SUMMARY
Background & aims
Dietary sodium, protein, acid precursors, and water have been linked to cyst growth in polycystic kidney disease; yet, no studies in patients have examined the feasibility of using a dietary intervention that controls all of these factors. The aim of this study was to determine if a diet, appropriate for persons of most ages, reduces the excretion of sodium, urea, acid, and decreases mean urine osmolality while gaining acceptance by patients with autosomal dominant polycystic kidney disease (ADPKD).
Methods
Twelve adults with ADPKD enrolled in a pre-post pilot feasibility study and served as their own controls. Individuals consumed their usual diet for one week then for four weeks followed an isocaloric diet lower in sodium and protein and higher in fruits, vegetables, and water. Three-day diet records and two 24-h urine samples were collected at baseline, week 2, and week 4 visits; blood pressure, weight, and serum were obtained at all three visits. A modified nutrition hassles questionnaire was completed on the last visit.
Results
During the dietary intervention, subjects (n = 11) consumed less sodium, protein, and dietary acid precursors 36%, 28%, and 99%, respectively, and increased fluid intake by 42%. Urinary sodium, urea, net acid excretion, osmoles, and osmolality decreased 20%, 28%, 20%, 37%, and 15%, respectively; volume increased 35%. Urine changes were in accord with the diet record. Ninety-one percent of participants reported that none of the hassles were worse than “somewhat severe”, and most participants felt “somewhat confident” or “very confident” that they could manage the new diet.
Conclusions
A majority of adult patients with ADPKD successfully prepared and followed a composite diet prescription with decreased sodium, protein, acid precursors, and increased fluid intake. This trail was registered at ClinicalTrials.gov (NCT01810614).
Keywords: Polycystic kidney disease, Renal nutrition, Net acid excretion, Potential renal acid load, Dietary acid load, Net endogenous acid production
1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder that affects ~600,000 individuals in the United States and ~12.5 million people worldwide [1]. The clinical hallmark of ADPKD is the development of cysts in individual renal tubules that enlarge progressively throughout life. Although cysts begin forming during fetal development, the glomerular filtration rate (GFR) usually stays within an apparently normal range for several decades owing to compensatory hyperfiltration [2]. Meanwhile, cysts increase in number and volume, causing serious injury to blood vessels and renal tubules. Recent evidence indicates that kidney volume predicts the likelihood of developing renal insufficiency over time, suggesting that the growth of cysts is linked to declining kidney function [3].
The disease phenotype is highly variable causing differing rates of cyst growth even among members of the same family [4]. Dietary factors appear to account for some of the variability in this rate of renal enlargement; a process that appears to be hastened by the excessive intake of salt and animal-sourced protein [5,6]. Additionally, increased urinary acid excretion accelerates cyst growth in experimental animals and leads to a more rapid decline in kidney function in patients with moderately advanced chronic progressive renal diseases, including ADPKD [5,7–14]. Acid excretion is largely driven by high intakes of animal-sourced dietary protein, but can be reduced by base-producing fruits and vegetables [15]. Increasing fluid intake has also been shown to reduce kidney weight (% of total body weight) by 27–30% by lowering plasma levels of arginine vasopressin (AVP); a result achieved when reaching a urine osmolality of <290 mosm/kg H2O [16]. In view of this evidence, we aimed to determine if a relatively complex dietary prescription targeting dietary salt, protein, acid precursors, and fluid intake would be meaningfully adopted by adult patients with ADPKD.
2. Materials and methods
2.1. Study population
Twelve subjects with a certain diagnosis of ADPKD confirmed by family history, magnetic resonance, computed tomography or ultrasound imaging were enrolled [1]. Subjects were recruited from the University of Kansas Medical Center Polycystic Kidney Disease clinic during routine visits between May 2013 and January 2014. Inclusion in the study required a blood pressure <135/85 mmHg, stable weight and clinical biochemistry, a diet history of >30 mEq/ day of net endogenous acid production (NEAP), and an estimated GFR (eGFR) of >30 ml/min/1.73 m2 based on the MDRD-EPI equation [17]. Subjects were excluded if they used pharmaceuticals or dietary restrictions/enhancements for preexisting medical conditions not associated with standard of care for ADPKD or if they had been prescribed medications that affect acid/base status. A physical examination was performed by a physician to confirm medical appropriateness to participate. Written informed consent was obtained prior to study enrollment. This study was approved by the Human Subject Committee at the University of Kansas Medical Center. This trial was registered at clinicaltrials.gov (NCT01810614). A physician co-investigator monitored data and safety.
2.2. Study design
Subjects were enrolled for a five-week study that included an enrollment visit, three study visits (baseline, 2 weeks, 4 weeks) and two follow-up phone calls (Fig. 1). Study visits occurred in the clinical and translational science unit. Each subject submitted a three-day diet record (three days prior to study visits regardless of weekday or weekend), two consecutive 24-h urine collections (two days prior to study visit), and a study nurse recorded blood pressures, anthropometrics, and drew blood samples at each visit. All subjects received instructions on how to modify their diet and served as their own controls in this pre-post pilot feasibility study.
Fig. 1.
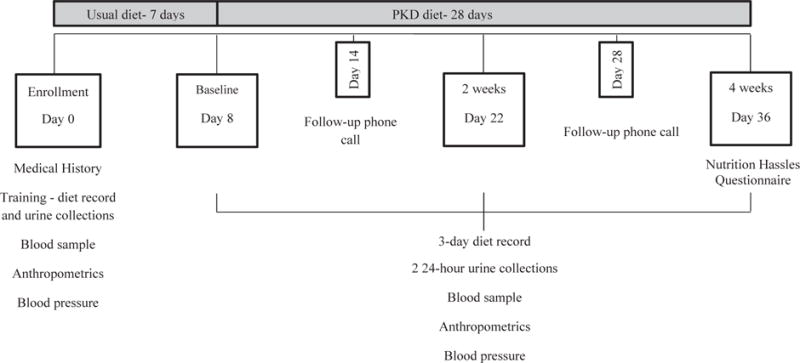
Diagram of the study protocol, study visit days, and data collected at visits. PKD, polycystic kidney disease.
