N19, N40) (Geisler et al. 1958; Picton et al. 1974; Davis and Hirsh 1979; Ozdamar and Kraus 1983), represents the earliest components of the auditory evoked response (AER) with generators in auditory cortex (AC) (Celesia 1976; Scherg and Von Cramon 1986; Liégeois-Chauvel et al. 1994) and is thus crucial for understanding early cortical processing in audition. Furthermore, the MLR has also been used to assess temporal acuity of auditory cortex (Rupp et al. 2002a), peripheral auditory function (Rupp et al. 2002b; Atcherson and Moore 2014), functional integrity of the ascending auditory pathway (Musiek et al. 1984; Fifer and Sierra-Irizarry 1988; Kraus and McGee 1990; Alain et al. 2013), and depth of anesthesia (Plourde 2006), making it an important tool in both research and clinical settings.
Unlike the primary visual and somatosensory cortices, where afferent projections derive exclusively from the contralateral hemifield (Wall and Dubner 1972; Rodieck 1979) resulting in highly lateralized cortical responses to lateralized stimuli (Buchner et al. 1994; Clark et al. 1994), the primary AC receives substantial ipsilaterally derived projections (Brugge 2013). These can be either excitatory or inhibitory, while contralaterally derived projections are thought to be exclusively excitatory (Phillips and Gates 1982). Whether such functional neuroanatomical gradients nevertheless result in lateralized MLRs remains unclear. Although it is well established that later components of the auditory evoked response—especially the N1—can be lateralized in response to both monaural (Scherg and Von Cramon 1986; Woldorff et al. 1999; Hine and Debener 2007; Königs and Gutschalk 2012) and lateralized binaural (McEvoy et al. 1993; McEvoy et al. 1994; Palomäki et al. 2005; Johnson and Hautus 2010; Königs and Gutschalk 2012) stimuli, previous studies examining the MLR are inconclusive. While some have reported balance for monaural stimulation (Peters and Mendel 1974; Scherg and Von Cramon 1986; Kileny et al. 1987; Jacobson and Grayson 1988), others have indicated varying degrees of contralateral dominance for the N19 (Woods and Clayworth 1985), the N19-P30 pair (Woods et al. 1987), the P30 and P50 (Mäkelä et al. 1994), or the P50-N100 pair (Pantev et al. 1986), but not for binaural stimuli lateralized by interaural time differences (ITDs) (McEvoy et al. 1994).
Apart from providing new insight into early cortical auditory processing, establishing the laterality of the auditory MLR would assist in understanding the relationship between the dendritic currents measured by M/EEG (Lopes da Silva 2013) and the fMRI BOLD signal, which is thought to reflect a combination of synaptic activity and spiking (Singh 2012). In response to monaural stimulation, fMRI typically shows strong contralateral dominance, often double in amplitude for contralateral compared to ipsilateral stimuli (Scheffler et al. 1998; Woldorff et al. 1999; Jäncke et al. 2002; Langers et al. 2005; Gutschalk and Steinmann 2015). In response to ITD-lateralized sounds, fMRI shows little or no contralateral dominance (Woldorff et al. 1999; von Kriegstein et al. 2008; McLaughlin et al. 2015). Given that the long-latency components of the AER are only slightly lateralized in response to either monaural or lateralized binaural stimuli, and if auditory fMRI activity is more strongly coupled to earlier, faster components of the AER (i.e., the MLR), this would suggest that the MLR might be strongly lateralized in response to monaural sounds.
Utilizing whole-head magnetoencephalography in 10 normal-hearing listeners, we reexamined lateralization of the MLR in response to both monaural and binaural, ITD-lateralized click stimuli. After it was found that the SN10—a late, infrequently observed midbrain response (Davis and Hirsh 1979; Hashimoto 1982)—was consistently present in our listeners, we used this response in conjunction with those from cortex to examine another discrepancy between fMRI and M/EEG data. Specifically, early components of the AER tend to show binaural responses that are approximately equal to the sum of their monaural counterparts, i.e., little to no binaural interaction (but see Riedel and Kollmeier 2002; Riedel and Kollmeier 2006; Junius et al. 2007), while later components tend to show sub-additivity (Pratt 2013). In contrast, the lone fMRI study examining this question showed monaural activity that was itself larger than its binaural counterpart—i.e., strong sub-additivity—at every structure examined, down to and including the inferior colliculus (IC) (Krumbholz et al. 2005). We therefore evaluated if the later brainstem response potentially shows a pattern that is more similar to fMRI results.
Methods
Ethics Statement
All procedures were approved by the Institutional Review Board at the University Hospital Heidelberg in accordance with the Declaration of Helsinki, and all participants provided written informed consent prior to participation.
Listeners
Eleven healthy listeners with no reported history of hearing disorders were recruited for the study (five males; 20–43 years of age, mean 25.6). One female listener was excluded from analysis due to low signal-to-noise ratio of the averaged evoked responses, leaving five male and five female listeners in the remaining analyses.
Stimuli and Procedure
All sound stimuli were generated in MATLAB (The Mathworks, Natick, MA) and stored as 32-bit wav files at a sampling rate of 48 kHz. The digital sound files were converted to analog waveforms by an on-board sound card (RME DIGI96/8 PAD) and freestanding DA converter (RME ADI-8 DS, RME, Haimhausen, Germany) controlled by SoundMexPro software (SoundMexPro, Oldenburg, Germany) in the MATLAB environment. The analog stimuli then passed through a programmable attenuator (TDT PA5) before being amplified (TDT HB7, Tucker-Davis Technologies, Alachua, FL, USA) and presented to listeners via ER-3 insert earphones (Etymotic Research, Elk Grove Village, IL, USA). Note that our choice of ER-3 earphones effectively low-pass filtered our click stimuli at approximately 3 kHz—this is unlikely to have affected our results.
The stimuli used in the study were sequences of clicks with interclick intervals ranging between 97 and 137 ms. Each click consisted of four “up” samples at a sampling rate of 48 kHz with one of five conditions: (1) monaural left, (2) monaural right, (3) dichotic with a −604.17-μs ITD (29 samples at 48 kHz), (4) dichotic with a 604.17-μs ITD, or (5) diotic. The left- and right-leading ITD stimuli were used to determine whether perceived lateralization or lateralization, per se, is the crucial factor underlying lateralized MLRs. Six thousand instances of each click condition were presented to each listener in random order, split across two blocks (3000 instances per condition per block). The recording time needed for each block was approximately 30 min, for a total recording time of approximately 1 h. Stimuli were presented at 96-dB peak-equivalent SPL, which was indicated by pilot studies to be a comfortable listening level.
Data Acquisition
MEG was recorded using a whole-head system comprised of two orthogonal planar gradiometers at each of 61 locations around the head (Ahonen et al. 1993) and digitized at a sampling rate of 1000 Hz with an online band-pass filter between 0.03 and 330 Hz. Stimulus trigger pulses were produced by the remaining six channels of the same eight-channel sound card used to present the sound stimuli and recorded by the MEG acquisition system. Thus, temporal resolution of stimulus-locked averaging was limited only by the 1000-Hz sampling rate of the MEG acquisition. Prior to the MEG session, the locations of 32 points around the head, referenced to the coordinate system defined by three cardinal points (left and right preauricular points and the nasion), were digitized using the Polhemus tracker system (Colchester, VT, USA). Participants’ head position within the dewar was measured by four head position-indicator coils placed on the left and right mastoids and forehead. During the recording, participants watched a silent movie of their choosing and were instructed to remain as still as possible.
Preprocessing
MEG data were analyzed using BESA 5.1 (BESA GmbH, Gräfelfing, Germany). Bad channels, i.e., channels with either no signal or excessive noise, were rejected by visual inspection. The data were then divided into epochs spanning from 10 ms before to 70 ms after click onset and binned according to click condition; epochs containing large artifacts were automatically rejected using a gradient criterion (<2 % of all epochs, on average). The epochs were then baseline corrected by subtracting the mean signal in the baseline period (at 0 ms and from 0 to 10 ms for brainstem and cortical responses, respectively) and averaged. A grand average comprised of all conditions was additionally created for both source analysis (see below) and display purposes.
