Abstract
In rodents and humans, testicular cells, similar to other mammalian cells, are supported by actin-, microtubule (MT)- and intermediate filament-based cytoskeletons to regulate spermatogenesis during the epithelial cycle. However, most of the published findings in the literature are limited to studies that visualize these cytoskeletons in the seminiferous epithelium during spermatogenesis. Few are focus on the underlying molecular mechanism that regulates their organization in the epithelium in response to changes in the stages of the epithelial cycle remains largely explored. Functional studies in the last decade have begun to focus on the role of binding proteins that regulate these cytoskeletons, and some interesting data have been rapidly emerging in the field. Since the actin- and intermediate-based cytoskeletons have been recently reviewed, herein we focus on the MT-based cytoskeleton for two reasons. First, besides serving as a structural support cytoskeleton, MT is known to serve as the track to support and facilitate the transport of germ cells, such as preleptotene spermatocytes connected in clones and elongating/elongated spermatids during spermiogenesis across the blood-testis barrier (BTB) and the adluminal compartment, respectively, during spermatogenesis. While these cellular events are crucial to the completion of spermatogenesis, they have been largely ignored in the past. Second, MT-based cytoskeleton is working in concert with the actin-based cytoskeleton to provide structural support to the transport of intracellular organelles across the cell cytosol, such as endosome-based vesicles, and residual bodies, phagosomes in Sertoli cells, to maintain the cellular homeostasis in the seminiferous epithelium. We critically evaluate some recent published findings herein to support a hypothesis regarding the role of MT in conferring germ cell transport in the seminiferous epithelium.
Keywords: testis, spermatogenesis, seminiferous epithelial cycle, Sertoli cells, germ cells, meiosis, spermiogenesis, seminiferous tubule, germ cell transport
Introduction
Spermatogenesis is the biological process through which undifferentiated germ cells develop into haploid sperm. It takes place in the seminiferous epithelium of the seminiferous tubules which are the functional unit in the mammalian testis that produces an upward of 200 million spermatozoa per day in a human male from puberty at ~12 years of age [1, 2]. This enormous cellular output thus illustrates there are extensive but tightly coordinated cellular events that take place in the seminiferous tubules. The seminiferous epithelium is physically divided into two compartments, basal and adluminal (apical), by the blood-testis-barrier (BTB). The BTB is one of the many blood-tissue barriers in the mammalian body that creates an exclusive microenvironment in the seminiferous epithelium, namely the adluminal compartment wherein meiosis I/II and post-meiotic spermatid development take place, segregating these events from systemic circulation [3]. Undifferentiated spermatogonia, and differentiated spermatogonia, such as type A and type B, reside in the basal compartment. This is where type B spermatogonia differentiate into preleptotene spermatocytes, which are the germ cells that must be transported across the BTB at stage VII–VIII of the epithelial cycle in rodents to enter the adluminal compartment to further differentiate and develop into late stage spermatocytes to prepare for meiosis I/II during spermatogenesis [4]. Since germ cells are immotile cells, lacking locomotive apparatus such as filopodia and lamellipodia found in fibroblasts and macrophages, they cannot independently move across the seminiferous epithelium, and rely solely on the Sertoli cell for their transport across the BTB and the adluminal compartment to prepare for their eventual release into the tubule lumen during spermiation.
The seminiferous epithelium is comprised of only two cell types, Sertoli cells and germ cells, unless infiltrated by macrophages in the basal and/or adluminal compartment of the seminiferous tubules during pathological conditions such as inflammation induced by orchitis [5,6] or following exposure to environmental toxicants (e.g., MEHP) [7]. Due to the unique association between Sertoli cells and germ cells at specific stages of their development in the seminiferous epithelium during spermatogenesis, the epithelium can be divided into stages I–XII, I–XIV, and I–VI in the mouse, rat and human testis respectively, known as the epithelial cycle of spermatogenesis [2, 8, 9]. The epithelial cycle of the mouse testis consists of stages I–XII and is shown in Figure 1 [10]. As noted in Figure 1, the germ cell types that associate with the Sertoli cell in each stage are different. Sertoli cells, also known as nurse cells, are in continuous contact with germ cells, providing structural and nutritional support throughout the seminiferous epithelial cycle of spermatogenesis [11]. When cultured in vitro, Sertoli cells are motile cells, as they are capable of traversing the nitrocellulose or polyester filters of transwell bicameral units [12], analogous to fibroblasts, macrophages and metastatic tumor cells. However, Sertoli cells in vivo are relatively static and polarized as evidenced by the location of the nuclei close to the basement membrane throughout the epithelial cycle. During the cycle, each Sertoli cell remains in close contact with ~30–50 germ cells [13] by sending its cytoplasmic processes to engulf germ cells at different stages of their development across the entire seminiferous epithelium so that these cells remain in close contacts with one another. The Sertoli cell is able to provide germ cells structural and nutritional support via an extensive and dynamic cytoskeletal network [14]. Similar to other mammalian cell types, Sertoli cells have a cytoskeletal network made up of actin microfilaments, intermediate filaments, and microtubules. While the actin-based cytoskeleton in the seminiferous epithelium is the best studied structural component, the molecular mechanism(s) by which actin microfilaments is regulated to confer their plasticity remains unknown, and how the actin network interacts with microtubules and intermediate filaments to confer cytoskeletal dynamics during the epithelial cycle remains virtually unexplored.
Figure 1. The seminiferous epithelial cycle of spermatogenesis in the mouse testis.
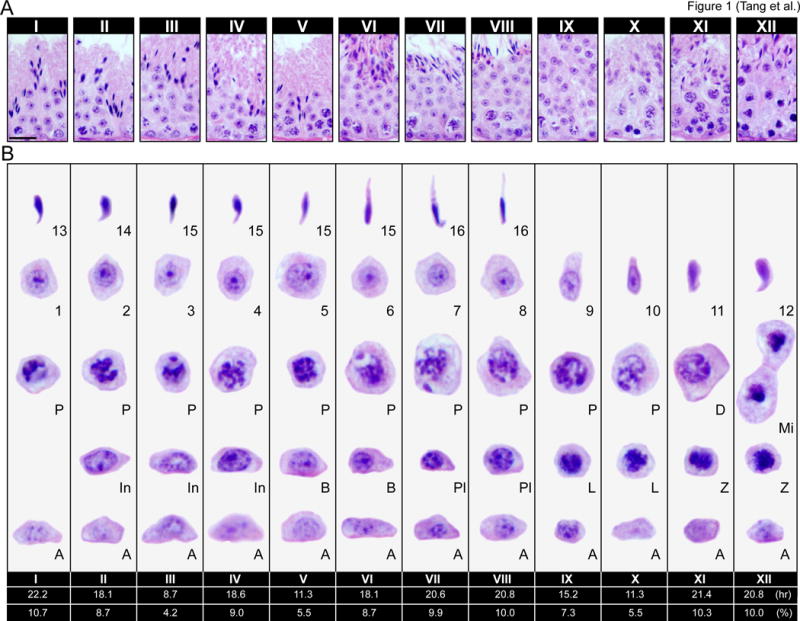
(A) Normal mouse testis cross-sections were stained with hematoxylin and eosin (H&E) to visualize stages I–XII of the seminiferous epithelial cycle. Each stage depicts the unique cellular associations between germ cells and Sertoli cell as spermatogonial stem cells progressively develop into spermatids. Scale bar, 60 μm, which applies to other micrographs in the same panel. Each tubule has 4–5 layers of different germ cell types which are shown in (B) for each typical stage of the epithelial cycle. The duration (in hr) and frequency (in %) of each stage in a single spermatogenic (or epithelial) cycle (completion of stages I–XII in ascending order) is listed below the respective stages as earlier reported [10]. Rodents undergo the spermatogenic cycle 4.5 times to complete spermatogenesis which refers to the development of haploid sperm (i.e., fully developed elongated spermaitds) from undifferentiated spermatogonia; and so, the duration of spermatogenesis in the mouse is ~40 days [8, 10, 129] and one epithelial (or spermatogenic cycle) is ~8.6 day (i.e., ~207 hr). Type A spermatogonia (A), intermediate spermatogonia (In), and type B (B) spermatogonia all undergo mitosis. Type B spermatogonia undergo mitosis to form preleptotene [72] spermatocyctes which are the only germ cells to be transported across the BTB. Preleptotene spermatocytes progressively develop into diplotene (D) spermatocytes, which enter diakinesis of meiosis I [8], once secondary spermatocytes form, they quickly enter meiosis II to generate haploid spermatids. Spermatogonia (type A; In, Intermediate; type B); spermatocytes (Pl, preleptotene; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; Mi, meiotic division); round spermatids (1–8); elongate spermatids (9–16).
