Abstract
Studies have demonstrated associations between cardiovascular factors and Alzheimer’s disease (AD) with minimal focus on other neurodegenerative diseases. Utilizing cross-sectional data from 17,532 individuals in the National Alzheimer’s Coordinating Center, Uniform Data Set, we compared presence of cardiovascular factors (body mass index-BMI, atrial fibrillation, hypertension, hyperlipidemia, and diabetes) in individuals carrying a diagnosis of: Probable AD (ProbAD), Possible AD, VaD, dementia with Lewy bodies (DLB), frontotemporal dementia, Parkinson’s disease, progressive supranuclear palsy, or corticobasal degeneration, to normal individuals. Generalized linear mixed models were fitted with age, gender, and cardiovascular factors as fixed effects and Alzheimer’s Disease Centers as random effects. In late life, only BMI of ProbAD and DLB subjects were statistically significantly lower than normals (p values < 0.001). When accounting for co-linearity within cardiovascular factors, low BMI was a comorbidity of certain dementia etiologies as compared to normals. These data support a concept of disease-specific associations with certain cardiovascular factors.
Keywords: Alzheimer’s disease, dementia with Lewy bodies, Parkinson’s disease, Corticobasal degeneration, progressive supranuclear palsy, Body Mass Index
INTRODUCTION
It is apparent through many epidemiological studies, including but not limited to the Honolulu Asia Aging Study, the Religious Orders Study, 90+ study, and previous National Alzheimer’s Coordinating Center (NACC) database studies, that the presence or absence of cardiovascular factors are associated with risk for cognitive impairment and dementia, especially Alzheimer’s disease (AD) and vascular dementia (VaD).1–10 In midlife, these factors may increase dementia risk. However, the association between cardiovascular factors in elderly subjects, and especially in those that are already cognitively impaired, and subsequent expression of dementia may decrease or even reverse.9–15 This perhaps may be due to the pathology underlying cognitive impairment is already well established in these individuals and could lead to certain cardiovascular comorbidity profiles.
While the association of cardio- and cerebrovascular disease with VaD is easily appreciated, hypothetical molecular mechanisms have also been advanced that may explain altered associations of AD to those with cardiovascular disease or a predisposition towards it, including increased oxidative stress, deranged insulin signaling, hypercholesterolemia-induced neuronal membrane changes, and circulating inflammatory mediators.2, 4, 16 In comparison, there has been minimal focus on whether cardiovascular factors might also have differential associations for other neurodegenerative diseases, such as dementia with Lewy bodies (DLB) and frontotemporal dementias (FTD), despite the fact that underlying DLB and/or FTD associated pathologies may be collectively responsible for up to 35% of all cases of clinical dementia.17, 18 Furthermore, hampering an analysis of individual cardiovascular factors is the extensive co-linearity amongst them; separating the effects of these factors from each other is necessary in order to truly understand specific associations with diseases.
It is critical to investigate whether specific cardiovascular factors have divergent rates of occurrence in neurodegenerative diseases in late life, as this may reflect on the pathogenesis of disease-specific etiologies and identify potential interventions. Establishing the relative timing of these processes will provide clues as to which may be risk factors and which may be disease effects. The current study utilizes cross-sectional late life data from NACC to determine whether associations of certain cardiovascular factors (atrial fibrillation, hypercholesterolemia, hypertension, diabetes, and BMI) in AD and VaD are also present in other neurodegenerative conditions including DLB, FTD, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), Parkinson’s disease (PD), or prion disease (Prion) as compared to a group of elderly individuals with no evidence of cognitive impairment, dementia, or parkinsonism (normals). The overarching hypothesis of the current study is that cardiovascular factors associated with AD in late life will not be associated with other neurodegenerative diseases. This hypothesis is in support of the contention that it is through specific mechanisms that certain cardiovascular factors are a comorbidity of AD, and there is not a global association of cardiovascular factors with all neurodegenerative diseases.
