Abstract
Sensorineural hearing loss (SNHL) is one of the most common diseases, accounting for about 90% of all hearing loss. Leading causes of SNHL include advanced age, ototoxic medications, noise exposure, inherited and autoimmune disorders. Most of SNHL is irreversible and managed with hearing aids or cochlear implants. Although there is increased understanding of the molecular pathophysiology of SNHL, biologic treatment options are limited due to lack of noninvasive targeted delivery systems. Obstacles of targeted inner ear delivery include anatomic inaccessibility, biotherapeutic instability, and nonspecific delivery. Advances in nanotechnology may provide a solution to these barriers. Nanoparticles can stabilize and carry biomaterials across the round window membrane into the inner ear, and ligand bioconjugation onto nanoparticle surfaces allows for specific targeting. A newer technology, nanohydrogel, may offer noninvasive and sustained biotherapeutic delivery into specific inner ear cells. Nanohydrogel may be used for inner ear dialysis, a potential treatment for ototoxicity-induced SNHL.
Keywords: Nanomedicine, nanohydrogel, targeted delivery, ototoxicity, nanodialysis, inner ear
Graphical abstract
1. Introduction
The inner ear consists of the cochlea and vestibule, and it is a crucial drug delivery target for the treatment of hearing and balance disorders, respectively. Routes for drug entry into the inner ear include the systemic circulation, which feeds the labyrinthine artery that supplies the cochlea and vestibule, and the round window membrane (RWM), which connects the middle ear with the inner ear. Anatomic challenges for drug delivery into the inner ear include the blood-inner ear barrier, limited labyrinthine artery supply, and difficult access to the RWM. Mechanistic challenges for drug delivery into the inner ear include variations in RWM permeability, biotherapeutic instability, uncontrolled drug elimination, and nonspecific drug delivery. While inner ear diseases have historically been treated with systemic therapies, local intratympanic (IT) injection can offer more efficient and less systemically toxic drug delivery. Hydrogel, another localized drug delivery system, has the additional advantages of sustained and controlled drug release, which can reduce inter-patient variability in drug elimination while maximizing drug diffusion. Although these forms of drug delivery have been used successfully in the treatment of various inner diseases, such as sudden sensorineural hearing loss (SHL), autoimmune inner ear disease (AIED), and Meniere’s disease, they can neither stabilize newer therapeutics, including biomaterials, nor target therapies to specific cell types within the inner ear. Nanotechnology may be able to address these current limitations and offer a noninvasive, sustained and targeted drug delivery system.
1.1 Systemic Drug Delivery
Systemic drug delivery can be accomplished via the intravenous, intramuscular or oral route, and it has been successfully used to treat inner ear diseases such as sudden hearing loss (SHL), autoimmune inner ear disease (AIED), and Meniere’s disease. Oral corticosteroids are commonly prescribed for the treatment of SHL, defined as a 30 decibels or greater hearing loss in 3 consecutive frequencies over less than 72 hours [1]. A pioneering study by Wilson et al demonstrated that oral corticosteroids could effectively reverse SHL with a recovery rate of 61%, as compared with a placebo recovery rate of 32% [2]. Additional studies have since demonstrated SHL recovery rates of 57–66% with oral corticosteroid use [3,4]. More long-term courses of oral corticosteroids are used in the treatment of AIED, which presents as progressive bilateral asymmetric sensorineural hearing loss (SNHL) occurring over weeks to months, with possible vestibular deficit [5–7]. The current treatment protocol for AIED is 4 weeks of high-dose oral prednisone and subsequent tapering to the lowest possible dose while maintaining therapeutic effect; the rate of hearing preservation using this regimen is reportedly between 53–70% [6]. Like SHL and AIED, Meniere’s disease has also been successfully treated with systemic drug therapy. Aminoglycosides, which were historically delivered intra-muscularly, are indicated for the treatment of vertigo in patients with who have failed more conservative medical therapies [1]. Although inherently ototoxic, well-titrated doses of aminoglycosides can preferentially ablate vestibular structures that are responsible for balance, thereby reducing vertigo symptoms in Meniere’s disease patients [8,9]. However, even with current titration methods, hearing loss associated with treatment is not uncommon.
Though drug delivery to the inner ear can be achieved via systemic administration, limited local blood supply and poor penetration of the blood-inner ear barrier often results in subtherapeutic local concentrations. Administration of large doses of medication is required to produce the desired therapeutic effect, often leading to severe toxicities. Systemic corticosteroids can lead to hypertension, hyperglycemia, osteoporosis and immunosuppression, along with more long-term adrenal suppression at high doses [1]. In a prospective study by Alexander et al of AIED patients receiving oral prednisone, 17.6% of patients developed of hyperglycemia in a dose-dependent manner and 39% of patients experienced weight gain of 10 pounds or more. Although the hyperglycemia appeared to be reversible, the weight gain persisted even after drug discontinuation [7]. Aminoglycoside toxicity, too, can produce hearing loss and persistent disequilibrium [9–11]. Although certain aminoglycosides, such as streptomycin and gentamicin, are more vestibulotoxic than cochleotoxic, high doses can lead to cochlear damage and sensorineural hearing loss [8–10].
1.2 Intratympanic Drug Delivery
To improve the efficacy and reduce the adverse effects of systemic drug delivery, more localized drug delivery systems were developed to target inner ear disease. Schuknecht et al was the first to introduce intratympanic (IT) injection as a means of delivering streptomycin into the inner ear for the successful treatment of Meniere’s disease patients [12]. IT injection of a drug into the middle ear space allows for drug diffusion across the round window membrane (RWM) into the inner ear. In bypassing the labyrinthine artery and blood-inner ear barrier, IT injection provides a more direct and efficient approach as compared to systemic drug administration. In fact, drug concentrations measured in the inner ear fluids, perilymph and endolymph, are significantly higher with IT injection than with oral or parenteral administration [6,13]. Not only can IT injection more efficiently deliver drugs into the inner ear, it can also avoid many of the side effects associated with systemic therapy.
Due to these advantages and the relative ease of performing the procedure in the office, IT injection of corticosteroids and aminoglycosides is now commonly used for treatment of SHL and Meniere’s disease. [1,13]. For example, IT corticosteroid injections can be used as primary or salvage therapy for SHL and for patients with contraindications for systemic corticosteroid therapy [14]. A large randomized prospective trial by Rauch et al found that primary IT corticosteroid therapy and primary oral corticosteroid therapy were equally effective at restoring hearing in patients with SHL, although the latter was associated with systemic side effects such as hyperglycemia and appetite changes [15]. A more recent study by Filipo et al found that while IT and oral corticosteroid therapy produced similar improvement in the pure tone averages of patients with SHL, IT injection led to significantly better hearing threshold improvement, suggesting the therapeutic superiority of primary IT injection [4]. IT corticosteroid injection for the treatment of AIED is less well-studied, and the few reports published involve AIED patients who have failed or could not tolerate systemic steroid and/or methotrexate therapy. While high dose oral corticosteroids continue to be the standard of care for AIED, IT corticosteroids have shown promise as at least a salvage treatment option for these patients [6].