There are two conditions that are necessary to support germ cell development, namely structural and nutritional support of germ cells by the Sertoli cell, and the presence of a functional BTB to create a unique microenvironment in the adluminal compartment to allow the occurrence of meiosis I/II and the subsequent post-meiotic spermatid development. In the testis, the ectoplasmic specialization (ES) is a unique actin-rich anchoring junction found in between the Sertoli-Sertoli cell interface (called the basal ES) at the BTB, and in between the Sertolispermatid (steps 8–16 or steps 8–19 spermatids in the mouse or rat testes) interface (called the apical ES) [15]. The ES is comprised of F-actin bundles in between the Sertoli cell plasma membrane and cisternae of the endoplasmic reticulum. Unlike most other mammalian cell junctions, the ES is not a static structure as it undergoes dynamic changes to accommodate spermatid movement across the seminiferous epithelium. F-actin dynamics at the ES has been extensively studied, and to date a host of actin regulatory proteins has been uncovered which are implicated in regulating ES dynamics [16]. Despite these findings, it is still unclear how germ cells are physically transported across the epithelium. It has been proposed that F-actin at the apical ES serves as the vehicle to transport germ cells [17]. Earlier studies on the microtubule cytoskeleton in the seminiferous epithelium propose that microtubules serve as the tracks for germ cells to be transported and involving microtubule-specific motor proteins such as kinesin [18–21], analogous to the function of microtubules to serve as the tracks in other mammalian epithelia [22]. Herein, we discuss the roles of the microtubule cytoskeleton in germ cell development and transport. We also introduce new concepts which may serve as a basis for future studies.
Structure and function of microtubules (MT)
Microtubules (MTs) are dynamic polymers composed of protofilaments of α- and β-tubulin [23]. A single protofilament is made up of α- and β-tubulin heterodimers arranged in a head to tail manner, leading to the intrinsically polar nature of MTs, with α-tubulin located at what is designated the minus end and β-tubulin at the plus end (Figure 2A, B) [24, 25]. While polymerization of MTs can occur at both ends, the rate of growth differs in which the plus end is the site of fast growth whereas the minus end is the site of slow growth. A major reason why slow growth occurs at the minus end is because it is the end that anchors the MT, namely in the microtubule organizing center (MTOC) [26]. The centrosome is the main MTOC found in the cell and is localized near the nucleus. MTs grow out from the centrosome towards the plasma membrane; and in non-dividing cells, MT distribution in the cytoplasm radiates out from the centrosome providing an organized MT-based network for the cell [27].
Figure 2. Microtubule dynamics.
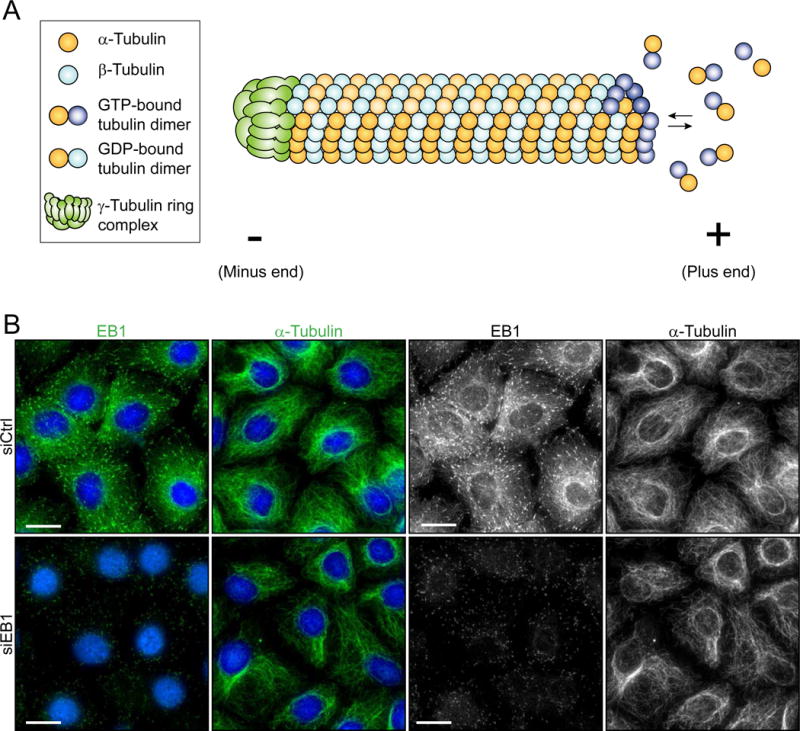
(A) Microtubules are essential for proper cellular function. This diagram depicts a side-view of a MT. MTs are comprised of protofilaments, which are polymers of α/β-tubulin heterodimers arranged in a head-to-tail manner. MTs are intrinsically polar and have 2 ends which are designated plus (fast growing) and minus (slow growing) end. The γ-tubulin ring complex is located at the minus end and it is responsible for catalyzing MT nucleation [26]. Though polymerization can occur at both ends, the rate of polymerization at the plus end exceeds that of the minus end. GTP-bound tubulin heterodimers are added to the growing end of a MT. When additional GTP-bound tubulin heterodimers are added, previously incorporated GTP-bound β-tubulin is hydrolyzed to GDP-β-tubulin. (B) MT dynamics is regulated by MT associated proteins (MAPs) such as EB1. Cultured Sertoli cells were treated with siRNA duplexes targeting EB1 (siEB1) versus non-targeting negative control siRNA duplexes (siCtrl) that serve as the corresponding control. Normal EB1 (top left panel) and α-tubulin (top second panel) (green fluorescence or grayscale – micrographs in grayscale are the same images as the green fluorescence images to better depict changes in EB1 and α-tubulin organization in these Sertoli cells) distribution throughout Sertoli cells is seen in cells treated with non-targeting control siRNA (siCtrl). However, after EB1 knockdown (siEB1) as visualized by diminished fluorescence (see left top panel vs. left bottom panel), α-tubulin organization in the Sertoli cells was disrupted (second panel). For instance, MTs were found to be retracted from the cell cortex and localized more intensely around the cell nucleus. These results show that EB1 is necessary for normal MT organization and dynamics in the Sertoli cell. Scale bar, 25 μm, which applies to the other image of the same panel.
A unique property of MTs is dynamic instability, which is a term that describes their polymerization and depolymerization behavior whereby they can rapidly alternate between phases of growth and shrinkage [28, 29]. The α- and β-tubulins in a heterodimer are each bound to a GTP (Figure 2A). The GTP bound to β-tubulin can be hydrolyzed to GDP as additional GTP bound dimers are added to a growing MT (Figure 2A) [25]. However, depending on the dynamic state of the MT it is possible that the rate of GTP hydroysis may lag behind the rate at which GTP bound tubulin is added to the growing end. Thus resulting in an accumulation of GTP bound tubulin at the growing end, which is referred to as the GTP-tubulin cap. This cap functions to protect the growing end from immediate depolymerization, since GDP tubulin readily dissociates [30, 31]. When a MT depolymerizes, its rate of hydrolysis supersedes the rate of GTP tubulin addition to the growing end; this switch from growth to shrinkage is called catastrophe [28]. Tightly controlled regulation of dynamic MTs is required for numerous processes in the cell. For example, MTs are essential for structural support of the cell, vesicle and organelle transport, chromosome segregation, intracellular trafficking, cell motility, and cell polarity [32]. Additionally MT dysfunction/dysregulation is involved in human diseases, such as cancer and neurodegenerative disorders like Alzheimer’s disease, and can potentially lead to cell death [33, 34].
Regulation of microtubules
Microtubules are dynamic structures involved in a diverse array of cellular functions and must be tightly regulated for proper function in the cell such as MTs found in Sertoli cells (Figure 3). One way MTs are regulated is via posttranslational modifications (PTMs), which are referred to as changes in the functional property of a protein. Generally, PTMs are modifications that occur on MTs, after polymerization, and rarely on free tubulin monomers [35]. Some of the most common MT PTMs are: acetylation (a marker of MT stability/age), phosphorylation (regulation of polymerization), and tyrosination/detyrosination (a marker of dynamic state of MTs) [36, 37]. Another way they can be regulated is through MT regulatory proteins such as microtubule-associate proteins (MAPs) and plus-end tracking proteins (+TIPs), as well as motor proteins [38].