Source Analysis
The 32 digitized head points were used to approximate the position of a spherical head model for each listener. The source configuration used to model the grand-averaged MEG waveforms in each listener consisted of three equivalent current dipoles, one in each auditory cortex on the superior temporal plane and one in the midbrain fixed to be just below the approximate location of the inferior colliculi using the BESA template brain (Table 1). The cortical dipoles were fit to the peak of the P30—a vertex-positive (in EEG) component of the MLR occurring approximately 30 ms post stimulus—after inspection of sensor-level waveforms indicated that it was the largest and most consistent component across listeners. Subsequently, the orientation of the midbrain dipole (whose location was fixed) was fit to the peak of the SN10, an infrequently observed negativity occurring at 10 ms post stimulus. The resulting dipole models were then used to construct spatially filtered source waveforms for all conditions in all listeners and saved as ASCII files for further analysis using Python. Note that the polarity of our cortical source waveforms is in reference to the surface of auditory cortex, which is also consistent with how such potentials are typically recorded at the vertex in EEG.
Table 1.
Talairach coordinates (in mm) for the two AC sources and dipole orientation for the midbrain source. Mean ± s.e.m. (standard error of the mean across listeners). Note that i hat, j hat, and k hat denote vector strengths (with values ranging from zero to one) away from the sagittal, coronal, and axial planes, respectively
| x | y | z | i hat | j hat | k hat | |
|---|---|---|---|---|---|---|
| Left AC | −44.3 ± 1.7 | −23.7 ± 3.1 | 5.6 ± 3.8 | 0.01 ± 0.06 | 0.64 ± 0.06 | 0.72 ± 0.04 |
| Right AC | 44.4 ± 2.3 | −23.0 ± 2.2 | 8.0 ± 1.9 | −0.06 ± 0.05 | 0.56 ± 0.07 | 0.77 ± 0.04 |
| Midbrain | 0 | −35.2 | −15.9 | 0.01 ± 0.08 | 0.70 ± 0.00 | −0.66 ± 0.02 |
The data were then low-pass filtered at 150 Hz using a zero phase-shift, second-order Butterworth filter, detrended, and baseline corrected. For detrending, the epoch-length linear trend (as determined by a least-squares fit to the data) was subtracted from each epoch. The amplitudes and latencies of each of four successive MLR peaks in the cortical source waveforms were then measured in the following intervals: N19 (15–25 ms), P30 (25–35 ms), N40 (35–45 ms), and P50 (45–55 ms). As no high-pass filter was used, amplitudes for each component were referenced to the immediately preceding component (i.e., P13-N19, N19-P30, P30-N40, and N40-P50), which was necessary for putatively negative-going components that were positive if surrounded by large positive components (the N40, in particular). Finally, the amplitude and latency of the SN10 in the midbrain source was measured as the negative peak occurring between 5 and 15 ms post stimulus.
To examine whether the resulting midbrain activity might instead have reflected unaccounted-for cortical activity, we also analyzed the data using a classical two-dipole model (one source in each AC). The resulting AC source waveforms were nearly identical to those from the three-dipole model (data not shown). Thus, we present only the results from the three-dipole model.
Lateralization Indices
To quantify the amount of lateralization exhibited by the MLR, we computed lateralization indices for each component pair in each of two ways. The first, which is consistent with the index used by previous literature, was defined for each ear as the difference between the right and left AC responses, i.e., LatEar = (|Right AC| − |Left AC|)/(|Right AC| + |Left AC|). The second is defined in each hemisphere as the response elicited by the ipsilateral stimulus subtracted from that elicited by the contralateral stimulus, divided by their sum, i.e., LatHemi = (|Contra| − |Ipsi|)/(|Contra| + |Ipsi|). By computing lateralization within a hemisphere across the two ears, this latter index eliminates confounds of hemispheric asymmetry, which can be quite strong for tonal stimuli in auditory cortex (with typically right-ear > left-ear responses) due in large part to anatomical differences resulting in differing amounts of field cancelation (Shaw et al. 2013).
Binaural Difference Responses
To examine binaural interaction in both cortical and subcortical waveforms, we computed binaural difference waveforms by subtracting the mean of the responses to binaural conditions from the sum of responses to monaural conditions (van Olphen et al. 1978; Dobie and Norton 1980; Levine 1981; Wrege and Starr 1981). The binaural difference response gives an indication of the linear additivity of monaural responses, and prominent deflections in the binaural difference response are thought to reflect neuronal activity specific to binaural stimulation (Dobie and Berlin 1979; Gaumond and Psaltikidou 1991). To quantify the binaural difference response, we computed average amplitudes within 2.5 ms of each of three grand average peaks in both the cortical and subcortical waveforms. For the cortex (midbrain), these peaks were at 12, 27, and 53 ms (10, 19, and 34 ms). Note that the waveforms from left and right AC were collapsed prior to computing these amplitudes.
Statistical Analysis
Peak amplitudes and latencies were evaluated using a repeated-measures analysis of variance (ANOVA) framework in R (http://www.r-project.org/). When appropriate, additional ANOVAs and post hoc paired comparisons were performed (see the “Results” section). In order to balance and simplify the statistical analysis, the peak amplitude and latency values from the diotic control condition were excluded after it was found that they did not differ from either of the ITD-lateralized binaural conditions. Subsequently, for the cortical sources, the peak amplitude values from each of the four component pairs (P13-N19, N19-P30, P30-N40, and N40-P50) were entered into separate three-way ANOVAs with hemisphere (left vs. right), type (monaural vs. binaural), and side (contralateral vs. ipsilateral) as factors. The same analysis on latency values was performed but on individual components rather than component pairs. The peak and latency values from the midbrain source were evaluated for the SN10 component only, using a one-way ANOVA with condition as the lone factor. For peaks in the binaural difference waveforms, single-sample, two-sided t tests were used to evaluate whether each peak was significantly different from zero.
Results
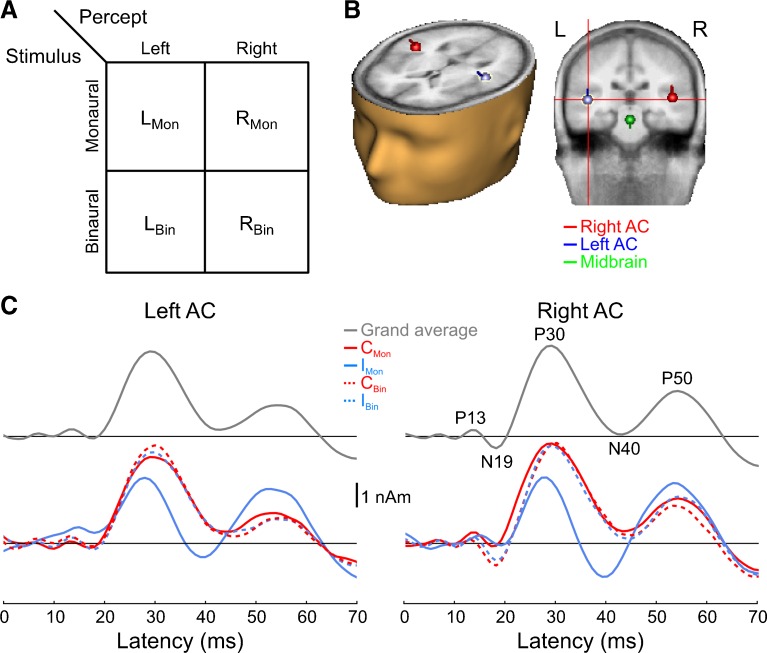
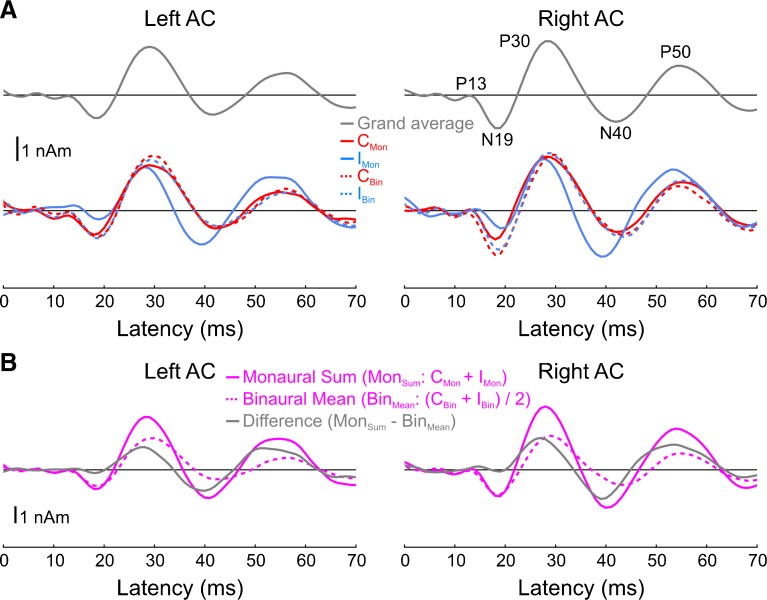
Grand-average source waveforms displayed the characteristic components of the MLR, including the P13, N19, P30, N40, and P50 (Fig. 1). All conditions elicited nearly identical responses, with the exceptions being (i) the qualitatively different response elicited by the monaural, ipsilateral stimulus (IMon) and (ii) the larger N19 elicited by binaural vs. monaural stimuli, particularly in right AC. Visual inspection of the blue, IMon, waveform in Figure 1C suggests that the difference between this waveform and those elicited by the remaining three conditions manifested itself as (i) a smaller (relative to the preceding P13), delayed N19; (ii) a smaller, earlier P30; (iii) an N40, or at least a stronger one; and (iv) a larger P50.