Although IT delivery is efficient and has reduced the toxicities associated with systemic drug delivery, it continues to demonstrate inconsistency in delivering standard doses of drugs into the inner ear. Because IT injection relies on diffusion for the drug to reach the inner ear from the middle ear, its success is directly related to the amount of drug that comes into contact with the RWM. Any drug that is not in contact with the RWM is eliminated via the Eustachian tube. Differences in RWM permeability can thus lead to variable rates of drug retention and elimination. This inconsistency is evident in the application of IT aminoglycosides for Meniere’s disease treatment. As mentioned, aminoglycosides are inherently ototoxic and can cause hearing loss and permanent disequilibrium at inappropriately high doses. Early studies of IT streptomycin delivery for Meniere’s disease showed that although vertigo was relieved in the majority of patients, hearing loss developed in greater than 25% of patients [12,16]. In an effort to reduce toxicity, the clinical endpoint of therapy changed over time from complete ablation to alteration of the number of vertiginous attacks, and various timing and dosage regimens of IT gentamicin injection were tested [1,17]. A meta-analysis by Chia et al evaluated the efficacy of vertigo control and hearing loss rate of scheduled drug delivery, including various timing and dosage regimens, as compared to drug titration. They concluded that the titration method had the best rate of vertigo control (96.3%) while multiple daily dosage had the highest rate of hearing loss (34.7%) [18]. The superiority of the titration method, in which drug delivery is terminated with onset of vestibular symptoms, changes in vertigo, or hearing loss, as compared with scheduled dosing emphasizes the significant inter-patient variability seen with IT drug delivery.
The hearing toxicity associated with current IT aminoglycoside injection indicates that this approach cannot achieve targeted inner ear drug delivery. Instead of seeking a drug that is purely vestibulotoxic, it would be prudent to develop a drug delivery system that specifically carries the drug to the cell population of interest. The ideal drug delivery system for Meniere’s disease would target the vestibular hair cells, which sense and transduce positional displacement, and the dark cells of the stria vascularis and planum semilunatum, which produce the endolymph associated with vertiginous symptoms [18].
1.3. Hydrogel Delivery System
To address the inconsistency and inter-patient variability of IT drug delivery, hydrogel was developed as alternative form of localized drug delivery. Paulson et al first introduced the chitosan-glycerophosphate (CGP) hydrogel delivery system, which aimed to reduce variation in drug elimination by providing more sustained and controlled drug delivery into the inner ear [19]. The CGP hydrogel is applied, as a liquid, directly onto the round window niche (RWN), where it then solidifies at body temperature to maximize RWM contact. CGP hydrogel is composed of a porous matrix, which is degraded over time by middle ear lysozymes to generate slow and continuous drug release. Studies have since shown that the CPG hydrogel system can steadily release drugs such as dexamethasone and gentamicin into the perilymph of the inner ear [19,20]. In functional studies of mice who received CPG hydrogel-delivered gentamicin, the sustained release of relatively low drug concentrations per day led to longer vestibular suppression and less risk of hearing loss as compared with the IT injection group [20]. Not only does the CPG-hydrogel system offer a more efficient and predictable release mechanism, it can be further regulated by the enzyme chitosanase. Chitosanase can break down the chitosan chains in the hydrogel through a glycolytic reaction to cause faster drug elimination via the Eustachian tube [21]. In the case of gentamicin hydrogel administration, chitosanase can act as an additional safety measure to prevent hearing loss in the event of an unanticipated drug reaction.
1.4 Nanoparticle Delivery
While the methods discussed above can provide noninvasive, sustained and regulated drug delivery into the inner ear, they cannot deliver newer therapeutics, including biomaterials, which are relatively unstable within extracellular compartments. Furthermore, these methods cannot by themselves target delivery to specific locations or cell types in the inner ear. Nanoparticle technology has therefore been of great interest due to its stabilizing effect on biomaterials and potential for targeted therapeutic delivery. Please refer to section 4 for further discussion about the applications of nanoparticle drug delivery for treatment of inner ear disease.
2. Inner Ear Anatomy and Physiology
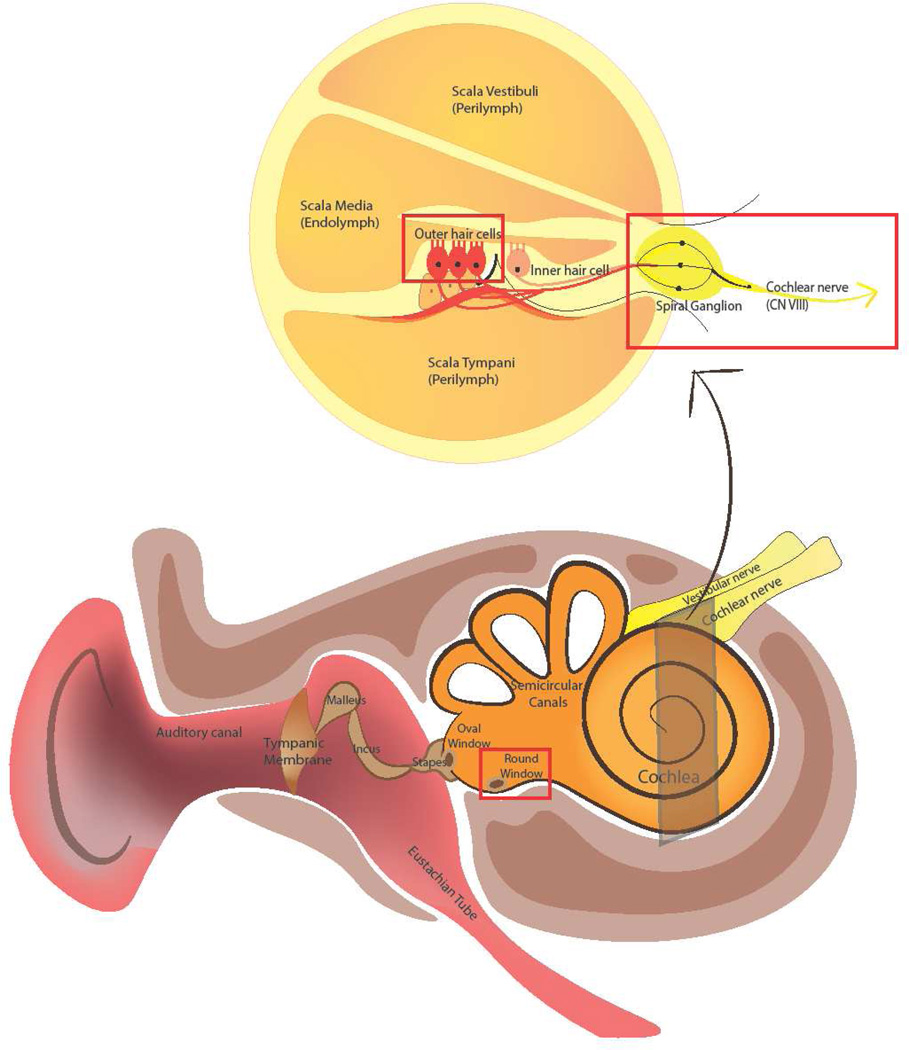
The inner ear consists of the cochlea and the vestibule, which are responsible for hearing and balance, respectively. These are exquisitely delicate organs encased in the hardest bone in the body with limited pathways for access. Efforts to target therapy into the inner ear are further challenged by the need for noninvasive access, as invasion of the inner ear leads to irreversible sensorineural hearing loss (SNHL). Figure 1 illustrates the anatomy of ear and the location of the round window, a crucial site of entry for drug delivery into the inner ear.