METHODS
Clinical subject selection
The Uniform Data Set (UDS) is composed of standardized clinical evaluations collected at all Alzheimer’s Disease Centers (ADCs) funded by the National Institute on Aging. The UDS includes information on demographics, behavioral symptoms, cognitive testing, medical history, family history, clinical impressions, and diagnoses.19, 20 The present analysis includes data from 34 federally funded ADCs. Subject data were collected under Institutional Review Board approval from the 34 ADCs; the current study was approved by the Institutional Review Board at the Banner Sun Health Research Institute. Detailed description and documentation of the UDS and the NACC database can be found at the following URL: (www.alz.washington.edu/). We utilized data from the NACC Uniform Data Set (September 2014 data freeze) that consisted of demographic and clinical data on 30,953 subjects from their last recorded visits.
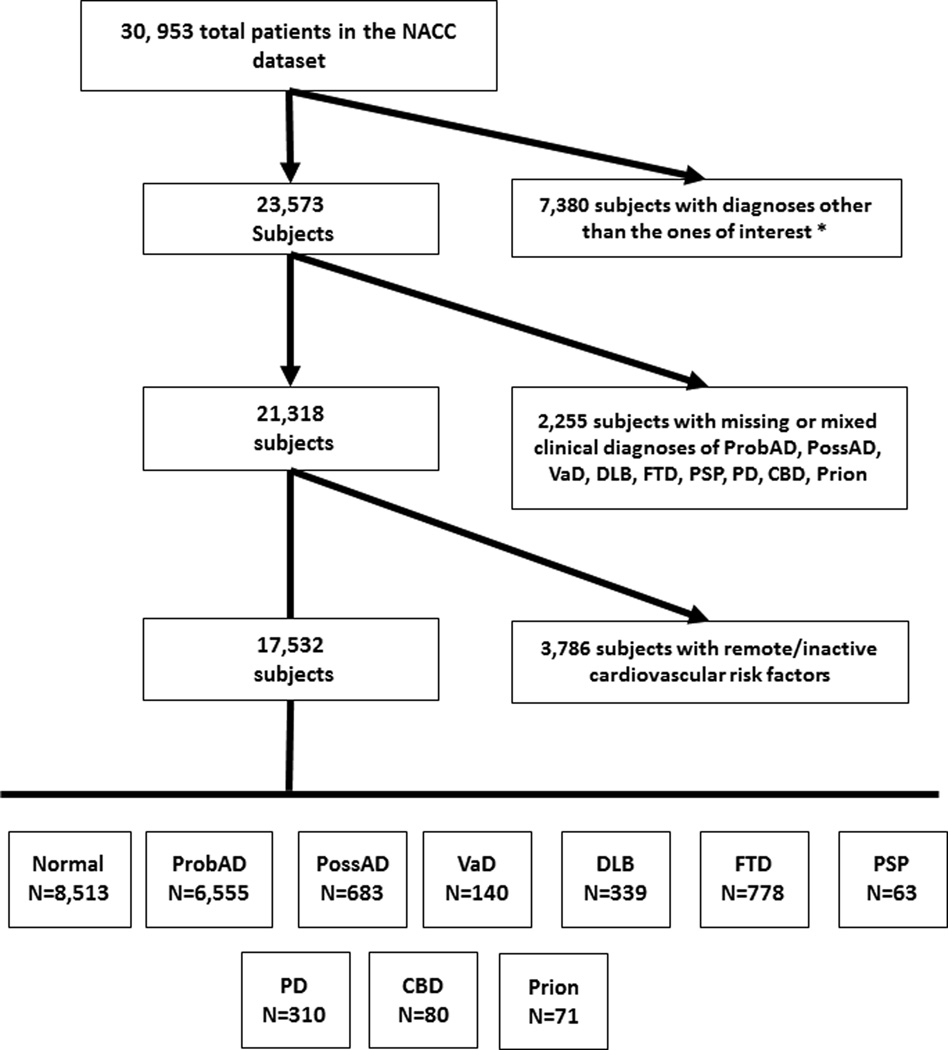
Figure 1 provides a flow chart of inclusion/exclusion criteria. Individuals were excluded if they had diagnoses other than the ones of interest at their last visit: alcohol dementia, dementia undefined, mild cognitive impairment, Huntington’s disease, cognitive dysfunction due to illness, hydrocephalus, brain injury, Down’s syndrome, cognitive dysfunction from medical illnesses, cognitive dysfunction from medications, brain cancer and other major psychiatric illnesses. To better evaluate disease specific mechanisms, individuals were included only if they had a single clinical diagnosis of one the following: Normal cognition, CBD, DLB, FTD, PD, AD, Prion, PSP and VaD. For example, patients with AD and contributing VaD were excluded (i.e., mixed dementia, or mixed neurodegenerative diagnoses). Furthermore, due to the relatively rare nature of FTD subtypes, and thus, smaller sample sizes, the FTD group was collapsed and comprised of patients with any one of the following primary diagnoses: primary progressive aphasia, progressive nonfluent aphasia, all forms of semantic dementia, and the behavioral variant of FTD. Lastly, due to the focus on late life manifestations of cardiovascular factors in persons with a neurological diagnosis, we excluded individuals who had diabetes, atrial fibrillation, hypertension, and hyperlipidemia that were listed as remote/inactive on their UDS form since ages at onset for these conditions are not recorded.