Figure 3. Basal ES and microtubules in cultured Sertoli cells with an established functional TJ-permeability barrier.
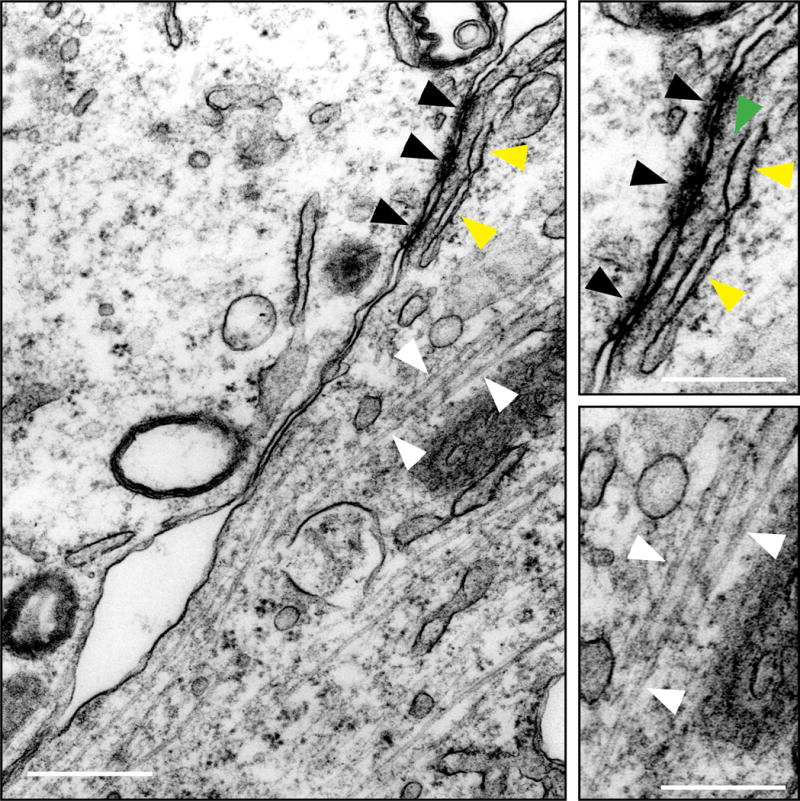
This is an electron micrograph showing the network of microtubules (MTs) near the interface between 2 Sertoli cells when these cells were cultured in vitro for 2 days with an established TJ-permeability barrier and prepared for electron microscopy. Black arrowheads illustrate the tight junctions (TJs), and the “kisses” typical of TJs are noted, whereas the yellow arrowheads illustrate the endoplasmic reticulum (ER) and some actin microfilament bundles (annotated by the green arrowhead that appear as dot-like structures because actin microfilaments lie perpendicular to the Sertoli cell plasma membrane) which are the basic components of the basal ES. Both the TJ and basal ES are magnified and shown on the top right panel as an inset. Microtubules (MTs) are also present near the Sertoli cell-cell interface as indicated by white arrowheads, which are also magnified and shown on the lower right panel as an inset. Scale bar, 1 μm; 0.5 μm in inset.
End-binding protein 1 (EB1)
EB1 is a highly conserved MT plus-end tracking protein (+TIP) (Figure 2B) known to regulate plus-end dynamics during cellular processes such as, mitosis and intracellular transport [39]. This ~30 kDa protein is one of 3 members of the EB family, EB2 and EB3 being the other members and was first discovered in a yeast two-hybrid system as an interacting protein of adenomatous polyposis coli (APC) protein, a product of the APC gene which when altered is involved in colorectal tumorigenesis [40–42]. EB1, like EB2 and EB3, is a dimeric protein containing two monomers that are dimerized via their coiled-coil domains at the C-terminus [43], and each polypeptide contains a calponin homology (CH) domain at the N-terminus conferring its ability to bind to MTs [43, 44]. Interestingly, calponin, which is a calcium binding protein, and the associated CH domain found in many proteins, are known to interact with actin and not MTs; EB1 was the first example of a protein containing a CH domain responsible for its binding to MTs [45, 46]. EB1 is one of the most widely studied +TIPs since it is a functionally diverse regulatory protein. As a +TIP, EB1 is predominantly localized to the plus-end of MTs, but can also be found along the length of MTs and at the centrosome [47]. Its location at the distal ends of MTs positions it at an ideal location to communicate at the cell cortex and interact with other regulatory proteins. In the testis, EB1 colocalizes with seminiferous epithelial microtubules and is found at both the apical and basal ES. A recent study using a Sertoli cell in vitro system revealed that EB1 plays a critical role in regulation of not only MT dynamics, but also actin dynamics in Sertoli cells [48], suggesting that EB1 may be a protein that induces cross-talk and coordination between actin- and MT-based cytoskeletons in the testis. In this study, EB1 was silenced through the use of siRNA, causing BTB disruption, decrease in MT polymerization, and disorganization of MT and actin microfilament cytoskeletons [48]. Sertoli cells treated with EB1 siRNA exhibited a MT distribution different from control cells (Figure 2B). Unlike in control cells, where MTs are distributed throughout the cytosol extending to the cell periphery, MTs of EB1 knockdown cells retracted from the cell cortex/edge and preferentially surrounded the cell nuclei. This observation suggests that EB1 is required for proper MT distribution in the Sertoli cell. In vivo, Sertoli cells are in continuous contact with differentiating germ cells (GC), and must therefore be able to undergo changes in its shape to adapt to changes in GC throughout the epithelial cycle. MTs are dynamic polymers, which in part are responsible for changes in Sertoli cell morphology. In addition to providing structural support for the cell, MTs also serve roles in intracellular trafficking. Since knockdown of EB1 caused MTs to retract from the cell edge, it is likely that the knockdown of EB1 would affect delivery of essential nutrients and other regulatory proteins that are required to maintain cell function at the cell cortex. This possibility is indeed supported by the observation that a knockdown of EB1 in Sertoli cell epithelium with a functional TJ-barrier led to internalization of TJ- (e.g., CAR, ZO-1) and basal ES- (e.g., N-cadherin, ß-catenin) proteins from the cell-cell interface into the cell cytosol [48].
Interestingly, loss of EB1 in Sertoli cells also led to changes in F-actin dynamics (Figure 2B). Knockdown caused alteration in F-actin organization as actin microfilaments were no longer found distributed across the cell cytosol and became truncated [48]. Distribution of adhesion proteins that use actin microfilaments for attachment, such as CAR and N-cadherin were mislocalized at the Sertoli cell-cell interface as noted above, this thus further de-stabilized the Sertoli cell TJ-barrier, leading to its disruption [48]. These results suggest that EB1 plays a critical role in the regulation of Sertoli cell dynamics via both the MT and actin networks, confirming findings in other epithelia that EB1 serves as a point of crosstalk between the two cytoskeletons [39, 49]. Based on these findings, it is likely that EB1 plays a major role in regulating spermatogenesis, however further studies using an in vivo approach are necessary to further elucidate the role of EB1.
Formins
Formins are a family of proteins that promote actin polymerization, enabling the cell to carry out a variety of cellular functions that depend on a dynamic cytoskeletal network. This family of proteins is a diverse one as eukaryotes have numerous formin genes; for example, mammalian formins alone are encoded by 15 different genes [50, 51]. Formins are large dimeric multi-domain proteins, and are characterized by their formin homology 1 and 2 (FH1 and FH2) domains located at the C-terminus, both of which interact with actin [51]. The FH2 domain is the most conserved formin domain and functions in mediating actin nucleation by binding to the barbed end (fast growing end) of an actin filament [52, 53]. FH2 association with the barbed end of an actin filament promotes the addition of actin subunits, thus resulting in elongation of an actin microfilament [51–53]. In epithelia, formins are involved in cell migration, cytokinesis, endocytosis, morphogenesis, and cell adhesion [54, 55]. A recent study uncovered the function of formin family member, formin 1, in the testis [56]. In this study, formin 1 knockdown was achieved via siRNA both in vitro and in vivo. First, the in vitro model was used to study formin 1 function in the regulation of Sertoli cell dynamics. Results from in vitro silencing of formin 1 revealed that this protein is crucial to maintain actin dynamics as evidenced by decreases in both actin bundling and actin polymerization capability of Sertoli cells. Additionally, in vitro knockdown caused a disruption in the TJ-barrier as basal ES proteins were mislocalized. Based on these findings, an in vivo model was then used to further study formin 1 function in the testis. Knockdown of formin 1 in the testis further confirmed that its absence causes the mislocalization of basal ES proteins, and also, disruption of the F-actin network both at the basal and apical ES. Disruption of F-actin network at the apical ES led to defects in polarity and transport of spermatids with mis-oriented spermatids trapped inside the seminiferous epithelium. This study reveals that formin 1 is necessary for regulation of the BTB and apical ES, as loss of the protein resulted in disruption of BTB, spermatid polarity, and spermiation.