Figure 1. Ear Anatomy and Sites for Targeted Inner Ear Delivery.
Sound enters the auditory canal and delivers mechanical energy to the middle ear via vibration of the tympanic membrane and subsequently, the malleus, incus and stapes. Vibration of the stapes against the oval window allows for sound to enter the inner ear and cause fluid wave propagation within the perilymph of the cochlea. Movement of perilymph leads to deflection of hair cells and conversion of mechanical into electrical energy, which can stimulate neuronal cells along with the auditory pathway from the spiral ganglion to the auditory cortex.. The round window is one of the two openings to access the inner ear from the middle ear and it is sealed by the round window membrane. The round window membrane is surrounded by a bony pouch called the round window niche. Data collected recently demonstrate that different substances, including antibiotics, steroids, local anesthetics, and biomaterials can traverse the membrane into perilymph of the inner ear.
2.1 Sound transduction pathway
To reach the inner ear, sound traverses the auditory canal where mechanical energy causes vibration of the tympanic membrane at its medial extent. This vibration is transmitted across the middle ear ossicles, the malleus, incus, and stapes, amplifying the energy received by the inner ear via lever effects. The energy is further amplified (17:1) due to the difference in the surface area of the tympanic membrane and oval window [6].
Sound energy is transmitted to the cochlea as the stapes footplate vibrates on the oval window, one of the structures that connects the middle ear with the inner ear. The cochlea is a spiral apparatus with approximately 2.5 turns that contains three fluid-filled longitudinal tracts: the scala vestibule, scala media and scala tympani [22]. The scala vestibule and scala tympani carry perilymph, while the scala media carries endolymph. Sound vibrations along the basilar membrane of the cochlea generate fluid waves within these cochlear tracts, and the differential structural composition of the cochlea along its length causes maximal vibration by higher frequencies at the base and lower frequencies at the apex. The mechanical energy of basilar membrane vibration is converted into electrical energy via surface mechanoreceptors on highly specialized hair cells [23,24]. Each hair cell has apical extensions called stereocilia, which respond to fluid deflection by opening transduction channels and causing hair cell depolarization. Hair cell depolarization then triggers depolarization of the bipolar neurons of the spiral ganglion, which collectively form the cochlear division of CN VIII. The cochlear nerve travels along the internal auditory canal and eventually synapses with other neuronal cells in the central nervous system [24,23]. Dysfunction or damage at any point along this neuronal pathway can lead to hearing loss or dysfunction. Thus, current research is aimed at finding biomaterials that will increase neuronal survival and potentiate neurite outgrowth as a means of treating specific forms of neural hearing loss. [25–28].
2.2 Cochlear diffusion of drugs via the round window membrane
Current inner ear drug delivery strategies rely on another connection between the middle and inner ear, the round window, which is covered by a three-layered, semi-permeable round window membrane (RWM). The RWM is composed of an outer epithelial layer, middle fibrous layer, and an inner epithelial layer [22]. As demonstrated with IT injection, hydrogel delivery and nanoparticle delivery (see sections 1.2–1.4), the round window has been a successful entryway for inner ear drug delivery. The exact pharmacokinetics of drug delivery across the human RWM are difficult to study, due to the risk of permanent hearing loss when sampling inner ear fluids. However, animal studies and computer simulations have provided greater understanding and quantification of drug delivery into the inner ear [29–31].
Drug dispersal into the inner ear is influenced by multiple factors, including RWM permeability, the rate of drug diffusion within the perilymph, and the rate of drug clearance. RWM permeability depends upon solute characteristics as well as local biochemical mediators, RWM thickness, and presence of RWM inflammation or injury [22]. Smaller, cationic particles preferentially pass through the RWM, and reduced RWM thickness increases permeability [32]. Local biochemical mediators, such as leukotrienes, prostaglandins, and histamine, can alter RWM permeability; in fact, histamine has been shown to facilitate uptake of intratympanically injected dexamethasone [22,33]. Similarly, the local inflammatory state of the RWM can affect its permeability. Goycoolea et al found that initial otitis media infection led to increased RWM permeability, while later stages of infection led to decreased permeability due to granulation tissue formation and RWM thickening [32,34]. Nordang et al found that inflammation secondary to topical application of saline or hydrocortisone can, over time, lead to local edema and membrane leakage of the RWM, which in turn increases RWM permeability [35]. This finding suggests that any intratympanic injection can lead to inflammatory changes that may subsequently alter RWM permeability for that drug [22]. The optimal delivery system to potentially reduce RWM permeability variations would offer slow and sustained drug release to prevent drastic local environmental changes. The hydrogel system, as discussed in section 1.3, may be able to accomplish this goal.
Once a drug crosses the RWM into the inner ear, further distribution depends upon the rate of drug diffusion within the perilymph and the rate of drug clearance. Salt and Ma administered a marker ion, trimehtylphneylammonoium (TMPA) across RWM of guinea pigs to study inner ear drug diffusion, and found that TMPA concentration was mainly determined by its rate of diffusion and its rate of clearance from the perilymph [29]. Furthermore, they found that TMPA unevenly distributed throughout the turns of the cochlea, with the highest concentration at the base and the lowest at the apex. Interestingly, additional studies by Salt suggested that it was not possible to achieve uniform drug distribution along the cochlea, and that even prolonged drug administration did not change the concentration gradient [30]. While the concentration gradient could not be eliminated, overall inner ear drug concentration could be maximized by increasing the time the drug remains in the middle ear, which increases diffusion relative to clearance [31]. This finding highlights the importance of a sustained drug delivery system that can maintain therapeutic doses of drug within the inner ear. Moreover, the persistence of the base-apex concentration gradient generates a need for targeted inner ear delivery such that a drug may preferentially bind to apical cells without affecting basilar cells. These challenges may be addressed by the hydrogel system and nanotechnology, respectively.
3. Nanoparticle Delivery
Nanoparticles (NPs) have been created from a variety of materials, generally ranging from tens to several hundred nanometers in diameter, and can be customized to encapsulate various therapeutic agents of interest [36]. Customization of NP attributes can allow for noninvasive application, drug stabilization, controlled release, and surface modification for specific targeting [37]. Many NP systems have been developed for inner ear drug delivery, including poly(d,l-lactide-co-glycolide acid) (PLGA) nanoparticles (NPs), magnetic NPs, lipid NPs, liposomes, polymersomes, hydroxyapatite NPs, and silica NPs [36]. Local administration of nanoparticles has been shown to successfully deliver biomaterials into the inner ear via the RWM, although there has been limited research on targeted NP delivery into the inner ear [38–41].