2.3. Three-day diet records
Diet records included a detailed accounting of all food and beverage consumed, the amounts consumed, the method of food preparation, and, if homemade, the recipe that was used. The amount of food consumed was measured by weight on an electronic food scale or by typical volumetric household measures. If neither of these methods of measurement were available, participants were asked to estimate portion sizes using picture books that were provided. Diet records were analyzed at each visit using the Nutrition Data for System Research (NDSR version 2012) [18]. To improve collection of dietary data, probing techniques and a multiple pass system were used during study visits to gather information that subjects may have omitted or recorded incorrectly [19].
2.4. Urine measurements
Participants were instructed at the enrollment visit on how to collect 24-h urine samples. Instructions included detailed methods for properly recording start and stop times and when to switch to the second 24-h collection. Written instructions were also provided. Timed 24-h urine collections were completed on the two days prior to each visit and coincided with the last two days of the diet record. Urine collections were kept on ice or placed in a refrigerator throughout the duration of each timed collection. Urine volume was determined from the weight of the collected sample. Aliquots of urine were centrifuged, and stored immediately at −20 C for the later measurement of urinary acid excretion [20]. The remaining urine was sent immediately to the University of Kansas Hospital lab for analysis of electrolytes, urea, creatinine, bicarbonate, and osmolality.
2.5. Other measurements
Height was measured without shoes using a stadiometer and weight was recorded with participants without shoes in street clothes. Subjects were supine for the measurement of blood pressure. Electrolytes, blood urea nitrogen (BUN), creatinine, uric acid, glucose, and bicarbonate were determined in serum samples by the hospital laboratory.
2.6. Diet and urine calculations
2.6.1. Diet
Three-day diet records were used to calculate net endogenous acid production from the formula NEAP = PRAL + organic acids (OA) and the mean recorded. NEAP is used to predict the amount of acid excreted in the urine [21,22]. The first term, PRAL, estimates the potential net acid or base contribution a food or liquid would make when combusted by whole body metabolism [23]. The daily PRAL was calculated from all food and drink consumed over the day by estimating the dietary intake of anions (phosphorus and sulfur) and cations (potassium, calcium, and magnesium) from diet records using nutrient profiles from NDSR; sulfur was estimated based on the normative content of methionine and cysteine derived from protein in the diet. The fractional absorption of nutrients by the intestines, the dissociation of phosphate, and ionic valences are all taken into account in Remer’s method for determining PRAL [21,23]. Daily consumption of these anions and cations was entered into an excel program developed by the research team that accounts for the variables listed in Remer’s equation to generate the daily PRAL of the diet. The second component of the equation, OA, is estimated from body surface area according to Berkemeyer and Remer [24]. Given the time frame and stable body weight in this study, we assumed that OA excretion was also stable.
2.6.2. Urine
Net acid excretion (NAE) is a direct measure of acid/alkali excretion in the urine and is determined from the sum of titratable acid + ammonium − bicarbonate [15]. NEAP predicts acid in the urine and should approximate NAE. NAE was measured within 3 months of sample collection using standard methods [20].
2.7. Diet prescription
The experimental diet was tailored for individual subjects based on their baseline dietary intake of sodium, protein and NEAP (derived from three-day diet records) and measurements of urine osmolality and urine volume obtained from two 24-h urine collections (Table 1) [18,22,25]. The test diet was followed for the next four-weeks. At baseline, subjects received detailed instruction from a registered dietitian about limiting the intake of salt and protein while increasing the intake of fruits, vegetables, and fluid.
Table 1.
Diet prescription.a
| Diet component | Target level | Focus |
|---|---|---|
| Dietary Acids | Reduce NEAP by 50% (minimum 40 mEq/d) | Increased fruits and vegetables were prescribed using a new fruit and vegetable point system. 1 point = 1 mEq reduction in NEAP. |
| Protein | 0.8–1.0 g/kg body weight/d | A standard protein point system was prescribed to limit total protein intake, primarily animal protein. |
| Sodium | 1–1.5 mmol/kg body weight | Daily limits were apportioned to limits for each meal and snack based on the individual’s usual dietary pattern. Sodium reduction guidelines from the American Heart Association were also provided [26]. |
| Fluid | Reduce urine osmolality to 285 mosm/kg H2O | Subjects were given exact volumes of fluid to drink throughout the day to reduce urine osmolality to target level. |
NEAP, net endogenous acid production.
2.7.1. Sodium
The daily limit for sodium in the test diet (1–1.5 mEq/kg) was apportioned over the course of the day based on the number of meals and snacks a participant typically ate. Each subject’s diet was uniquely modified in this manner to change their usual dietary pattern as little as possible. Subjects were informed of the sources of sodium in their diet, how to read food labels, and how to access nutrition information from restaurants and food companies online. Educational materials from the American Heart Association were provided to help educate subjects about dietary sodium [26].
2.7.2. Protein
Protein intake was limited to 0.8–1.0 g/kg body weight/day. To ease tracking, a protein point system was developed. Protein points were assigned to all protein-rich foods (meat, beans, dairy, protein-rich grains); one protein point was equal to approximately seven grams of protein (i.e., one ounce of meat, one cup of milk, or ½ cup dried beans). Participants were also taught how to convert food labels listing grams of protein to protein points. Participants were encouraged to make the majority of reductions in protein intake by eating less animal based protein sources.
2.7.3. Fruits and vegetables
The amount of fruits and vegetables prescribed was calculated to reduce NEAP by 50% (minimum 40 mEq/day) after accounting for their baseline fruit and vegetable intake. Because changes in NEAP are driven by changes in PRAL in adults with stable body weight, increasing the intake of fruits and vegetables decreases PRAL. Nutrient profiles from NDSR were used to calculate the PRAL per 100 g of food according to Remer [23]. These values were converted into the amount of PRAL reduction per serving of fruit or vegetable and given as a handout to the participants so they could track their intake (i.e., adding 40 points of fruits and vegetables per day is equivalent to reducing PRAL by 40 mEq). Participants were instructed on the number of points to eat daily from fruits and vegetables.
2.7.4. Fluids
Since the goal was to reduce the urine osmolality to that of plasma or below, we used baseline mean 24-h urine osmolality excretion to determine the total fluid intake that would be needed to decrease the mean urine osmolality to ≤285 mosm/kg H2O/d; an additional 20% was added to correct for insensible losses [25]. Assuming 1 kg fluid = 1000 ml of fluid, the daily fluid goal (mL/ d) = ((24-hr urine mosm/kg H2O/285 mosm/kg H2O) × total urine volume (mL/d)) × 1.2.