In addition to actin dynamics, formins can also regulate MT dynamics. Dia, the mammalian homolog of the Drosophila gene diaphanous, is a type of formin first discovered as a Rho effector; and studies have shown that it plays numerous roles in the mammalian cells including actin stress fiber formation, regulation of MTs, phagocytosis, formation of adherens junctions, and others [57, 58]. Two Dia isoforms, Dia1 and Dia2, are present in the testis and play a role maintaining structure of both Sertoli and germ cells during spermatogenesis [59]. In other epithelia it has been shown that Dia1 forms a complex with EB1 to promote MT stabilization [60,61]. Since both Dia1 and EB1 are present in the testis, it is conceivable that these two regulatory proteins act similarly to regulate spermatogenesis. Though both Dia1 and EB1 regulate actin and MT networks, their effects on one cytoskeleton may not necessarily depend on the other. For example, it has been shown that MT regulation by Dia is independent of its role in regulation of the actin cytoskeleton [62].
MAP/Microtubule affinity-regulating kinases (MARKs)
MARKs are functionally diverse MT regulating proteins, serving a number of different roles in the cell including: cell polarization, stabilization, cell cycle regulation, intracellular signaling and others [63–65]. There are four MARK isoforms known to date, namely MARK1, 2, 3, and 4, found in mammalian cells displaying similar sequence homology with MARK4 being the least similar [65]. MARKs are Par-1 family proteins and in addition to regulating MT dynamics, they are also implicated in regulation of cell polarity by working in concert with polarity proteins [63, 66, 67]. MARKs were recently reviewed and will not be discussed in detail here [68]. However it is important to note that MARK4 protein in the testis plays a role in the regulation of spermatogenesis [69]. This isoform is present at the BTB and may regulate desomosome at this site since coimmunoprecipitation results revealed that MARK4 structurally interacts with desmosomal armadillo protein plakophillin-2 (PKP2) in the testis [69]. In addition, an overexpression study of MARK4 in human fibroblasts revealed that overexpressed MARK4 co-localized with vimentin, suggesting that MARK4 may also regulate the intermediate filament network [70]. MARK4 is also present at the apical ES and is likely to facilitate protein trafficking events at the apical ES, where endocytic vesicle-mediated ES protein trafficking occurs since MTs are tightly associated with endosomal functions [22]. In addition, it is proposed that MARK4 may also regulate cross communication between the actin filaments and MTs at the apical ES. Recently, MARK4 in Drosophila and mammalian cells has been shown to be a negative regulator of mammalian target of rapamycin complex 1 (mTORC1) [71]. This complex is especially important for the modulation of protein synthesis, and has recently shown to be a regulator of the BTB [72, 73]. As kinases, MARKs are regulated by downstream effectors such as LKB1 (liver kinase B1) which is a Ser/Thr kinase known to regulate cell polarity and junctional complexes [74]. A recent study using a Sertoli cell specific LKB1 mutant mouse model revealed that loss of LKB1 led to defects in spermatogenesis, such as loss of SC polarity and dysregulation of junctional complexes [75]. These defects were attributed to downregulation of MARK and upregulation of mTOR signaling [75]. Collectively, these findings suggest that MARK4 in the testis may also function as a negative regulator of mTORC1 via its effects on F-actin and also MTs at the BTB.
Microtubule-based transport
Microtubule-based transport in neurons is one of the most widely studied intracellular transport mechanisms [22, 76]. This is due to the extensive polarized MT arrays of neuronal axons, which makes neurons a convenient model for studying transport. Sertoli cell MT arrays have been described as resembling neuronal MTs since they are polarized and arranged in dense bundles [77, 78] (Figure 3). Cargo transport in neurons is carried out via motor proteins on both the actin and MT cytoskeletons. Motor proteins are especially important for intracellular transport in neurons due to the unique shape of nerve cells. Neurons consist of three main parts: cell body, dendrites (structures that arise from the cell body), and axon (a long extension of the cell body, reaching up to a meter long in humans) [79, 80]. The majority of the proteins that are essential for neuron function originate from the cell body and thus depend on a highly controlled and differential transport system to ensure proper delivery to often distant and large sites [81]. Dynein and kinesin are the MT motor proteins involved in neuronal transport, and since both are present in the testis, understanding transport mechanisms in neurons and other cell types will aid in uncovering the transport functions of the MT cytoskeleton in the seminiferous epithelium.
Kinesin superfamily proteins (KIFs)
KIFs comprise a group of motor proteins that typically generate plus end directed movement along MTs, powered by ATP hydrolysis [82]. Conventionally, native state KIFs are composed of 2 heavy chains and 2 light chains, totaling in ~360–380 kDa [83, 84]. They have a conserved catalytic motor domain and the location of the motor domain within the molecule determines the preference for movement towards either MT plus or minus end [85, 86]. These enzymes are implicated in a whole range of biological processes, such as transport of organelles like endoplasmic reticulum (ER) and vesicles, mitosis, meiosis, and MT depolymerization [84, 85, 87]. KIFs are essential for directional transport of organelles and proteins in neurons during development, and in the adult brain [88].
During spermatogenesis, developing spermatids are moved from the apical to the basal region during stages IV–V of the epithelial cycle in the rat, and then transported back to the apical region at stages VI–VII until they line up near the luminal edge of the tubule lumen before spermiation at stage VIII [9]. The purpose and mechanism of this up-down-up movement of spermatids is still unclear. As previously stated, ER is a component of the ES and has been proposed to be a site of attachment between motor proteins, such as kinesin, along SC MTs to carry out translocation of adjacent spermatids [19]. Interestingly, KIF protein has been detected at the ES and was suspected to be an isoform of KIF20 [20]. Since that study in 2007, two testis-specific isoforms of KIF have been identified: KIF3A and KIF3B [89]. It has been proposed that KIF is responsible for transport of spermatids towards the basal region, where the plus-ends of SC MTs are located [90]. In Figure 3, an electron micrograph of cultured SCs, MTs can be seen arranged parallel to SC-SC interface. The localization of MTs along cell-cell interface further supports the idea of MTs serving as the track for translocation of spermatids and other organelles (e.g., endosomes, phagosomes). As described earlier, the apical ES consists of F-actin bundles sandwiched in between SC plasma membrane and cisternae of membrane-bound organelle, endoplasmic reticulum (ER). It is known that ER is pulled along MTs to be properly positioned within the eukaryotic cell [27]. Since ER is a component of the apical ES, it is likely that the ER present at this cell-cell junction is supported by the presence of MTs.
Cytoplasmic dynein
Cytoplasmic dynein is a minus end directed MT motor protein complex of high molecular weight, ranging from 1000–2000 kDa [91, 92]. Dynein is a member of the AAA+ (ATPases associated with diverse activities) superfamily, which is a protein family that exerts activity by re-configuring and translocating macromolecules [93, 94]. Similar to KIFs, dynein is also powered by ATP hydrolysis, but is not structurally related to them. This mechanoenzyme is used for intracellular transport and in axons generates retrograde transport [79, 88]. Before kinesin was discovered at the ES in the testis, cytoplasmic dynein had already been found to associate with ER and co-localize with actin at that site [95, 96]. Since dynein is a minus end directed motor protein, it has been hypothesized that it may be responsible for the translocation of spermatids along the SC MT tracks, especially during stages VI–VIII when spermatids are brought back to the apical region prior to their release at spermiation.