Certain classes of nanoparticles exhibit additional characteristics that offer unique diagnostic and therapeutic advantages. For example, superparamagnetic iron oxide nanoparticles (SPIONs), gold nanoparticles, and cationic polymer carriers may be used for bioimaging by MRI, confocal laser microscopy, two-photon luminescence, etc… [39,42–46] The inherent magnetic properties of SPIONs also allows for greater control of drug delivery and dispersion within the inner ear by application of an external magnet [42,43,47–49]. Nanoparticles such as cationic polymers and cationic liposomes offer non-viral vector options for gene therapy delivery, which reduces the immunogenicity, inflammatory responses, and risk of insertional mutagenesis [50–56]. Table 1 describes the current nanoparticle carrier systems available and applications for inner ear drug delivery.
Table 1.
Nanoparticle Carrier Systems for Inner Ear Delivery
| Nanoparticle (NP) Carrier System |
Characteristics | Advantages | Select Publications on NP Inner Ear Delivery |
|---|---|---|---|
| Poly(d,l-lactide-co-glycolide acid) (PLGA) nanoparticles [49,83–85] | Composed of biodegradable polymers Rate of degradation is regulated by the lactic:glycolic acid ratio and can promote sustained drug release |
Carry hydrophobic and hydrophilic molecules Sustained release and stabilization of drug during delivery |
Tamura et al (2005): local application of PLGA NPs in vivo allowed for delivery across the RWM into the perilymph [38] Wen et al (2014): PLGA NPs can deliver single and compound drugs via the RWM into the inner ear with sustained release and increased local bioavailability [86] |
| Superparamagnetic iron oxide nanoparticles (SPIONs) [42,48,49,83] | Composed of magnetite (Fe3O4) and/or maghemite (Fe2O3) | Delivery can be directed by external magnetic fields May be used for bioimaging by MRI |
Mondalek et al (2006): SPIONs can cross an in vitro tripartite model RWM under the influence of a magnetic field [42] Barnes et al (2007): SPIONs can cross RWMs of guinea pigs in vivo [43] Ge et al (2007): SPIONs embedded within PLGA NPs can be delivered into the chinchilla cochlea with wide distribution [39] |
| Lipid NPs [83,87] | Composed of a hydrophobic triglyceride core and an amphiphilic surfactant shell | Carry hydrophobic and hydrophilic molecules Sustained release and stabilization of drug during delivery Good for large scale manufacturing Low production cost |
Zou et al (2008): lipid NPs can cross the RWM via a paracellular mechanism to reach spiral ganglion cells, nerve fibers and spiral ligament fibrocytes in an in vivo rat model [40] Chen et al (2008, 2010): lipid NPs can deliver dexamethasone via intratympanic injection into the inner ear of guinea pigs [36,88] |
| Polymersomes [36,83,89] | Amphiphilic block copolymers that self-assemble to form vesicles in an aqueous environment | Carry hydrophobic and hydrophilic molecules Structurally similar to liposomes but with increased drug stabilization and sustained drug release Controlled drug release by using polymers reactive to specific external stimuli such as pH, temperature, or magnetic field |
Buckiova (2012): Both liposome and polymersome NPs are capable of carrying a disulfiram, a toxin, into the inner ear to cause spiral ganglion cell death with hearing loss measurable by a functional readout [37] See Section 4.2 for targeted polymersome drug delivery studies by Roy and Zhang [26,72,73] |
| Cationic polymers [50,55,56,90] | Examples include polyethylenimine (PEI), polyamidoamine dendrimer Composed of protonable amino nitrogen atoms (“proton sponge”), organelle-escape units, and degradable fragment Allows for release of gene therapy within endosomes without DNA degradation |
Can bind to negatively charged siRNA and DNA molecules to form polyplexes and be used for gene delivery Higher transfection efficiency than liposomes May be used for bioimaging |
Tan (2008): polyethylenimine can transfect the perilymph of the cochlea in an in vivo guinea pig model with sustained DNA delivery [50] Praetorius (2008): cationic lipids and dendrimers can transfect in the area of the organ of Corti in an in vitro and in vivo mouse model and cause expression of a reporter gene [91] |
| Liposomes [12,44, 55–58] | Vesicular structures similar to living cell membranes, formed by phospholipid organization into a bilayer structure Cationic liposomes created by adding cationic amine groups to neutral helper lipids |
Carry hydrophobic and hydrophilic molecules Sustained release and stabilization of drug during delivery Low production cost Cationic liposomes can bind to negatively-charged DNA molecules to form lipoplexes and be used for gene delivery |
Wareing et al (1999): intracochlear injection or infusion of a liposome-transgene complex can transfect cochlear cells with sustained transgene expression in an in vivo guinea pig model [51] Jero et al (2001): liposomes complexed with reporter humanized green fluorescent protein gene can be delivered across the RWM of mice in vivo and induce transgene expression within inner ear structures [52] Maeda et al (2007): liposomes complexed with plasmids expressing GJB2R75W, an allele that causes deafness, were delivered across on mouse RWMs, leading to positive transgene expression in the cochlea. Auditory brainstem response testing demonstrated that significant hearing loss was seen postdelivery, illustrating the functional effects of liposomal transgene delivery on hearing [53,94–96] Zuris et al (2014): cationic liposome delivery of Cre-recombinase and Cas9-based transcription activators into the mouse inner ear in vivo led to 90% Cre-mediated recombination and 20% Cas9-mediated genome modification within hair cells [54] |
| Gold nanoparticles [44–46,97] | Composed of gold atoms | Carry hydrophobic and hydrophilic molecules Plasmon resonance, can be used for imaging with confocal laser microscopy, two-photon luminescence, and optical coherence tomography |
There are no studies in the literature on gold nanoparticle delivery into the inner ear to date, but gold nanoparticles have been successfully used for targeted drug delivery in cancer cells |
| Nanohydrogel [21,74] | Combination of the CGP-hydrogel system and nanoparticle carriers | Can carry a variety of nanoparticles, which can encompass both hydrophobic and hydrophilic molecules Significantly more sustained release mechanism than individual nanoparticle carriers Release can be controlled by the enzyme, chitosanase |
Lajud (2014): liposomal NPs delivered via nanohydrogel in vitro persisted under physiological conditions for at least 2 weeks, and NPs could be detected in the scala media [74] See section 4.2 for additional discussion of the nanohydrogel system |
4. Targeting nanoparticle delivery
Targeted therapy involves the addition of surface ligands onto nanoparticles. The efficacy of these surface ligands depends on their stability and specificity. Bioconjugation allows for the creation of stable peptide bonds, thus anchoring and immobilizing ligands to the nanoparticle surface. Many bioconjugation techniques have been described in the literature including maleimide, N-hydroxysuccinimide, and carboiimide-based chemistries [57], but few have been found to consistently and effectively add ligands with the correct orientation, surface density, and site-specificity [58,59]. We will briefly review two more recently developed techniques, expressed protein ligation (EPL) and click chemistry.