For patients with baseline mean urine osmolality equal to plasma or less we prescribed sufficient water to maintain urine osmolality at baseline levels.
2.8. Nutrition hassles questionnaire analysis
This diet is formulated for lifetime use so understanding the participants’ views about the extent of daily stress associated with nutritional issues is important. We adapted the Nutrition Hassles Questionnaire (NHQ) to flesh out particular aspects of the diet, such as concerns about the prescribed diet, planning menus, and eating the proper amounts, as well as issues related to the manner in which the diet was tracked by participants, that might impede wide acceptance [27]. The NHQ is a set of 33 items designed to assess the perception of stress associated with the diet and is divided into three sections: hassles, uplifts, and self-efficacy. The hassles section includes 15 questions related to following the diet, each rated on a 0–3 scale (0 = no hassle and 3 = severe hassle). It includes questions aimed at uncovering issues associated with planning meals, preparing meals, and tracking intake of dietary components. The uplifts section includes six questions about events that made the participant feel good while following the diet and is rated on a 0–3 scale (0 = never and 3 = very often). The self-efficacy section includes 12 questions about how confidently a person identifies and selects appropriate foods for meals and snacks, prepares appropriate foods, and their ability to follow the diet prescription. This section is rated on a 5 point scale (1 = not confident at all and 5 = very confident).
2.9. Statistical analyses
A paired samples t-test was used to compare two week and four week visit data to baseline measurements. Mean results of the three day diet records, 24-h urine collections, and blood pressures were analyzed with SPSS software [28]. Data were managed by REDCap (CTSA Award # UL1TR000001). Differences were considered statistically significant at p ≤ 0.05.
3. Results
Baseline demographics data are listed in Table 2 of the 50 individuals approached about participation in this diet intervention, twelve subjects signed consents and were enrolled. No subjects withdrew from this study and no adverse events were reported. This cohort is a cross-section of middle-aged, ADPKD patients with well-preserved eGFR.
Table 2.
Baseline descriptive features.a
| Characteristic | Valueb |
|---|---|
| Number of participants, n (M/F) | 11 (4/7) |
| Age (y) | 47 ± 15 |
| Caucasian, % (n) | 91 (10) |
| African American, % (n) | 9 (1) |
| Height (cm) | 169.9 ± 9.5 |
| Weight (kg) | 71.3 ± 12.4 |
| BMI (kg/m2) | 24.9 ± 5.1 |
| Systolic Blood Pressure (mmHg) | 129 ± 11 |
| Diastolic Blood pressure (mmHg) | 77 ± 7 |
| eGFR (ml/min/1.73 m2) | 84 ± 25 |
BMI, Body Mass Index; eGFR, estimated glomerular filtration rate.
All results either mean ± SD or % (n), unless otherwise indicated.
3.1. Compliance
Of the 12 subjects enrolled, one subject failed to record 3-day diet records and improperly collected 24-h urine samples and was considered a failure, because data were unavailable or unreliable. The remaining 11 subjects attended every study visit and brought completed diet records and urine collections with them to each visit. Creatinine excretion at the two week and four week visits was ~7% below baseline; however, this decrease is likely related to the reduced intake of animal protein in the test diet and not incomplete collection of 24-h urine samples [29].
3.2. Baseline
3.2.1. Dietary intake
Diet records revealed that the subjects at baseline were already eating reasonably well-balanced amounts of protein, carbohydrates, and fat (Table 3); however, energy intake was slightly more than recommended based on body size. Intake of sodium and protein was somewhat higher than commonly recommended for ADPKD subjects, but meat is a prominent component of most diets in the Midwest. The intake of other mineral cations, including potential protons, was moderate except for potassium which was relatively low. Due to the relatively high animal-sourced protein and low potassium intake, baseline NEAP was acidic in all subjects (mean = 58 mEq/d). Baseline fluid intake was variable averaging 3.2 L a day which is in the upper range of conventional intake but not uncommon in ADPKD patients who are encouraged to drink extra water [30–33].
Table 3.
Dietary intake in 11 individuals with ADPKD by study visit.a
| Recommendedb | Baseline | 2 weeks | 4 weeks | |
|---|---|---|---|---|
| Nutrient intake | ||||
| Energy Intakec (kcal) | 1782–2138 | 2334 ± 416 | 2355 ± 425 | 2107 ± 399 |
| Fat (% of energy) | 20–35% | 34.6 ± 5.4 | 26.8 ± 7.2** | 25.2 ± 6.0*** |
| Cholesterol (mg/d) | NE | 327 ± 138 | 185 ± 82** | 192 ± 76* |
| Carbohydrates (% of energy) | 45–65% | 47.4 ± 7.9 | 59.1 ± 8.2*** | 61.6 ± 8.7*** |
| Fiber (g/d) | 21–38 | 20.4 ± 4.5 | 32.5 ± 7.4*** | 28.4 ± 6.8*** |
| Protein (% of energy) | 10–35% | 17.0 ± 4.1 | 12.3 ± 2.7*** | 13.7 ± 4.3*** |
| Protein (g/d) | Based on body weight | 97.8 ± 25.9 | 70.9 ± 12.9** | 69.9 ± 18.4** |
| Animal Protein (g/d) | NE | 68.5 ± 24.7 | 37.5 ± 15.2*** | 40.9 ± 20.3** |
| Plant Protein (g/d) | NE | 29.3 ± 7.2 | 33.3 ± 14.4 | 29.0 ± 6.8 |
| Protein (g/kg) | 0.8 | 1.39 ± 0.40 | 1.01 ± 0.19** | 0.99 ± 0.25** |
| Fruits and vegetables (g/d) | NE | 441 ± 258 | 1201 ± 355*** | 1214 ± 389*** |
| Fluids | ||||
| Fluids (mL/d) | 2700–3700 | 3229 ± 573 | 4628 ± 855*** | 4575 ± 1076** |
| Solute Intake | ||||
| Sodium (mEq/d) | 57–65 | 155.8 ± 45.7 | 102.8 ± 23.5** | 99.0 ± 25.8** |
| Sodium (mEq/kg) | – | 2.26 ± 0.87 | 1.45 ± 0.29** | 1.41 ± 0.37** |
| Potassium (mEq/d) | 120 | 74 ± 14 | 116 ± 21*** | 111 ± 24*** |
| Calcium (mEq/d) | 50–60 | 63 ± 26 | 68 ± 34 | 64 ± 32 |
| Magnesium (mEq/d) | 25.5–34.5 | 30 ± 7 | 39 ± 17 | 36 ± 17 |
| Phosphorus (mEq/d) | 45 | 94 ± 18 | 81 ± 13 | 75 ± 10** |
| Sulfur (mEq/d) | NE | 9 ± 3 | 6 ± 1*** | 6 ± 2*** |
| OA (mEq/d) | NE | 43.1 ± 3.8 | 43.0 ± 3.7 | 43.1 ± 3.7 |
| Dietary Acids | ||||
| PRAL (mEq/d) | NE | 14.9 ± 20.0 | −44.0 ± 24.0*** | −41.7 ± 27.9*** |
| NEAP (mEq/d) | NE | 57.9 ± 21.4 | −1.0 ± 23.6*** | 1.4 ± 28.0*** |
p 0.05 compared to baseline.