If this holds true, then much more work is needed to elucidate the mechanism by which spermatids are translocated through the epithelium by these motor proteins. In general, the information available regarding motor proteins presents their function in intracellular transport, via transport of vesicles and organelles. However, can this model apply to the translocation of entire cells, such as developing germ cells which are much larger in size than organelles, during spermatogenesis? An in vitro study in which spermatids with attached ES were isolated from the seminiferous epithelium of the rat testis provided additional evidence that MTs are involved in spermatid translocation [19]. In this study, isolated spermatids and attached ES were assayed for their ability to translocate fluorescently labeled MTs; and indeed, it was found that MTs could be moved along the ES [19]. However, it has been just over 15 years since that study and we still do not fully understand the mechanism of spermatid transport and how exactly motor proteins and the ES are involved. And if motor proteins work to translocate spermatids across the epithelium, do KIFs and dynein work independently or together as a team? Currently testis specific KIFs are plus end directed motors, but there is still the possibility that there may be other KIFs present which may operate in minus end directed transport. Since spermatids are translocated both apically and basally, do the respective motor proteins only associate with the ES when they are required to move the spermatid in the corresponding direction, or are they always present at the ES and by some mechanism are activated to overpower the other to change spermatid migration? Furthermore, what are the signaling proteins that direct the association with kinesins or dyneins to induce plus- or minus-end directed MT-mediated transport?
MT studies in the rat testis
Toxicological studies
To date, the mechanisms by which developing germ cells are transported across the seminiferous epithelium have not been fully elucidated. Currently, it is believed that the MT-based cytoskeleton of the Sertoli cell serves as the primary means for transporting developing germ cells due to its close association with spermatids at the apical ES and prominence at the Sertoli cell stalk. Interestingly, toxicology studies in the testes have revealed that a number of chemical agents that target MTs exerted their effects in the seminiferous epithelium to disrupt spermatogenesis. A recent review discusses the different histopathologies associated with Sertoli cell MT disruption [97]. Germ cell and epithelium sloughing, spermatid and residual body retention, and vacuolization are a few of the histopathologies observed in these studies. Sloughing of the epithelium is when the apical cytoplasm of the epithelium, which contains cohorts of germ cells, is shed into the lumen, and is oftentimes caused by MT depolymerizing agents, such as colchicine and carbendazim [98–100]. Another pathology, spermatid retention, describes the impaired movement of elongate spermatids due to disruption of Sertoli cell MTs caused by chemicals like colchicine, paclitaxel (Taxol), 2,5-hexandione and others [101]. Residual body retention is another result of MT disruption in the seminiferous epithelium [102]. Microtubules are involved in phagocytosis, which is the mechanism used by macrophages and neutrophils to remove pathogens. In the testis, phagocytosis also occurs in the seminiferous epithelium, but is executed by the Sertoli cells since macrophages are usually excluded from the seminiferous epithelium. Phagocytosis is an important “self-cleaning” process in which the Sertoli cell engulfs the residual body, or excess cytoplasm of a mature spermatid, from the apical region and transported that down to the basal region where it is degraded via the lysosomal pathway [103, 104]. Without MTs, the residual bodies will accumulate in the apical region as they can no longer be transported to the basal region. Vacuolization is another characteristic pathology due to MT disruption in the seminiferous epithelium [98, 105, 106]. The vacuoles are enlarged Sertoli cell endoplasmic reticulum, but how MT disruption causes this occurrence is unclear.
There are several types of cellular junctions present in the seminiferous epithelium, all of which are dependent on the Sertoli cell cytoskeleton. Tight junctions and basal ectoplasmic specialization (ES) between Sertoli cells at the BTB, and apical ES between spermatids (steps 8–16 or 8–19 in mouse or rat testes) and Sertoli cells, as well as tubulobulbar complex (TBC) at the BTB and also between Sertolilate spermatids are all junction types that comprise the seminiferous epithelium. These junction types involve interaction with actin microfilaments and microtubules to facilitate the movement of developing germ cells across the epithelium. In order to study transport of developing germ cells, one must first understand how cell junctions are regulated in the seminiferous epithelium. Studies using toxicant models, such as bisphenol A (BPA), cadmium, and perfluorooctanesulfonate (PFOS), have revealed that these environmental toxicants can target cell junctions in the testis to affect reproductive function [107–109]. In addition to environmental toxicants, some male contraceptives under development also have been shown to target cell junctions in the testis. One male contraceptive in particular, adjudin, has been studied extensively to describe the mechanisms by which it temporally disrupts spermatogenesis. This drug targets the actin cytoskeleton and affects adhesion between Sertoli and germ cells, leading to premature spermiation of mostly elongate/elongating spermatids [110]. Additionally, some non-specific effects of this drug are reported, such as: sloughing and vacuolization, which are both pathologies caused by the previously discussed MT targeting agents, and germ cell degeneration [97, 111]. There is reason to believe that adjudin may also target MTs. Figure 4 shows the staining of α-tubulin in tubules of adult male rats treated with adjudin (50 mg/kg b.w., by oral gavage) vs. control normal rat testes. In a side by side comparison, it is clear that the arrangement of tubulin across the seminiferous epithelium is disrupted 4 days after treatment with adjudin. By 4 days, MT bundles are no longer columnar in shape extending apically along the Sertoli cell stalks as noted in control testes. The MTs appear to have collapsed, possibly due to the absence of elongate/elongating spermatids and germ cells (Figure 4). Figure 5 is a schematic diagram illustrating the disruption of the MT and actin-based cytoskeletal networks following adjudin treatment that leads to defects in spermatid adhesion and also spermatid transport. It is already known that actin is a molecular target of adjudin [112], but whether MT bundle collapse occurs because tubulin is a direct target of adjudin, or is a consequence of the absence of germ cells and spermatids remains to be determined. However, sloughing of germ cells in particular elongating/elongated spermatids is likely the result of an actin-based cytoskeletal collapse since adhesion proteins utilize actin filaments for their attachment. If actin filaments remain intact, it is unlikely that spermatid sloughing would occur.
Figure 4. Microtubule disruption after treatment with adjudin.
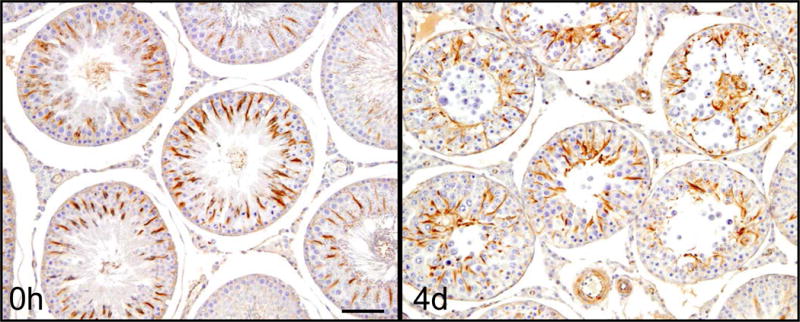
Rats were administered adjudin, a potential male contraceptive, at a dosage of 50 mg/kg b.w. via oral gavage. Rats were terminated after 4 days, testes removed, fixed, embedded and analyzed by IHC to observe morphological changes in cytoskeleton of seminiferous tubules. The image on the left is a cross section of a normal rat testis stained for α-tubulin. The arrangement of MTs at 0h in normal rat testes shows the typical spoke-like pattern in the Sertoli cell cytoplasm which thus serve as the track for the transport of spermatids and other organelles (e.g., phagosomes, endosomes). MTs lie at an almost 90° angle to the basement membrane of the tunica propria. However, 4 days after adjudin was administered, MT arrangement within the tubules is grossly disrupted, no longer displaying the typical spoke-like pattern. The MT spokes collapsed in concurrence with the loss of spermatids/germ cells, instead of forming columnar structures perpendicular to the basement membrane, MTs had become diffused within the epithelium and many of them are laid in parallel to the basement membrane of the tunica propria. Scale bar, 120 μm, which applies to the other micrograph in this panel.
Figure 5. Schematic diagram of effects of adjudin on Sertoli cell cytoskeleton in the seminiferous epithelium.
