Abstract
Background
The mechanistic underpinnings of sex differences in occurrence of depression and efficacy of antidepressant treatments are poorly understood. Here we examined the effects of isolation stress and the fast acting antidepressant ketamine, on anhedonia and depression-like behavior, spine density and synaptic proteins in male and female rats.
Methods
We used chronic social isolation stress (IS) paradigm to test the effects of ketamine (0, 2.5 and 5mg/kg) on behavior and levels of synaptic proteins Synapsin1, PSD95 and GluR1 in male and female rats in diestrus. mPFC spine density was also examined in males and in females that received ketamine either during diestrus or proestrus phase of their estrous cycle.
Results
Males showed anhedonia and depression-like behavior after 8 weeks of IS, concomitant with decreases in spine density and levels of Synapsin1, PSD95 and GluR1 in the mPFC; changes that were reversed by a single injection of ketamine (5 mg/kg). Females, after 11 weeks of IS, showed depression-like behavior but no signs of anhedonia. Although both doses of ketamine rescued depression-like behavior in female rats, the decline observed in synaptic proteins and spine density in IS and in diestrus females, could not be reversed by ketamine. Spine density was higher in females during proestrus than in diestrus.
Conclusions
Our findings implicate a role for synaptic proteins Synapsin1, PSD95 and GluR1, and mPFC spine density in the antidepressant effects of ketamine in IS males but not in IS females, suggesting dissimilar underlying mechanisms for efficacy of ketamine in the two sexes.
Six key words for indexing: Sex-difference, ketamine, social-isolation, mPFC, anhedonia, depression
Introduction
Interesting gender differences exist in a) the prevalence rate of depression (1, 2) b) the symptoms of depressive disorders (3, 4) and c) the efficacy of antidepressant medication (5–7). Depression is twice as prevalent amongst women as in men. Specific symptoms of depression such as anxiety, bulimia and suicidal ideation are more common in women than in men, who predominantly display symptoms of alcoholism and substance abuse (8, 9). Pre-clinical research has also shown robust sex differences in animal models of depression (10). In spite of the existence of compelling evidence for sex differences in depression, the mechanisms underpinning these remain largely unexplored. Studies have attributed behavioral differences between males and females to sexual dimorphism of the underlying neuronal circuitry and neuroendocrinal systems (11) which in turn have genetic (12) and epigenetic (13) underpinnings. Although some clinical studies have reported higher efficacy of SSRIs in women than in men (5), collectively, for both sexes, the current available antidepressants with slow onset of therapeutic effects, have serious limitations (14, 15). However, recently, ketamine the NMDA receptor antagonist classically used as an anesthetic has emerged as a fast acting antidepressant (16, 17). Acute injections of ketamine produce rapid antidepressant effects within few hours (18–20). Interestingly, previous studies from our lab on rats (21) and others on mice (22) have shown females to be more sensitive to ketamine than males. Indeed, a single injection of 2.5 mg/kg dose of ketamine induced antidepressant-like effect in female but not in male rats, that responded to doses of 5 mg/kg and above (21). To further investigate sex differences in antidepressant actions of ketamine, we examined its effects on a) anhedonia and depression-like behavior, b) spine density in the mPFC and c) molecular changes in the mPFC synaptoneurosomes, in male and female rats that were exposed to chronic social isolation stress (IS). Isolation stress is known to evoke depression and anhedonia-like behavior in rats (23). Previous studies from our lab have established the suitability of this stress paradigm for comparative studies between males and females (24), because, unlike chronic unpredictable (25) and chronic mild (26) stress paradigms that were employed to delineate molecular pathways affected by ketamine in male animals, chronic IS does not disrupt the estrous cycle in female animals. Our results highlight interesting differences between males and females in their response to IS and ketamine treatment and implicate alternative underlying mechanisms for efficacy of ketamine in male and female rats.
Materials and Methods
Animals
Adult male (250–270 g) and female (200–225 g) Sprague Dawley rats (Charles River, USA) were maintained either under pair-housed (PH) condition or in solitary cages under isolation stress (IS) on a 12 hour/12 hour light/dark cycle (lights on at 0500 h) with ad libitum access to food and water. All animal protocols were carried out in accordance with the NIH Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Florida State University.
Experimental designs
Effect of ketamine on anhedonia and depression-like behavior in male and female rats subjected to isolation stress
Male (n = 8/group) and female (n = 6/group) rats were housed either under pair housed (PH) condition or under isolation stress (IS). During this period of time, PH and IS groups of animals were tested on sucrose preference test (SPT) periodically (3 alternate days/week) to check for the emergence of behavioral abnormalities induced by IS. Once a significant decline in preference for sucrose was observed in the IS group, a decline that was stable and maintained over the next week, animals were injected with saline/ketamine (2.5, 5 mg/kg; Butler Schein Animal Health, Inc.) and tested for anhedonia-like behavior on the SPT after 3 hours (day1) and 27 hours (day2) and for depression-like behavior on the FST on the third day post injection (Figure 1A). Female rats were lavaged for determination of estrous cycle stage and those having consistent cycle lengths of 4–5 days were injected with ketamine and tested behaviorally during diestrus.