EPL refers to a native, site-specific chemical ligation between a recombinant protein (i.e. ligand) with a C-terminal thioester, and a peptide or protein with an N-terminal cysteine [60–62]. A C-terminal thioester can be added onto a targeting ligand through the use of auto-processing proteins called inteins. Inteins are placed between the ligand and an affinity tag. Following bacterial expression and affinity purification, the ligand is released from the affinity tag to create a reactive thioester at the C-terminus. The thioester can then react with any peptide containing an N-terminal cysteine. This reaction has been shown to be highly efficient when both functional groups are at high concentrations [63], but studies of EPL when applied to nanoparticles have shown less efficacy, which may be due to limited nanoparticle concentrations [57].
Click chemistry offers stereospecific and efficacious additions of ligands to nanoparticles through a Cu I -catalyzed terminal alkyne-azide cycloaddition. This method has been shown to be successful in the coupling of peptides and fluorescent tags to SPIONs and lipases to gold NPs [64–66], but there has been little data supporting the addition of larger proteins or antibodies to NPs using click chemistry. There are additional concerns that the click chemistry reaction may degrade or modify the antibody of interest [65].
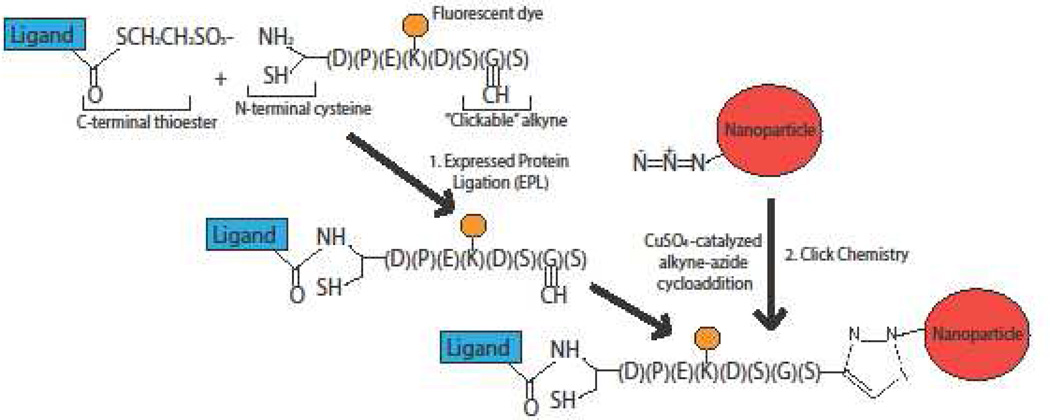
To address the shortcomings of EPL and click chemistry approaches, expressed protein ligation and click chemistry were recently combined into a single system that permits the conjugation of targeting ligands to nanoparticles in a site-specific and efficient manner [57]. Figure 2 illustrates the chemical mechanism for the combined EPL-click chemistry system, which produces stereospecific ligand attachment onto nanoparticle surfaces. This approach was recently adapted to also allow full-length antibodies to be conjugated onto nanoparticle surfaces [67].
Figure 2. Combined expressed-protein ligation (EPL) and click chemistry bioconjugation.
Expressed-protein ligation involves the site-specific reaction between a ligand bound to a C-terminal thioester and an alkynated fluorescent peptide (AFP) containing an N-terminal cysteine. The AFP contains a “clickable” alkyne that can react with the nanoparticle surface via copper-catalyzed alkyne-azide cycloaddition (i.e. click chemistry), allowing for stereospecific attachment of the ligand of interest to the nanoparticle.
5. Applications of nanotechnology to sensorineural hearing loss of inner ear disease
Sensorineural hearing loss (SNHL) is one of the most common sensory deficits in humans, affecting millions of people worldwide. Leading causes of this type of hearing loss include advanced age, ototoxic medications, noise exposure, inherited disorders, and immune disorders. Almost all SNHL is irreversible, and current management is limited to sound amplification devices and cochlear implants. Cochlear and retrocochlear pathology are responsible for SNHL. Nanotechnology provides the potential for a noninvasive and targeted approach to deliver biomaterials to specific structures within the inner ear for the treatment of SNHL.
5.1. Nanoparticle approach for noise induced hearing loss
Noise-induced hearing loss (NIHL) often causes permanent inner ear damage. The initial cell type affected by NIHL is the outer hair cell, which modulates incoming auditory signals. Damage to these outer hair cells may alter transduction of sound to the cochlear nerve, therefore causing SNHL.
One drug studied for the treatment of NIHL is the antioxidant edaravone. A recent study by Gao et al. looked at nanoparticle delivery of edaravone in vivo into the inner ear of guinea pigs with NIHL [68]. Edaravone has been used clinically in Japan for treatment of acute cerebral infarction via inhibition of hydroxyl free radicals, and studies have demonstrated the utility of edaravone in treating NIHL by the same mechanism [69,70]. Gao et al found that IT delivery of solid lipid nanoparticle (SLN)-encapsulated edaravone inhibited free radical generation in the cochlea and decreased hearing thresholds following noise-induced injury, although it did not demonstrate significant hearing recovery. This may be due to inefficient therapeutic effect and non-targeted drug delivery to the damaged cell sites.
Using identified biomarkers for targeted delivery can significantly improve drug delivery efficiency. Prestin is an electromotility protein that is solely expressed on the surface of outer hair cells (OHCs) [71]. Discovery of prestin as an outer hair cell (OHC) biomarker paved the way for targeted OHC drug delivery. Indeed, preliminary data has shown that prestin-targeted nanoparticles have increased specific binding to OHCs of mouse cochlear explants. The combination of prestin-targeted nanoparticles with edaravone for inner ear delivery may significantly increase delivery efficiency and may lead to better therapeutic effect.
5.2 Nanoparticle approach for different types of SNHL
Disruption to retrocochlear pathways, from the spiral ganglion cells (SGCs) to the brain, can also lead to SNHL. Although there is increased understanding of auditory neuronal survival biomolecular pathways, delivery of biomaterials to targeted sites has remained challenging. Many current studies are aimed at delivering biomaterials using nanotechnology, and targeting them specifically to these damaged cell sites, for the treatment of SNHL.
One study by Meyer et al demonstrated that the use of lipid NPs to deliver rolipram to SGCs in vitro increased neuronal survival and elongation of neurite outgrowth via inhibition of phosphodiesterase 4 in a dose-dependent manner. They showed that lipid NP-encapsulated rolipram led to higher SGC survival rates and neurite outgrowth than rolipram delivery alone [25]. While rolipram may be studied in the future as a potential therapeutic agent, the findings of this study could not be replicated in vivo, which the authors believe may be due to non-sustained and nonspecific rolipram delivery.
In order to target neuronal cells, Roy et al delivered nanoparticles conjugated with nerve growth factor-derived peptide in an in vitro inner ear model and found ligand-specific binding to Trks and p75 neurotrophin receptors of the SGCs, Schwann cells and nerve fibers [26]. Trks and p75 neurotrophin receptors are part of the signaling cascade regulating the survival of vestibular and cochlear neurons. The potential translational application of NP-based delivery was shown in a human ex vivo study done by the same group, which showed that the RWM of freshly frozen temporal bones is permeable to polymeric formulations of various sizes and chemical make ups [72]. Nerve growth factor derived peptide targeted NP delivery may play a critical role for the treatment of SGC lesion-based SNHL [27,28].