Fig. 1.
Paradigm and cortical source waveforms. A Stimulus conditions and experimental design. B Grand-average source locations/orientations overlaid onto BESA’s template brain/head surface. C Auditory cortex source waveforms for the grand average (all conditions collapsed; top panels) as well as each condition separately (bottom panels). Five main peaks of the MLR are visible: P13, N19, P30, N40, and P50. The solid (dashed) traces show the waveforms for the monaural (binaural) conditions. The response to the diotic click stimulus is not shown, after it was found that it did not differ from either of the remaining binaural conditions. C Mon contralateral monaural stimulus, I Mon ipsilateral monaural stimulus, C Bin contra-leaded binaural stimulus, I Bin ipsi-leading binaural stimulus.
Cortical Sources—Amplitude
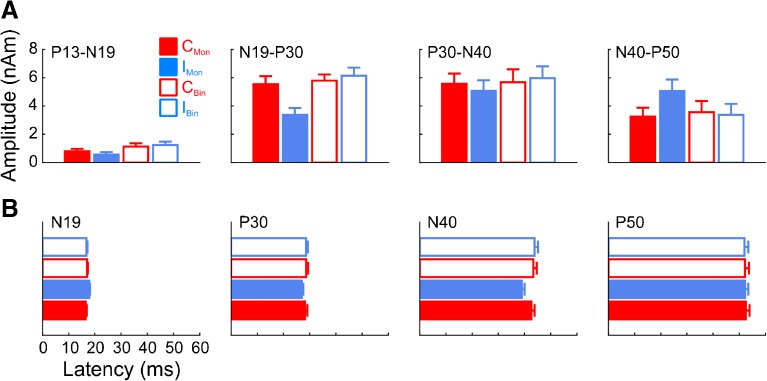
To quantify these effects, we computed peak amplitudes and latencies for each of the four components (component pairs, in the case of amplitude) and conditions (Fig. 2A). The most consistent effect was a stronger overall response in right AC compared to left AC, consistent with previous reports (main effect of hemisphere for each component pair, cf. Table 2). An additional main effect of type was observed for the P13-N19 and N19-P30. This seems real for the P13-N19 given that both monaural stimuli elicited smaller responses than either binaural stimuli (Fig. 2A, leftmost panel). For the N19-P30, however, this effect (as well as a main effect of side) was likely driven exclusively by the smaller response to the IMon condition; the CMon stimulus elicited a response nearly as large as either binaural stimuli. This was confirmed by a significant two-way interaction between type and side. For the P13-N19, we additionally observed a significant hemisphere-by-type interaction, suggesting that the difference between monaural and binaural responses was larger in one hemisphere. This was confirmed by subsequent two-way ANOVAs (one for each hemisphere), where it was found that the binaural/monaural difference in the P13-N19 amplitudes was larger and more robust in right AC, though both were significant (left AC: F1,9 = 7.6, P < 0.05; right AC: F1,9 = 42.8, P < 0.0005). For the P30-N40 pair, no significant effects were observed apart from the main effect of hemisphere. For the N40-P50 pair, we observed a significant type-by-side interaction, here driven by larger IMon responses compared to the other conditions (Fig. 2A, rightmost panel).
Fig. 2.
Cortical amplitude (A) and latency (B) quantifications for each of the four component pairs (for amplitude) or components (for latency). Error bars denote standard error of the mean across listeners. Legend as in Fig. 1. For display purposes, values were collapsed across hemispheres but analyzed separately statistically.
Table 2.
Component pair amplitude and latency statistics
| Amplitude | Latency | |||||||
|---|---|---|---|---|---|---|---|---|
| P13-N19 | N19-P30 | P30-N40 | N40-P50 | P13-N19 | N19-P30 | P30-N40 | N40-P50 | |
| Hemi (L vs. R) | 7.9* | 6.2* | 5.5* | 19.2** | 2.9, n.s. | 0.9, n.s. | 3.4· | 1.0, n.s. |
| Type (M vs. B) | 29.4*** | 20.6** | 0.8, n.s. | 1.5, n.s. | 4.1· | 8.7* | 25.3** | 0.2, n.s. |
| Side (C vs. I) | 2.0, n.s. | 14.6** | 0.9, n.s. | 10.7* | 20.2** | 2.9, n.s. | 22.0** | 0.0, n.s. |
| Hemi × type | 11.8* | 0.8, n.s. | 0.6, n.s. | 0.1, n.s. | 1.6, n.s. | 1.8, n.s. | 0.2, n.s. | 0.1, n.s. |
| Hemi × side | 1.2, n.s. | 0.1, n.s. | 0.2, n.s. | 0.9, n.s. | 0.5, n.s. | 0.1, n.s. | 0.4, n.s. | 0.3, n.s. |
| Type × side | 2.0, n.s. | 19.3** | 0.1, n.s. | 8.9* | 13.8** | 3.5· | 9.8* | 0.7, n.s. |
| 3-way interaction | 1.5, n.s. | 0.1, n.s. | 0.2, n.s. | 0.5, n.s. | 0.9, n.s. | 0.0, n.s. | 0.3, n.s. | 0.0, n.s. |
The value in each cell represents an F value with 1 and 9 ° of freedom in the numerator and denominator, respectively (i.e., F 1,9), reflecting the fact that there were two levels in each factor and 10 participants. Hemisphere: right vs. left. Type: monaural vs. binaural. Side: contralateral vs. ipsilateral
·P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001
Because one of the primary reasons for conducting the study was to examine MLR lateralization, we additionally performed paired amplitude comparisons, in each AC and for each component pair, between the contralaterally and ipsilaterally evoked responses for the monaural conditions. This analysis indicated significant lateralization in each AC for the N19-P30 (left AC: T9 = 2.7, P < 0.05, right AC: T9 = 4.6, P < 0.01), the N40-P50 (left AC: T9 = 2.6, P < 0.05, right AC: T9 = 2.9, P < 0.05), and a trend in right AC for the P13-N19 (T9 = 2.0, P = 0.075).
To further quantify MLR lateralization, we computed a lateralization index for each component pair in each of two ways (see the “Methods” section), the first defined for each ear (LatEar) and the second defined for each hemisphere (LatHemi; cf. Table 3). For the LatEar index, positive values indicate that responses are stronger in right AC, while negative values indicate larger responses in left AC. It is apparent from this index that right AC responses were generally larger than left AC responses, consistent with previous auditory MEG studies using tonal stimuli. For the LatHemi index, positive values indicate that responses are stronger in response to contralateral stimulation; negative values indicate responses that are stronger in response to ipsilateral stimuli. Here, the earlier component pairs had positive values indicating larger responses elicited by contralateral stimuli. This trend was reversed for the N40-P50 pair, reflecting larger ipsilaterally driven responses at those latencies. Differences between left and right AC lateralization indices were never statistically significant. As the latter index avoids the typical right > left asymmetry and thus yields values that are more consistent and easier to interpret in the context of lateralization, we focus our later discussion on it.
Table 3.
