Abstract
In the present study, the combined adjuvant effect of 7DW8-5, a potent α-GalCer-analog, and monophosphoryl lipid A (MPLA), a TLR4 agonist, on the induction of vaccine-induced CD8+ T-cell responses and protective immunity was evaluated. Mice were immunized with peptides corresponding to the CD8+ T-cell epitopes of a malaria antigen, a circumsporozoite protein of Plasmodium yoelii, and a tumor antigen, a Wilms Tumor antigen-1 (WT-1), together with 7DW8-5 and MPLA, as an adjuvant. These immunization regimens were able to induce higher levels of CD8+ T-cell responses and, ultimately, enhanced levels of protection against malaria and tumor challenges compared to the levels induced by immunization with peptides mixed with 7DW8-5 or MPLA alone. Co-administration of 7DW8-5 and MPLA induces activation of memory-like effector natural killer T (NKT) cells, i.e. CD44+CD62L−NKT cells. Our study indicates that 7DW8-5 greatly enhances important synergistic pathways associated to memory immune responses when co-administered with MPLA, thus rendering this combination of adjuvants a novel vaccine adjuvant formulation.
Keywords: Adjuvant, Glycolipid, NKT cells, CD1d, TLR4, Malaria vaccine, Cancer vaccine, WT-1, Circumsporozoite protein, Memory-like effector NKT cells
1. Introduction
Invariant natural killer T (iNKT) cells express an invariant TCR-α chain (Vα14Jα18 in mice and Vα24Jα18 in humans) and recognize endogenous and exogenous glycolipids, such as α-galactosylceramide (α-GalCer) and its analogs [1–4]. Their TCR is restricted to CD1d, a MHC-I-like molecule found on antigen presenting cells, such as dendritic cells (DCs) [5,6].
iNKT cells exert a very important role in bridging innate and adaptive immunity, which makes this subset an attractive target for the development of vaccine adjuvants [7,8]. iNKT cells have been shown to increase protective T-cell immunity upon activation with α-GalCer when co-administered with malaria vaccines [9]. This potent adjuvant effect is due in part to the fact that the activation of iNKT cells by α-GalCer also rapidly induces the full maturation of DCs in vivo and thereby acts as an adjuvant for both CD4+ and CD8+ T-cell immunity [7,10]. The Toll-like receptor (TLR) pathway has also been shown to participate in the interaction between iNKT cells and DCs [11]. Activation of the TLR pathway induces transcription of several components of the inflammatory response, such as factor NF-κB, interferon-regulatory factors (IRFs) and MAP kinases, which lead to the production of pro-inflammatory cytokines such as TNFα, IL-12, IL-6, and IL-1 by DCs [12–15]. Given the ability of the TLR pathway to shape humoral and cellular responses, TLR agonists have been proposed as vaccine adjuvants [14–18].
In an attempt to further increase the adjuvant effect of formulations based on TLR agonists, strategies that combine these with other potent activators of DCs and iNKT cells [19–21] have been evaluated. Given the co-operative effect of DCs and iNKT cells, focusing on these two is a promising strategy, as illustrated in Supplemental Fig. 1, for the development of better adjuvant formulations. In the present study, we intended to evaluate the combined effect of a potent glycolipid, 7DW8-5, α-GalCer analog [22], when utilized along with the agonist of TLR4, MPLA (monophosphoryl lipid A) (Supplemental Fig. 1). Our results demonstrate that the combination of 7DW8-5 and MPLA induces protective effector memory CD8+ T-cell responses to an immunodominant epitope of a Plasmodium yoelii circumsporozoite protein (PyCSP) [23], as well as to HLA-A2-restricted epitopes of a Wilms Tumor antigen-1 (WT-1) [24].
2. Materials and methods
2.1. Mice
BALB/c mice at 6 to 8 weeks old were purchased from The Jackson Laboratory (Bar Harbor, ME). HLA-A2-transgenic mice on a C57BL/6 (B6) background were purchased from Taconic (Hudson, NY). Mice were kept in proper conditions as stated in the regulations and guidelines of animal care at the Comparative Bioscience Center animal facility at Rockefeller University.
2.2. Parasites
P. yoelii (17XNL strain) sporozoites were obtained from dissected salivary glands of infected Anopheles stephensi mosquitoes, 2 weeks after infectious blood meal as described [25,26]. The mosquitoes were maintained in the Insectary at New York University School of Medicine.
2.3. Immunization
BALB/c mice were immunized three to five times with 3-week interval by intra-muscular (i.m.) injection with PyCSP-derived peptide, SYVPSAEQI [23], at 20 µg with and without different adjuvants in varied concentrations and diluted in PBS. HLA-A2+/+ β2m-transgenic mice in B6 background were immunized three times with 3-week interval by i.m. injection with 20 µg of HLA-A2-restricted WT-1-derived peptides, WH (SLGEQQYSV) and WT (CMTWNQMNL) [24], with and without different adjuvants in varied concentrations and diluted in PBS.
2.4. Cell lines
To prepare antigen-presenting cells (APCs) for the ELISpot assay, A20.2J cells (mouse B cell lymphoma) were grown to 1.0 × 106 cells/mL in complete RPMI-10 medium supplemented with 10% fetal bovine serum (FBS), antibiotics and 10 mM HEPES and kept at 37 °C in 5% CO2 in an incubator. EL-4 expressing HLA-A2 were grown in complete DMEM supplemented with 10% FBS, antibiotics and 10 mM HEPES and kept at 37 °C in 5% CO2. Cells were washed, resuspended in supplemented media at a concentration of 1 × 107 cells/mL and loaded with PyCSP-derived and WT-1-derived peptides in the previous section, as well as HIV gag (TLNAWVKVV) mock peptide as negative control, at 20 µg/mL and incubated for 2 h at 37 °C. After the incubation, cells were irradiated with 8000 rad (12 min) using an OPD irradiator. After irradiation, cells were washed one more time and resuspended in complete media at 1 × 106 cells/mL.
2.5. Generation of a C1498 cell line co-expressing WT-1 and HLA-A2
The WT-1-C1498 murine leukemia cell line, which is syngeneic to C57BL/6 mice, was established via transfection of C1498 with murine WT-1 cDNA [27,28]. The HLA-A2.1 (HHD) gene, which encodes an interspecies hybrid MHC-class I gene linked to a human β2-microglubulin (β2m), was amplified from AAV-A2 vector [29] and subsequently subcloned into pLPCX vector (Moloney MLV-based retroviral vector, Clontech, Mountain View, CA). The recombinant retrovirus encoding for the hybrid HLA-A2-β2m molecule was produced by transient transfection of the ectopic packaging cell line Platinum-E (Plat-E, Cellbiolabs, San Diego, CA), using Lipofectamine 2000 transfection reagent (Invitrogen, Life Technologies, San Diego, CA). Viral supernatants were harvested 48 and 72 h after transfection, concentrated and purified. Retroviral supernatants were then loaded onto Retronectin-coated, nontissue culture treated 24-well plates according to the manufacturer's instruction (Takara Bio Inc., Otsu, Japan). For transduction, mouse WT-1-C1498 cells were seeded and incubated for at least 48 h with the viral particles. Two days after transduction, the double positive population of HLA-A2 and β2m was sorted out using FACsAria Cell Sorter (BD Biosciences Immunocytometry Systems, Franklin Lakes, NJ) (Supplemental Fig. 2A) and cloned by limiting dilution in the presence of Puromycin (1 µg/mL). WT-1 expression stability was verified by both flow cytometric analysis (Supplemental Fig. 2A) and RT-PCR (Supplemental Fig. 2B), as described [27].
2.6. Sporozoite challenge and assessment of parasite burden in the liver
P. yoelii sporozoite challenge was performed as described [25,26]. Briefly, immunized as well as naïve mice were injected with 1 × 104 live P. yoelii sporozoites via tail vein. Forty-two hours after the challenge, when the parasites fully matured in the hepatocyte, livers were collected from the mice, and RNA was extracted. The parasite burden in the liver was determined by measuring parasite-specific ribosomal RNA using 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA). Parasite burden was described as a ratio of the absolute copy number of parasite ribosomal RNA to that of mouse GAPDH mRNA.