Figure 1.
Flow chart of inclusion and exclusion criteria used to define our series within the National Alzheimer’s Coordinating Center Uniform Data Set. *Diagnoses of exclusion: alcohol dementia, dementia undefined, mild cognitive impairment, Huntington’s disease, cognitive dysfunction due to medical illness, hydrocephalus, brain injury, Down’s syndrome, cognitive dysfunction from medications, brain cancer, and other major psychiatric illness.
Using these inclusion and exclusion criteria, 17,532 individuals were included and assigned to one of the following clinical diagnostic categories based on their last visit: probable AD (ProbAD; n=6,555), possible AD (PossAD; n=683), VaD (n=140), DLB (n=339), FTD (n=778), PD (n=310), PSP (n=63), CBD (n=80), or Prion (n=71). Subjects who had normal cognition at all visits (NACCNORM variable within the NACC UDS derived variables) and lacked a diagnosis of PSP, CBD, and PD were considered normal (normals, n=8,513) and served as the reference group.
Cardiovascular Factor Data
Cross-sectional data on cardiovascular factors were taken from the last recorded visit in the NACC database. Cardiovascular data included: BMI (weight in kg divided by height in centimeters squared), hypertension, diabetes, hyperlipidemia, and atrial fibrillation. Individuals with cardiovascular factors listed as recent/active were compared to the absent category. To provide more meaningful clinical applications, BMI was also divided into 4 categories: those who were underweight (BMI <18.5), had a normal BMI (18.5 <BMI < 25), overweight (25 <BMI < 30) and obese (BMI >30). Data on age at visit, sex, APOE ε4 carrier status, and smoking history (at least 100 lifetime cigarettes) were also noted.
Statistical Analysis
All analyses were conducted using SAS statistical software (Version 9.2 SAS Institute Inc., Cary, NC) and Systat (Systat, Inc., San Jose, CA). Categorical variables were assessed using chi-square tests for proportional differences. Continuous variables were analyzed using a one-way ANOVA. To address co-linearity within cardiovascular factors and understand relationships of cardiovascular factors (atrial fibrillation, hypercholesterolemia, hypertension, diabetes and BMI) to certain neurodegenerative diseases (CBD, DLB, FTD, PD, ProbAD, PossAD, PSP and VaD) and Prion as compared to normals, we fitted generalized linear mixed models (using PROC GLIMMIX in SAS). These models are fitted under the assumption that the outcome is influenced by both fixed and random effects. The fixed effects included sex, age at last visit, and all cardiovascular factors (atrial fibrillation, hypercholesterolemia, hypertension, diabetes, and BMI) analyzed.21 Although ADCs share common components and features, each ADC has unique research questions and methods 20 leading to clustered data; hence the individual ADCs were considered a random effect in the GLIMMIX model. Given that approximately 40% of APOE values were unavailable in our series; APOE genotype was not included in the models. Each disease (CBD, DLB, FTD, PD, ProbAD, PossAD, PSP, Prion, and VaD) was run separately as an outcome variable with normal cognition as the reference group. A False Discovery Rate 22 was used to correct for multiple comparisons setting an α level of 0.001.
RESULTS
Clinical Data and Demographics
Demographics for each group are listed in Table 1. Frequencies of cardiovascular factors and average BMIs for each group are listed in Table 2. Supplemental Figure 1 displays the relative frequency of the disease groups and normals in the series; normals and ProbAD being the most common diagnoses (49% and 37%, respectively).