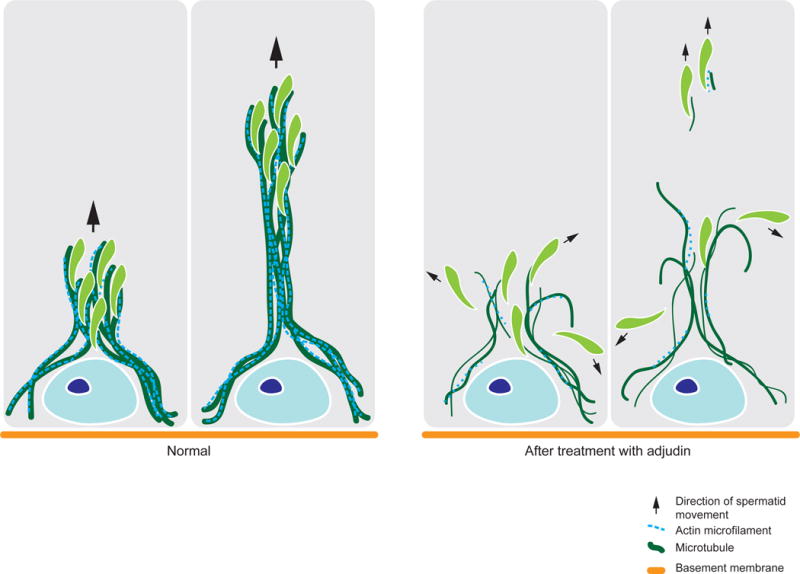
In normal Sertoli cells of the seminiferous epithelium, MTs serve as the tracks for the transport of developing germ cells across the BTB and the seminiferous epithelium throughout spermatogenesis. Actin microfilaments are also present along these tracks, serving as the support and vehicle for germ cell transport across the epithelium. Using adjudin as a model to study germ cell transport, we have provided evidence that germ cell transport is disrupted due to the loss of MT track and actin support as briefly summarized herein.
Microtubules and disease
The tight regulation of MTs is essential for maintenance of axonal transport and cell division. However when regulation of the two aforementioned MT related processes is compromised, it can lead to the development of neurodegenerative disease, such as Alzheimer’s disease (AD), and cancer, respectively. We briefly review some of these findings since these data provide insight into designing future experiments to unravel the role of MTs in spermatogenesis.
Alzheimer’s disease is a progressive and debilitating neurodegenerative disease that afflicts millions of people worldwide. According to the 2009 World Alzheimer Report, this number is predicted to triple by 2050 (www.alz.org). Currently, scientists are focused on investigating structural hallmarks of the AD brain such as, plaques (amyloid-β (Aβ) protein aggregates that accumulate in between nerve cells) and neurofibrillary tangles (hyperphosphorylated tau protein filament aggregates) [113–115]. Those with advanced stage AD can be identified by symptoms such as memory loss, behavioral and mood changes, confusion, and others (www.alz.org). It was believed that these symptoms were due to the accumulation of plaques and neurofibrillary tangles which interfere with proper neuronal communication and transport. However, amyloid-ß accumulations have also been found in the brains of cognitively healthy older individuals [116], suggesting that amyloid-ß accumulation is not the cause of AD. In fact, with the recent advances in medicine and brain imaging, there is no reliable method to diagnose, prevent or treat AD in humans. Tau is a MT-associated protein (MAP) which binds and stabilizes MTs. Physiologically, tau associates with MTs; however, in the AD brain, tau is hyperphosphorylated causing it to disengage from axonal MTs [114, 117]. When disengaged from axonal MTs, they aggregate and form neurofibrillary tangles resulting in destabilized axonal MTs [118]. Almost two decades earlier, MARK family proteins were discovered to be responsible for phosphorylation of tau [119–121]. Of the 4 human MARK isoforms, MARK4 expression was the highest and was found to co-localize with MTs mostly in the axon. A screening of MARK4 expression in different tissue types revealed that it is predominantly expressed in brain and testis [122]. Since MT stability is implicated in axonal/neuronal integrity, MT stabilizing agents may be a promising treatment for AD [118]. Thus, future studies should include better understanding of the MARKs in MT function, and their role in cross-talk with actin-based cytoskeleton. Furthermore, it is important to delineate the role of Tau in MT dynamics in Sertoli cell function.
The MT network also plays a pivotal role in cancer, a disease characterized by uncontrolled growth of cells. One major event of the cell cycle is mitosis, which is when chromosomes of a cell nucleus are separated into identical sets of chromosomes. Metaphase is a stage of mitosis when chromosomes are lined up at the cell equator and attach to spindle fibers made of MTs [123]. This stage is critical for the proper separation of chromosomes, to ensure that each daughter cell contains the identical chromosomes of the parent cell, and exemplifies just one of the complex roles MTs play in regulation of the cell cycle. However, when this regulation is upturned and cells begin to divide uncontrollably, cancer ensues. Because MTs play an insurmountable role in cell cycle regulation, they are used as a target in treatment of cancers. A number of drugs available target tubulin and are divided into two main classes: MT-stabilizing (i.e. taxanes) and MT-destabilizing (i.e. vinca alkaloids) drugs [124–126]. These drugs bind to tubulin (referred to as tubulin-binding agents, TBAs) and interfere with mitosis, which induces mitotic arrest and cell death [126, 127]. Microtubules are an attractive target for cancer therapies since they are tightly regulated dynamic structures involved in many cellular functions. As such, if a drug can be designed to specifically target the Sertoli cell MT network, it could be developed into a male contraceptive that would induce failure in organelle transport in Sertoli cells, impeding spermatogenesis. As noted earlier, actin is a cellular target of adjudin, but whether tubulin is as well remains to be investigated. However, it is known that both the F-actin and MT-networks are disrupted by adjudin. A better understanding on the mechanism of how adjudin works can provide new insights in the regulation of MT and F-actin dynamics in the testis.
Future perspectives and concluding remarks
The transport of germ cells across the seminiferous epithelium, such as the transport of preleptotene spermatocytes connected in clones across the BTB, and elongating/elongated spermatids across the adluminal compartment during spermiogenesis, is an intricate and tightly coordinated process. Despite decades of research on the topic of spermatogenesis [104, 128], we still do not have a clear picture on how exactly germ cells are translocated in the seminiferous epithelium. It is suspected that motor proteins, such as kinesin and dynein, are responsible for the movement of non-motile germ cells. But how is it possible that these proteins, which are regularly employed by the cell to transport nutrients and organelles within the cell, can transport entire cells, such as preleptotene spermatocytes and spermatids? For years, it has been accepted that the MT network provides the tracks along which germ cells/spermatids travel, but not much progress has been made to elucidate the exact mechanisms by which this occurs. Currently researchers in this field are focusing on the players involved in regulating MT dynamics, but how spermatids move throughout the epithelium is a question still left unanswered. What are mechanical forces generated to move these non-motile cells? Are motor proteins the answer? The transport of germ cells is unlike any other cellular movement known to date. A closer look into how this occurs would greatly benefit the understanding of the regulation of spermatogenesis and current issues such as infertility and male contraceptive development.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034 to C.Y.C.; U54 HD029990, Project 5 to C.Y.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests: Nothing to declare
References
- 1.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29:469–87. doi: 10.2164/jandrol.107.004655. [DOI] [PubMed] [Google Scholar]
- 3.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1363–434. [Google Scholar]
- 4.Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–28. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 5.Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, et al. Funcitonal and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J Pathol. 2008;215:108–17. doi: 10.1002/path.2328. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler K, Tardif S, Rival C, Luu B, Bui E, Del Rio R, et al. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc Natl Acad Sci USA. 2011;108:7511–6. doi: 10.1073/pnas.1017615108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy CJ, Stermer AR, Richburg JH. Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-Ethylhexyl) phthalate (MEHP) Biol Reprod. 2014;91:18. doi: 10.1095/biolreprod.113.115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 9.Xiao X, Mruk DD, Wong CK, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology (Bethesda) 2014;29:286–98. doi: 10.1152/physiol.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakberg EF. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956;99:507–16. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- 11.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 12.Mruk D, Zhu LJ, Silvestrini B, Lee WM, Cheng CY. Interactions of proteases and protease inhibitors in Sertoli-germ cell cocultures preceding the formation of specialized Sertoligerm cell junctions in vitro. J Androl. 1997;18:612–22. [PubMed] [Google Scholar]
- 13.Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat. 1983;167:163–79. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- 14.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 15.Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004;15:439–47. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Qian X, Mruk DD, Cheng YH, Tang EI, Han D, Lee WM, et al. Actin binding proteins, spermatid transport and spermiation. Seminars in cell & developmental biology. 2014;30:75–85. doi: 10.1016/j.semcdb.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee NP, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update. 2004;10:349–69. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- 18.Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell. 1989;21:361–79. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 19.Beach SF, Vogl AW. Spermatid translocation in the rat seminiferous epithelium: coupling membrane trafficking machinery to a junction plaque. Biol Reprod. 1999;60:1036–46. doi: 10.1095/biolreprod60.4.1036. [DOI] [PubMed] [Google Scholar]