2.7. Tumor challenge
WT-1+HLA-A2+C1498 tumorigenic cell lines were grown in complete RPMI 1640 (Sigma Aldrich) supplemented with 10% FBS, penicillin (100 U/mL) and streptomycin (100 µg/mL) and pyruvic acid (1%) at 37 °C. The selection was performed with puromycin (100 µg/mL). To induce tumor growth, 3 × 106 WT-1+HLA-A2+C1498 cells in 100 µL PBS were injected subcutaneously into the right flank of the HLA-A2 transgenic mice immunized previously with peptides and adjuvants alone or in combination. Tumor challenge was performed 14 days after vaccination. Tumor growth was monitored up to 50 days after subcutaneous injection of WT-1+HLA-A2+C1498 cells.
2.8. ELISpot assay
The numbers of PyCSP-specific, IFN-γ-secreting CD8+ T-cells among splenocytes obtained from immunized BALB/c mice and WT-1-specific IFN-γ-secreting CD8+ T-cells in the splenocytes of immunized HLA-A2 transgenic mice were determined by an ELISpot assay as previously described [23,25,26], with some modifications. Briefly, after splenocytes were prepared from spleen collected from mice 14 days after immunization, they were co-cultured the peptide-loaded APCs for 24 h at 37 °C on the ELISpot plate pre-coated with IFN-γ antibody, as previously described. Then the ELISpot plate was incubated with biotinylated anti-mouse IFN-γ antibody, followed by incubation with avidin-conjugated with horseradish peroxidase. Finally, the spots were developed after adding ELISpot substrate (Biolegend, San Diego, USA), as described [23,25,26]. The assays were run in duplicates for each mouse sample and 5 × 105 splenocytes/well were incubated with irradiated peptide-loaded or unloaded APCs at the ratio of 2:1 (splenocyte:APC). In order to identifying the number of IFN-γ-secreting CD8+ T cells for each well, the mean number of spots (for duplicates) counted in the wells incubated with splenocytes together peptide-loaded APCs was subtracted by the mean number of spots (for duplicates) counted in the wells incubated with splenocytes and unloaded APCs.
2.9. Assessing memory phenotypes and tetramer+ T-cells by a flow cytometric analysis
Upon lysing red blood cells, splenocytes were prepared as abovementioned. After washing the cells twice, splenocytes were blocked for 5 min on ice using inactivated normal mouse sera supplemented with anti-CD16/CD32 (clone 93 - BioLegend). After that, cells were stained for 40 min on ice in the dark with the following antibodies: anti-mouse CD3 (clone SK7 - BioLegend), anti-mouse CD4 (BioLegend), anti-mouse CD8 (clone SK1 - BioLegend), anti-mouse NK1.1 (BioLegend) anti-mouse CD44 (BioLegend), anti-mouse CD62L (BioLegend), anti-mouse CD11a (BioLegend), SYVPSAEQI-loaded tetramer. After staining, cells were washed twice with PBS containing 2% FBS, fixed with 1% paraformaldehyde, and analyzed using a BD LSR II (BD Biosciences, Franklin Lakes, NJ). For intracellular cytokine staining, splenocytes were stimulated for 6–12 h using the synthetic malaria and WT-1-specific peptides listed above, TLNAWVKVV (HIV gag) mock peptide as negative control or PMA-ionomycin (as a positive control) in the presence of brefeldin at 37 °C. The assays were performed as previously described [30]. Briefly, after blocking with the anti-mouse CD16/CD32 antibody, cells were stained for surface markers with antibodies against CD3, CD4, CD8, CD11a, CD44 and CD62L as mentioned. Next, lymphocytes were permeabilized with perm/wash solution (BD Biosciences), stained with the FITC-labeled anti-mouse IFN-γ antibody, anti-mouse Granzyme B antibody, or FITC Mouse IgG1 κ Isotype control antibody (Biolegend), fixed with 1% paraformaldehyde, and the staining profiles were acquired using a BD LSR II (BD Biosciences), using FACS DIVA software. The data analyses were performed using FlowJo Software version 10.0.6 (Tree Star Inc., Ashland, OR, USA), as previously described [4,5].
2.10. Statistical analysis
To compare the levels of different parameters induced by or associated with the different combinations of adjuvants, ANOVA one-way with Dunnett's post-test was performed to compare non-stimulated and peptide-stimulated paired samples. All statistical analyses were performed using GraphPad Prism software version 5.0.
3. Results
3.1. PyCSP-specific CD8+ T-cell response and protection against P. yoelii sporozoites challenge upon vaccination with PyCSP peptide together with or without 7DW8-5 and TLR agonists
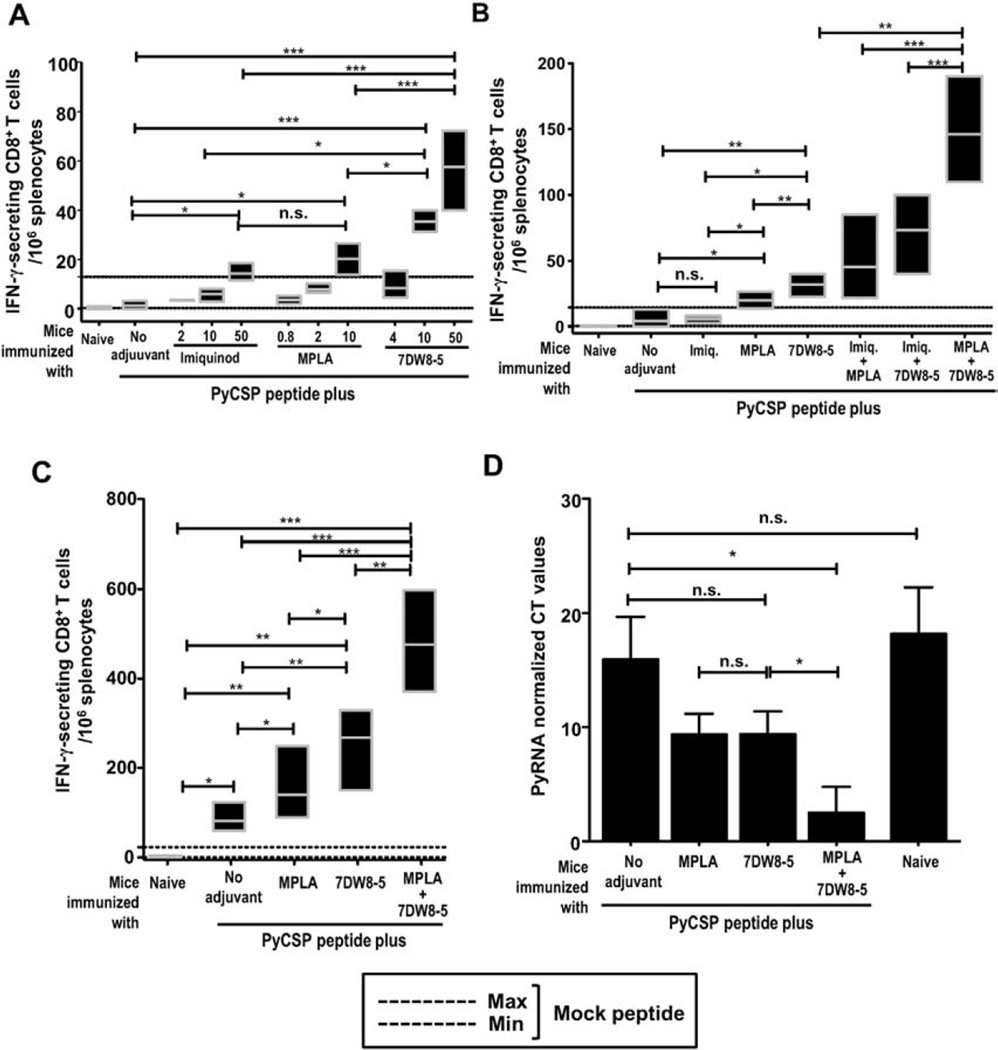
To evaluate the adjuvant effect of 7DW8-5 and TLR agonists on PyCSP-specific CD8+ T-cell response, BALB/c mice were immunized 3 times with 3-week interval with SYVPSAEQI peptide, corresponding to an immunodominant CD8 epitope of the PyCSP, along with different concentrations of 7DW8-5, MPLA and Imiquimod. Two weeks after the last immunization, splenocytes were collected and the level of PyCSP-specific CD8+ T-cell response was determined by an ELISpot assay. Fig. 1A shows a dose-dependent adjuvant effect of the glycolipid 7DW8-5, TLR4 (MPLA) and TLR7 (Imiquimod) agonists on the PyCSP-specific CD8+ T-cell response induced by vaccination with PyCSP-derived peptide. MPLA is able to induce PyCSP-specific CD8+ T-cell response at a lower concentration range (0.8–10 µg) compared to Imiquimod and 7DW8-5 (2–50 µg); however, the glycolipid (7DW8-5) is able to induce 4–8 times higher PyCSP-specific CD8+ T-cell response compared to TLR4 (MPLA) and TLR7 (Imiquimod) agonists, when these three compounds were evaluated at the same concentration of 10 µg/mouse (p < 0.01).