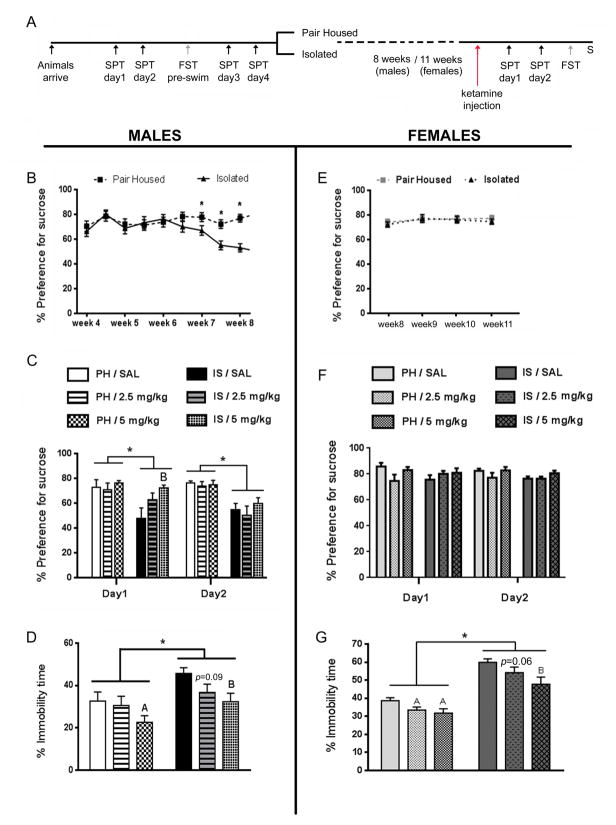
Figure 1.
Isolation stress and ketamine affect male and female rats differently, in tests for anhedonia and depression-like behavior. (A) Schematic representation of the experimental design. Animals were tested for their baseline behaviors on the sucrose preference test (SPT) and pre-swim for the forced swim test (FST), prior to grouping into pair-housed (PH) and isolation stress (IS). PH and IS rats lived in respective housing conditions for 8 weeks (males) or 11 weeks (females). Ketamine was injected after week 8 in males and week 11 in females when they were in diestrus. SPT was performed 3 hours (day1) and 27 hours (day2) after ketamine injection, followed by FST on the next day for male rats and the next 1–2 days for females (when they were in diestrus). Animals were sacrificed (S) 1 hour after FST. (B) IS induced a significant and stable decline in sucrose preference after 7 weeks in male rats. (C) This deficit could be rescued by an acute injection of ketamine (5mg/kg) on day1 of SPT, but not on day2. (D) IS also evoked an increase in immobility time, indicative of depression-like behavior in the FST in males. A single injection of ketamine (5mg/kg) reduced immobility time significantly in the FST in both PH and IS male rats. (E) IS did not evoke a decline in sucrose preference in the female rats even after 11 weeks of stress. (F) Ketamine did not increase preference for sucrose in female PH and IS rats, at either dose (2.5 and 5mg/kg). (G) IS evoked a significant increase in immobility time, in the FST in females rats. A single injection of both doses of ketamine (2.5 and 5mg/kg) caused a reduction in immobility time in the FST in PH as well as IS female rats. Data are mean ± SEM for percent preference for sucrose in SPT and percent immobility time in FST [n = 8/group (males), 6/group (females)]. (*p<0.05, Ap<0.05 compared to PH/SAL, Bp<0.05 compared to IS/SAL, repeated measures ANOVA for SPT and two way ANOVA for FST, Fisher’s post-hoc test)
Effect of ketamine on mPFC spine density in male rats and during different phases of estrous cycle in female rats subjected to isolation stress
To investigate effects of ketamine on spine density in animals exposed to IS, bilateral stereotactic surgeries were performed to infuse HSV- GFP into the mPFC of PH and IS rats of both sexes (n = 4/group). Female rats were lavaged to determine estrous cycle stage. 3–4 days after surgery, rats were injected with saline/ketamine (2.5, 5 mg/kg). Female rats were injected with ketamine either on diestrus when gonadal hormone levels are lowest or on proestrus when gonadal hormone levels are at peak. Rats were perfused 3 hours post drug injections. GFP immunohistochemistry was performed to detect fluorescence. Dendrites belonging to the apical tuft of layer V pyramidal neurons of the mPFC were imaged using Zeiss Confocal microscope under 63X oil objective. Spines were counted using Neurolucida Explorer.
Effect of ketamine on synaptic proteins in male and female rats subjected to isolation stress
PH and IS male (n = 6/group) and female (n = 5/group) rats that underwent behavioral testing, were sacrificed 1 hour after FST and brain tissues were collected for Western blot assay of synaptic proteins; Synapsin1, Post synaptic density protein 95 (PSD95) and Glutamate receptor 1 (GluR1) on synaptoneurosomal fractions isolated from mPFC.
Behavioral tests
Sucrose preference test (SPT)
The SPT, a two-bottle choice paradigm was performed as described earlier (21, 27). Rats were habituated to drinking from two bottles prior to testing. For testing of baseline preference for sucrose and for the SPT after the period of stress, rats were given access to two pre-weighed bottles, one of which contained water and the other contained 0.25% sucrose for the first 2 hours of the dark cycle. The bottles were weighed at 17:00 and 19:00 hours and the preference for sucrose over water was used as a measure of anhedonia.