Lateralization indices for each source and component pair from the monaural conditions. By ear, a positive (negative) index indicates that the response was stronger in right (left) AC. By hemisphere, a positive (negative) index indicates that the response was stronger in response to contralateral (ipsilateral) stimulation
| P13-N19 | N19-P30 | P30-N40 | N40-P50 | |
|---|---|---|---|---|
| LatEar | ||||
| Left ear | 0.39 ± 0.10 | 0.35 ± 0.07 | 0.20 ± 0.07 | 0.02 ± 0.11 |
| T 9 = 3.2* | T 9 = 4.7** | T 9 = 2.5* | T 9 = 0.14, n.s. | |
| Right ear | −0.02 ± 0.17 | −0.14 ± 0.06 | 0.06 ± 0.10 | 0.49 ± 0.05 |
| T 9 = −0.10, n.s. | T 9 = −2.2· | T 9 = 0.53, n.s. | T 9 = 7.7*** | |
| LatHemi | ||||
| Left AC | 0.19 ± 0.15 | 0.25 ± 0.09 | 0.07 ± 0.09 | −0.26 ± 0.11 |
| T 9 = 1.2, n.s. | T 9 = 2.7* | T 9 = 0.73, n.s. | T 9 = −2.3* | |
| Right AC | 0.26 ± 0.13 | 0.24 ± 0.05 | 0.08 ± 0.09 | −0.22 ± 0.07 |
| T 9 = 1.9· | T 9 = 4.8** | T 9 = 0.88, n.s. | T 9 = −2.9* | |
We did not observe any significant differences between the left and right AC indices, indicating similar bias in each AC for either contralateral (in the case of the N19 and/or P30) or ipsilateral (N40 and/or P50) responses. T values below each index indicate whether it differed significantly from zero
·P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001
Cortical Sources—Latency
The statistical pattern of results for component latencies is summarized primarily by a type-by-side interaction for the N19, P30, and N40 components, driven by (i) a later N19 and (ii) earlier P30s and N40s for the IMon condition (cf. Table 2); no significant latency effects were observed for the P50 (Fig. 2B).
Midbrain Source
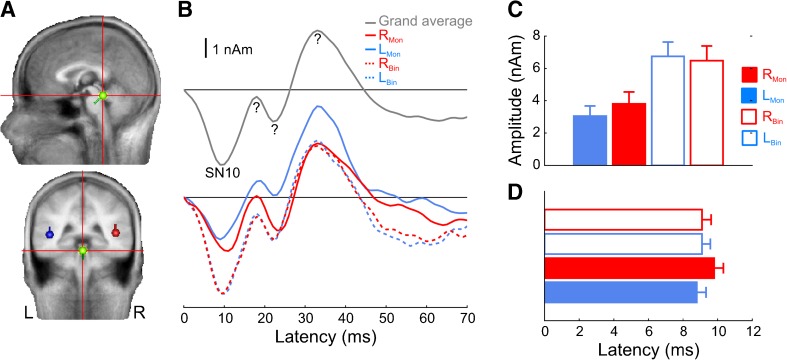
Figure 3 shows the midbrain grand-averaged source orientation (panel A) and waveform (panel B, upper panel), the waveforms for each of the four conditions averaged separately (panel B, lower panel), and amplitude and latency quantifications (panels C and D, respectively). The SN10 is clearly present as a negative deflection in the waveforms around 10 ms and was highly consistent across individuals in terms of both dipole orientation and waveform morphology. Two-way ANOVAs computed on the peak amplitude and latency values for the SN10 indicated that amplitude was significantly larger for binaural vs. monaural stimulation (F1,9 = 50.9, P < 0.0001); there was no significant effect of latency (F1,9 = 1.4, n.s.). Neither amplitude nor latency was significantly affected by whether the stimulus was presented to the left or right ear, and there were no significant interactions. Later deflections were also clearly present in the midbrain source at 18, 22, and 33 ms. As we neither hypothesized their occurrence nor set out to examine them, and given their temporal overlap with active cortical sources, we did not analyze them in detail. However, we return to them in the discussion.
Fig. 3.
Midbrain source analysis and amplitude/latency quantifications. A Average source orientation. B Grand-averaged source waveforms collapsed across all conditions (top panel) and for each of the five stimulus conditions separately (bottom panel). The SN10 is clearly visible as a negative-going deflection at ∼10 ms. R Mon right-ear monaural stimulus, L Mon left-ear monaural stimulus, R Bin right-leaded binaural stimulus, L Bin left-leading binaural stimulus. Question marks denote components of questionable source origin. C, D SN10 amplitude and latency quantifications for all conditions. Legend same as in B. Error bars denote standard error of the mean across listeners.
Binaural Difference Responses
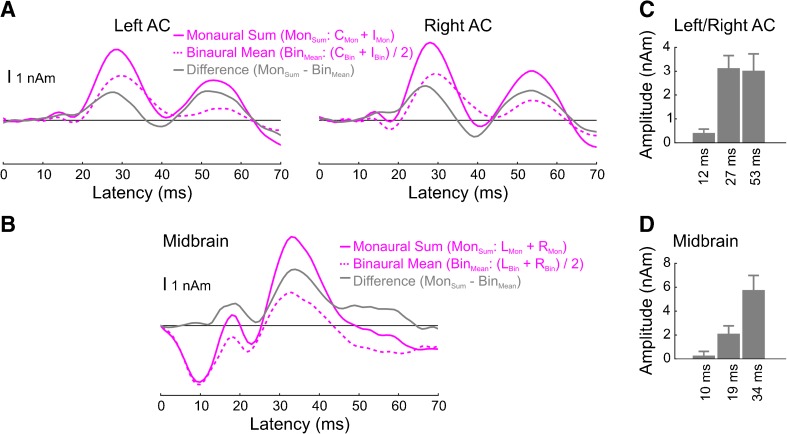
Binaural difference responses are shown in Figure 4. For the cortical sources, three positive-going peaks in binaural difference waveforms (gray traces) can be observed, peaking at 12, 27, and 53 ms. Because the binaural difference responses from left and right AC were similar, we averaged them before computing peak amplitudes and statistics. The resulting peaks at 27 and 53 ms were significantly different from zero (27 ms: T9 = 5.5, P < 0.001; 53 ms: T9 = 4.0, P < 0.01). There was also a trend for the peak at 12 ms (T9 = 1.9, P = 0.09), though a supplemental analysis using narrower band-pass filters (20–150 Hz as opposed to 0–150 Hz) did not reveal such a peak in the binaural difference waveforms (Fig. 5). Although there is likely substantial binaural interaction at around the time of the N40 given the fact that the IMon condition was the only condition to definitely elicit this deflection (cf. Fig. 1C), this interaction does not manifest itself in the binaural difference waveform given the facts that (i) the response of the IMon condition is phase advanced relative to any of the other conditions and (ii) the IMon and CMon traces are of opposite polarities at this latency. The binaural interaction at this latency appears stronger if we apply a 20-Hz high-pass filter to the waveforms and results in a significant peak in the binaural difference waveform at 39 ms (T9 = −4.2, P < 0.01). Finally, although the amplitudes of the later two peaks (at 27 and 53 ms) appear similar, it is worth noting that the binaural difference response itself (gray traces) was larger than the mean binaural response (dashed pink traces).
Fig. 4.
Binaural difference (BD) responses for the cortical (A) and subcortical (B) sources and their amplitude quantifications (C, D). The binaural response is the average of the two binaural ITD conditions (C Bin and I Bin for A, R Bin and L Bin for B), and the monaural sum response is the sum of the two monaural conditions (C Mon + I Mon for panel A, R Mon + L Mon for panel B). Note that both the peak amplitudes shown in panel C and the associated statistical tests were computed after collapsing across the two hemispheres.
Fig. 5.
Cortical source waveforms and binaural difference responses after applying a 20-Hz HPF. A Grand-average (top panels) and individual condition (bottom panels) source waveforms (B). Corresponding binaural difference responses.
The N19 appeared to behave in a fundamentally different, supra-additive manner. That is, the N19 peak of the binaural mean response is more negative than the N19 of the sum of the two monaural conditions. In our opinion, this likely reflects either (i) the fact that peaks at both 12 and 27 ms (likely corresponding to the P13 and P30) show sub-additivity, perhaps making the N19 of the monaural sum artificially positive, or (ii) the difficulty in obtaining sufficient SNR for these early, small components. Indeed, when using the same 20-Hz high-pass filter that was used to evaluate the N40, the 12-ms peak in the binaural difference waveform is not statistically different from zero (T9 = 0.4, n.s.), and the binaural difference waveform is completely flat until the P30 latency, suggesting linear additivity of the N19.