Fig. 1.
PyCSP-specific CD8+ T-cell response and protection against P. yoelii sporozoite challenge upon i.m. immunization with a PyCSP peptide mixed with adjuvants. (A) Groups of BALB/c mice (n = 5) received a single dose of i.m. immunization with a PyCSP-derived peptide (SYVPSAEQI; 20 µg) mixed with different doses of 7DW8-5, MPLA or Imiquimod. Two weeks later, splenocytes were collected from immunized as well as naïve mice, and the level of PyCSP-specific CD8+ T-cell response was determined by ELISpot assay. In (A–C), results of IFN-γ-secreting cells/million splenocytes were expressed as mean bars and error for each group. (B) Groups of BALB/c mice (n = 5) received a single dose of i.m. immunization with a PyCSP peptide mixed with 7DW8-5, MPLA, or Imiquimod alone or in combination. Two weeks later, splenocytes were collected from immunized as well as naïve mice, and the level of PyCSP-specific CD8+ T-cell response was determined by ELISpot assay. (C) Groups of BALB/c mice (n = 5) received three doses of i.m. immunization with a PyCSP peptide alone, or with a PyCSP peptide mixed with 7DW8-5 and/or MPLA with 3-week interval. Two weeks after the last immunization, splenocytes were collected from immunized as well as naïve mice, and the level of PyCSP-specific CD8+ T-cell response was determined by ELISpot assay. (D) Groups of BALB/c mice (n = 6) received three doses of i.m. immunization with a PyCSP peptide alone, or with a PyCSP peptide mixed with 7DW8-5 and/or MPLA with 3-week interval. Two weeks after the last immunization, immunized as well as non-immunized naïve mice (n = 6) were challenged with live P. yoelli sporozoites, and 42-hours later, livers were collected and the amounts of P. yoelii-specific ribosomal RNA (PyrRNA) in the liver were determined by qRT-PCR. The results are expressed as normalized PyrRNA copy number. In Fig. 1, all the experiments were repeated at least three times. Statistical significance was displayed as ***, **, or *, if the p value is <0.001, <0.01, or <0.05, respectively. The abbreviation - n.s. - stands for “not significant” and was assigned if p ≥ 0.05.
Considering the potential adjuvant effects of 7DW8-5 and the TLR agonists in inducing PyCSP-specific CD8+ T-cell response after peptide immunization, we sought to verify the potential adjuvant effect of the combination of 7DW8-5 and the TLR agonists. Fig. 1B shows the synergic adjuvant effect of 7DW8-5 and a TLR4 agonist (MPLA) in enhancing the level of peptide-specific IFN-γ-secreting CD8+ T-cell response induced after a single round of peptide immunization. The level of PyCSP peptide-specific CD8+ T-cell response enhanced by the combination of 7DW8-5 and MPLA is statistically higher than that enhanced by 7DW8-5 alone (p < 0.01) (Fig. 1B). There was no significant increase in the level of PyCSP-specific CD8+ T-cell response, when mice were immunized with PyCSP peptide mixed with both 7DW8-5 and the TLR7 agonist (Imiquimod) (Fig. 1B).
When mice were given 3 immunizing doses, the adjuvant effects of 7DW8-5 and MPLA became even more apparent. A group of mice received PyCSP peptide immunization mixed with both 7DW8-5 and MPLA, as an adjuvant, induced 3–5-fold higher level of PyCSP-specific CD8+ T-cell response than that received PyCSP peptide alone (Fig. 1C). It is noteworthy that when splenocytes were collected from BALB/c mice 2 weeks after receiving two doses of 7DW8-5 administration with 3-week interval, the adjuvant alone failed to induce IFN-γ response, as determined by an ELISpot assay (Supplemental Fig. 3).
To determine the level of protective immunity induced by PyCSP peptide-based vaccine in combination with 7DW8-5 and MPLA, as an adjuvant, BALB/c mice received 3 immunizing doses with 3-week interval. Twelve days after the last immunization, both immunized and non-immunized groups of BALB/c mice were challenged intravenously with 1 × 104 live P. yoelii sporozoites. Then the livers were collected from challenged mice, and the levels of parasite burden in the liver were determined by qRT-PCR. As shown in Fig. 1D, peptide immunization with 7DW8-5 and MPLA combined induced a highest level of protective immunity that significantly (p < 0.05) inhibited the parasite development in the liver, whereas peptide immunization with either 7DW8-5 or MPLA alone failed to mount a protective immunity that could significantly inhibit the parasite burden in the liver.