Forced swim test (FST)
The FST, a two day procedure was performed as described previously (28, 29). On day 1 and day2, rats were placed in 30 × 45 cm Plexiglas cylinders filled with water, maintained at 25 °C for 15 and for 5 minutes respectively and their behaviors were videotaped, and analyzed for immobility time by a scorer blind to the treatment conditions. Immobility was defined as minimum movement required for remaining afloat (30). Female rats received the pre-test and test when they were in the diestrus stage of their cycle, when the levels of gonadal hormones are at their lowest. Therefore the FST test was conducted 3 days after ketamine injection in male rats, and 3–5 days after ketamine injection in female rats.
Virus delivery and immunohistochemistry for GFP staining
To visualize dendrites and spines in the pre-limbic (PL) region of the mPFC, both male and female rats were given bilateral stereotactic injections of the HSV- GFP viral vector [p1005+ HSV plasmid expressing GFP under the control of CMV promoter] following standard methods (31). Animals were anesthetized using Isoflurane (Henry Schein Animal Health, OH, USA) during surgeries. Bregma coordinates used for the surgery were as follows: +3.1 mm anterior, +/− 0.6 mm lateral, and 3.9 mm ventral to bregma. The virus was delivered at a rate of 0.1 μl/min using 24-gauge syringe needles (Hamilton), for a total volume of 1.0 μl per animal, on each side. The animals were sacrificed 3–4 days after viral infection when transgene expression is maximal.
Coronal sections (50 μm) of the mPFC, encompassing the site of HSV-GFP injection, were generated using a Vibratome (Leica Microsystems, Germany). To visualize the spines, immunohistochemistry for GFP was performed on the sections. Briefly; after blocking (5% normal goat serum and 0.3% Triton X) sections were incubated overnight with Chicken-anti-GFP (1:500; Abcam), washed and incubated with Alexa488-conjugated goat-anti-chicken (1:1000; Life Technologies) for 3 hours. Labeled pyramidal neurons of the PL were imaged under 63x oil objective (Zeiss Plan-Apochromat, NA = 1.40) of a Zeiss LSM880 confocal microscope. The fluorescent tag was excited using an Argon/Krypton 488nm laser line. For spine density analysis, Z-stacks of images were obtained, consisting of 2–4 scans at 3x optical zoom, 0.5 μm step sizes.
Spine density measurements
Spine density was sampled in the proximal segment [a segment known to be affected by chronic stress exposure (32)] of the apical tuft dendrites of layer V pyramidal neurons of the PL region of the mPFC. Dendritic spines were qualitatively classified into one of the following three types: mushroom, thin and stubby. Spine density was analyzed using Neurolucida Explorer (version 9, MBF Bioscience) and expressed as the number of spines per 10 μm dendritic segments. Dendritic branches, 30–50 μm long, from the branch point, were included in the analysis. Consistent with previously reported spine density analyses (32), ~12 neurons from each rat, (n = 4 rats in each treatment group) were quantified. In cases where the established criteria for inclusion in analyses were not met by the visible neurons of both hemispheres, data was obtained from neurons only in one of the two hemispheres.
Synaptoneurosome preparation and western blot
The prelimbic region of the mPFC was tissue punched in a cryostat and frozen at −80 °C until further processing. Synaptoneurosomal fractions were prepared and collected from the tissue as previously described (21, 32). Briefly, tissue was homogenized in a solution containing 0.32 M sucrose, 20 mM HEPES (pH 7.4), 1 mM EDTA and protease inhibitors and centrifuged at 4 °C for 10 minutes at 2800 rpm. The pellet (nuclear fraction) was removed. The supernatant was centrifuged at 4 °C for 10 minutes at 12,000 rpm and the pellet (crude synaptoneurosomal fraction) was collected and sonicated in 50 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 2 mM EDTA, and protease inhibitors. Equal concentrations of proteins (10 mg) were loaded into 12% Acrylamide gel for electrophoresis. Immunoblots were incubated overnight with Synapsin1 (1:1000), PSD95 (1:1000), GluR1 (1:500) or GAPDH (1:5000) antibodies (Cell Signalling Technology), washed and incubated for 1 hour with goat anti-rabbit IR-Dye680LT (Li-COR Biosciences; 1:20,000) fluorescent secondary antibody, and visualized using Odyssey infrared imaging system (Li-COR Biosciences). Quantification was performed using NIH ImageJ software.
Statistical analysis
Data was analysed using repeated measures and two-way analysis of variance (ANOVA) followed by Fisher’s post-hoc wherever appropriate. p values <0.05 were considered statistically significant.
Results
Effect of ketamine on anhedonia and depression-like behavior in male and female rats subjected to isolation stress
Male IS rats started showing a significant decline in sucrose preference from week-7 onwards, which was stable across week-8 [effect of housing condition; F(1,46)=50.1, p<0.05] (Figure 1B). In male rats, the IS evoked decline in sucrose preference [housing; F(1,42)=7.76, p<0.05] was completely rescued by an acute injection of ketamine (5mg/kg) on day1 of SPT [effect of treatment; F(2,42)=3.24, p<0.05] but not on day2 (Figure 1C). IS also elicited an increase in immobility time [housing; F(1,42)=9.68, p<0.05], indicative of depression-like behavior in the FST in male rats (Figure 1D). Ketamine (5mg/kg) reduced immobility time significantly in the FST in male rats [treatment; F(1,42)= 4.77, p<0.05]. The 2.5mg/kg dose of ketamine did not improve behavior in the SPT and FST in males, consistent with previous findings (21).