For the midbrain source, no significant difference was observed in the latency range of the SN10 (T9 = 0.51, P = 0.62). In contrast, two later peaks at 19 and 34 ms were both significantly different from zero (19 ms: T9 = 2.8, P < 0.05; 34 ms: T9 = 4.4, P < 0.01). However, given the difficulty of measuring deep brain activity with MEG and the fact that these later peaks co-occurred with cortical sources, it is not clear whether they truly arose from the midbrain.
Discussion
The present findings demonstrate a robust contralateral dominance of the P30 in response to monaural stimulation but no significant lateralization for ITD-lateralized stimulation. Unexpectedly, the subsequent N40 and P50 peaks showed ipsilateral instead of contralateral dominance. Furthermore, the data show that the SN10, an infrequently observed brainstem response, can be reliably recorded with MEG using tailored source analysis methods.
Generators and Lateralization of the MLR
Like many previous M/EEG, intracranial, and lesion-effect studies, our data clearly implicate primary auditory cortex as the main generator of the MLR (Celesia 1976; Kraus et al. 1982; Özdamar et al. 1982; Scherg and Von Cramon 1986; Liégeois-Chauvel et al. 1994; Hashimoto et al. 1995; Kuriki et al. 1995; Gutschalk et al. 1999; Godey et al. 2001; Rupp et al. 2002b; Yvert et al. 2005; Parkkonen et al. 2009). Lateralization of the MLR in response to monaural stimulation, however, has historically been controversial, with some EEG studies reporting lateralization (Woods and Clayworth 1985; Woods et al. 1987; Cacace et al. 1990; Kaseda et al. 1991) and others reporting balance (Peters and Mendel 1974; Scherg and Von Cramon 1986; Kileny et al. 1987; Jacobson and Grayson 1988). Consistent with human intracranial recordings (Celesia 1976), MEG has shown lateralization, both of the P30 (Mäkelä et al. 1994; Yvert et al. 2001) and the related auditory steady-state response (ASSR) (Ross et al. 2005; Gutschalk et al. 2012), which reflects overlapping middle-latency responses (Gutschalk et al. 1999; Bohórquez and Ozdamar 2008).
Based on the present data, the P30 evoked by monaural clicks seems clearly lateralized, with a contralateral-greater-than-ipsilateral amplitude difference of approximately 40 % (i.e., a lateralization index of 0.25), consistent with previous ASSR data (Ross et al., 2005; Gutschalk et al., 2012). Numerically, the N19 showed similarly contralateral responses (cf. Table 3). However, these effects did not reach statistical significance, perhaps due to the relatively small size of the component and the difficulty in obtaining a clean baseline. Thus, N19 lateralization may well be somewhat smaller or larger than that observed here. The latter is suggested by the high-passed filtered data (Fig. 5), which enhances contralateral dominance of the N19 while attenuating contralateral dominance of the P30. Similar differences in data-analysis setups might have biased whether the N19 (Woods and Clayworth 1985) or P30 (Mäkelä et al. 1994) was found to be contralaterally dominant previously.
Somewhat unexpected was the finding of ipsilateral dominance of the subsequent N40 and P50 components. To our knowledge, such an inverse lateralization effect has only been observed as a numerical trend for the N40 (Woods and Clayworth 1985) and in a subset of participants for the P50 (Mäkelä et al. 1994), and others have found the opposite effect, i.e., N40 and P50 responses that were larger for monaural stimuli presented contralaterally (Pantev et al. 1986; Mäkelä et al. 1994; Yvert et al. 2001). Such variable results for the later MLR components could in part be due to the presence of multiple P50 generators (Yvert et al. 2001), with some showing contralateral dominance and others showing ipsilateral dominance. The version of the P50 measured by a given study is likely related to details of the acoustic stimulation used.
While contralaterally dominant MLR responses might be expected given contralateral dominance of excitatory projections in the ascending auditory pathway, the ipsilateral dominance we observed for the N40/P50 is less intuitive. One explanation could be that the N40/P50 receives different input than the P30, perhaps inhibition from subcortical nuclei (Glendenning et al. 1992; Stecker et al. 2015) or excitation from the opposite hemisphere via the corpus callosum. Evidence for the latter idea comes from a recent MEG/fMRI study using 8-Hz steady-state responses, which reflects overlapping P50s (Gutschalk and Steinmann 2015). Though not the case in all participants, that study observed a trend for larger ipsilaterally driven responses that was reversed in a participant with callosal agenesis. Interestingly, the P50 (but not the P30) evoked by monaural stimulation is suppressed in the presence of a contralateral masking noise (Ozdamar and Bohórquez 2008), further suggesting that the P50 receives different input than the P30.
Note that we are not suggesting that different components reflect either exclusively excitatory or inhibitory post-synaptic potentials (PSPs); indeed, excitation and inhibition occur simultaneously in auditory cortex, at least in rodents (Wehr and Zador 2003). Accordingly, the remaining dissociation of stronger contralateral dominance in fMRI as compared to MEG might reflect a greater relative sensitivity of fMRI towards either excitatory PSPs or high-frequency synaptic activity, i.e., high gamma, an aspect of neural activity that is known to correlate strongly with the BOLD signal (Mukamel et al. 2005; Singh 2012) and which is difficult to observe with M/EEG. While detailed microelectrode data on this issue are lacking, what data are available show multiple laminar response profiles in primary auditory cortex of awake nonhuman primates (Reser et al. 2000; Steinschneider et al. 2008), suggesting that the phenomenon is likely complex and multi-faceted.
Measuring the SN10 with MEG
Though it appeared consistently across participants in our data, prior MEG evidence for the SN10 is sparse (Hashimoto et al. 1995; Kuriki et al. 1995). It is more commonly observed with EEG (Picton et al. 1974; Davis and Hirsh 1979; Dobie and Norton 1980; Woods and Clayworth 1985; Tawfik and Musiek 1991; McPherson and Starr 1993) and likely arises from the midbrain given that (i) human intracranial recordings near the inferior colliculus show such a response (Hashimoto 1982; Møller and Jannetta 1982; Kadoya et al. 1998) and (ii) there are no simultaneous deflections in the cortex, either in the present study or in thalamic or cortical potentials recorded intracranially (Yvert et al. 2002). To our knowledge, only one previous MEG study has provided direct evidence of a response at 10 ms (Kuriki et al. 1995) and in only two of four participants. However, the source of that deflection was localized to the same vicinity as the other MLR components. The reason the SN10 was consistently observed in the present study may have to do with the fact that the location of our midbrain dipole was fixed and only its orientation fit. This, combined with the fact that the two cortical dipoles accounted for most of the activity arising from AC, perhaps allowed us to pull out midbrain activity that produces only minor field deflections outside the head.
Binaural Additivity
While binaural difference waves revealed that the SN10 showed near-linear summation, the P30 and subsequent waves of the MLR showed increased proclivity for binaural interaction, as in previous studies (Pratt 2013). In fact, the waveforms elicited by binaural and contralateral monaural stimuli were almost identical, resulting in a binaural difference response whose morphology was very similar to the response elicited by ipsilateral monaural stimuli. The N19 was the only cortical component that increased significantly for binaural vs. contralateral, monaural stimulation. In the binaural difference waves, however, there remained a sustained displacement around the N19 latency. The N19 thus appears to be more linear than the P30, though likely not as linear as the SN10, baseline issues notwithstanding. Later deflections of the midbrain source appeared very binaural, though it is difficult to say with certainty that these deflections are not thalamic (Yvert et al. 2002) or projections of unaccounted-for cortical activity (Scherg and Von Cramon 1986; Gutschalk et al. 1999) given that they co-occur with active cortical sources. However, intracranial potentials with similar latencies and morphology as the first of these deflections (at 18 ms), and perhaps the second (at 22 ms), have been observed in the human IC (Hashimoto 1982).