p 0.01 compared to baseline.
p 0.001 compared to baseline.
Values are means ± SDs. ADPKD, autosomal dominant polycystic kidney disease; AI, adequate intake; AMDR, acceptable macronutrient dietary range; DRI, dietary recommended intake; NE, Not established; NEAP, net endogenous acid production; OA, organic acids; PRAL, potential renal acid load.
Acceptable Macronutrient Dietary Range, Adequate Intake (ranges based on gender and age ranges), or Recommended Dietary Allowances (ranges based on gender and age ranges) as appropriate.
Based on 25–30 kcal/kg actual body weight.
3.2.2. Urine excretion
The extent to which the diet records reported what was actually consumed was examined in the urine recovery of key solutes at baseline (Tables 3 and 4). We did not expect perfect correspondence, because subjects were expected to make timed collections and record diet consumption; however, the recoveries of sodium (95.9%), urea (85.4%), and potassium (88.8%) were excellent. Urine volume was 558 ml less than the intake reported in the diet record, likely due to insensible losses during the summer months. The mean urine osmolality at baseline was skewed with 8 of 11 greater than plasma (285 mosm/kg H2O).
Table 4.
Urine constituents in 11 individuals with ADPKD by study visit.a
| Baseline | 2 weeks | 4 weeks | |
|---|---|---|---|
| Urinary Solute Excretion | |||
| Sodium (mEq/d) | 149.4 ± 36.3 | 118.7 ± 42.9* | 120.3 ± 35.5* |
| Potassium (mEq/d) | 65.9 ± 12.7 | 101.9 ± 31.4*** | 102.5 ± 23.9*** |
| Calcium (mEq/d) | 8 ± 3 | 6 ± 3 | 6± 2** |
| Magnesium (mEq/d) | 8 ± 2 | 8 ± 2 | 8± 3 |
| Chloride (mEq/d) | 147 ± 31 | 128 ± 42 | 130 ± 32 |
| Phosphorus (mEq/d) | 50 ± 13 | 43 ± 11* | 47 ± 8 |
| Sulfurb (mEq/d) | 41 ± 8 | 31 ± 8*** | 32 ± 8*** |
| Urea Nitrogen, (g/d) | 11.17 ± 2.39 | 7.83 ± 2.47*** | 8.14 ± 2.30*** |
| Uric Acid (mg/dL) | 573 ± 99 | 527 ± 120 | 559 ± 87 |
| Creatinine (mg/d) | 1600 ± 294 | 1491 ± 254* | 1495 ± 213* |
| Urine osmoles (mosm/d) | 843 ± 121 | 718 ± 153** | 726 ± 118* |
| Urine osmolality (mosm/kg) | 335 ± 84 | 205 ± 55*** | 216 ± 70*** |
| Fluids | |||
| Urine volume (mL) | 2671 ± 740 | 3642 ± 849*** | 3598 ± 1005* |
| Urine acid-base excretion | |||
| Urine pHc (units) | 5.90/6.06 | 6.26/6.90 | 6.21/6.78 |
| Bicarbonate (mEq/d) | 1.50 ± 1.20 | 3.45 ± 2.51** | 3.91 ± 2.85** |
| Net Acid Excretion (mEq/d) | 37.8 ± 14.3 | 18.1 ± 17.0*** | 16.6 ± 22.5*** |
p ≤ 0.05 compared to baseline.
p ≤ 0.01 compared to baseline.
p ≤ 0.001 compared to baseline.
Values are means ± SDs. ADPKD, autosomal dominant polycystic kidney disease.
Urinary sulfur was estimated based on an average content of methionine (2.4%) and cysteine (2.0%) from protein intake based on Remer’s equation [23]. Protein intake was calculated from the urea excretion [47].
Mean/median.
3.3. Treatment
3.3.1. Intake
Subjects eating the test diet had an insignificant decrease in calorie intake, a reduction in dietary fat and protein related to the lower animal protein intake, and an increase in carbohydrates, related to the higher intake of fruits and starchy vegetables (Table 3). All macro nutrients remained within acceptable ranges.
Subjects also reached the goals for sodium (≤1.5 mmol/kg), protein (≤1 g/kg), and fluid intake (urine osmolality ≤ 285 mosm/ kg) tailored for each patient; changes that were sustained throughout the experimental period. Potassium intake increased 150% from baseline; there was no change in calcium or magnesium. With the increase in potassium and the reduction in protein, NEAP decreased from baseline by ~58 mEq/d at the two week and four week visits.
3.3.2. Urine excretion
Sodium excretion decreased (−20%) and potassium excretion increased (+155%) reflecting the changes in the dietary prescription. Urea excretion and urinary calcium decreased 28% and 29%, respectively, in response to the decrease in dietary protein intake [34]. Urine magnesium excretion remained stable throughout the study. NAE decreased 46% below baseline and was accompanied by a sustained rise in urine pH of 0.33/0.78 (mean/median) units (Table 4). Urine volume increased 35% above baseline consistent with a 43% increase in fluid consumption and mean osmolality declined reaching the 285 mosm/kg H2O target in 10/11 subjects (Tables 3 and 4).