Surprisingly, IS females showed no decline in sucrose preference even after 11 weeks of isolation (Figure 1E). A single injection of ketamine did not have an effect on sucrose preference in female rats, at either dose (Figure 1F). However, IS evoked a significant increase in immobility time in the FST [F(1,65)=74.39, p<0.05] and a single injection of both doses of ketamine elicited a reduction in immobility time in PH as well as in IS female rats [F(2,65)=7.95, p<0.05] (Figure 1G). Efficacy of both, the lower (2.5 mg/kg) as well as the higher (5 mg/kg) doses of ketamine in reversing the behavioral deficits observed in FST in females, strengthens our hypothesis that female rats are more sensitive to the antidepressant effects of ketamine than their male counterparts.
Effect of ketamine on mPFC spine density in male rats and during different phases of estrous cycle in female rats subjected to isolation stress
To investigate the effect of IS on mPFC spine density, we counted spine numbers by confocal imaging of the apical tuft of pre-labelled layer V pyramidal neurons of the mPFC (Figure 2B). Our results showed that 8 weeks of IS, induced a significant decline in spine density in the proximal segment of the apical tuft in male rats [housing; F(1,18)=9.68, p<0.05] (Figure 2C, D). This deficit was completely reversed by the 5 mg/kg but not the 2.5 mg/kg dose of ketamine [treatment; F(2,18) =3.16, p<0.05] (Figure 2D), consistent with the improvement in behavior observed in male rats in SPT and FST with the same dose. Further, analysis of spine morphology revealed a decline in mushroom [housing; F(1,18) =13.10, p<0.05] and thin [housing; F(1,18)=4.5, p<0.05] spine density in male IS rats (Figure 2E, F). Ketamine (5 mg/kg) increased the density of thin (treatment; F(2,18)=2.45, p<0.05), but not mushroom spines (treatment; F(2,18)=1.52, p=0.24) in male rats (Figure 2E, F).
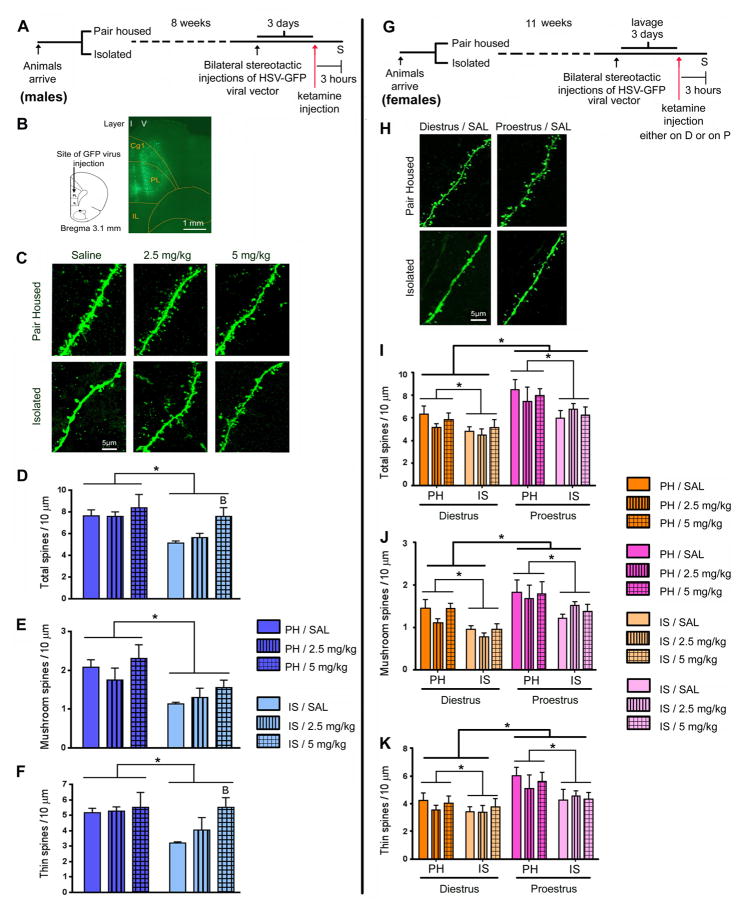
Figure 2.