3.2. WT-1-specific CD8+ T-cell response and protection against WT-1 tumor challenge in HLA-A2 transgenic mice upon vaccination with WT-1 peptides together with or without 7DW8-5 and MPLA
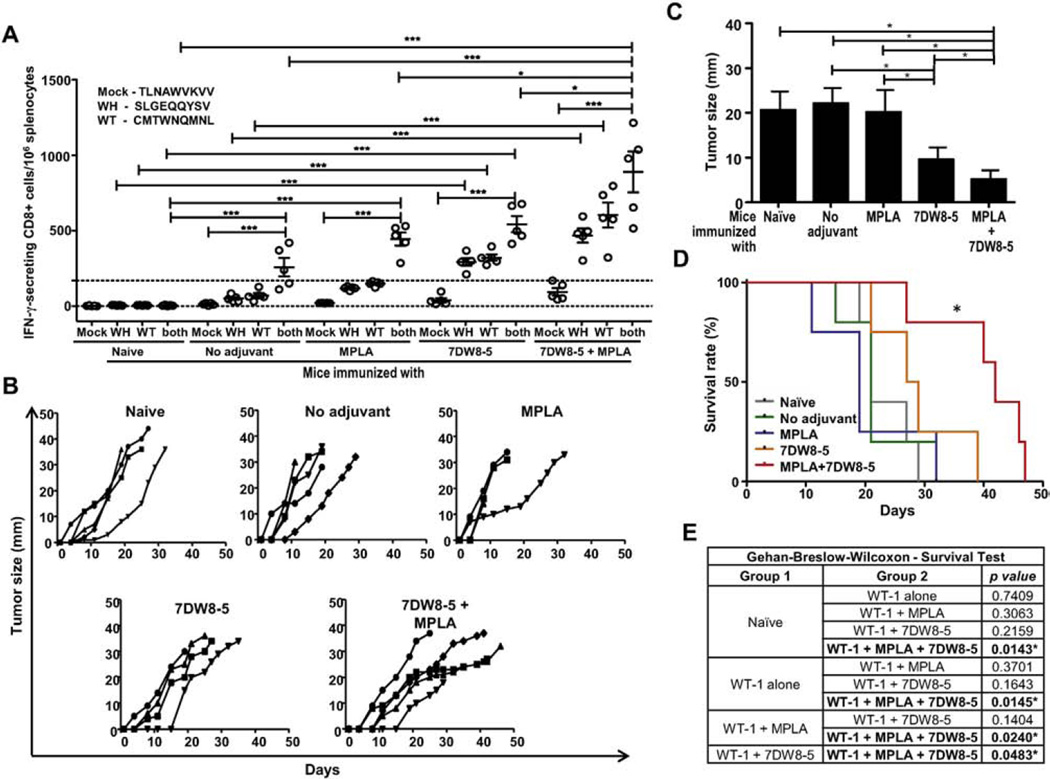
To further validate the adjuvant effect of 7DW8-5 and the TLR4 agonist, MPLA, a different vaccine platform comprised of tumor-derived peptides that contain HLA-A2-restricted CD8 epitopes was utilized. The two peptides correspond to different epitopes of the WT-1 cancer antigen, SLGEQQYSV and CMTWNQMNL. Because the WT-1 peptides correspond to HLA-A2-restricted CD8 epitopes, HLA-A2 transgenic B6 mice were immunized 3 times with the peptides alone, or the peptides together with 7DW8-5 and MPLA, individually and in combination. Two weeks after the last immunization, splenocytes were collected, and the levels of WT-1-specific CD8+ T-cell response were determined by an ELISpot assay. Fig. 2A shows that the levels of IFN-γ-secreting HLA-A2-restricted, WT-1-specific CD8+ T-cell response were significantly (p < 0.05) higher in mice that received immunization with WT-1 peptides mixed together with combined adjuvants compared to those not only in mice immunized with the peptides alone, but also in mice immunized with the peptides together with either adjuvant. Fig. 2B and C show the results for tumor size developed in naïve, as well as immunized mice, upon tumor challenge. Mice immunized with WT-1 peptides together with combined adjuvants grew significantly (p < 0.05) smaller tumors (Fig. 2C) compared with those of mice immunized with WT-1 peptides mixed with either adjuvant alone at day 15 post tumor challenge. Furthermore, co-administration of 7DW8-5 and MPLA with WT-1 peptides enhanced the level of protective anti-tumor immunity, resulting in a significant (p < 0.05) increase in survival rate of mice, compared to that of mice immunized with WT-1 peptides mixed with either MPLA or 7DW8-5 separately (Fig. 2D and E).
Fig. 2.
WT-1-specific CD8+ T-cell responses and protection against WT-1 tumor challenge upon i.m. immunization with WT-1-derived peptides mixed with two adjuvants, 7DW8-5 and MPLA. (A) Groups of HLA-A2 transgenic mice with B6 background (n = 5) received three doses of i.m. immunization with pooled HLA-A2-restricted WT-1-derived peptides, WH (SLGEQQYSV) and WT (CMTWNQMNL), with 7DW8-5 (10 µg) and/or MPLA (2 µg). (A) Two weeks after the last immunization, splenocytes were collected from immunized as well as naïve mice, and the level of CD8+ T-cell response specific for separated or pooled WT-1 peptides was determined by ELISpot assay. (B) Two weeks after the last immunization, immunized as well as naïve mice (n = 4–5) were subcutaneously challenged with WT-1+HLA-A2+C1498 tumor cells, and the size (diameters in millimeters) of the tumor growth was measured up to 50 days. (C) Cross-sectional comparison of tumor size (diameters in millimeters) among immunized mouse groups (WT-1 peptides with or without adjuvants) as well as a naive mouse group at day 15 post challenge with WT-1+HLA-A2+ C1498 tumor cells. (D) Survival rate of immunized mouse groups as well as a naïve mouse group after challenge with WT-1+HLA-A2+ C1498 tumor cells. (E) Results for the comparisons of survival curves registered for groups of mice consisting of a non-immunized, naïve mouse group, as well as groups immunized with WT-1 peptides with or without adjuvants. Statistical analyses of survival rates were performed using Gehan-Breslow Wilcoxon test. In Fig. 2, experiments were repeated twice. Statistical significance was displayed as ***, **, or *, if the p value is <0.001, <0.01, or <0.05, respectively.
3.3. Activation of NKT cells and increased CD11a expression in CD44+ effector NKT cells upon vaccination with peptides co-administered with 7DW8-5 and MPLA
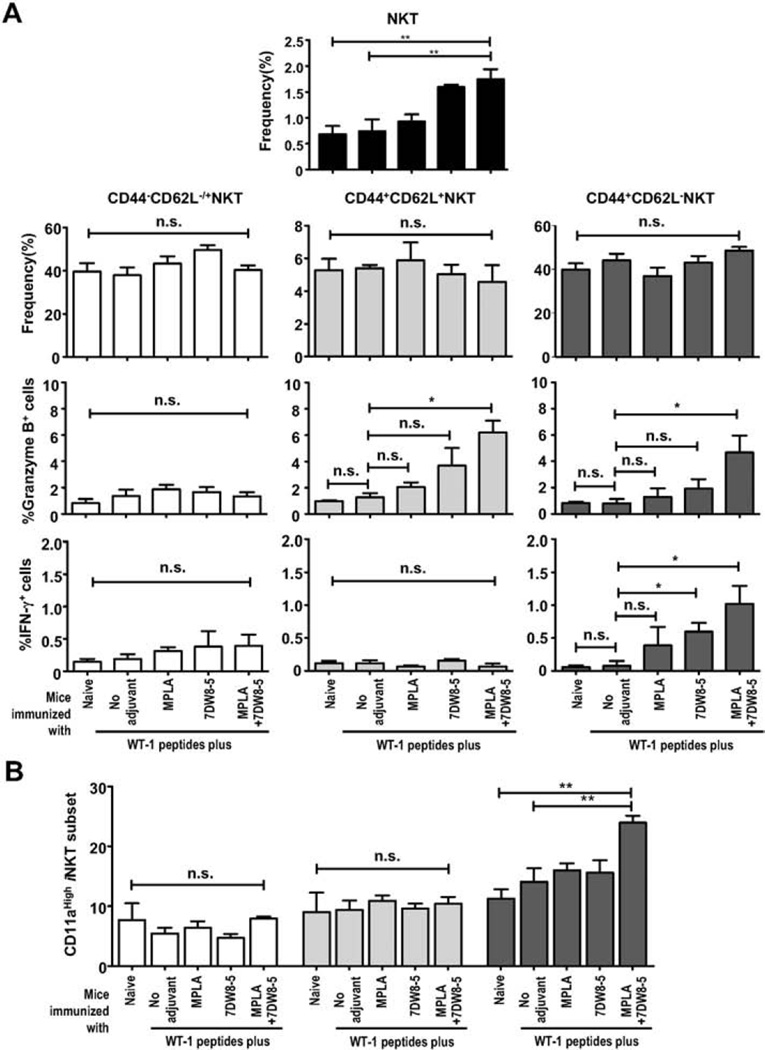
In order to understand the modifications on NKT-cell biology after repeated exposure to 7DW8-5 and TLR4 ligand (MPLA) separately and in combination, we have evaluated NKT-cell (SSClowCD3+/low CD8−NK1.1+) capacity on producing IFN-γ and granzyme B, as well as the percentages of NKT-cell subsets expressing memory markers (CD44 and CD62L). The NKT-cell subsets CD44−CD62L−/+, CD44+-CD62L+ and CD44+CD62L− were defined as naïve, central, and effector NKT cells, respectively (Supplemental Fig. 4). The percentages of each of the NKT cell subsets did not change in mice immunized with the adjuvants separately or in combination; however, the percentage of the total NKT cell population increased (Fig. 3A). The percentage of granzyme B+ central or effector NKT cells was significantly higher in mice immunized with the WT-1 peptides mixed with 2 adjuvants than in mice immunized with the peptides without adjuvant. The percentage of IFN-γ-secreting effector NKT cells was significantly higher in mice immunized with the peptides mixed with 2 adjuvants and even in mice immunized with the peptides and 7DW8-5 alone than that in mice immunized with the peptides without adjuvant. The percentage of IFN-γ+-naïve or central NKT cells did not increase after repeated doses of the adjuvant combination compared to that in mice received peptides immunization without adjuvant. Activation on effector NKT cells, but not other subsets of NKT cells, was highest as evident by the increase of CD11ahigh expression upon peptides immunization with 7DW8-5 and MPLA combined, compared to that upon peptides immunization with either adjuvant alone or without adjuvant (Fig. 3B, Supplemental Fig. 4). It is noteworthy that iNKT cells are specific to 7DW8-5 in the context of CD1d molecules, but not to the peptides in the context of MHC-1 molecules. Therefore, the WT-1 peptides would not induce specific activation of NKT cells. Altogether, these results demonstrate that multiple doses of co-administration of 7DW8-5 and MPLA (regardless of peptide immunization) induce functional proinflammatory memory-like effector NKT cells.