3.4. Other findings
Body weight and BMI did not change whereas systolic blood pressure tended to decrease during treatment possibly responding to the decrease in sodium intake and increases in potassium intake (Table 5). BUN was significantly reduced reflecting the decreased animal-sourced protein intake and serum potassium levels increased significantly secondary to the increased intake of fruits and vegetables.
Table 5.
Serum components, anthropometrics, and blood pressures in 11 individuals with ADPKD by study visit.a
| Baseline | 2 weeks | 4 weeks | |
|---|---|---|---|
| Serum | |||
| Sodium (mEq/L) | 137 ± 2 | 138 ± 2 | 136 ± 1 |
| Potassium (mEq/L) | 3.8 ± 0.3 | 4.0 ± 0.3 | 4.1 ± 0.3** |
| Chloride (mEq/L) | 104 ± 2 | 103 ± 2 | 103 ± 2 |
| CO2 (mmol/L) | 27 ± 2 | 27 ± 2 | 28 ± 2 |
| Anion Gap (mEq/L) | 7 ± 2 | 7 ± 3 | 6 ± 2 |
| Glucose (mg/dL) | 83 ± 9 | 86 ± 10 | 87 ± 13 |
| BUN (mg/dL) | 20 ± 8 | 15 ± 8*** | 15 ± 9** |
| Creatinine (mg/dL) | 0.97 ± 0.24 | 0.98 ± 0.32 | 0.98 ± 0.28 |
| eGFR (ml/min/1.73 m2) | 84 ± 25 | 85 ± 27 | 83 ± 26 |
| Uric Acid (mg/dL) | 4.8 ± 1.6 | 4.7 ± 1.5 | 4.8 ± 1.5 |
| Calcium (mg/dL) | 9.7 ± 0.3 | 9.7 ± 0.5 | 9.7 ± 0.3 |
| Blood Pressure | |||
| Systolic (mmHg) | 129 ± 11 | 120 ± 12* | 123 ± 11 |
| Diastolic (mmHg) | 77 ± 7 | 77 ± 8 | 78 ± 8 |
| Anthropometrics | |||
| Weight (kg) | 71.3 ± 12.4 | 71.1 ± 12.1 | 71.3 ± 12.3 |
| BMI (kg/m2) | 24.9 ± 5.1 | 24.8 ± 5.0 | 24.9 ± 5.1 |
p ≤ 0.05 compared to baseline.
p ≤ 0.01 compared to baseline.
p 0.001 compared to baseline.
Values are means ± SDs. ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
3.5. Nutrition hassles questionnaire
3.5.1. Hassles
Overall, only one participant reported any of the hassles as “moderately severe” and no hassles were reported as “extremely severe” (Table 6). “Somewhat severe” hassles that are noteworthy include planning meals, tracking fruits and vegetables, tracking protein points, tracking fluid intake, and cooking differently. Interestingly, 9 out of 11 (82%) of participants reported that preparing meals was “not a hassle” while only 3 individuals (36%) felt that planning meals was “not a hassle” indicating that planning ahead was more of a hassle than actually preparing the meals.
Table 6.
Hassles questionnaire responses in 11 individuals with ADPKD.a
| Hassles | Number (%)
|
|||
|---|---|---|---|---|
| Not a hassle | Somewhat severe | Moderately severe | Extremely severe | |
| Planning meals | 4 (36) | 7 (64) | 0 | 0 |
| Not enough money for food | 10 (91) | 1 (9) | 0 | 0 |
| Preparing meals | 9 (82) | 2 (18) | 0 | 0 |
| Tracking fruits and vegetable points | 6 (55) | 4 (36) | 1 (9) | 0 |
| Tracking protein points | 4 (36) | 6 (55) | 1 (9) | 0 |
| Tracking fluids for the day | 6 (55) | 5 (45) | 0 | 0 |
| Limiting sodium in the diet | 8 (73) | 2 (18) | 1 (9) | 0 |
| Other individuals in your house won’t eat what is prepared | 8 (73) | 2 (18) | 1 (9) | 0 |
| Cooking differently | 7 (64) | 4 (36) | 0 | 0 |
| Not enough time to plan meals | 9 (82) | 2 (18) | 0 | 0 |
| Not enough time to prepare meals | 8 (73) | 2 (18) | 1 (9) | 0 |
| Concerns about other’s perceptions of your diet | 10 (91) | 1 (9) | 0 | 0 |
| Not fully understanding what to do | 10 (91) | 1 (9) | 0 | 0 |
| Grocery shopping | 9 (82) | 2 (18) | 0 | 0 |
| Using handouts that were given to follow diet | 9 (82) | 2 (18) | 0 | 0 |
| Uplifts | No uplifts | Somewhat often | Moderately often | Extremely often |
|---|---|---|---|---|
| Meeting family responsibilities | 4 (36) | 0 | 3 (27) | 4 (36) |
| Meeting your responsibilities | 6 (55) | 1 (9) | 0 | 4 (36) |
| Eating out | 4 (36) | 5 (45) | 1 (9) | 1 (9) |
| Cooking for someone else | 5 (45) | 1 (9) | 0 | 5 (45) |
| Being well-prepared | 6 (55) | 0 | 1 (9) | 4 (36) |
| Helping to improve someone else’s health | 5 (45) | 1 (9) | 3 (28) | 2 (18) |
| Self-efficacy | Not at all confident | Somewhat not confident | Neutral | Somewhat confident | Very confident |
|---|---|---|---|---|---|
| Identify appropriate food for meals and snacks | 0 | 0 | 0 | 6 (55) | 5 (45) |
| Choose appropriate foods to satisfy hunger | 0 | 1 (9) | 3 (27) | 4 (36) | 3 (27) |
| Prepare variety of fruits and vegetables | 0 | 2 (18) | 1 (9) | 5 (45) | 3 (27) |
| Provide meat as a side dish instead of an entree | 0 | 1 (9) | 4 (36) | 2 (18) | 4 (36) |
| Ask for help from family and friends when needed | 1 (9) | 1 (9) | 1 (9) | 3 (27) | 5 (45) |
| Limit protein foods in the diet/follow restriction | 0 | 0 | 1 (9) | 6 (55) | 4 (36) |
| Limit sodium in meals | 0 | 1 (9) | 1 (9) | 2 (18) | 7 (64) |
| Prepare high fruit/vegetable point meals | 0 | 1 (9) | 1 (9) | 4 (36) | 5 (45) |
| Prepare recipes that incorporated fruits/vegetables | 0 | 0 | 0 | 7 (64) | 4 (36) |
| Educate others about high fruit/vegetable point foods | 0 | 2 (18) | 1 (9) | 5 (45) | 3 (27) |
| Drink prescribed fluids | 0 | 1 (9) | 2 (18) | 2 (18) | 6 (55) |
| Eat prescribed fruits/vegetables points | 0 | 1 (9) | 2 (18) | 2 (18) | 6 (55) |
n (%) (all such values). ADPKD, autosomal dominant polycystic kidney disease.