Ketamine rescued IS evoked mPFC spine density deficits in males but not in female rats. (A) Schematic representation of the experimental design with male rats. Animals were grouped into PH and IS. After 8 weeks of stress, bilateral stereotactic injections of HSV-GFP were performed. After 3 days, when virus expression was at its maximum, animals were injected with ketamine. 3 hours after ketamine injection, animals were sacrificed (S). (B) Map of PL and IL regions in the mPFC of the rat, showing the site of injection of the HSV-GFP virus. GFP fluorescence was detected by immunostaining of sections. Shown is a low magnification (10x) fluorescence image of a GFP expressing mPFC section. Green fluorescence can be seen both in layer I (apical tuft) and in layer V (cell body) of the PL region of the mPFC. (C) Representative images are shown of high magnification Z-stack projections of proximal segments of the layer V pyramidal cell apical tuft dendrites of male rats (scale bar: 5 μm). (D) IS decreased spine density in the proximal segment of the apical tuft. This deficit was completely reversed by ketamine treatment (5 mg/kg) in male rats. Analysis of spine morphology revealed a decline in (E) mushroom and (F) thin spine density in IS animals. A single injection of ketamine (2.5 and 5 mg/kg) increased the density of (F) thin, but not (E) mushroom spines in the mPFC of male rats. (G) Schematic representation of the experimental design with female rats. Animals were grouped into PH and IS. Animals were lavaged for tracking of estrus cycle. After 11 weeks of grouping, bilateral stereotactic injections of HSV-GFP were performed. After 3 days, animals were injected with ketamine, either during diestrus (D) or proestrus (P). 3 hours after ketamine injection, animals were sacrificed (S). (H) Representative images are shown of high magnification Z-stack projections of apical tuft dendrites in female rats (scale bar: 5 μm). (I–K) IS evoked a significant decline in the density of spines of all sub categories in female rats irrespective of the cycle stage (effect of housing condition). (I) Total spine density, (J) mushroom as well as (K) thin spine densities were significantly lower in both PH and IS groups of female rats during diestrus than in proestrus (effect of cycle stage). However, in female rats, ketamine did not cause an increase in spine density in the mPFC. Density of spines was analyzed using Neurolucida Explorer (version 9; MBF Bioscience). Results are the mean ± SEM (~12 cells from four rats in each group; *p<0.05, Bp<0.05 compared to IS/SAL, two-way ANOVA, Fisher’s post-hoc test).
We also investigated the effect of ketamine on spine density changes evoked by IS in female rats. Female rats were lavaged for tracking of estrous cycle. After 11 weeks of stress, bilateral stereotactic injections of HSV-GFP were performed. After 3–4 days, animals were injected with saline/ketamine (2.5, 5 mg/kg) either during diestrus or during proestrus and sacrificed 3 hours later (Figure 2G). IS evoked significant declines in the densities of total (F(1,36) =8.83, p<0.05), mushroom (F(1,36) =15.49, p<0.05) and thin spines (F(1,36) =5.46, p<0.05) in female rats irrespective of the cycle stage (main effect of housing condition) (Figure 2I–K). Total (F(1,36)=18.87, p<0.05), mushroom (F(1,36)=18.66, p<0.05) and thin spine (F(1,36)=12.97, p<0.05) densities were significantly lower in both PH and IS female rats during diestrus than in proestrus (main effect of cycle stage) (Figure 2I–K). However, in female rats, ketamine did not alter spine density in the mPFC (Figure 2I–K). Density of stubby spines remained unaltered in males (F(1,18)=0.22, p=0.8) and females (F(1,36)=0.28, p=0.59) under all conditions.
Effect of ketamine on synaptic proteins in male and female rats subjected to isolation stress
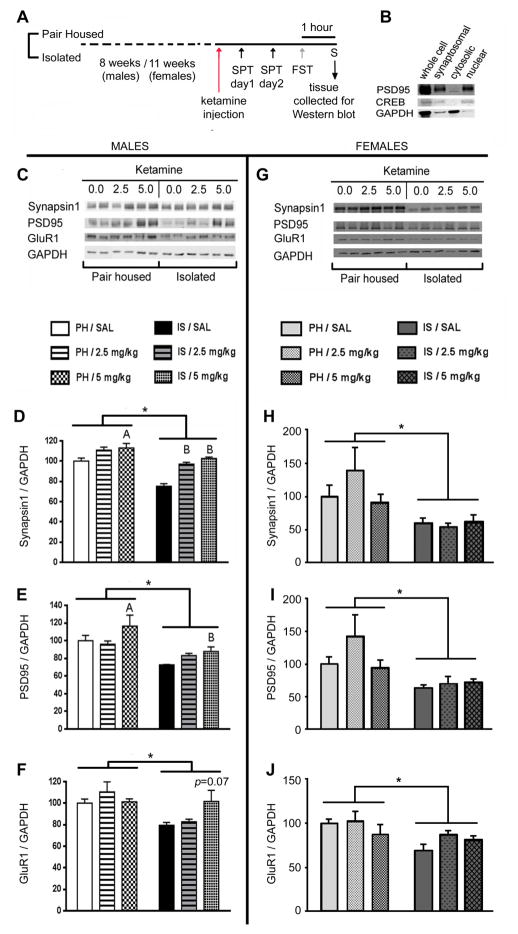
We examined the influence of IS on specific synaptic proteins in synaptoneurosome preparations of the mPFC (Figure 3A, B). Our results showed that levels of Synapsin1 (F(1,30)=47.31, p<0.05) PSD95 (F(1,30)=19.02, p<0.05) and GluR1(F(1,32)=9.26, p<0.05), were significantly reduced in the mPFC of IS males (Figure 3C–F). A single injection of ketamine (5mg/kg) reversed the decline observed in the levels of Synapsin1 (F(2,30)=27.39, p<0.05) and PSD95 (F(2,30)=3.37, p<0.05) and attenuated the decline in GluR1(F(2,32)=1.70, p=0.07) in male rats (Figure 3C–F), consistent with our results in behavioral and spine density experiments. Similarly, declines in the levels of Synapsin1 (F(1,26)=12.67, p<0.05), PSD95 (F(1,26)=11.94, p<0.05) and GluR1 (F(1,6)=8.17, p<0.05) were also observed in the mPFC of IS females (Figure 3G–J). However, unlike in the males, ketamine did not elevate the levels of these proteins in the females, consistent with ketamine’s lack of effect on SPT and spine density in female rats.
Figure 3.