Fig. 3.
Analysis of NKT-cell memory subsets. Splenocytes were obtained from a group (n = 5) of naïve HLA-A2 transgenic mice (B6 background), as well as groups (n = 5) of mice received five doses of i.m. immunization with two WT-1 peptides, or with WT-1 peptides mixed with 7DW8-5 and/or MPLA. Then the percentages of CD44−CD62L+/− (naïve); CD44+CD62L+ (central); CD44+CD62L− (effector) and total NKT-cell subsets were analyzed by FACS. Results are expressed as the percentages of total splenocytes. In (A), the percentages of the NKT-cell subsets, Granzyme B+-cells and IFN-γ+-cells among CD44−CD62L+/−, CD44+CD62L+ and CD44+CD62L− NKT cells were demonstrated. In (B), the percentage of CD11a+ cells among the subsets pre-defined as CD44−CD62L+/−, CD44+CD62L+ and CD44+CD62L− among total NKT cells was shown. In this figure, experiments were repeated twice. Statistical significance was displayed as ***, **, or *, if the p value is <0.001, <0.01, or <0.05, respectively. The abbreviation - n.s. - stands for “not significant” and was assigned if p ≥ 0.05.
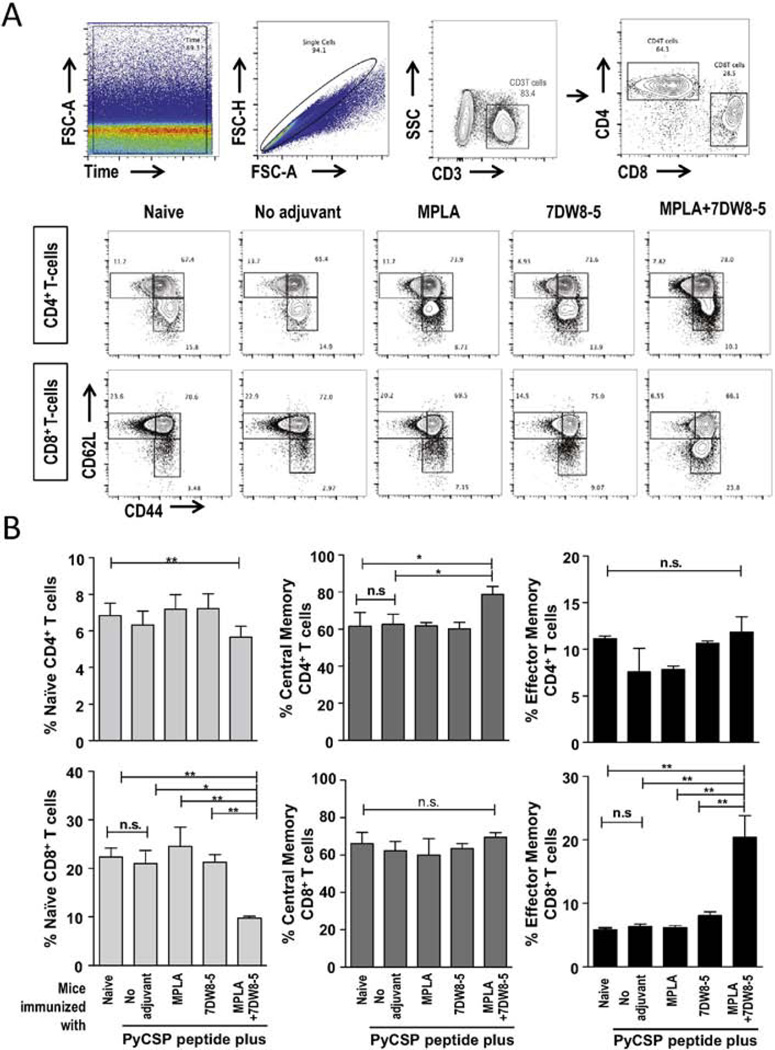
3.4. Decreased naïve T-cells and increased effector memory cd8+ T-cells and central memory CD4+ T-cells upon vaccination with a peptide co-administered with 7DW8-5 and MPLA
To investigate whether the adjuvant combination of 7DW8-5 and MPLA is able to elicit expansion of memory T-cell responses, we evaluated the phenotypic aspects of CD4+ and CD8+ T-cells after peptide immunization with the adjuvant combination. For that, the percentage of CD4+ and CD8+ T cells and the percentages of these subsets expressing memory markers (CD44 and CD62L) were assessed by FACS (Fig. 4A). The subsets CD44−CD62L−/+, CD44+CD62L+ and CD44+CD62L− were defined as naïve, central and effector CD4+ and CD8+ T cells, respectively. The results show that the co-administration of peptide with both 7DW8-5 and MPLA decreases the percentages of naïve CD4+ and CD8+ T-cells but significantly increases the percentages of central memory CD4+ T cells and effector memory CD8+ T cells, when compared to those of mice immunized with peptide without adjuvant or with either adjuvant alone (Fig. 4B).
Fig. 4.
CD4+ and CD8+ T-cell memory phenotypes elicited upon i.m. immunization with a PyCSP peptide mixed with 7DW8-5 and MPLA. Splenocytes were obtained from BALB/c mice after three doses of i.m. immunization with a PyCSP peptide alone, or with a PyCSP peptide mixed with 7DW8-5 and/or MPLA. Splenocytes from a group of non-immunized mice (n = 5) were used as a negative control. Then the percentages of CD44−CD62L+/− (naïve); CD44+CD62L+ (central) and CD44+CD62L− (effector) CD4+ and CD8+ T-cell subsets, were analyzed by FACS. (A) A gate on Time versus FSC was built to exclude carry over events from the previous sample. After that, a singlet gate on FSC-Area versus FSC-height was set to remove doublets. From singlets, T-cells were selected by CD3 versus SSC plot, followed by CD4 versus CD8 plot. CD4+ versus CD8+ T-cells were evaluated for CD62 versus CD44 expression. The subsets CD44−CD62L−/+, CD44+CD62L+ and CD44+CD62L− were defined as naïve, central and effector CD4+ and CD8+ T-cells, respectively. (B) The results are expressed in percentages. In this figure, experiments were repeated twice. Statistical significance was displayed as ***, **, or *, if the p value is <0.001, <0.01, or <0.05, respectively. The abbreviation - n.s. - stands for “not significant” and was assigned if p ≥ 0.05.