Taken together, these results are broadly consistent with previous M/EEG studies (Dobie and Norton 1980; Debruyne 1984; Woods and Clayworth 1985; McPherson et al. 1989; McPherson and Starr 1993; Polyakov and Pratt 1995; Junius et al. 2007), though our data generally show a larger increase of interaction with component latency. The long-latency N1 response, not measurable in the present study due to the short ISI, shows yet more interaction (Pantev et al. 1986; Königs and Gutschalk 2012). This would seem to suggest a hierarchy of binaural interaction (Pratt 2013). However, fMRI work has observed substantial binaural interaction down to the IC (Krumbholz et al. 2005), with individual monaural responses themselves as large or larger than binaural responses. While wave V of the ABR also shows binaural interaction, the interaction is small relative to the size of the peak (Riedel and Kollmeier 2002; Riedel and Kollmeier 2006; Junius et al. 2007). The binaural interaction of putative later midbrain components subsequent to the SN10 would therefore better match with the fMRI results, because the interaction would be stronger based on the source activity in this study, but the nature of these waves remains speculative at this point. Thus, instead of an anatomical hierarchy, currently available data suggest that monaural and binaural information is processed in parallel at various nuclei throughout the auditory pathway, with monaural information being reflected by the linear or near-linear additivity of the SN10 and N19 and binaural information marked by BOLD activity as well as wave V (and possibly late midbrain components). However, details of the N19’s binaural additivity and the nature of late-brainstem waves remain to be determined.
Spatial Representation in the Auditory Pathway
The lone factor determining MLR morphology in our data was the presence or absence of stimulation in the contralateral ear. This suggests that the representation of acoustic space, per se, in primary auditory cortex is such that it does not strongly manifest itself in the population-level activity reflected by the MLR. This is consistent with previous M/EEG studies, in which the MLR has shown sensitivity neither to ITDs (McEvoy et al. 1994) nor to externalized percepts generated by head-related transfer functions (Junius et al. 2007). It is also consistent with human fMRI data, which has shown that primary auditory cortex is highly sensitive to both ear of stimulation (Woods et al. 2010; Stecker et al. 2015; Gutschalk and Steinmann 2015) and interaural level differences (ILDs) (McLaughlin et al. 2015; Stecker et al. 2015), but not ITD (von Kriegstein et al. 2008; McLaughlin et al. 2015). Although ITD sensitivity has been found in human auditory cortex, it is generally located in more posterolateral areas (von Kriegstein et al. 2008; McLaughlin et al. 2015) and in long- as opposed to middle-latency components of the AER (McEvoy et al. 1993; Salminen et al. 2009; Salminen et al. 2010; Magezi and Krumbholz 2010; Gutschalk et al. 2012) (for reviews, see Salminen et al. 2012; Gutschalk 2014). In any case, the population-level representation of ITD at the level of the midbrain might be fundamentally different from that found in primary AC (Thompson et al. 2006; von Kriegstein et al. 2008; Belliveau et al. 2014; Vonderschen and Wagner 2014; Yao et al. 2015), though this is in need of further clarification.
Acknowledgments
The authors would like to thank Barbara Burghardt and Esther Tauberschmidt for assistance with the data collection as well as two anonymous reviewers for helpful comments. This work was supported by the German Federal Ministry of Education and Research (BMBF) grant number 01EV0712 to AG.
Compliance with Ethical Standards
Ethics Statement
All procedures were approved by the Institutional Review Board at the University Hospital Heidelberg in accordance with the Declaration of Helsinki, and all participants provided written informed consent prior to participation.
Contributor Information
Andrew R. Dykstra, Phone: +49 (0) 6221 56 5196, Email: andrew.dykstra@med.uni-heidelberg.de
Alexander Gutschalk, Phone: +49 (0) 6221 56 36811, Email: alexander.gutschalk@med.uni-heidelberg.de.
References
- Ahonen AI, Hämäläinen MS, Kajola MJ, et al. 122-channel squid instrument for investigating the magnetic signals from the human brain. Phys Scr. 1993;T49A:198–205. doi: 10.1088/0031-8949/1993/T49A/033. [DOI] [Google Scholar]
- Alain C, Roye A, Arnott SR (2013) Middle- and long-latency auditory evoked potentials: what are they telling us on central auditory disorders? In: Celesia GG (ed) Handbook of Clinical Neurophysiology, Vol. 10 - Disorders of Peripheral and Central Auditory Processing. Elsevier, Amsterdam, pp 177–199
- Atcherson SR, Moore PC. Are chirps better than clicks and tonebursts for evoking middle latency responses? J Am Acad Audiol. 2014;25:576–583. doi: 10.3766/jaaa.25.6.7. [DOI] [PubMed] [Google Scholar]
- Belliveau LAC, Lyamzin DR, Lesica NA. The neural representation of interaural time differences in gerbils is transformed from midbrain to cortex. J Neurosci. 2014;34:16796–16808. doi: 10.1523/JNEUROSCI.2432-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohórquez J, Ozdamar O. Generation of the 40-Hz auditory steady-state response (ASSR) explained using convolution. Clin Neurophysiol. 2008;119:2598–2607. doi: 10.1016/j.clinph.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Brugge JF (2013) Anatomy and physiology of auditory pathways and cortex. In: Celesia GG (ed) Handbook of Clinical Neurophysiology, Vol. 10 - Disorders of Peripheral and Central Auditory Processing. Elsevier, Amsterdam, pp 25–59
- Buchner H, Fuchs M, Wischmann H-A, et al. Source analysis of median nerve and finger stimulated somatosensory evoked potentials: multichannel simultaneous recording of electric and magnetic fields combined with 3d-MR tomography. Brain Topogr. 1994;6:299–310. doi: 10.1007/BF01211175. [DOI] [PubMed] [Google Scholar]
- Cacace AT, Satya-Murti S, Wolpaw JR. Human middle-latency auditory evoked potentials: vertex and temporal components. Electroencephalogr Clin Neurophysiol. 1990;77:6–18. doi: 10.1016/0168-5597(90)90012-3. [DOI] [PubMed] [Google Scholar]
- Celesia GG. Organization of auditory cortical areas in man. Brain. 1976;99:403–414. doi: 10.1093/brain/99.3.403. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp. 1994;2:170–187. doi: 10.1002/hbm.460020306. [DOI] [Google Scholar]
- Davis H, Hirsh SK. A slow brain stem response for low-frequency audiometry. Audiology. 1979;18:445–461. doi: 10.3109/00206097909072636. [DOI] [PubMed] [Google Scholar]
- Debruyne F. Binaural interaction in early, middle and late auditory evoked responses. Scand Audiol. 1984;13:293–296. doi: 10.3109/01050398409042139. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Berlin CI. Binaural interaction in brainstem-evoked responses. Arch Otolaryngol. 1979;105:391–398. doi: 10.1001/archotol.1979.00790190017004. [DOI] [PubMed] [Google Scholar]
- Dobie RA, Norton SJ. Binaural interaction in human auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1980;49:303–313. doi: 10.1016/0013-4694(80)90224-2. [DOI] [PubMed] [Google Scholar]
- Fifer RC, Sierra-Irizarry B. Clinical applications of the auditory middle latency response. Am J Otol. 1988;9(Suppl):47–56. [PubMed] [Google Scholar]
- Gaumond RP, Psaltikidou M. Models for the generation of the binaural difference response. J Acoust Soc Am. 1991;89:454–456. doi: 10.1121/1.400482. [DOI] [PubMed] [Google Scholar]
- Geisler CD, Frishkopf LS, Rosenblith WA. Extracranial responses to acoustic clicks in man. Science. 1958;128:1210–1211. doi: 10.1126/science.128.3333.1210. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN, Hutson KA, Masterton RB. Acoustic chiasm V: inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol. 1992;319:100–122. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf J, et al. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Clin Neurophysiol. 2001;112:1850–1859. doi: 10.1016/S1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Gutschalk A. MEG Auditory Research. In: Supek S, Aine CJ, editors. Magnetoencephalography: from signals to dynamic cortical networks. Berlin Heidelberg: Springer; 2014. pp. 679–711. [Google Scholar]