Another study done by Zhang et al looked at a different targeting approach to SGCs and cochlear nerve [73]. They delivered polymersome-nanoparticles conjugated with the Tet1 peptide into the inner ear through both IT injection and cochleostomy. They found that this peptide binds specifically to trisialoganglioside clostridial toxin receptors on SGCs and the cochlear nerve. Interestingly, they found that a cochleostomy approach worked for targeted delivery to the SGCs and cochlear nerve while IT injection did not. Much like the Meyer study, these authors concluded that an IT injection approach could not efficiently deliver the nanoparticles across the RWM to the SGCs and cochlear nerve of the inner ear. Although a cochleostomy approach demonstrated nanoparticle delivery to the SGCs and auditory nerve, this approach is an invasive procedure to the inner ear and it may worsen the existing pathology.
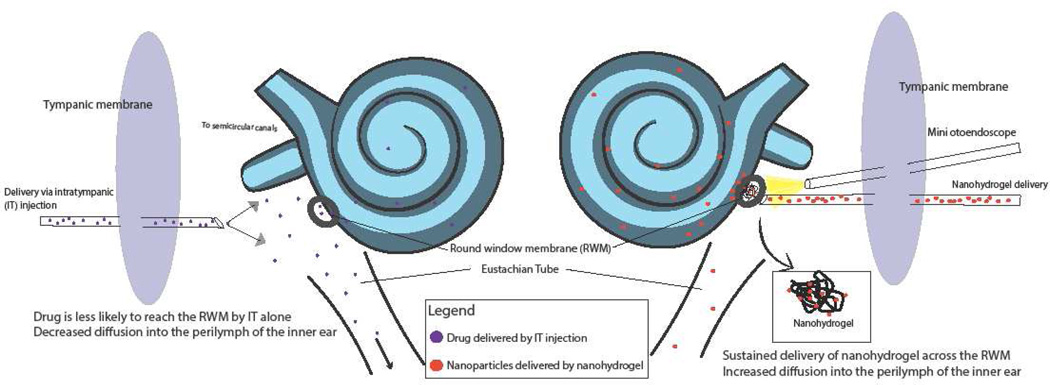
In summary, although different targeted nanoparticle delivery systems demonstrate promising results, the major limitations of non-sustained and/or invasive inner ear delivery hinder translational application. To address this problem, Li et al has proposed a nanohydrogel delivery system, which combines nanotechnology with a hydrogel delivery system. They have shown that liposomal NPs delivered via nanohydrogel in vitro persisted under physiologic conditions for at least 2 weeks, thus demonstrating sustained release. Furthermore, they found that these NPs could be delivered in vivo across the mouse RWM to reach structures in the scala media [74]. Another unique feature of this delivery approach is that it is noninvasive to the inner ear, which is crucial for clinical application. The combination of this nanohydrogel with targeting techniques will potentially be capable of delivering therapeutic biomaterials into specific molecular-cellular sites of the inner for the treatment of SNHL with different pathologies. Figure 3 provides a schematic representation of a functionalized nanoparticle delivered by the chitosan-glycerophosphate hydrogel system, specifically targeted to an outer hair cell.
Figure 3. Nanohydrogel delivery into the inner ear.
Schematic overview of targeted nanoparticle delivery using a chito system (nanohydrogel) to bring therapeutic biomaterials to specific cellular structures within the inner ear. A schematic diagram of a functionalized NP is shown, with targeting ligands on the surface that can specifically bind to prestin, an electromot chitosan-glycerophosphate (CGP) hydrogel electromotility protein solely expressed on outer hair cells
5.3 Nanodialysis for Cisplatin-induced ototoxicity
Cisplatin-induced ototoxicity is not uncommon and results in permanent inner ear damage. It is a well-known obstacle to the administration of cisplatin-based chemotherapies, which are commonly used to treat head and neck cancers, along with lung and genitourinary cancers. Some studies suggest that the rates of cisplatin-induced ototoxicity may be as high as 94%, with ototoxicity occurring in one third of patients who have received a single dose of cisplatin and worsen with further treatment [75]. It has been shown that cisplatin reaches the inner ear within minutes of systemic treatment and that outer hair cells are more severely affected than inner hair cells [76]. There are currently no treatments to prevent or reverse cisplatin-induced ototoxicity. Hearing loss that results from cisplatin-induced ototoxicity is irreversible; therefore, prophylactic or early treatment is a crucial component for optimized treatment regimens.
Prevention of cisplatin-induced ototoxicity has been demonstrated with reducing agents, such as glutathione, which decreases oxidative damage to the inner ear by free radicals [77–79]. Glutathione and other reducing agents have been studied using systemic and IT delivery before cisplatin administration and have been shown to decrease hearing loss. However, not only are these drugs limited by inefficient inner ear delivery, they cannot be removed from the inner ear once they are complexed with the toxin. Accumulation of these drug-toxin complexes may cause secondary toxicity to the inner ear.