Table 1.
Demographic characteristics for each clinical diagnostic group.
| Normal | ProbAD | PossAD | DLB | VaD | FTD | PD | PSP | CBD | Prion | P value † | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N total | 8,513 | 6,555 | 683 | 339 | 140 | 778 | 310 | 63 | 80 | 71 | |
| % Female | 66.2% | 58.5% | 57.4% | 23.6% | 56.4% | 41.7% | 29.9% | 45.3% | 54.3% | 40.9% | <0.0001 |
| Age at visit, yrs.* |
73±11.2 | 77±9.9 | 78±10.1 | 74±7.8 | 78±10.0 | 66±9.5 | 74±10.1 | 71±9.4 | 67±7.4 | 60±10.6 | <0.0001 |
| Smoking History | 46.1% | 42.6% | 47.4% | 47.1% | 49.3% | 41.1% | 43.0% | 44.4% | 46.9% | 34.3% | <0.0001 |
| APOE 4 carrier | 33.8% | 63.3% | 45.1% | 47.0% | 28.4% | 37.0% | 27.8% | 27.9% | 24.5% | 33.3% | <0.0001 |
Abbreviations: ProbAD, Probable Alzheimer’s disease; PossAD, Possible Alzheimer’s disease; DLB, dementia with Lewy bodies; VaD, vascular dementia; FTD, frontotemporal dementia; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; CBD, corticobasal degeneration; Prion, prion disease
Age at visit is listed as Mean±standard deviation
P-values are from ANOVA (age) and chi-squared test for a difference in proportion across all groups.
Table 2.
Body mass index (mean ± standard deviations) and frequencies of cardiovascular factors for each clinical diagnostic group.
| Normal | ProbAD | PossAD | DLB | VaD | FTD | PD | PSP | CBD | Prion | |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI | 27±5.5 | 26±4.9 | 27±5.4 | 26±4.4 | 29±6.9 | 28±5.1 | 26±4.8 | 27±4.5 | 26±5.1 | 24±5.0 |
| Hypertension | 54.1% | 54.2% | 58.4% | 50.0% | 87.1% | 40.8% | 44.1% | 54.7% | 34.6% | 42.3% |
| Hypercholesterolemia | 53.7% | 53.1% | 58.7% | 50.6% | 75.7% | 47.6% | 44.1% | 46.9% | 46.9% | 38.0% |
| Diabetes | 13.5% | 12.5% | 15.6% | 9.5% | 30.7% | 9.5% | 8.4% | 10.9% | 4.9% | 9.9% |
| Atrial Fibrillation | 6.4% | 5.9% | 6.7% | 9.2% | 18.6% | 2.8% | 6.1% | 3.1% | 3.7% | 1.4% |
Abbreviations: ProbAD, Probable Alzheimer’s disease; PossAD, Possible Alzheimer’s disease; DLB, dementia with Lewy bodies; VaD, vascular dementia; FTD, frontotemporal dementia; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; CBD, corticobasal degeneration; Prion, prion disease; BMI, Body mass index
Presence of Cardiovascular Factors across Clinical Groups
Summaries of results from the generalized linear mixed models comparing each disease group to normals adjusted for age at visit, sex, and cardiovascular factors, are listed in Table 3. In the current series, individuals with a diagnosis of ProbAD were more likely to have recent/active atrial fibrillation at the time of their last visit as compared to normals (P<0.0001). Furthermore, individuals with a diagnosis of DLB and ProbAD were more likely to have lower BMI than normals (P values <0.0001). As expected, given diagnostic criteria for VaD,2 persons who had recent/active hypertension at the time of their last visit were more likely to have a diagnosis of VaD than normal (P <0.001). There were no other statistically significant associations meeting the prescribed α level. Furthermore, there were several statistically significant associations between demographic variables such as gender and age at visit with each cardiovascular factor; odds ratios as well as 95% confidence intervals and corresponding p-values are listed in Supplemental Table 1.
Table 3.