- 20.Vaid KS, Guttman JA, Singaraja RR, Vogl AW. A kinesin is present at unique sertoli/spermatid adherens junctions in rat and mouse testes. Biol Reprod. 2007;77:1037–48. doi: 10.1095/biolreprod.107.063735. [DOI] [PubMed] [Google Scholar]
- 21.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs KL, Greensmith L, Schiavo G. Regulation of axonal transport by protein kinases. Trends Biochem Sci. 2015;40:597–610. doi: 10.1016/j.tibs.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Nogales E. Structural insights into microtubule function. Annual review of biochemistry. 2000;69:277–302. doi: 10.1146/annurev.biochem.69.1.277. [DOI] [PubMed] [Google Scholar]
- 24.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 25.Downing KH, Nogales E. Tubulin structure: insights into microtubule properties and functions. Current opinion in structural biology. 1998;8:785–91. doi: 10.1016/s0959-440x(98)80099-7. [DOI] [PubMed] [Google Scholar]
- 26.Petry S, Vale RD. Microtubule nucleation at the centrosome and beyond. Nature cell biology. 2015;17:1089–93. doi: 10.1038/ncb3220. [DOI] [PubMed] [Google Scholar]
- 27.Cooper GM. Microtubule Motors and Movements. 2nd. Sunderland, MA: Sinauer Associates; 2000. [Google Scholar]
- 28.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–42. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 29.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Weisenberg RC, Deery WJ, Dickinson PJ. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976;15:4248–54. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- 31.Wilson L, Farrell KW. Kinetics and steady state dynamics of tubulin addition and loss at opposite microtubule ends: the mechanism of action of colchicine. Ann N Y Acad Sci. 1986;466:690–708. doi: 10.1111/j.1749-6632.1986.tb38452.x. [DOI] [PubMed] [Google Scholar]
- 32.van der Vaart B, Akhmanova A, Straube A. Regulation of microtubule dynamic instability. Biochem Soc Trans. 2009;37:1007–13. doi: 10.1042/BST0371007. [DOI] [PubMed] [Google Scholar]
- 33.Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr Opin Cell Biol. 2001;13:41–8. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 34.Bahmanyar S, Nelson WJ, Barth AI. Role of APC and its binding partners in regulating microtubules in mitosis. Adv Exp Med Biol. 2009;656:65–74. doi: 10.1007/978-1-4419-1145-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–72. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wloga D, Gaertig J. Post-translational modifications of microtubules. J Cell Sci. 2010;123:3447–55. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 38.Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan KT. TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. The Journal of cell biology. 2005;171:197–200. doi: 10.1083/jcb.200509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–7. [PubMed] [Google Scholar]
- 41.Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, et al. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:601–13. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 42.Su LK, Qi Y. Characterization of human MAPRE genes and their proteins. Genomics. 2001;71:142–9. doi: 10.1006/geno.2000.6428. [DOI] [PubMed] [Google Scholar]
- 43.Sen I, Veprintsev D, Akhmanova A, Steinmetz MO. End binding proteins are obligatory dimers. PLoS One. 2013;8:e74448. doi: 10.1371/journal.pone.0074448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Molecular cell. 2007;27:976–91. doi: 10.1016/j.molcel.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bu W, Su LK. Characterization of functional domains of human EB1 family proteins. J Biol Chem. 2003;278:49721–31. doi: 10.1074/jbc.M306194200. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi I, Ikura M. Crystal structure of the amino-terminal microtubule-binding domain of end-binding protein 1 (EB1) J Biol Chem. 2003;278:36430–4. doi: 10.1074/jbc.M305773200. [DOI] [PubMed] [Google Scholar]
- 47.Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–6. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats – an in vitro study. Endocrinology. 2014:en20141720. doi: 10.1210/en.2014-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schober JM, Kwon G, Jayne D, Cain JM. The microtubule-associated protein EB1 maintains cell polarity through activation of protein kinase C. Biochemical and biophysical research communications. 2012;417:67–72. doi: 10.1016/j.bbrc.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schonichen A, Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–63. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Breitsprecher D, Goode BL. Formins at a glance. J Cell Sci. 2013;126:1–7. doi: 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards M, Zwolak A, Schafer DA, Sept D, Dominguez R, Cooper JA. Capping protein regulators fine-tune actin assembly dynamics. Nat Rev Mol Cell Biol. 2014;15:677–89. doi: 10.1038/nrm3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, et al. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–5. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 54.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nature Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–51. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li N, Mruk DD, Wong CK, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplasmic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology. 2015:en20151161. doi: 10.1210/en.2015-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, et al. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–56. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thumkeo D, Shinohara R, Watanabe K, Takebayashi H, Toyoda Y, Tohyama K, et al. Deficiency of mDia, an actin nucleator, disrupts integrity of neuroepithelium and causes periventricular dysplasia. PLoS One. 2011;6:e25465. doi: 10.1371/journal.pone.0025465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mironova E, Millette CF. Expression of the diaphanous-related formin proteins mDia1 and mDia2 in the rat testis. Dev Dyn. 2008;237:2170–6. doi: 10.1002/dvdy.21622. [DOI] [PubMed] [Google Scholar]
- 60.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nature Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 61.Eng CH, Huckaba TM, Gundersen GG. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol Biol Cell. 2006;17:5004–16. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–36. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matenia D, Mandelkow EM. The tau of MARK: a polarized view of the cytoskeleton. Trends Biochem Sci. 2009;34:332–42. doi: 10.1016/j.tibs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 64.Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biochem Sci. 1998;23:307–11. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- 65.Trinczek B, Brajenovic M, Ebneth A, Drewes G. MARK4 is a novel microtubule-associated proteins/microtubule affinity-regulating kinase that binds to the cellular microtubule network and to centrosomes. J Biol Chem. 2004;279:5915–23. doi: 10.1074/jbc.M304528200. [DOI] [PubMed] [Google Scholar]
- 66.Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 67.Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E. MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol. 2004;167:99–110. doi: 10.1083/jcb.200401085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocr. 2013;217:R13–23. doi: 10.1530/JOE-12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, et al. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012;2:117–26. doi: 10.4161/spmg.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rovina D, Fontana L, Monti L, Novielli C, Panini N, Sirchia SM, et al. Microtubule-associated protein/microtubule affinity-regulating kinase 4 (MARK4) plays a role in cell cycle progression and cytoskeletal dynamics. Eur J Cell Biol. 2014;93:355–65. doi: 10.1016/j.ejcb.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Guan KL. Microtubule-associated protein/microtubule affinity-regulating kinase 4 (MARK4) is a negative regulator of the mammalian target of rapamycin complex 1 (mTORC1) J Biol Chem. 2013;288:703–8. doi: 10.1074/jbc.C112.396903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mok KW, Mruk DD, Cheng CY. Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the “Yin” and “Yang” effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2. Int Rev Cell Mol Biol. 2013;301:291–358. doi: 10.1016/B978-0-12-407704-1.00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanwar PS, Kaneko-Tarui T, Zhang L, Teixeira JM. Altered LKB1/AMPK/TSC1/TSC2/mTOR signaling causes disruption of Sertoli cell polarity and spermatogenesis. Hum Mol Genetics. 2012;21:4394–405. doi: 10.1093/hmg/dds272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–38. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 77.O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Sem Cell Dev Biol. 2014;30:45–54. doi: 10.1016/j.semcdb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Vogl AW, Weis M, Pfeiffer DC. The perinuclear centriole-containing centrosome is not the major microtubule organizing center in Sertoli cells. Eur J Cell Biol. 1995;66:165–79. [PubMed] [Google Scholar]