3.5.2. Uplifts
Of the 11 participants, five experienced uplifts such as cooking for someone else, and meeting family and one’s own responsibilities whereas six did not (Table 6).
3.5.3. Self-efficacy
Ten out of 11 individuals felt “somewhat confident” or “very confident” that they could manage the new diet (Table 6).
4. Discussion
The relatively complex diet prescription used in this trial was designed to mitigate risk factors that have recently been shown to associate with the development of large cystic kidneys that progress at accelerated rates to renal failure. None of the individual components in the diet is unique; however, the limitation of sodium and animal-sourced protein intake together with the increased ingestion of fruits, vegetables and water has not been evaluated previously in patients with ADPKD. Poor compliance with single component dietary regimens, e.g. sodium, is relatively high in the population at large, so it would be reasonable to suppose that adding more elements to the prescription would further undermine meaningful acceptance. In this respect, ADPKD patients differ from the usual. Many watched a parent or a sibling proceed along the path to kidney failure, dialysis and renal transplantation and appear to be primed to follow medical regimens that may slow the course of the disease. We were pleased to see, with the exception of one person, how well the participants in this study mastered the complexities of diet preparation and record keeping with only modest stress based on their responses to the hassles questionnaire.
Dietary sodium intake has been extensively evaluated in hypertensive disorders and limitations in the diet to a varying degree have been shown to have an important role in bringing blood pressure into the normal range [35,36]. The hypertension commonly found in ADPKD has an extracellular fluid volume-dependent component involving sodium retention as well as increased renin release secondary to the effects of cysts on renal arterioles. In the current study, the reduction in sodium intake was associated with a tendency for systolic blood pressure to decrease, although remaining in the normal range. For reasons that are just now being unraveled, high sodium intake is associated with larger kidneys and a faster decline in renal function in ADPKD making sodium a lifestyle factor worthy of strict control [6,37].
The intake of animal-sourced protein has also been shown to affect cyst growth [38]. In the Consortium for Radiologic Imaging of PKD (CRISP) study, ADPKD patients who excreted the largest amounts of urea, a surrogate for protein intake, had larger kidneys that progressed more rapidly to renal failure [6,38]. The mechanisms that render increased animal-sourced protein intake harmful to polycystic kidneys are unknown. In the current study, animal-sourced protein intake was reduced but not to extreme levels. To do otherwise would likely defeat overall compliance and put patients at risk of protein-energy wasting.
Normal kidneys buffer proton excretion with ammonium and phosphate, keeping the concentration of hydrogen ions in plasma and urine relatively low and less likely to injure nephrons. Increased acid excretion accelerated the progression of ADPKD in an animal model of the disorder and the administration of alkali reversed the downward trend [5,8]. Recent studies of chronic progressive renal insufficiency in patients with hypertensive nephropathy approaching the end-stage of the disease have exposed the harmful effects of hydrogen ion excretion in patients eating regular diets containing abundant acid precursors [9,39]. These findings were recently validated in a nationally representative cohort of individuals with chronic kidney disease showing that individuals with higher dietary acid intake progress to kidney failure more rapidly than their low dietary acid consuming peers [14]. The mechanisms by which hydrogen ions stimulate renal cyst growth are unknown; however, in light of these studies it seems rational to reduce the amount of dietary acids precursors. In the current study, we show that patients with ADPKD will modify the intake of fruits, vegetables, and animal-sourced protein sufficient to decrease the excretion of protons in the urine for at least four weeks. NEAP was significantly reduced in the diet and NAE was significantly reduced in the urine, corresponding metrics confirming that the diet prescription met the goal of reducing the excretion of protons (Tables 3 and 4). In view of the excellent recovery of other dietary components, we cannot account for finding the change in NAE (−20.5 mEq) only 36% as great as NEAP (−57.5 mEq/d). Interestingly, similar discrepancies between NEAP and NAE have previously been found in clinical trials even when urine collections were performed in a clinical trials unit [9,15]. The discrepancy between NEAP and NAE may reside in the estimate of endogenous organic acid (OA) contributions to NEAP. As proposed by Remer et al., a greater percentage error may occur in diets with a considerable intake of fruit since they contain certain acids that are incompletely metabolized which are not taken into account in the estimation of OA [23].
AVP is a major extrinsic factor that influences cyst growth in PKD [31,40]. An inhibitor targeting the AVP-V2 receptor slowed disease progression in patients with ADPKD [41]. Studies in an animal model of PKD lead us to think that reducing plasma AVP levels by increasing the intake of water might have a similar effect on cyst growth [16]. Urine osmolality levels exceeding plasma values (~285 mosm/kg H2O) indicate that circulating levels of AVP are high enough to increase water reabsorption in collecting ducts. In the current study, baseline mean 24-h urine osmolality in ADPKD patients ranged from 170 to 480 mosm/kg H2O; values determined throughout the day or night could be higher or lower than the 24-h mean value, but in general they run above plasma levels in the vast majority of patients [6,42]. All of the subjects reduced urine osmolality below their individual baseline values and in 91% osmolality was reduced below that of plasma, changes that would certainly be associated with lowered levels of plasma AVP perfusing renal tubules and cysts. Achieving an average 24-h osmolality goal of 285 mosm/kg H2O seems reasonable in the short term until formal clinical trials produce results indicating that lower levels of urine osmolality are more protective and can be achieved without harm.