Results from generalized linear mixed models for associations of cardiovascular factors for each neurodegenerative disease as compared to normals.
| Hypertension | Diabetes | BMI | Atrial Fibrillation | Hypercholesterolemia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-value | Estimate | SE | p-value | Estimate | SE | p-value | Estimate | SE | p-value | Estimate | SE | p-value | |
| ProbAD | −0.04 | 0.04 | 0.38 | −0.04 | 0.06 | 0.51 | −0.05 | 0.09 | <0.0001 | −0.44 | 0.09 | <0.0001 | −0.03 | 0.04 | 0.55 |
| PossAD | 0.00 | 0.10 | 0.99 | 0.11 | 0.14 | 0.42 | −0.01 | 0.01 | 0.26 | −0.35 | 0.19 | 0.07 | 0.15 | 0.10 | 0.14 |
| DLB | −0.17 | 0.16 | 0.29 | −0.39 | 0.25 | 0.12 | −0.08 | 0.02 | <0.0001 | 0.02 | 0.27 | 0.95 | 0.10 | 0.15 | 0.50 |
| VaD | 1.22 | 0.30 | <0.0001 | 0.65 | 0.23 | 0.005 | 0.00 | 0.02 | 0.92 | 0.80 | 0.28 | 0.004 | 0.52 | 0.24 | 0.03 |
| PSP | 0.17 | 0.15 | 0.26 | −0.19 | 0.21 | 0.36 | −0.01 | 0.01 | 0.48 | −0.29 | 0.29 | 0.32 | −0.03 | 0.14 | 0.81 |
| CBD | 0.04 | 0.15 | 0.80 | −0.12 | 0.21 | 0.55 | −0.02 | 0.01 | 0.17 | −0.09 | 0.28 | 0.76 | −0.03 | 0.14 | 0.82 |
| Prion | 0.02 | 0.09 | 0.80 | 0.00 | 0.13 | 0.99 | −0.01 | 0.01 | 0.23 | −0.03 | 0.18 | 0.85 | −0.02 | 0.09 | 0.85 |
| PD | −0.20 | 0.15 | 0.18 | −0.23 | 0.26 | 0.38 | −0.05 | 0.02 | 0.006 | −0.09 | 0.29 | 0.75 | −0.30 | 0.15 | 0.05 |
| FTD | −0.09 | 0.13 | 0.42 | −0.10 | 0.16 | 0.55 | 0.00 | 0.01 | 0.87 | −0.35 | 0.25 | 0.17 | 0.00 | 0.11 | 0.97 |
Abbreviations: SE, standard error. NOTE: Age at visit, gender, and all cardiovascular factors listed above were listed as fixed effects in the generalized mixed models. Separate models for each disease group as the output variable were run having normals as the reference group. A False Discovery Rate was used to correct for multiple comparisons setting an α level of 0.001.
BMI by Category
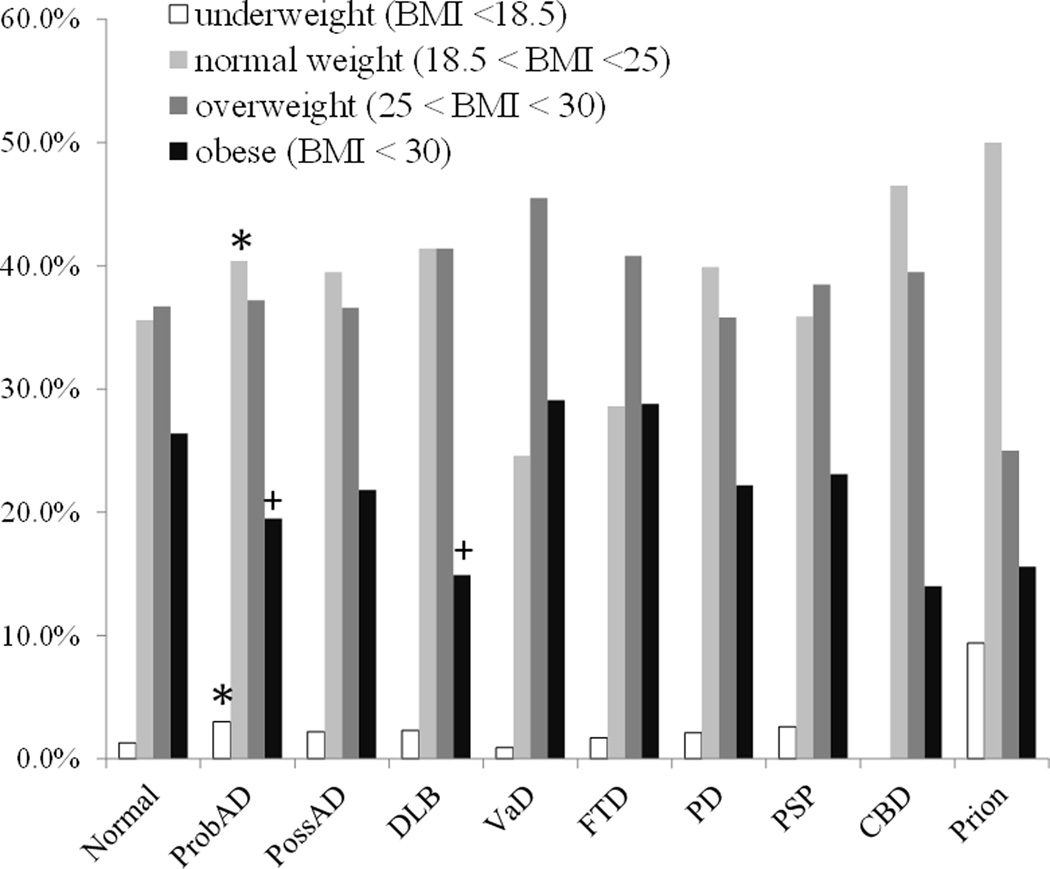