Ketamine rescued IS evoked decline in synaptic protein levels in males but not in female rats. (A) Schematic representation of the experimental design. Animals were grouped into PH or IS. After 8 weeks (males) or 11 weeks (females) of stress, animals were injected with ketamine (females were injected when they were in diestrus). After completion of behavioral tests(i.e 3 days in males and 3–5 days for females) after ketamine injection, animals were sacrificed and brains were collected for Western blot assays. (B) Synaptoneurosomal fractions collected for Western blot assays were enriched in post synaptic density proteins such as PSD-95. (C) Representative Western blot showing that IS decreases levels of Synapsin1, PSD95 and GluR1 in the mPFC of male rats, and the ability of a single injection of ketamine to reverse this effect. IS evoked a significant decline in levels of (D) Synapsin1, (E) PSD95 and (F) GluR1in male rats; deficits that could be rescued by a single injection of ketamine (5mg/kg). (G) Representative Western blot showing that IS decreases levels of Synapsin1, PSD95 and GluR1 in the mPFC of female rats too, but a single injection of ketamine does not reverse this effect. IS evoked a significant decline in levels of (H) Synapsin1, (I) PSD95 and (J) GluR1 in female rats; however, these deficits could not be rescued by a single injection of ketamine. Levels of individual proteins were quantified using NIH ImageJ. Values represent mean ± SEM (n = 5–7/group) Levels of GAPDH were quantitated to control for differences in amounts of protein loading. (*p<0.05, Ap<0.05 compared to PH/SAL, Bp<0.05 compared to IS/SAL, two-way ANOVA, Fisher’s post-hoc test)
Discussions
The consequences of social isolation stress are distinct and different, based on the time and duration of administration (33). Studies have investigated the effect of early-life (34–37), and adolescent (38, 39) social isolation of varying time-lengths on behavior and underlying mechanisms. Here we showed for the first time, the effect of chronic social isolation (8–12 weeks) administered during adulthood, on behavior, spine density and synaptic molecules in the mPFC in male as well as in female rats. Our study is unique because it incorporates the variable of sex, which had not been taken into consideration in previous reports. We further investigated the effect of ketamine, in male and female rats exposed to chronic IS in adulthood. The effect of ketamine and IS on spine density was studied in diestrus as well as in proestrus females, giving us further insights on the influence of ovarian hormones on spine density in ketamine treated IS rats. Our experimental design enabled us to unveil several differences between males and females exposed to the same stressor (albeit for different exposure time) and treatment paradigm.
Our results showed that while 7 weeks of IS in adulthood evoked a strong anhedonia-like behavior in male rats, in the female rats it did not. This result is in agreement with a previous report in which anhedonia-like behavior was not observed in female Wistar rats that underwent chronic IS in adulthood (40). Clinical (41) and preclinical studies (42) have shown that circulating levels of estrogen in females can modulate the threshold for sweetness detection and preference for sucrose. Given that the female rats used in our experiment for SPT had undisrupted estrous cycles, the effect of IS alone on sucrose preference, in the presence of fluctuating levels of ovarian hormones remained unresolved. Future experiments examining sucrose preference during specific stages of the estrous cycle, or in overectomized and hormone-supplemented rats may provide a better understanding of the influence of ovarian hormones on anhedonia-like behavior in IS females. In the FST, although both sexes showed depression-like behavior after chronic IS, male rats responded only to the higher dose of ketamine, while the females responded to both (2.5 mg/kg and 5 mg/kg). This result corroborated previous findings from our lab which demonstrated that female rats have higher sensitivity towards ketamine and respond to the lower dose, to which the males do not (21). Additionally, in the FST, significant improvements in behavior with ketamine were observed also in the PH groups. PH males injected with the higher dose and PH females injected with either dose of ketamine showed significant lowering of immobility time in the FST. While the lower dose of ketamine did not influence the behavior of either sex in the SPT, a prominent sex difference in the efficacy of this dose emerged in the FST. Given that FST is a stressful behavioral test, the unmasking of a sex difference particularly in the FST may have resulted from the additional/acute stress experienced during the test.