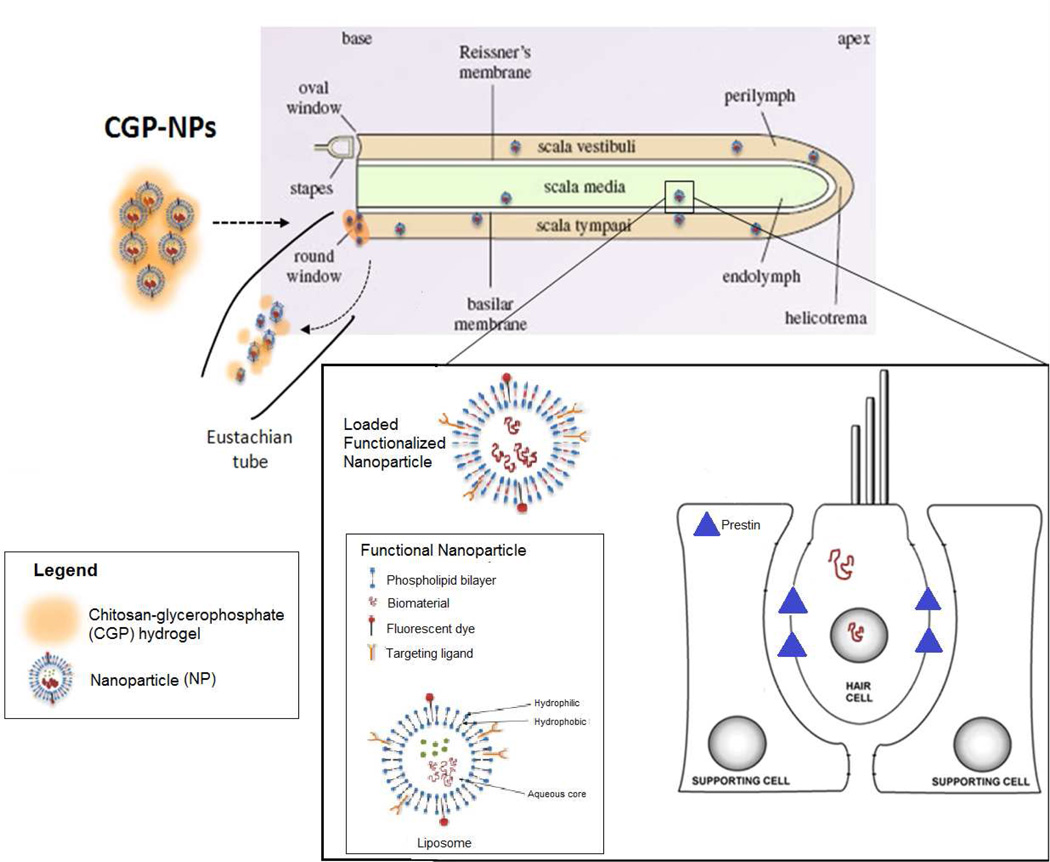
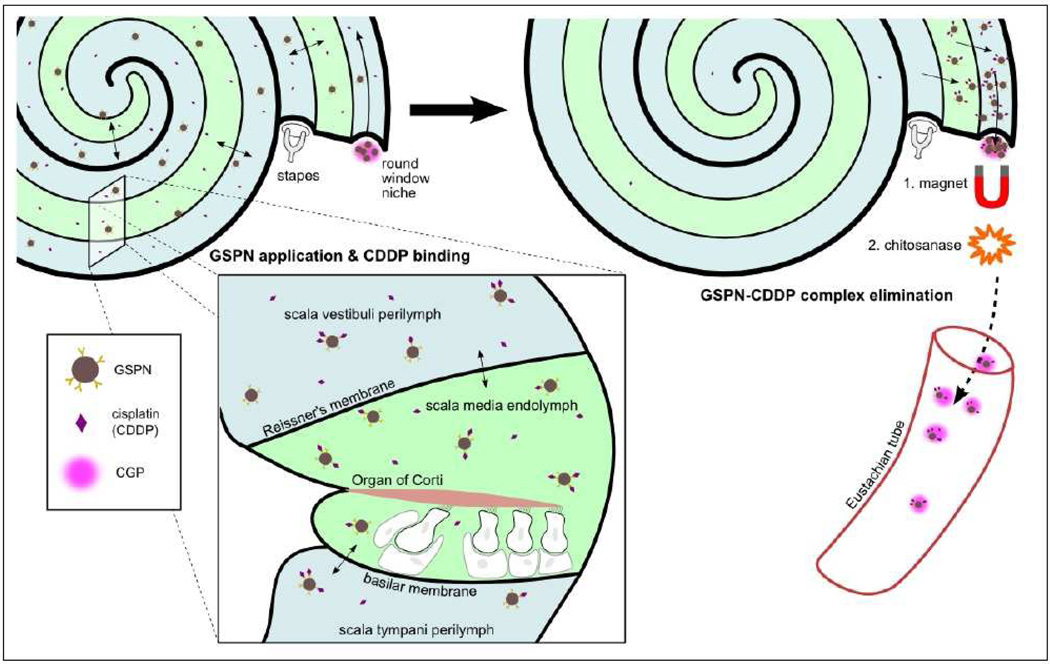
Nanotechnology may offer a noninvasive solution to the treatment of cisplatin-induced ototoxicity. As discussed above (section 3.2), biologically coated SPIONs are able to traverse the RWM for delivery into the inner ear. More importantly, their delivery can be controlled by the application of an external magnetic field. Subsequent removal of the magnet leads to dispersion by Brownian motion such that these SPIONs can distribute evenly throughout the perilymph. Furthermore, pegylated coating of SPIONs can accommodate bioconjugation of surface ligands such as glutathione, which has been shown to bind the desired target: cisplatin [80,81]. Once the SPIONs bind to cisplatin, the external magnet can be redirected to remove the complexes from the inner ear. This process, as illustrated in Figure 4, is known as nanodialysis.
Figure 4. Application of nanodialysis system for potential treatment of cisplatin-induced ototoxicity.
Glutathione-conjugated polyethylene glycol-polycaprolactone SPIO-encapsulated nanoparticles (GPSNs) are delivered into the inner ear using chitosan-glycerophosphate (CPG) hydrogel. GPSNs disperse within the fluid compartments of the inner ear to sequester cisplatin particles. An external magnet is applied to draw out the cisplatin-GPSN complexes and chitosanase is added to degrade the CPG hydrogel, thus removing the complexes from the inner ear by elimination through the Eustachian tube.
Preliminary experiments studying the glutathione-SPION delivery system have been promising. In vivo experiments confirmed that fluorescently tagged SPIONs can be passed through the RWM into the inner ear and retrieved from the cochlea using an external magnet. Furthermore, glutathione-bound agarose magnetic beads have been successful at sequestering cisplatin. Future experiments will include conjugation of glutathione to SPIONs for delivery into the inner ear for cisplatin-binding and subsequent removal using an external magnet.
6. Conclusion and Future Directions
Efficient targeted inner ear delivery is the key for successful treatment of sensorineural hearing loss of different pathologies. To date, there is a lack of an efficient, noninvasive and sustained delivery system that can both stabilize therapeutic biomaterials and target them to specific structures within the inner ear.
Modern nanotechnology offers a promising tool for efficient and noninvasive inner ear drug and biomaterial delivery. Nanoparticle systems such as PLGA nanoparticles, lipid nanoparticles, liposomes, and polymersomes have been shown to carry a variety of drugs and biomaterials across the round window membrane into the inner ear in in vitro models, although with more limited success in in vivo animal models.
Furthermore, the development of the combined EPL-click chemistry bioconjugation system allows for ligands to be effectively and stereo-specifically attached onto nanoparticle surfaces, creating a mechanism for targeted delivery. Recent studies of specific inner ear biomarkers and pathways, such as prestin and the neurotrophin family receptors have shown promise for targeted therapy of sensorineural hearing loss. Future research may focus on even more site-specific inner ear delivery systems. It has already been shown that distinct types of voltage-gated potassium channels are expressed in a gradient manner from the base to the apex in mice cochlea, and that these ion channel expressions are controlled by brain-derived neurotropic factor (BDNF) and neurotrophin-3 (NT-3). A nanoparticle delivery system could be used to deliver these neurotrophic factors or even ion channel proteins and transgenes in the cochlea in vivo [82]. Being able to target specific sites and cell types of the cochlea, may translate to distinct treatments for high frequency or low frequency SNHL, which may prevent damage to nonpathological areas and cells.
Although there have been successful attempts at targeted nanoparticle delivery of biomaterials to the inner ear in vitro, the findings have not been consistent in vivo. As discussed above, the Meyer study was only able to deliver rolipram in vitro, not in vivo, and the Zhang study was only able to deliver the Tet1 peptide via invasive cochleostomy, not intratympanic injection. The findings highlight the underlying limitations of current targeted nanoparticle delivery systems: non-sustained drug release and invasive approaches for delivery. One possible way to resolve these issues is to use the nanohydrogel delivery system, as the hydrogel provides sustained release of biomaterials across the RWM noninvasively. Studies on the use of the nanohydrogel system for targeted therapeutic delivery into the inner ear are currently underway.