- Gutschalk A, Steinmann I. Stimulus dependence of contralateral dominance in human auditory cortex. Hum Brain Mapp. 2015;36:883–896. doi: 10.1002/hbm.22673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschalk A, Mase R, Roth R, et al. Deconvolution of 40 Hz steady-state fields reveals two overlapping source activities of the human auditory cortex. Clin Neurophysiol. 1999;110:856–868. doi: 10.1016/S1388-2457(99)00019-X. [DOI] [PubMed] [Google Scholar]
- Gutschalk A, Brandt T, Bartsch A, Jansen C. Comparison of auditory deficits associated with neglect and auditory cortex lesions. Neuropsychologia. 2012;50:926–938. doi: 10.1016/j.neuropsychologia.2012.01.032. [DOI] [PubMed] [Google Scholar]
- Hashimoto I. Auditory evoked potentials from the human midbrain: slow brain stem responses. Electroencephalogr Clin Neurophysiol. 1982;53:652–657. doi: 10.1016/0013-4694(82)90141-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto I, Mashiko T, Yoshikawa K, et al. Neuromagnetic measurements of the human primary auditory response. Electroencephalogr Clin Neurophysiol Potentials Sect. 1995;96:348–356. doi: 10.1016/0168-5597(95)00004-C. [DOI] [PubMed] [Google Scholar]
- Hine J, Debener S. Late auditory evoked potentials asymmetry revisited. Clin Neurophysiol. 2007;118:1274–1285. doi: 10.1016/j.clinph.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Grayson AS. The normal scalp topography of the middle latency auditory evoked potential Pa component following monaural click stimulation. Brain Topogr. 1988;1:29–36. doi: 10.1007/BF01129337. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Wüstenberg T, Schulze K, Heinze H. Asymmetric hemodynamic responses of the human auditory cortex to monaural and binaural stimulation. Hear Res. 2002;170:166–178. doi: 10.1016/S0378-5955(02)00488-4. [DOI] [PubMed] [Google Scholar]
- Johnson BW, Hautus MJ. Processing of binaural spatial information in human auditory cortex: neuromagnetic responses to interaural timing and level differences. Neuropsychologia. 2010;48:2610–2619. doi: 10.1016/j.neuropsychologia.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Junius D, Riedel H, Kollmeier B. The influence of externalization and spatial cues on the generation of auditory brainstem responses and middle latency responses. Hear Res. 2007;225:91–104. doi: 10.1016/j.heares.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Kadoya C, Henry T, Beydoun A, et al. Middle latency auditory evoked potentials recorded from human temporal lobes and near the upper brainstem. In: Hashimoto I, Kakigi R, et al., editors. Recent advances in human neurophysiology. Amsterdam: Elsevier; 1998. pp. 256–264. [Google Scholar]
- Kaseda Y, Tobimatsu S, Morioka T, Kato M. Auditory middle-latency responses in patients with localized and non-localized lesions of the central nervous system. J Neurol. 1991;238:427–432. doi: 10.1007/BF00314648. [DOI] [PubMed] [Google Scholar]
- Kileny P, Paccioretti D, Wilson AF (1987) Effects of cortical lesions on middle-latency auditory evoked responses (MLR). [DOI] [PubMed]
- Königs L, Gutschalk A. Functional lateralization in auditory cortex under informational masking and in silence. Eur J Neurosci. 2012;36:3283–3290. doi: 10.1111/j.1460-9568.2012.08240.x. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T. Clinical applications of the middle latency response. J Am Acad Audiol. 1990;1:130–133. [PubMed] [Google Scholar]
- Kraus N, Ozdamar O, Hier D, Stein L (1982) Auditory middle latency responses (MLRs) in patients with cortical lesions. [DOI] [PubMed]
- Krumbholz K, Schönwiesner M, Rübsamen R, et al. Hierarchical processing of sound location and motion in the human brainstem and planum temporale. Eur J Neurosci. 2005;21:230–238. doi: 10.1111/j.1460-9568.2004.03836.x. [DOI] [PubMed] [Google Scholar]
- Kuriki S, Nogai T, Hirata Y. Cortical sources of middle latency responses of auditory evoked magnetic field. Hear Res. 1995;92:47–51. doi: 10.1016/0378-5955(95)00195-6. [DOI] [PubMed] [Google Scholar]
- Langers DRM, van Dijk P, Backes WH. Lateralization, connectivity and plasticity in the human central auditory system. Neuroimage. 2005;28:490–499. doi: 10.1016/j.neuroimage.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Levine RA. Binaural interaction in brainstem potentials of human subjects. Ann Neurol. 1981;9:384–393. doi: 10.1002/ana.410090412. [DOI] [PubMed] [Google Scholar]
- Liégeois-Chauvel C, Musolino A, Badier JM, et al. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol Potentials Sect. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron. 2013;80:1112–1128. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Magezi DA, Krumbholz K. Evidence for opponent-channel coding of interaural time differences in human auditory cortex. J Neurophysiol. 2010;104:1997–2007. doi: 10.1152/jn.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä JPP, Hämäläinen M, Hari R, et al. Whole-head mapping of middle-latency auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol. 1994;92:414–421. doi: 10.1016/0168-5597(94)90018-3. [DOI] [PubMed] [Google Scholar]
- McEvoy L, Hari R, Imada T, Sams M. Human auditory cortical mechanisms of sound lateralization: II. Interaural time differences at sound onset. Hear Res. 1993;67:98–109. doi: 10.1016/0378-5955(93)90237-U. [DOI] [PubMed] [Google Scholar]
- McEvoy L, Mäkelä JP, Hämäläinen M, Hari R. Effect of interaural time differences on middle-latency and late auditory evoked magnetic fields. Hear Res. 1994;78:249–257. doi: 10.1016/0378-5955(94)90031-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin SA, Higgins NC, Stecker GC. Tuning to binaural cues in human auditory cortex. J Assoc Res Otolaryngol. 2015 doi: 10.1007/s10162-015-0546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson DL, Starr A. Binaural interaction in auditory evoked potentials: brainstem, middle- and long-latency components. Hear Res. 1993;66:91–98. doi: 10.1016/0378-5955(93)90263-Z. [DOI] [PubMed] [Google Scholar]
- McPherson DL, Tures C, Starr A. Binaural interaction of the auditory brain-stem potentials and middle latency auditory evoked potentials in infants and adults. Electroencephalogr Clin Neurophysiol Potentials Sect. 1989;74:124–130. doi: 10.1016/0168-5597(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Møller AR, Jannetta PJ. Evoked potentials from the inferior colliculus in man. Electroencephalogr Clin Neurophysiol. 1982;53:612–620. doi: 10.1016/0013-4694(82)90137-7. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Musiek FE, Geurkink NA, Weider DJ, Donnelly K. Past, present, and future applications of the auditory middle latency response. Laryngoscope. 1984;94:1545–1553. doi: 10.1288/00005537-198412000-00002. [DOI] [PubMed] [Google Scholar]
- Ozdamar O, Kraus N. Auditory middle-latency responses in humans. Audiology. 1983;22:34–49. doi: 10.3109/00206098309072768. [DOI] [PubMed] [Google Scholar]
- Ozdamar O, Bohórquez J (2008) Suppression of the P(b) (P(1)) component of the auditory middle latency response with contralateral masking. Clin Neurophysiol 119:1870–1880. doi:10.1016/j.clinph.2008.03.023 [DOI] [PubMed]
- Özdamar Ö, Kraus N, Curry F. Auditory brain stem and middle latency responses in a patient with cortical deafness. Electroencephalogr Clin Neurophysiol. 1982;53:224–230. doi: 10.1016/0013-4694(82)90027-X. [DOI] [PubMed] [Google Scholar]
- Palomäki KJ, Tiitinen H, Mäkinen V, et al. Spatial processing in human auditory cortex: the effects of 3D, ITD, and ILD stimulation techniques. Brain Res Cogn Brain Res. 2005;24:364–379. doi: 10.1016/j.cogbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Pantev C, Lütkenhöner B, Hoke M, Lehnertz K. Comparison between simultaneously recorded auditory-evoked magnetic fields and potentials elicited by ipsilateral, contralateral and binaural tone burst stimulation. Audiology. 1986;25:54–61. doi: 10.3109/00206098609078369. [DOI] [PubMed] [Google Scholar]