Figure 2 demonstrates the frequencies of individuals in each disease category who fell within ranges for underweight, normal BMI, overweight, or were obese at the time of their last visit. Given a set α level of 0.001, compared to normals, those with ProbAD were more likely to be underweight (3% vs. 1%, p<0.001). Subjects with ProbAD were more likely to be within the normal BMI range compared to normals (40% vs. 36%, p<0.001). Subjects with ProbAD and DLB were significantly less likely to be obese when compared to normals (20%, 15%, and 26%, respectively, p values <.001 for both comparisons). There were no differences across any groups in the overweight category (overall p value = 0.27).
Figure 2.
Frequencies of subjects in each disease group who were considered underweight, normal weight, overweight and obese at the time of their last visit. * Significantly greater than normals, + significantly less than normals.
DISCUSSION
This study used cross-sectional clinical data from the NACC UDS to investigate several neurodegenerative diseases and their association with the presence or absence of late life cardiovascular factors as compared to normals. Our findings support a hypothesis that associations between late life cardiovascular factors and neurodegenerative disease may be restricted to AD and VaD, and possibly DLB. The findings also support the emerging understanding, convincingly demonstrate by several independent groups, that in late life once an individual has dementia, cardiovascular factors are often less prevalent.3, 5, 7, 10, 12, 23, 24 This is critical since certain factors, such as BMI may be a disease specific comorbidity.
Much evidence for the relationships of cardiovascular factors to neurodegenerative diseases have been derived from studies of AD, or from studies in which AD was most likely the dominant cause of dementia or cognitive impairment (for review 9). This is one of the first studies to investigate how late life non-AD neurodegenerative diseases might be associated with certain cardiovascular factors. These results demonstrate a specific association of AD and DLB with cardiovascular factors, especially with respect to BMI. As autopsy studies have shown that 80% or more of subjects with DLB also meet neuropathological criteria for AD;25 hence, it is quite possible that the DLB association may be due to underlying AD. Given individuals already presented with the specific neurodegenerative disease, (AD, DLB) these data suggest that lower BMI may be a comorbidity of certain dementia subtypes.