Several studies have linked chronic stress exposure to decline in spine density and myelination in the mPFC (32, 37, 43–45). Recently, ketamine was shown to elicit a rapid reversal of chronic unpredictable stress (CUS) evoked decline in spine density in the mPFC in male mice (32). Given the large body of literature that implicates mPFC and dendritic spines as possible substrates for the action of stress, we examined the effects of IS and ketamine on mPFC spine density in male and female (diestrus and proestrus) rats. Previous studies have shown that spine density in the female mPFC decreases during diestrus, when the circulating levels of estrogen and progesterone are low, and increases during proestrus when the hormone levels are high, in cycling female rats (46). In our study, a similar pattern of spine density fluctuations was witnessed in the mPFC of female rats even after 11 weeks of IS. It was indeed interesting to note lower spine densities in both PH and IS females during diestrus, than during the proestrus phase of the cycle. Clinical depression is also reported more commonly in women during phases of life when levels of estrogen and progesterone are at their lowest, such as during the post-partum and menopausal phase (47). Our results add to the existing pool of knowledge that advocates a direct correlation between hormonal levels, spine density and mood. Ketamine evoked a rapid (within 3 hours) and complete reversal of the IS mediated decline in spine density in male rats. While the decline in total spine density was a cumulative effect of the decline in thin spines and mushroom spines, the increase in spine density by ketamine within 3 hours, was mediated mostly through an increase in thin spines (new, immature spines), while the mushroom spines (established, mature) remained unaffected. Previous studies have shown increases in number of mushroom spines in the mPFC of CUS exposed mice, after 24 hours of ketamine treatment (32). Although our results show a rapid increase in the number of thin, immature spines within 3 hours of ketamine treatment, it remains to be investigated whether a longer delay after ketamine treatment (24 hours) would enable maturation of the thin spines into mushroom spines in IS rats. It would also be interesting to investigate mushroom spine densities in male rats after 3 days of ketamine treatment, given the fact that we observed a rescue of PSD95 levels, a marker for mature spines 3 days post ketamine injections. On the other hand, ketamine had no effect on spine density in the mPFC of female rats. Studies in the past have shown that effects of stress on spine density are circuit, sex and stressor specific (48–50). For example, while 10 days of restraint stress elicited no change in spine density in mPFC neurons that project to the basolateral amygdala (BLA) in male rats (51), the same stressor increased spine density in BLA projecting neurons of mPFC layer III in female rats (52). However, past studies have not investigated sex effects on the action of antidepressants on spine density. Our result demonstrates for the first time that sex differences exist in the effects of stress and antidepressants on spine density. However, one of the limitations of this study is the fact that animals tested for spine density analysis, were not examined for behavioural changes induced by IS.
Observing stress effects on spine density in the mPFC encouraged us to investigate levels of synaptic proteins in the mPFC of IS rats. We examined the levels of well characterized pre and post-synaptic molecules, Synapsin1, PSD95 and GluR1 that have previously shown a decline in the male mPFC following chronic stress (32, 53) and in post-mortem studies on MDD patients [Synapsin1; (54), PSD95; (55), GluR1; (56)]. Our results demonstrated sex differences in the long term (3 days) effects of acute ketamine injection on synaptic proteins. A single injection of ketamine was effective in reversing the IS evoked decline in synaptic proteins in the mPFC of male, but not female rats. Increases in Synapsin1, PSD95 and GluR1 levels in the mPFC of male mice exposed to CUS were previously shown with ketamine and considered crucial for spinogenesis and the rapid antidepressant effects of ketamine (32). Although our results in male rats are in agreement with Li et al, we hypothesize the existence of alternate underlying mechanisms for the behavioral efficacy of ketamine in female rats. Another study implicated reduction in eEF2 kinase activity which triggers increases in BDNF translation in the hippocampus in the rapid antidepressant effects of ketamine in male mice (26). Examining the effect of ketamine on the eEF2-BDNF pathway in the hippocampus of female rats was beyond the scope of this study. However, we speculate the involvement of other brain areas such as the hippocampus, and/or pathways in the antidepressant effects of ketamine in female rats. Very recent studies have shown sex differences in expression of several NMDA and AMPA receptor subunit genes in post-mortem mPFC tissue of patients suffering from MDD (57) and in rats with a history of prenatal chronic mild stress (53). An altered AMPA to NMDA receptor function balance has also been implicated in rapid antidepressant effects of ketamine in male mice (58). Future experiments examining possible sex differences in the expression of glutamate receptor genes and AMPA receptor activity in specific neuronal circuits may provide further insights for differential efficacy of ketamine in male and female rats.
In conclusion, our findings highlight a) sex differences in the effect of chronic IS on behavior, spine density and synaptic proteins, b) sex differences in the action of ketamine on behavior, spine density and synaptic proteins in animals subjected to chronic IS and c) in females, estrous cycle stage specific differences in the effect of chronic IS on spine density in the mPFC. We implicate a role for mPFC synaptic proteins and spine maturation in the antidepressant effects of ketamine in male rats subjected to IS in adulthood. Our results indicate that the efficacy of ketamine is probably mediated via dissimilar underlying mechanisms in the two sexes, and opens up several avenues for investigations in the future.