- Parkkonen L, Fujiki N, Mäkelä JP. Sources of auditory brainstem responses revisited: contribution by magnetoencephalography. Hum Brain Mapp. 2009;30:1772–1782. doi: 10.1002/hbm.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JF, Mendel MI. Early components of the averaged electroencephalic response to monaural and binaural stimulation. Int J Audiol. 1974;13:195–204. doi: 10.3109/00206097409071677. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Gates GR. Representation of the two ears in the auditory cortex: a re-examination. Int J Neurosci. 1982;16:41–46. doi: 10.3109/00207458209147600. [DOI] [PubMed] [Google Scholar]
- Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials. I: Evaluation of components. Electroencephalogr Clin Neurophysiol. 1974;36:179–190. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- Plourde G. Auditory evoked potentials. Best Pract Res Clin Anaesthesiol. 2006;20:129–139. doi: 10.1016/j.bpa.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Polyakov A, Pratt H. Three-channel Lissajous’ trajectory of the binaural interaction components of human auditory middle-latency evoked potentials. Hear Res. 1995;82:205–215. doi: 10.1016/0378-5955(94)00178-S. [DOI] [PubMed] [Google Scholar]
- Pratt H. Sensory ERP components. In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. Oxford: Oxford University Press; 2013. pp. 89–114. [Google Scholar]
- Reser DHH, Fishman YI, Arezzo JC, Steinschneider M. Binaural interactions in primary auditory cortex of the awake macaque. Cereb Cortex. 2000;10:574–584. doi: 10.1093/cercor/10.6.574. [DOI] [PubMed] [Google Scholar]
- Riedel H, Kollmeier B. Comparison of binaural auditory brainstem responses and the binaural difference potential evoked by chirps and clicks. Hear Res. 2002;169:85–96. doi: 10.1016/S0378-5955(02)00342-8. [DOI] [PubMed] [Google Scholar]
- Riedel H, Kollmeier B. Interaural delay-dependent changes in the binaural difference potential of the human auditory brain stem response. Hear Res. 2006;218:5–19. doi: 10.1016/j.heares.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Rodieck RW. Visual pathways. Annu Rev Neurosci. 1979;2:193–225. doi: 10.1146/annurev.ne.02.030179.001205. [DOI] [PubMed] [Google Scholar]
- Ross B, Herdman AT, Pantev C. Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cereb Cortex. 2005;15:2029–2039. doi: 10.1093/cercor/bhi078. [DOI] [PubMed] [Google Scholar]
- Rupp A, Gutschalk A, Hack S, Scherg M. Temporal resolution of the human primary auditory cortex in gap detection. Neuroreport. 2002;13:2203–2207. doi: 10.1097/00001756-200212030-00008. [DOI] [PubMed] [Google Scholar]
- Rupp A, Uppenkamp S, Gutschalk A, et al. The representation of peripheral neural activity in the middle-latency evoked field of primary auditory cortex in humans. Hear Res. 2002;174:19–31. doi: 10.1016/S0378-5955(02)00614-7. [DOI] [PubMed] [Google Scholar]
- Salminen NH, May PJC, Alku P, Tiitinen H. A population rate code of auditory space in the human cortex. PLoS One. 2009;4:e7600. doi: 10.1371/journal.pone.0007600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen NH, Tiitinen H, Yrttiaho S, May PJC. The neural code for interaural time difference in human auditory cortex. J Acoust Soc Am. 2010;127:EL60–EL65. doi: 10.1121/1.3290744. [DOI] [PubMed] [Google Scholar]
- Salminen NH, Tiitinen H, May PJC. Auditory spatial processing in the human cortex. Neuroscientist. 2012;18:602–612. doi: 10.1177/1073858411434209. [DOI] [PubMed] [Google Scholar]
- Scheffler K, Bilecen D, Schmid N, et al. Auditory cortical responses in hearing subjects and unilateral deaf patients as detected by functional magnetic resonance imaging. Cereb Cortex. 1998;8:156–163. doi: 10.1093/cercor/8.2.156. [DOI] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D. Evoked dipole source potentials of the human auditory cortex. Electroencephalogr Clin Neurophysiol. 1986;65:344–360. doi: 10.1016/0168-5597(86)90014-6. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Hämäläinen MS, Gutschalk A. How anatomical asymmetry of human auditory cortex can lead to a rightward bias in auditory evoked fields. Neuroimage. 2013;74:22–29. doi: 10.1016/j.neuroimage.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Singh KD. Which “neural activity” do you mean? fMRI, MEG, oscillations and neurotransmitters. Neuroimage. 2012;62:1121–1130. doi: 10.1016/j.neuroimage.2012.01.028. [DOI] [PubMed] [Google Scholar]
- Stecker GC, McLaughlin SA, Higgins NC. Monaural and binaural contributions to interaural-level-difference sensitivity in human auditory cortex. Neuroimage. 2015;120:456–466. doi: 10.1016/j.neuroimage.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinschneider M, Fishman YI, Arezzo JC. Spectrotemporal analysis of evoked and induced electroencephalographic responses in primary auditory cortex (A1) of the awake monkey. Cereb Cortex (New York, NY 1991) 2008;18:610–625. doi: 10.1093/cercor/bhm094. [DOI] [PubMed] [Google Scholar]
- Tawfik S, Musiek FE. SN10 auditory evoked potential revisited. Am J Otol. 1991;12:179–183. [PubMed] [Google Scholar]
- Thompson SK, von Kriegstein K, Deane-Pratt A, et al. Representation of interaural time delay in the human auditory midbrain. Nat Neurosci. 2006;9:1096–1098. doi: 10.1038/nn1755. [DOI] [PubMed] [Google Scholar]
- van Olphen AF, Rodenburg M, Verwey C. Distribution of brain stem responses to acoustic stimuli over the human scalp. Audiology. 1978;17:511–518. doi: 10.3109/00206097809072611. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Griffiths TD, Thompson SK, McAlpine D. Responses to interaural time delay in human cortex. J Neurophysiol. 2008;100:2712–2718. doi: 10.1152/jn.90210.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderschen K, Wagner H. Detecting interaural time differences and remodeling their representation. Trends Neurosci. 2014;37:289–300. doi: 10.1016/j.tins.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Wall PD, Dubner R. Somatosensory pathways. Annu Rev Physiol. 1972;34:315–336. doi: 10.1146/annurev.ph.34.030172.001531. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Tempelmann C, Fell J, et al. Lateralized auditory spatial perception and the contralaterality of cortical processing as studied with functional magnetic resonance imaging and magnetoencephalography. Hum Brain Mapp. 1999;7:49–66. doi: 10.1002/(SICI)1097-0193(1999)7:1<49::AID-HBM5>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DL, Clayworth CC. Click spatial position influences middle latency auditory evoked potentials (MAEPs) in humans. Electroencephalogr Clin Neurophysiol. 1985;60:122–129. doi: 10.1016/0013-4694(85)90018-5. [DOI] [PubMed] [Google Scholar]
- Woods DL, Clayworth CC, Knight RT, et al. Generators of middle- and long-latency auditory evoked potentials: implications from studies of patients with bitemporal lesions. Electroencephalogr Clin Neurophysiol. 1987;68:132–148. doi: 10.1016/0168-5597(87)90040-2. [DOI] [PubMed] [Google Scholar]
- Woods DL, Herron TJ, Cate AD, et al. Functional properties of human auditory cortical fields. Front Syst Neurosci. 2010;4:155. doi: 10.3389/fnsys.2010.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrege K, Starr A. Binaural interaction in human auditory brainstem evoked potentials. Arch Neurol. 1981;38:572–580. doi: 10.1001/archneur.1981.00510090066008. [DOI] [PubMed] [Google Scholar]
- Yao JD, Bremen P, Middlebrooks JC. Transformation of spatial sensitivity along the ascending auditory pathway. J Neurophysiol. 2015;113:3098–3111. doi: 10.1152/jn.01029.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvert B, Crouzeix A, Bertrand O, et al. Multiple supratemporal sources of magnetic and electric auditory evoked middle latency components in humans. Cereb Cortex. 2001;11:411–423. doi: 10.1093/cercor/11.5.411. [DOI] [PubMed] [Google Scholar]
- Yvert B, Fischer C, Guénot M, et al. Simultaneous intracerebral EEG recordings of early auditory thalamic and cortical activity in human. Eur J Neurosci. 2002;16:1146–1150. doi: 10.1046/j.1460-9568.2002.02162.x. [DOI] [PubMed] [Google Scholar]
- Yvert B, Fischer C, Bertrand O, Pernier J. Localization of human supratemporal auditory areas from intracerebral auditory evoked potentials using distributed source models. Neuroimage. 2005;28:140–153. doi: 10.1016/j.neuroimage.2005.05.056. [DOI] [PubMed] [Google Scholar]