3.5. Increased granzyme B+CD11ahigh central and effector memory, as well as IFN-γ+ effector memory CD8+ T cells, upon vaccination with a peptide co-administered with 7DW8-5 and MPLA
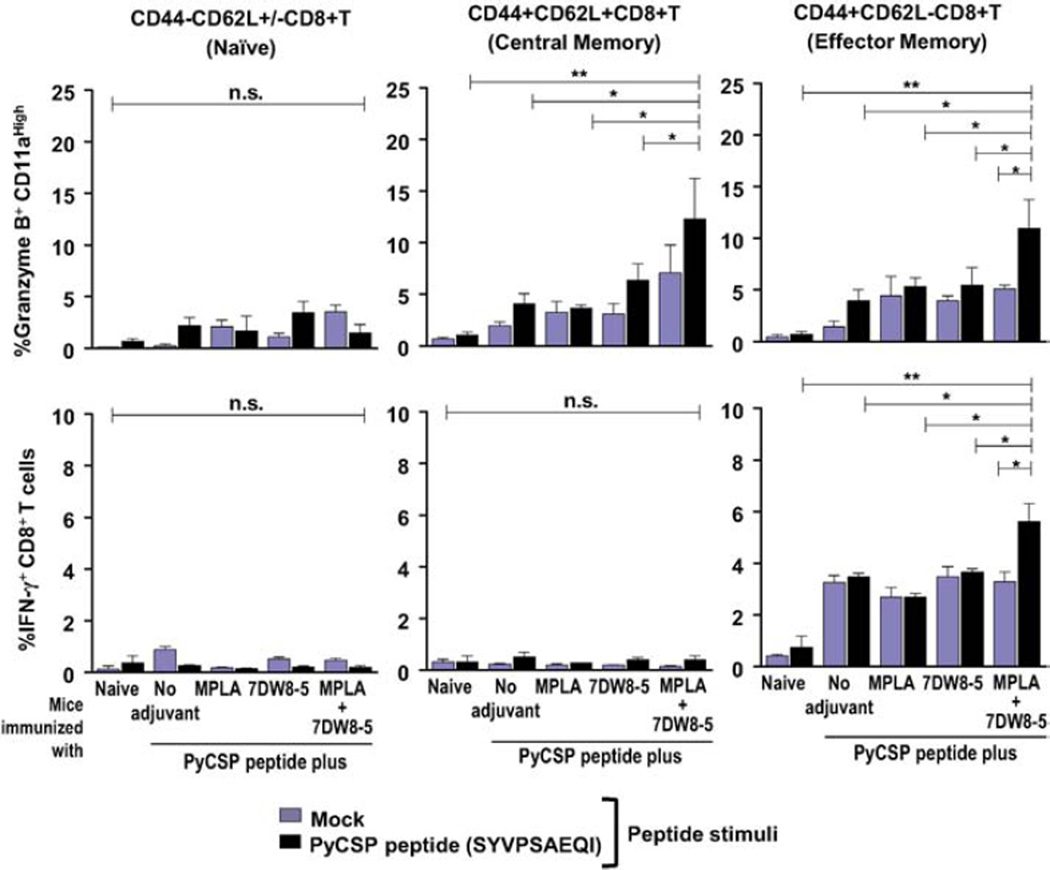
In addition, the functional aspects of the memory CD8+ T-cell subsets were evaluated by isolating splenocytes from mice immunized with SYVPSAEQI malaria peptide together with or without adjuvants, followed by briefly culturing them with the peptide in vitro. Afterwards, the percentages of IFN-γ and granzyme-B-producing CD8+ T cells, as well as the percentage of these subsets expressing memory markers (CD44 and CD62L), was assessed by FACS (Supplemental Fig. 5). The naïve CD8+ T-cell subset does not significantly respond to peptide stimulation, as no differences were observed for granzyme B or IFN-γ production (Fig. 5). However, the percentages of granzyme B+ CD11ahigh central and effector memory CD8+ T-cells, as well as the percentage of IFN-γ+ effector memory CD8+ T-cells, significantly increased within splenocytes isolated from mice which were immunized with the malaria peptide together with both 7DW8-5 and MPLA compared to that from mice immunized with either adjuvant alone or without adjuvant, upon in vitro stimulation with the malaria peptide (Fig. 5).
Fig. 5.
Function of CD8+ T-cell memory subsets elicited after i.m. immunization with a PyCSP peptide mixed with 7DW8-5 and MPLA. Splenocytes were obtained from BALB/c mice after three doses of i.m. immunization with a PyCSP peptide alone, or with a PyCSP peptide mixed with 7DW8-5 and/or MPLA. Splenocytes from a group of non-immunized mice (n = 5) were used as a negative control. Then the percentage of Granzyme B+-CD11ahigh cells (A) IFN-γ+-cells (B) among CD8+ T-cells among naïve (CD44−CD62L+/−), central (CD44+CD62L+) and effector (CD44+CD62L−) subsets, respectively, were analyzed by FACS. In this figure, experiments were repeated twice. Statistical significance was displayed as ***, **, or *, if the p value is <0.001, <0.01, or <0.05, respectively. The abbreviation - n.s. - stands for “not significant” and was assigned if p ≥ 0.05.
3.6. Increased Tet+ population within effector memory T-cells upon vaccination with a peptide co-administered with 7DW8-5 and MPLA
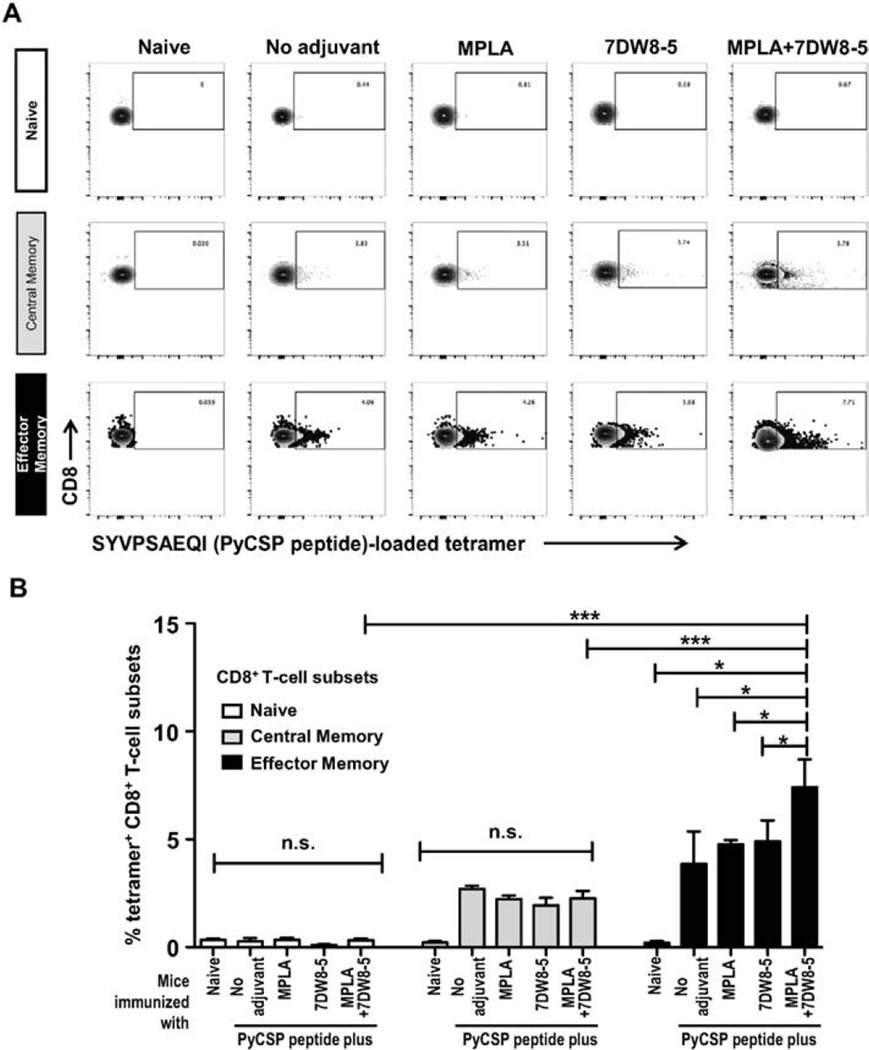
In order to confirm the results showing the enhancement of peptide vaccine-induced PyCSP-specific CD8+ T-cell response by co-administration of 7DW8-5 and MPLA (Fig. 1), we also performed a tetramer assay. When we measured the percentage of SYVPSAEQI-loaded tetramer positive (Tet+) cells by FACS analysis (Fig. 6A), we found that the percentage of SYVPSAEQI-loaded Tet+ effector memory CD8+T cells was significantly higher in mice immunized with the malaria peptide together with both 7DW8-5 and MPLA adjuvants than that in mice immunized with the peptide together with either adjuvant alone or without adjuvant (Fig. 6B). This increase was not observed with Tet+ cells in naïve and central memory CD8+ T cells subsets (Fig. 6B).