The current study illustrates that in most patients with ADPKD good to excellent compliance can be expected when modest limitations of salt and protein are combined with an increased intake of fruits, vegetables, and fluids. Low sodium, low protein, and weight loss diets are notoriously difficult to maintain over the long term and the increased water drinking prescription to reduce urinary stone formation and shedding is commonly ignored [43–46]. To counter these tendencies, in the current study we offered a palatable diet, relatively simple to prepare, coupled with intense counseling at the outset. One of the 12 enrollees attended all of the sessions but was unable to record dietary information or collect urine samples and we excluded the subject from the final analysis. In the remaining 11 subjects, >90% reported that none of the dietary hassles were worse than “somewhat severe”, the lowest form of severity on the questionnaire, and the majority of patients felt at least “somewhat confident” in their knowledge and abilities to follow the diet. We think a treatment plan like this that frequently reinforces the importance of the diet as long-term protection from kidney harm would likely gain acceptance by most patients with ADPKD given the seriousness of the condition at the end stage.
Limitations in this study include the small sample size and short duration dictated by funding constraints, and a lack of kidney function markers that could monitor renal damage and changes in function over such a short interval of time. Despite these concerns we thought a pilot study of this relatively complex prescription was needed to determine if it was reasonable to proceed to a more expansive and expensive clinical trial of sufficient length to establish efficacy in respect to disease progression. We think the results support the design and execution of a larger scale controlled clinical trial to determine if modifying dietary components will slow the progression of ADPKD. Strengths in this study include being the first to address the utility of attacking multiple dietary targets in ADPKD in conjunction with multiple 24-h urine collections to verify diet records in an outpatient setting.
In conclusion, the experimental diet tested successfully in this study targets factors known to accelerate the progression of ADPKD. Based on the urinary verification of dietary limits and the good acceptance by the participants, we think this new PKD diet will help to justify a controlled clinical trial to determine the extent to which dietary modifications may ameliorate hypertension, renal pain, hematuria and the progression of biomarkers of renal dysfunction in children and adults.
Acknowledgments
The investigators thank the study participants for their valuable contributions to the study and Brenda Magenheimer for her assistance in training staff to perform the appropriate lab analyses.
Funding source
This work was supported by the Frontiers Pilot and Collaborative Studies Funding Program and the University of Kansas Medical Center Research Institute Clinical Pilots Program (JG, JMHR, DKS), the Abbott Nutrition Award funded through the Academy of Nutrition and Dietetics (JMT), and the University of Kansas Medical Center Kidney Institute’s Research and Development fund (JJG). Support for J. Hamilton-Reeves was provided by KL2 training grant KL2TR000119 a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research (JMHR). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. The sponsors were not involved in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Abbreviations
- ADPKD
autosomal dominant polycystic kidney disease
- AI
adequate intake
- AMDR
acceptable macronutrient dietary range
- AVP
arginine vasopressin
- BMI
body mass index
- BUN
blood urea nitrogen
- CRISP
consortium for radiologic imaging of polycystic kidney disease
- DRI
dietary recommended intake
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- NAE
net acid excretion
- NE
Not established
- NEAP
net endogenous acid production
- NDSR
Nutrition Data for System Research
- NHQ
Nutrition Hassles Questionnaire
- OA
organic acids
- PKD
polycystic kidney disease
Footnotes
Statement of authorship
JMT, JMHR, DKS, CAG, SEC, DEW, and JJG designed the research; JMT, CC, and JJG conducted the research; JMT and JJG performed statistical analyses; JMT, JMHR, DKS, CAG, SEC, DEW, and JJG wrote the manuscript; JMT and JJG had primary responsibility for final content. All authors read and approved the final manuscript.
Disclaimers
No disclaimers to be listed.
Conflict of interest
The authors have no potential conflicts of interest.
References
- 1.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477–85. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 2.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241:F85–93. doi: 10.1152/ajprenal.1981.241.1.F85. [DOI] [PubMed] [Google Scholar]
- 3.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, et al. Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2012;7:479–86. doi: 10.2215/CJN.09500911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–98. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 5.Cowley BD, Jr, Grantham JJ, Muessel MJ, Kraybill AL, Gattone VH., 2nd Modification of disease progression in rats with inherited polycystic kidney disease. Am J Kidney Dis. 1996;27:865–79. doi: 10.1016/s0272-6386(96)90525-9. [DOI] [PubMed] [Google Scholar]
- 6.Torres VE, Grantham JJ, Chapman AB, Mrug M, Bae KT, King BF, Jr, et al. Potentially modifiable factors affecting the progression of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2011;6:640–7. doi: 10.2215/CJN.03250410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee T, Crews DC, Wesson DE, Tilea A, Saran R, Rios Burrows N, et al. Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol. 2014;15:137. doi: 10.1186/1471-2369-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanner GA, Tanner JA. Citrate therapy for polycystic kidney disease in rats. Kidney Int. 2000;58:1859–69. doi: 10.1111/j.1523-1755.2000.00357.x. [DOI] [PubMed] [Google Scholar]
- 9.Goraya N, Simoni J, Jo C, Wesson DE. Dietary acid reduction with fruits and vegetables or bicarbonate attenuates kidney injury in patients with a moderately reduced glomerular filtration rate due to hypertensive nephropathy. Kidney Int. 2012;81:86–93. doi: 10.1038/ki.2011.313. [DOI] [PubMed] [Google Scholar]
- 10.Goraya N, Wesson DE. Acid-base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens. 2012;21:552–6. doi: 10.1097/MNH.0b013e328356233b. [DOI] [PubMed] [Google Scholar]
- 11.Goraya N, Wesson DE. Is dietary acid a modifiable risk factor for nephropathy progression? Am J Nephrol. 2014;39:142–4. doi: 10.1159/000358602. [DOI] [PubMed] [Google Scholar]
- 12.Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010;78:1128–35. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
- 13.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010;77:617–23. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee T, Crews DC, Wesson DE, Tilea AM, Saran R, Rios-Burrows N, et al. High dietary acid load predicts ESRD among adults with CKD. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remer T, Manz F. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 1994;59:1356–61. doi: 10.1093/ajcn/59.6.1356. [DOI] [PubMed] [Google Scholar]
- 16.Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. J Am Soc Nephrol. 2006;17:2220–7. doi: 10.1681/ASN.2006030251. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.SF S. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products- A research perspective. J Food Comp Anal. 2001;14:315–22. [Google Scholar]
- 19.Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Chan JC. The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem. 1972;5:94–8. doi: 10.1016/s0009-9120(72)80014-6. [DOI] [PubMed] [Google Scholar]
- 21.Remer T, Dimitriou T, Manz F. Dietary potential renal acid load and renal net acid excretion in healthy, free-living children and adolescents. Am J Clin Nutr. 2003;77:1255–60. doi: 10.1093/ajcn/77.5.1255. [DOI] [PubMed] [Google Scholar]
- 22.Frassetto LA, Lanham-New SA, Macdonald HM, Remer T, Sebastian A, Tucker KL, et al. Standardizing terminology for estimating the diet-dependent net acid load to the metabolic system. J Nutr. 2007;137:1491–2. doi: 10.1093/jn/137.6.1491. [DOI] [PubMed] [Google Scholar]
- 23.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95:791–7. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 24.Berkemeyer S, Remer T. Anthropometrics provide a better estimate of urinary organic acid anion excretion than a dietary mineral intake-based estimate in children, adolescents, and young adults. J Nutr. 2006;136:1203–8. doi: 10.1093/jn/136.5.1203. [DOI] [PubMed] [Google Scholar]
- 25.Wang CJ, Creed C, Winklhofer FT, Grantham JJ. Water prescription in autosomal dominant polycystic kidney disease: a pilot study. Clin J Am Soc Nephrol. 2011;6:192–7. doi: 10.2215/CJN.03950510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Heart Association. Shaking the Salt Habit. (Accessed February 2013 at http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/PreventionTreatmentofHighBloodPressure/Shaking-the-Salt-Habit_UCM_303241_Article.jsp).