For a disease specific molecular explanation, one may look at hypothalamic neurofibrillary tangles that are known to occur very early in the Alzheimer’s disease process.26, 27 The hypothalamus has been known to exert central control over peripheral glucose, fat, and energy metabolism.28 Neurofibrillary pathologies may cause disruptions in appetite, or perhaps, given AD has been associated with lower levels of brain glucose uptake and metabolic dysfunction,29 stores within the periphery may be depleted to compensate for brain metabolic demand, leading to lower BMI. More detailed research is needed to confirm this hypothesis. Other hypotheses to account for the association of lower BMI once AD is present may revolve around non-specific effects of neurodegenerative diseases, such as mental stress of life, nihilistic apathy that may exist once a patient has a terminal disease such as dementia, decreases in activities of daily living (i.e., grocery shopping, cooking) and loss of recall and understanding due to cognitive dysfunction, that may lead to under nutrition.13, 30–32 The current study revealed that BMI was not associated with PD, Prion, FTD, PSP, or CBD after adjusting for confounders such as age. Another hypothesis concerns the hyposmia often observed in prodromal stages of AD; however, early hyposmia is also present in other diseases such as PD,33–36 which did not show any significant association with BMI. The absence of significant relationships between cardiovascular factors and other dementias may support that certain neuropathological features may be sufficient for the development of the dementia. In contrast, the significant relationships between certain cardiovascular factors and AD suggest that cerebrovascular disease could potentially contribute to the development of dementia.37 Cardiovascular damage has been reported to be common within the setting of neuropathologically confirmed AD.4, 38, 39 Some studies have even suggested that cardiovascular damage is associated with accelerated cognitive decline within the setting of AD.38, 40 Furthermore, a study conducted by our colleagues suggests that AD is more closely related to cerebrovascular disease rather than coronary artery disease.12 Another possibility for the discrepant associations between cardiovascular factors, AD and non-AD dementias may be related to group differences in severity and duration of dementia, as both a more severe cognitive deficit and a longer disease duration are likely to result in more weight loss and greater reversal or loss of cardiovascular factors. Further analysis investigating the relationship of BMI to dementia severity and progression is warranted.
There are numerous strengths to utilizing NACC data to answer questions like the ones posed in this study. The standardized data collection protocols used across ADCs, as well as the large number of individuals in each disease group provide reliable estimates of cardiovascular factors for those in each disease group and allow for statistical models such as the ones presented here. Identification of the associations of cardiovascular factors among different neurodegenerative diseases may have important implications, including the possibility of an AD-specific molecular mechanism and the possibility of prevention or slowing of disease progression through lifestyle modifications and medications. There are some limitations that should be mentioned with respect to this study. Due to many of the ADCs' focus on elderly subjects, the current study was unable to analyze these factors earlier in life. Also, persons who die in middle age or younger old age, e.g., due to more severe vascular disease, would generally not have been included in this study. Furthermore, although the current study would have liked to analyze autopsy confirmed diagnosis, neuropathology data were available for a minimal percentage of the reported series (less than 10%), and these small numbers caused quasi-separation during the modeling process and result in either inconsistent results or non-convergence, hence the use of the much larger clinical dataset.
Despite these limitations, these results support a concept of a disease-specific late life association with BMI. Given the cross-sectional nature of our study, we cannot readily determine causality. Although the current study provides evidence of lower BMI being a comorbidity of certain dementias, future longitudinal studies spanning from midlife to death, investigating the concordance of clinical and neuropathological diagnoses in the context of cardiovascular conditions accounting for co-linearity are warranted.
Supplementary Material
Percentages of individuals with each disease group and individuals who were considered normal within the series.
ACKNOWLEDGEMENTS
The authors would like to Drs. Stephen Hawes, Lilah Besser, and Leslie Phillips and for their helpful aid. This study was supported by National Institute on Aging grants to the National Alzheimer's Coordinating Center, subcontract: National Alzheimer’s Coordinating Center Junior Investigator Award. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD).
Footnotes
All authors report no conflicts of interest with relation to this study.