Acknowledgments
This work was funded by National Institute of Health Grant R01-MH087583 and RO1-MH099085 to Mohamed Kabbaj.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC. Epidemiology of women and depression. Journal of affective disorders. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 2.Holden C. Sex and the suffering brain. Science (New York, NY) 2005;308:1574. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- 3.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Chaplin TM, Hong K, Bergquist K, Sinha R. Gender Differences in Response to Emotional Stress: An Assessment Across Subjective, Behavioral, and Physiological Domains and Relations to Alcohol Craving. Alcoholism: Clinical and Experimental Research. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 6.Young EA, Kornstein SG, Marcus SM, Harvey AT, Warden D, Wisniewski SR, et al. Sex differences in response to citalopram: a STAR* D report. Journal of psychiatric research. 2009;43:503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thase ME, Frank E, Kornstein SG, Yonkers KA. Gender differences in response to treatments of depression. Gender and its Effects on Psychopathology. 2000:103–129. [Google Scholar]
- 8.Frank JB, Weihs K, Minerva E, Lieberman DZ. WOMEN’S MENTAL HEALTH IN PRIMARY CARE: Depression, Anxiety, Somatization, Eating Disorders, and Substance Abuse. Medical Clinics of North America. 1998;82:359–389. doi: 10.1016/s0025-7125(05)70611-8. [DOI] [PubMed] [Google Scholar]
- 9.Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, et al. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Comprehensive Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. British journal of pharmacology. 2014;171:4595–4619. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosgrove KP, Mazure CM, Staley JK. Evolving Knowledge of Sex Differences in Brain Structure, Function, and Chemistry. Biological Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. The Journal of Neuroscience. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodes GE. Sex, stress, and epigenetics: regulation of behavior in animal models of mood disorders. Biol Sex Differ. 2013;4:1. doi: 10.1186/2042-6410-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperor’s new drugs: an analysis of antidepressant medication data submitted to the US Food and Drug Administration. Prevention & Treatment. 2002;5:23a. [Google Scholar]
- 15.Lader M. Limitations of current medical treatments for depression: disturbed circadian rhythms as a possible therapeutic target. European neuropsychopharmacology. 2007;17:743–755. doi: 10.1016/j.euroneuro.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 17.Covvey JR, Crawford AN, Lowe DK. Intravenous ketamine for treatment-resistant major depressive disorder. Annals of Pharmacotherapy. 2012;46:117–123. doi: 10.1345/aph.1Q371. [DOI] [PubMed] [Google Scholar]
- 18.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 19.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of general psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Voort JLV, Ballard ED, et al. Ketamine’s Antidepressant Efficacy is Extended for at Least Four Weeks in Subjects with a Family History of an Alcohol Use Disorder. International Journal of Neuropsychopharmacology. 2014:pyu039. doi: 10.1093/ijnp/pyu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Wallace DL, Han M-H, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature neuroscience. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrier N, Kabbaj M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Hormones and behavior. 2012;61:678–685. doi: 10.1016/j.yhbeh.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biological Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-f, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duclot F, Hollis F, Darcy MJ, Kabbaj M. Individual differences in novelty-seeking behavior in rats as a model for psychosocial stress-related mood disorders. Physiology & behavior. 2011;104:296–305. doi: 10.1016/j.physbeh.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrier N, Kabbaj M. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biological psychiatry. 2012;71:642–651. doi: 10.1016/j.biopsych.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology. 2010;211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- 30.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioural pharmacology. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, et al. Optogenetic inhibition of cocaine seeking in rats. Addiction biology. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (New York, NY) 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall FS. Social deprivation of neonatal, adolescent, and adult rats has distinct neurochemical and behavioral consequences. Critical reviews in neurobiology. 1998;12:129–162. doi: 10.1615/critrevneurobiol.v12.i1-2.50. [DOI] [PubMed] [Google Scholar]
- 34.Brenes JC, Rodriguez O, Fornaguera J. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacology Biochemistry and Behavior. 2008;89:85–93. doi: 10.1016/j.pbb.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Ferdman N, Murmu R, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behavioural brain research. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiology & behavior. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain research. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 38.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 39.Haj-Mirzaian A, Amiri S, Kordjazy N, Rahimi-Balaei M, Haj-Mirzaian A, Marzban H, et al. Blockade of NMDA receptors reverses the depressant, but not anxiogenic effect of adolescence social isolation in mice. European Journal of Pharmacology. 2015;750:160–166. doi: 10.1016/j.ejphar.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Mileva GR, Bielajew C. Environmental manipulation affects depressive-like behaviours in female Wistar-Kyoto rats. Behavioural Brain Research. 2015;293:208–216. doi: 10.1016/j.bbr.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 41.Than TT, Delay ER, Maier ME. Sucrose threshold variation during the menstrual cycle. Physiology & behavior. 1994;56:237–239. doi: 10.1016/0031-9384(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 42.Green AD, Barr AM, Galea LAM. Role of estradiol withdrawal in ‘anhedonic’ sucrose consumption: A model of postpartum depression. Physiology & behavior. 2009;97:259–265. doi: 10.1016/j.physbeh.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, et al. Repeated Stress Induces Dendritic Spine Loss in the Rat Medial Prefrontal Cortex. Cerebral Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 44.Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC neuroscience. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J-R, Yan Y-T, Wang T-J, Chen L-J, Wang Y-J, Tseng G-F. Gonadal Hormones Modulate the Dendritic Spine Densities of Primary Cortical Pyramidal Neurons in Adult Female Rat. Cerebral Cortex. 2009;19:2719–2727. doi: 10.1093/cercor/bhp048. [DOI] [PubMed] [Google Scholar]
- 47.Douma S, Husband C, O’Donnell M, Barwin B, Woodend A. Estrogen-related Mood Disorders: Reproductive Life Cycle Factors. Advances in Nursing Science. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 48.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shors T, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. European Journal of Neuroscience. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 51.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-Induced Dendritic Remodeling in the Prefrontal Cortex is Circuit Specific. Cerebral Cortex. 2009;19:2479–2484. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen Promotes Stress Sensitivity in a Prefrontal Cortex–Amygdala Pathway. Cerebral Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Ma Y, Hu J, Cheng W, Jiang H, Zhang X, et al. Prenatal chronic mild stress induces depression-like behavior and sex-specific changes in regional glutamate receptor expression patterns in adult rats. Neuroscience. 2015;301:363–374. doi: 10.1016/j.neuroscience.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aston C, Jiang L, Sokolov B. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Molecular psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- 57.Gray A, Hyde T, Deep-Soboslay A, Kleinman J, Sodhi M. Sex differences in glutamate receptor gene expression in major depression and suicide. Molecular psychiatry. 2015;20:1057–1068. doi: 10.1038/mp.2015.91. [DOI] [PubMed] [Google Scholar]
- 58.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]