Fig. 6.
Assessment of specific CD8+ T-cell responses elicited after i.m. immunization with PyCSP-derived peptide mixed with 7DW8-5 and MPLA. Splenocytes were obtained from BALB/c mice after three doses of i.m. immunization with a PyCSP peptide alone, or with a PyCSP peptide mixed with 7DW8-5 and/or MPLA, and the tetramer assay was performed. Splenocytes from a group of non-immunized mice (n = 5) were used as a negative control. In (A), flow cytometric plots of CD8 versus SYVPSAEQI-loaded tetramer are shown for all immunization schemes as well as non-immunized controls after selection of naïve (CD44−CD62L+/−), central (CD44+CD62L+) and effector (CD44+CD62L−) subsets as described. In (B), the percentage of SYVPSAEQI-loaded tetramer+ T-cells among naïve (CD44−CD62L+/−), central (CD44+CD62L+) and effector (CD44+CD62L−) CD8+ T-cells subsets, respectively was measured by FACS. The cell subsets were indicated by the colors (naïve –white; central – gray; effector – black). In this figure, experiments were repeated twice and in duplicates. Statistical significance was displayed as ***, **, or *, if the p value is <0.001, <0.01, or <0.05, respectively. The abbreviation - n.s. - stands for “not significant” and was assigned if p ≥ 0.05.
4. Discussion
Our group has recently identified a α-GalCer analog, called 7DW8-5, which has a higher binding affinity to CD1d than α-GalCer and potently activates iNKT cells. This compound has been proven a valuable candidate for future vaccine adjuvant formulations [22,31]. However, after continuous stimuli, iNKT cells may become unresponsive to glycolipid presentation leading to an in situ anergic state [32,33] mediated by immature DCs. In this regard, using the combination of iNKT-cell activators with a DC maturation stimulus may lead to a long-lasting activation of iNKT cells, i.e. induction of memory-like iNKT cells, with different functional and phenotypic features yet to be understood. Therefore, combining two potent immune activators, such as CD1d ligands and TLR ligands, may be an interesting strategy for improving the adjuvant effect of iNKT-cell activation, as illustrated in Supplemental Fig. 1. In fact, the combined effect of iNKT-cell activators and TLR agonists has previously been demonstrated and employed on the immunotherapy of infectious diseases [19–21].
In the present study, we evaluated the combined effect on inducing memory responses after stimulation with the potent glycolipid, 7DW8-5, when utilized along with the agonists for TLR4 (MPLA) and TLR7 (Imiquimod) using peptides as a vaccine platform. Our results demonstrate that the combination of 7DW8-5 and MPLA, as an adjuvant, for a vaccine based on peptides containing P. yoelii circumsporozoite and WT-1 immunodominant epitopes indeed induce stronger, specific effector memory CD8+ T-cell responses, as well as central memory CD4+ T-cell responses, compared to either adjuvant alone.
An important concern that rises during the design of a cancer vaccine is the fact that live attenuated vectors may reactivate during immunosuppression. This concept opens the perspective for the generation of protein/peptide-based cancer vaccines and safe adjuvant formulations to boost the peptide-specific cytotoxic CD8+ T-cell responses instead of antibody-mediated immunity. The Wilms tumor protein, WT-1, is a widely recognized tumor antigen that is aberrantly expressed in myeloid and lymphoid leukemia [34–36], making this protein an attractive target for the development of cancer vaccines. Previous reports have demonstrated that selected WT-1 peptides induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1+ leukemia [37,38]. Recently, treatment involving chemotherapy and DCs pulsed with WT-1-specific MHC class I/II-restricted epitopes was associated with disease stability in advanced pancreatic cancer, demonstrating the potential of WT-1 peptides in therapeutic strategies in addition to preventive immunization [39]. In fact, the National Cancer Institute performed a pilot project to develop a well-vetted, priority-ranked list of cancer vaccine target antigens based on predefined and preweighted objective criteria in which WT-1 antigen scored the highest priority [40]. This supports our present study that proposes a new formulation of 7DW8-5 and MPLA adjuvants utilized along with HLA-A2-restricted WT-1 peptides in mice transgenic for HLA-A2, which has been proven to be useful in inducing strong CD8+ T-cell responses and protection against tumor challenge. This particular combination of peptides and adjuvants also increased the potency of the in vivo cytotoxic CD8+ T-cell responses and enhanced the protective effect of these peptides against both WT-1+ tumor challenge and against challenge with freshly isolated live P. yoelii sporozoites.
WT-1 peptide vaccinations have been shown to induce CD4+ and CD8+ T-cell responses in patients with mesothelioma and non-small cell lung cancer, and for that, adjuvants such as Montanide adjuvant and GM-CSF are necessary to induce the responses [41]. GM-CSF is a well-known adjuvant; however, this cytokine may also stimulate the growth of tumor cells. In contrast, a number of studies have documented the protective role of α-GalCer-activated NKT cells in direct anti-tumor immunity. Upon activation, NKT cells can rapidly produce many diversed immunoregulatory cytokines. Ligand-activated Vα14+ NKT cells were proven to kill tumor cells directly through a CD1d− Vα14+ T-cell receptor-independent, NK-like mechanism in mice [42, 43]. Given that cancer vaccines may also be employed for therapeutic purposes in which a specific CD8+ T-cell response would be beneficial with combating tumor cells in specific tumor sites, the direct and specific anti-tumor effect of the glycolipid highly advises for its usage in cancer vaccine adjuvant formulations.
Interestingly, our results demonstrate that the combination of 7DW8-5 and MPLA induces functional Th1 memory-like effector NKT cells after repeated exposure. The memory-like effector NKT-cell subset is able to produce robust pro-inflammatory and cytolytic responses as demonstrated by secretion of IFN-γ and granzyme B. These functional evidences, in addition to the fact that the memory-like NKT cells increase CD11a expression, indicate that they are activated without being led to an anergic status even after repeated stimulation [32,33]. This effector NKT-cell subset demonstrated a high correlation with the development of highly specific CD8+ T cells, which demonstrates that NKT cells may bridge the development of functional cytolytic and protective responses. In addition to the T-cell responses, the direct antitumor properties of this Th1 memory-like NKT-cell subset could act synergistically to the WT-1-specific CD8+ T-cell responses and increase the therapeutic effect of this vaccine formulation for treating advanced stage tumors. In fact, these results are corroborated by recent findings that indicate memory properties in NKT-cell subsets [44–46]. The ancient paradigm of immunological memory being regarded as a unique feature of the adaptive immune response mediated in an antigen-specific manner by T and B lymphocytes has been challenged previously and no longer stands the trial of evidence [47]. Persistent IFN-γ-producing iNKT-cell response was previously reported after stimulation with α-GalCer-loaded DCs [44]. Prolonged activation of iNKT cells by CD1d ligand-loaded DCs has demonstrated the surprising presence of effector memory-like iNKT cells in the lung of mice [46].
All the experiments in the current study were conducted in mice with 2 different strains, with one wild-type BALB/c mouse strain and another HLA-A2 transgenic mouse strain with C57BL/6 background. With regards to the clinical applications of MPLA and 7DW8-5, MPLA has already been tested in combination with other adjuvants and a vaccine in humans. The most advanced malaria vaccine candidate, RTS,S/AS01, also called Mosquirix, contains MPLA as one of the key adjuvants. This vaccine has shown to be effective in a phase III trial and has been licensed for use in endemic countries by the European Medicines Agency [48]. 7DW8-5 was recently tested by our group in a non-human primate (rhesus monkey) model. In fact, we have tested the adjuvant effect of 7DW8-5 for an adenovirus-based malaria vaccine in rhesus macaques and found that 7DW8-5 could enhance malaria-specific CD8+T-cell responses without showing notable reactogenicity [49].