REFERENCES
- 1.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 4.White L, Launer L. Relevance of cardiovascular risk factors and ischemic cerebrovascular disease to the pathogenesis of Alzheimer disease: a review of accrued findings from the Honolulu-Asia Aging Study. Alzheimer Dis Assoc Disord. 2006;20:S79–S83. doi: 10.1097/00002093-200607001-00012. [DOI] [PubMed] [Google Scholar]
- 5.Buchman AS, Wilson RS, Bienias JL, et al. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 6.Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- 7.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain. 2013;136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peltz CB, Corrada MM, Berlau DJ, et al. Cognitive impairment in nondemented oldest-old: prevalence and relationship to cardiovascular risk factors. Alzheimers Dement. 2012;8:87–94. doi: 10.1016/j.jalz.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer's disease, and dementia. J Alzheimers Dis. 2015;43:739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 10.Gu Y, Scarmeas N, Cosentino S, et al. Change in body mass index before and after Alzheimer's disease onset. Curr Alzheimer Res. 2014;11:349–356. doi: 10.2174/1567205010666131120110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman AS, Schneider JA, Wilson RS, et al. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 12.Beach TG, Maarouf CL, Brooks RG, et al. Reduced clinical and postmortem measures of cardiac pathology in subjects with advanced Alzheimer's Disease. BMC Geriatr. 2011;11:3. doi: 10.1186/1471-2318-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Mulley GP, Losowsky MS. Why are Alzheimer patients thin? Age Ageing. 1988;17:21–28. doi: 10.1093/ageing/17.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson M, Wang J, Davis DR, et al. Co-morbidity associated with dementia. Am J Alzheimers Dis Other Demen. 2002;17:73–78. doi: 10.1177/153331750201700210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf-Klein GP, Siverstone FA, Brod MS, et al. Are Alzheimer patients healthier? J Am Geriatr Soc. 1988;36:219–224. doi: 10.1111/j.1532-5415.1988.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 16.Kokjohn TA, Maarouf CL, Roher AE. Is Alzheimer's disease amyloidosis the result of a repair mechanism gone astray? Alzheimers Dement. 2012;8:574–583. doi: 10.1016/j.jalz.2011.05.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34:561–566. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]
- 18.Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4:771–780. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- 19.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 21.Dobson AJ. An Introduction to Generalized Linear Models, Second Edition. CRC Press; 2001. [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 23.Tolppanen AM, Ngandu T, Kareholt I, et al. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 24.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dugger BN, Adler CH, Shill HA, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord. 2014;20:525–529. doi: 10.1016/j.parkreldis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 27.Schultz C, Ghebremedhin E, Braak H, et al. Neurofibrillary pathology in the human paraventricular and supraoptic nuclei. Acta Neuropathol. 1997;94:99–102. doi: 10.1007/s004010050679. [DOI] [PubMed] [Google Scholar]
- 28.Valdearcos M, Xu AW, Koliwad SK. Hypothalamic inflammation in the control of metabolic function. Annu Rev Physiol. 2015;77:131–160. doi: 10.1146/annurev-physiol-021014-071656. [DOI] [PubMed] [Google Scholar]
- 29.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 30.Markus HS, Tomkins AM, Stern GM. Increased prevalence of undernutrition in Parkinson's disease and its relationship to clinical disease parameters. J Neural Transm Park Dis Dement Sect. 1993;5:117–125. doi: 10.1007/BF02251202. [DOI] [PubMed] [Google Scholar]
- 31.Abbott RA, Cox M, Markus H, et al. Diet, body size and micronutrient status in Parkinson's disease. Eur J Clin Nutr. 1992;46:879–884. [PubMed] [Google Scholar]
- 32.Jaafar AF, Gray WK, Porter B, et al. A cross-sectional study of the nutritional status of community-dwelling people with idiopathic Parkinson's disease. BMC Neurol. 2010;10:124. doi: 10.1186/1471-2377-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adler CH. Premotor symptoms and early diagnosis of Parkinson's disease. Int J Neurosci. 2011;121(Suppl 2):3–8. doi: 10.3109/00207454.2011.620192. [DOI] [PubMed] [Google Scholar]
- 34.McKinnon J, Evidente V, Driver-Dunckley E, et al. Olfaction in the elderly: a cross-sectional analysis comparing Parkinson's disease with controls and other disorders. Int J Neurosci. 2010;120:36–39. doi: 10.3109/00207450903428954. [DOI] [PubMed] [Google Scholar]
- 35.Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry. 1991;148:357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- 36.Mesholam RI, Moberg PJ, Mahr RN, et al. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- 37.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer's disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 39.Corder EH, Ervin JF, Lockhart E, et al. Cardiovascular damage in Alzheimer disease: autopsy findings from the Bryan ADRC. J Biomed Biotechnol. 2005;2005:189–197. doi: 10.1155/JBB.2005.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentages of individuals with each disease group and individuals who were considered normal within the series.