- 79.Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 80.Kevenaar JT, Hoogenraad CC. The axonal cytoskeleton: from organization to function. Frontiers in molecular neuroscience. 2015;8:44. doi: 10.3389/fnmol.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 82.Kozielski F, Sack S, Marx A, Thormahlen M, Schonbrunn E, Biou V, et al. The crystal structure of dimeric kinesin and implications for microtubule-dependent motility. Cell. 1997;91:985–94. doi: 10.1016/s0092-8674(00)80489-4. [DOI] [PubMed] [Google Scholar]
- 83.Bloom GS, Wagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988;27:3409–16. doi: 10.1021/bi00409a043. [DOI] [PubMed] [Google Scholar]
- 84.Drummond DR. Regulation of microtubule dynamics by kinesins. Sem Cell Dev Biol. 2011;22:927–34. doi: 10.1016/j.semcdb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 85.Vale RD, Fletterick RJ. The design plan of kinesin motors. Ann Rev Cell Dev Biol. 1997;13:745–77. doi: 10.1146/annurev.cellbio.13.1.745. [DOI] [PubMed] [Google Scholar]
- 86.Hunter AW, Wordeman L. How motor proteins influence microtubule polymerization dynamics. J Cell Sci. 2000;113(Pt 24):4379–89. doi: 10.1242/jcs.113.24.4379. [DOI] [PubMed] [Google Scholar]
- 87.Walczak CE, Gayek S, Ohi R. Microtubule-depolymerizing kinesins. Ann Rev Cell Dev Biol. 2013;29:417–41. doi: 10.1146/annurev-cellbio-101512-122345. [DOI] [PubMed] [Google Scholar]
- 88.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–80. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 89.Huang CJ, Huang CC, Chang CC. Association of the testis-specific TRIM/RBCC protein RNF33/TRIM60 with the cytoplasmic motor proteins KIF3A and KIF3B. Mol Cell Biochem. 2012;360:121–31. doi: 10.1007/s11010-011-1050-8. [DOI] [PubMed] [Google Scholar]
- 90.Redenbach DM, Vogl AW. Microtubule polarity in Sertoli cells: a model for microtubule-based spermatid transport. European journal of cell biology. 1991;54:277–90. [PubMed] [Google Scholar]
- 91.King SM. The dynein microtubule motor. Biochim Biophys Acta. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 92.Johnson KA, Wall JS. Structure and molecular weight of the dynein ATPase. J Cell Biol. 1983;96:669–78. doi: 10.1083/jcb.96.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 94.Roberts AJ, Kon T, Knight PJ, Sutoh K, Burgess SA. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol. 2013;14:713–26. doi: 10.1038/nrm3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller MG, Mulholland DJ, Vogl AW. Rat testis motor proteins associated with spermatid translocation (dynein) and spermatid flagella (kinesin-II) Biol Reprod. 1999;60:1047–56. doi: 10.1095/biolreprod60.4.1047. [DOI] [PubMed] [Google Scholar]
- 96.Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113(Pt 12):2167–76. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- 97.Johnson KJ. Testicular Histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4 doi: 10.4161/21565562.2014.979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Markelewicz RJ, Jr, Hall SJ, Boekelheide K. 2,5-hexanedione and carbendazim coexposure synergistically disrupts rat spermatogenesis despite opposing molecular effects on microtubules. Toxicol Sci. 2004;80:92–100. doi: 10.1093/toxsci/kfh140. [DOI] [PubMed] [Google Scholar]
- 99.Nakai M, Hess RA. Morphological changes in the rat Sertoli cell induced by the microtubule poison carbendazim. Tissue Cell. 1994;26:917–27. doi: 10.1016/0040-8166(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 100.Allard EK, Johnson KJ, Boekelheide K. Colchicine disrupts the cytoskeleton of rat testis seminiferous epithelium in a stage-dependent manner. Biology of reproduction. 1993;48:143–53. doi: 10.1095/biolreprod48.1.143. [DOI] [PubMed] [Google Scholar]
- 101.Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, et al. 2,5-hexanedione-induced testicular injury. Annu Rev Pharmacol Toxicol. 2003;43:125–47. doi: 10.1146/annurev.pharmtox.43.100901.135930. [DOI] [PubMed] [Google Scholar]
- 102.Boekelheide K, Hall SJ. 2,5-hexanedione exposure in the rat results in long-term testicular atrophy despite the presence of residual spermatogonia. J Androl. 1991;12:18–26. [PubMed] [Google Scholar]
- 103.Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci. 1987;513:1–15. doi: 10.1111/j.1749-6632.1987.tb24994.x. [DOI] [PubMed] [Google Scholar]
- 104.Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In: Griswold MD, editor. Sertoli Cell Biology. 2nd. Amsterdam: Elsevier; 2015. pp. 333–383. DOI: http://dx.doi.org/10.1016/B978-0-12-417047-6.00012.0. [Google Scholar]
- 105.Chapin RE, Morgan KT, Bus JS. The morphogenesis of testicular degeneration induced in rats by orally administered 2,5-hexanedione. Exp Mol Pathol. 1983;38:149–69. doi: 10.1016/0014-4800(83)90082-5. [DOI] [PubMed] [Google Scholar]
- 106.Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol. 2007;35:719–27. doi: 10.1080/01926230701481931. [DOI] [PubMed] [Google Scholar]
- 107.Wan HT, Mruk DD, Wong CK, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr(407): an in vitro study. Endocrinology. 2014;155:249–62. doi: 10.1210/en.2013-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao X, Mruk DD, Tang EI, Wong CK, Lee WM, John CM, et al. Environmental toxicants perturb human Sertoli cell adhesive function via changes in F-actin organization mediated by actin regulatory proteins. Hum Reprod. 2014;29:1279–91. doi: 10.1093/humrep/deu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng CY, Wong EW, Lie PP, Li MW, Su L, Siu ER, et al. Environmental toxicants and male reproductive function. Spermatogenesis. 2011;1:2–13. doi: 10.4161/spmg.1.1.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng CY, Lie PP, Wong EW, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–7. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng CY. Toxicants target cell junctions in the testis: Insights from the indazole-carboxylic model. Spermatogenesis. 2014;4 doi: 10.4161/21565562.2014.981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mruk DD, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis. 2011;1:137–46. doi: 10.4161/spmg.1.2.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–90. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 114.Mandelkow E. Alzheimer’s disease. The tangled tale of tau. Nature. 1999;402:588–9. doi: 10.1038/45095. [DOI] [PubMed] [Google Scholar]
- 115.Annaert W, De Strooper B. A cell biological perspective on Alzheimer’s disease. Ann Rev Cell Dev Biol. 2002;18:25–51. doi: 10.1146/annurev.cellbio.18.020402.142302. [DOI] [PubMed] [Google Scholar]
- 116.Jagust W. Is amyloid-beta harmful to the brain? Insights from human imaging studies. Brain. 2015 doi: 10.1093/brain/awv326. (in press; pii:awv326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361–84. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 118.Ballatore C, Brunden KR, Huryn DM, Trojanowski JQ, Lee VM, Smith AB., 3rd Microtubule stabilizing agents as potential treatment for Alzheimer’s disease and related neurodegenerative tauopathies. J Med Chem. 2012;55:8979–96. doi: 10.1021/jm301079z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 120.Jenkins SM, Johnson GV. Microtubule/MAP-affinity regulating kinase (MARK) is activated by phenylarsine oxide in situ and phosphorylates tau within its microtubule-binding domain. J Neurochem. 2000;74:1463–8. doi: 10.1046/j.1471-4159.2000.0741463.x. [DOI] [PubMed] [Google Scholar]
- 121.Mandelkow EM, Schweers O, Drewes G, Biernat J, Gustke N, Trinczek B, et al. Structure, microtubule interactions, and phosphorylation of tau protein. Ann N Y Acad Sci. 1996;777:96–106. doi: 10.1111/j.1749-6632.1996.tb34407.x. [DOI] [PubMed] [Google Scholar]
- 122.Schneider A, Laage R, von Ahsen O, Fischer A, Rossner M, Scheek S, et al. Identification of regulated genes during permanent focal cerebral ischaemia: characterization of the protein kinase 9b5/MARKL1/MARK4. J Neurochem. 2004;88:1114–26. doi: 10.1046/j.1471-4159.2003.02228.x. [DOI] [PubMed] [Google Scholar]
- 123.Nicklas RB. The forces that move chromosomes in mitosis. Annu Rev Biophys Biophys Chem. 1988;17:431–49. doi: 10.1146/annurev.bb.17.060188.002243. [DOI] [PubMed] [Google Scholar]
- 124.Mukhtar E, Adhami VM, Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther. 2014;13:275–84. doi: 10.1158/1535-7163.MCT-13-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou J, Giannakakou P. Targeting microtubules for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2005;5:65–71. doi: 10.2174/1568011053352569. [DOI] [PubMed] [Google Scholar]
- 126.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 127.Gan PP, McCarroll JA, Po’uha ST, Kamath K, Jordan MA, Kavallaris M. Microtubule dynamics, mitotic arrest, and apoptosis: drug-induced differential effects of betaIII-tubulin. Mol Cancer Ther. 2010;9:1339–48. doi: 10.1158/1535-7163.MCT-09-0679. [DOI] [PubMed] [Google Scholar]
- 128.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]