All in all, our present study demonstrates that 7DW8-5 combined with a TLR4 agonist, MPLA, displays a potent adjuvant effect and enhances the levels of specific CD8+ T-cell responses and protective immunity to malaria and cancer. We hope that our current results may be able to facilitate the clinical development of combined 7DW8-5 and MPLA adjuvants for the purpose of enhancing the efficacy of peptide-based vaccines in the near future.
Supplementary Material
Acknowledgments
The authors would like to thank Cristina Fernández-Arias for her assistance and Vincent Sahi for assistance with flow cytometry. This work was supported by a grant from NIH AI070258 (M.T.).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clim.2016.04.014.
Contributor Information
Jordana G. Coelho-dos-Reis, Email: jordana.reis@cpqrr.fiocruz.br.
Moriya Tsuji, Email: mtsuji@adarc.org.
References
- 1.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 2.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Proc Natl Acad Sci USA. 2005;102:1351–1356. doi: 10.1073/pnas.0408696102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 5.Lantz O, Bendelac A. An invariant T cell receptor α chain is used by a unique subset of MHC class I specific CD4+ and CD4−8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nature Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 7.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi SK, Lang GA, Devera TS, Johnson AM, Kovats S, Lang ML. Differential contribution of dendritic cell CD1d to NKT cell-enhanced humoral immunity and CD8+ T cell activation. J Leukoc Biol. 2012;9:783–790. doi: 10.1189/jlb.1111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni RR, Villanueva AI, Elawadli I, Jayanth P, Read LR, Haeryfar SM, Sharif S. Costimulatory activation of murine invariant natural killer T cells by toll-like receptor agonists. Cell. Immunol. 2012;277:33–43. doi: 10.1016/j.cellimm.2012.06.002. (2012) [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, Avalos AM, Ploegh HL. Accessory molecules for Toll-like receptors and their function. Nat Rev Immunol. 2012;12:168–179. doi: 10.1038/nri3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durand V, Wong SY, Tough DF, Le Bon A. Shaping of adaptive immune responses to soluble proteins by TLR agonists: a role for IFN-alpha/beta. Immunol Cell Biol. 2004;82:596–602. doi: 10.1111/j.0818-9641.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- 15.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trumpfheller C, Caskey M, Nchinda G, Longhi MP, Mizenina O, Huang Y, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci USA. 2008;105:2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahl-Hennig C, Eisenblätter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;A5:e1000373. doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caskey M, Lefebvre F, Filali-Mouhim A, Cameron MJ, Goulet JP, Haddad EK, et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J Exp Med. 2011;208:2357–2366. doi: 10.1084/jem.20111171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci USA. 2007;104:20490–20495. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McWilliams JA, Sanchez PJ, Haluszczak C, Gapin L, Kedl RM. Multiple innate signaling pathways cooperate with CD40 to induce potent, CD70-dependent cellular immunity. Vaccine. 2010;28:1468–1476. doi: 10.1016/j.vaccine.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmakar S, Bhaumik SK, Paul J, De T. TLR4 and NKT cell synergy in immunotherapy against visceral leishmaniasis. PLoS Pathog. 2012;8:e1002646. doi: 10.1371/journal.ppat.1002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues EG, Zavala F, Eichinger D, Wilson JM, Tsuji M. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J Immunol. 1997;158:1268–1274. [PubMed] [Google Scholar]
- 24.Oka Y, Elisseeva OA, Tsuboi A, Ogawa H, Tamaki H, Li H, et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms' tumor gene (WT1) product. Immunogenetics. 2000;51:99–107. doi: 10.1007/s002510050018. [DOI] [PubMed] [Google Scholar]
- 25.Ophorst OJ, Radosević K, Havenga MJ, Pau MG, Holterman L, Berkhout B, et al. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect Immun. 2006;74:313–320. doi: 10.1128/IAI.74.1.313-320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiratsuchi T, Rai U, Krause A, Worgall S, Tsuji M. Replacing adenoviral vector HVR1 with a malaria B cell epitope improves immunogenicity and circumvents preexisting immunity to adenovirus in mice. J. Clin. Invest. 2010;120:3688–3701. doi: 10.1172/JCI39812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oka Y, Udaka K, Tsuboi A, Elisseeva OA, Ogawa H, Aozasa K, et al. Cancer immunotherapy targeting Wilms' tumor gene WT1 product. J. Immunol. 2000;164:1873–1880. doi: 10.4049/jimmunol.164.4.1873. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima H, Oka Y, Tsuboi A, Tatsumi N, Yamamoto Y, Fujiki F, et al. Enhanced tumor immunity of WT1 peptide vaccination by interferon-β administration. Vaccine. 2012;30:722–729. doi: 10.1016/j.vaccine.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Li X, Coelho-dos-Reis JG, Wilson JM, Tsuji M. An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T-cells in mice. PLoS One. 2014;9:e88205. doi: 10.1371/journal.pone.0088205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nixon DF, Townsend AR, Elvin JG, Rizza CR, Gallwey J, McMichael AJ. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–487. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 31.Padte NN, Li X, Tsuji M, Vasan S. Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin. Immunol. 2011;140:142–151. doi: 10.1016/j.clim.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J. Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue K, Sugiyama H. WT 1 and leukemia. Rinsho Ketsueki. 1995;36:552–558. [PubMed] [Google Scholar]
- 35.Nishioka M, Tanemura A, Nishida S, Nakano A, Tsuboi A, Oji Y, et al. Vaccination with WT-1 (Wilms' tumor gene-1) peptide and BCG-CWS in melanoma. Eur. J. Dermatol. 2012;22:258–259. doi: 10.1684/ejd.2011.1619. [DOI] [PubMed] [Google Scholar]
- 36.Molldrem JJ. What T cells see in WT-1. Blood. 2012;120:1540–1541. doi: 10.1182/blood-2012-06-433979. [DOI] [PubMed] [Google Scholar]
- 37.Xue SA, Gao L, Hart D, Gillmore R, Qasim W, Thrasher A, et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood. 2005;106:3062–3067. doi: 10.1182/blood-2005-01-0146. [DOI] [PubMed] [Google Scholar]
- 38.Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O'Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012;120:1633–1636. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms' tumor 1 (WT1)-specific MHC Class I/II-restricted epitopes for pancreatic cancer. Clin Cancer Res. 2014;20:4228–4239. doi: 10.1158/1078-0432.CCR-14-0314. [DOI] [PubMed] [Google Scholar]
- 40.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krug LM, Dao T, Brown AB, Maslak P, Travis W, Bekele S, et al. WT1 peptide vaccinations induce CD4 and CD8 T cell immune responses in patients with mesothelioma and non-small cell lung cancer. Cancer Immunol. Immunother. 2010;59:1467–1479. doi: 10.1007/s00262-010-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth MJ, Godfrey DI. NKT cells and tumor immunity-a double-edged sword. Nat Immunol. 2000;1:459–460. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 44.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–874. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with α-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007;178:2853–2861. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu K, Sato Y, Shinga J, Watanabe T, Endo T, Asakura M, et al. KLRG+ invariant natural killer T cells are long-lived effectors. Proc Natl Acad Sci USA. 2014;111:12474–12479. doi: 10.1073/pnas.1406240111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, et al. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 49.Padte NN, Boente-Carrera M, Andrews CD, McManus J, Grasperge BF, Gettie A, Coelho-dos-Reis JG, Li X, Wu D, Bruder JT, Sedegah M, Patterson N, Richie TL, Wong CH, Ho DD, Vasan S, Tsuji M. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS ONE. 2013;8(10):e78407. doi: 10.1371/journal.pone.0078407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.