- 27.Hatton DC, Haynes RB, Oparil S, Kris-Etherton P, Pi-Sunyer FX, Resnick LM, et al. Improved quality of life in patients with generalized cardiovascular metabolic disease on a prepared diet. Am J Clin Nutr. 1996;64:935–43. doi: 10.1093/ajcn/64.6.935. [DOI] [PubMed] [Google Scholar]
- 28.IBM Corp. Released. IBM SPSS statistics for Windows. Version 19.0. Armonk, NY: IBM Corp; 2010. [Google Scholar]
- 29.Wiegmann TB, Zlomke AM, MacDougall ML, Kipp DE. Controlled changes in chronic dietary protein intake do not change glomerular filtration rate. Am J Kidney Dis. 1990;15:147–54. doi: 10.1016/s0272-6386(12)80512-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang CJ, Grantham JJ, Wetmore JB. The medicinal use of water in renal disease. Kidney Int. 2013;84:45–53. doi: 10.1038/ki.2013.23. [DOI] [PubMed] [Google Scholar]
- 31.Grantham JJ. Rationale for early treatment of polycystic kidney disease. Pediatr Nephrol. 2014 doi: 10.1007/s00467-014-2882-8. [DOI] [PubMed] [Google Scholar]
- 32.Torres VE, Harris PC. Strategies targeting cAMP signaling in the treatment of polycystic kidney disease. J Am Soc Nephrol. 2014;25:18–32. doi: 10.1681/ASN.2013040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clin J Am Soc Nephrol. 2009;4:1140–50. doi: 10.2215/CJN.00790209. [DOI] [PubMed] [Google Scholar]
- 34.Hu JF, Zhao XH, Parpia B, Campbell TC. Dietary intakes and urinary excretion of calcium and acids: a cross-sectional study of women in China. Am J Clin Nutr. 1993;58:398–406. doi: 10.1093/ajcn/58.3.398. [DOI] [PubMed] [Google Scholar]
- 35.Ecder T, Edelstein CL, Fick-Brosnahan GM, Johnson AM, Duley IT, Gabow PA, et al. Progress in blood pressure control in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36:266–71. doi: 10.1053/ajkd.2000.8970. [DOI] [PubMed] [Google Scholar]
- 36.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, et al. Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371:2255–66. doi: 10.1056/NEJMoa1402685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He FJ, MacGregor GA. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertens. 2009;23:363–84. doi: 10.1038/jhh.2008.144. [DOI] [PubMed] [Google Scholar]
- 38.Ogborn MR, Sareen S. Amelioration of polycystic kidney disease by modification of dietary protein intake in the rat. J Am Soc Nephrol. 1995;6:1649–54. doi: 10.1681/ASN.V661649. [DOI] [PubMed] [Google Scholar]
- 39.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010;78:303–9. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Wu Y, Ward CJ, Harris PC, Torres VE. Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol. 2008;19:102–8. doi: 10.1681/ASN.2007060688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gattone VH, 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med. 2003;9:1323–6. doi: 10.1038/nm935. [DOI] [PubMed] [Google Scholar]
- 42.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, et al. Tolvaptan in autosomal dominant polycystic kidney disease: three years’ experience. Clin J Am Soc Nephrol. 2011;6:2499–507. doi: 10.2215/CJN.03530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ortner Hadziabdic M, Mucalo I, Hrabac P, Matic T, Rahelic D, Bozikov V. Factors predictive of drop-out and weight loss success in weight management of obese patients. J Hum Nutr Diet. 2015;28(Suppl. 2):24–32. doi: 10.1111/jhn.12270. [DOI] [PubMed] [Google Scholar]
- 44.McCauley LR, Dyer AJ, Stern K, Hicks T, Nguyen MM. Factors influencing fluid intake behavior among kidney stone formers. J Urol. 2012;187:1282–6. doi: 10.1016/j.juro.2011.11.111. [DOI] [PubMed] [Google Scholar]
- 45.Cianciaruso B, Pota A, Pisani A, Torraca S, Annecchini R, Lombardi P, et al. Metabolic effects of two low protein diets in chronic kidney disease stage 4–5–a randomized controlled trial. Nephrol Dial Transpl. 2008;23:636–44. doi: 10.1093/ndt/gfm576. [DOI] [PubMed] [Google Scholar]
- 46.McMahon EJ, Campbell KL, Mudge DW, Bauer JD. Achieving salt restriction in chronic kidney disease. Int J Nephrol. 2012;2012:720429. doi: 10.1155/2012/720429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masud T, Manatunga A, Cotsonis G, Mitch WE. The precision of estimating protein intake of patients with chronic renal failure. Kidney Int. 2002;62:1750–6. doi: 10.1046/j.1523-1755.2002.00606.x. [DOI] [PubMed] [Google Scholar]