There is still much to learn about nanoparticles beyond their use as effective carrier and targeting agents. Certain classes of nanoparticles display additional properties that may be used in the future for the treatment of cisplatin-induced ototoxicity. As discussed, SPIONs have magnetic properties that allow their motion to be controlled by an applied external magnetic field. This property can be used for the treatment of cisplatin-induced ototoxicity, which involves removing cisplatin particles that have accumulated within the inner ear. Current studies are aimed at attaching glutathione, a ligand known to bind to cisplatin, onto SPIONs for delivery into the inner ear. The glutathione will bind to cisplatin within the inner ear, and the SPION-glutathione-cisplatin complex can subsequently be retrieved using an external magnet.
Taken together, the current research on targeted nanohydrogel delivery and nanodialysis are quite promising with strong translational potential. We predict in the future that the targeted nanohydrogel delivery system can effectively and noninvasively deliver therapeutic drugs and biomaterials to specific pathologic sites and cell types of the inner ear. As a consequence, it may repair defective cells and molecules in specific areas of the inner ear responsible for SNHL. We hope that the nanodialysis system can be effectively used for detoxification of different types of ototoxicity. This may greatly benefit patients who cannot compromise their critical treatment modalities despite the high potential for ototoxicity. Future progress can be achieved by combining modern nanotechnology with advancing knowledge of the biomolecular basis of sensorineural hearing loss.
Acknowledgments
This work was funded through a National Institute of Health (NIH) grant: 1R01DC014464-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCall AA, Swan EE, Borenstein JT, Sewell WF, Kujawa SG, McKenna MJ. Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear. 2010;31:156–165. doi: 10.1097/AUD.0b013e3181c351f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss. A double-blind clinical study. Arch. Otolaryngol. 1980;106:772–776. doi: 10.1001/archotol.1980.00790360050013. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Lee J, Yuan C, Chen R. Oral steroid treatment for idiopathic sudden sensorineural hearing loss. Saudi Med. J. 2015;36:291–296. doi: 10.15537/smj.2015.3.9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filipo R, Attanasio G, Russo FY, Cartocci G, Musacchio A, De Carlo A, et al. Oral versus short-term intratympanic prednisolone therapy for idiopathic sudden hearing loss. Audiol. Neurootol. 2014;19:225–233. doi: 10.1159/000360069. [DOI] [PubMed] [Google Scholar]
- 5.Ruckenstein MJ. Autoimmune inner ear disease. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12:426–430. doi: 10.1097/01.moo.0000136101.95662.aa. [DOI] [PubMed] [Google Scholar]
- 6.Buniel MC, Geelan-Hansen K, Weber PC, Tuohy VK. Immunosuppressive therapy for autoimmune inner ear disease. Immunotherapy. 2009;1:425–434. doi: 10.2217/imt.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander TH, Weisman MH, Derebery JM, Espeland MA, Gantz BJ, Gulya AJ, et al. Safety of High-Dose Corticosteroids for the Treatment of Autoimmune Inner Ear Disease. Otol. Neurotol. 2009;30:443–448. doi: 10.1097/MAO.0b013e3181a52773. [DOI] [PubMed] [Google Scholar]
- 8.Graham MD, Sataloff R, Kemink J. Titration streptomycin therapy for bilateral Meniere’s disease : A preliminary report. 1984;92:440–447. doi: 10.1177/019459988409200413. [DOI] [PubMed] [Google Scholar]
- 9.Balyan FR, Taibah A, De DG, Aslan A, Falcioni M, Russo A, et al. Titration streptomycin therapy in Meniere’s disease: long-term results. Otolaryngol. Head Neck Surg. 1998;118:261–266. doi: 10.1016/S0194-5998(98)80028-4. doi:S0194599898000291 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Selimoglu E. Aminoglycoside-Induced Ototoxicity. Curr. Pharm. Des. 2007;13:119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 11.Langman AW, Kemink JL, Graham MD. Titration streptomycin therapy for bilateral Meniere’s disease. Follow-up report. Ann Otol Rhinol Laryngol. 1990;99:923–926. doi: 10.1177/000348949009901113. [DOI] [PubMed] [Google Scholar]
- 12.Schuknecht HF. Ablation therapy in the management of Menière’s disease. Acta Otolaryngol. Suppl. 1957;132:1–42. [PubMed] [Google Scholar]
- 13.Jackson LE, Silverstein H. Chemical perfusion of the inner ear. Otolaryngol. Clin. North Am. 2002;35:639–653. doi: 10.1016/s0030-6665(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre PP, Staecker H. Steroid perfusion of the inner ear for sudden sensorineural hearing loss after failure of conventional therapy: a pilot study. Acta Otolaryngol. 2002;122:698–702. http://www.ncbi.nlm.nih.gov/pubmed/12484644. [PubMed] [Google Scholar]
- 15.Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, et al. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. Jama. 2011;305:2071–2079. doi: 10.1001/jama.2011.679. [DOI] [PubMed] [Google Scholar]
- 16.Silverstein H, Hyman SM, Feldbaum J, Silverstein D. Use of streptomycin sulfate in the treatment of Meniere’s disease. Otolaryngol. Neck Surg. 1984;92:229–232. doi: 10.1177/019459988409200218. [DOI] [PubMed] [Google Scholar]
- 17.Berryhill WE, Graham MD. Chemical and physical labyrinthectomy for Meniere’s disease. Otolaryngol. Clin. North Am. 2002;35:675–682. doi: 10.1016/s0030-6665(02)00025-7. [DOI] [PubMed] [Google Scholar]
- 18.Chia SH, Gamst AC, Anderson JP, Harris JP. Intratympanic gentamicin therapy for Ménière’s disease: a meta-analysis. Otol. Neurotol. 2004;25:544–552. doi: 10.1097/00129492-200407000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Paulson DP, Abuzeid W, Jiang H, Oe T, O’Malley BW, Li D. A novel controlled local drug delivery system for inner ear disease. Laryngoscope. 2008;118:706–711. doi: 10.1097/MLG.0b013e31815f8e41. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Heldrich J, Wang H, Yamashita T, Miyamoto S, Li A, et al. A controlled and sustained local gentamicin delivery system for inner ear applications. Otol. Neurotol. 2010;31:1115–1121. doi: 10.1097/MAO.0b013e3181eb32d1. [DOI] [PubMed] [Google Scholar]
- 21.Lajud Sa, Han Z, Chi FL, Gu R, Nagda Da, Bezpalko O, et al. A regulated delivery system for inner ear drug application. J. Control. Release. 2013;166:268–276. doi: 10.1016/j.jconrel.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 22.Banerjee A, Parnes LS. The biology of intratympanic drug administration and pharmacodynamics of round window drug absorption. Otolaryngol. Clin. North Am. 2004;37:1035–1051. doi: 10.1016/j.otc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Flint P, Haughley B, Lund V, Niparko J, Richardson M, Robbins KT, et al. Cummings Otolaryngology: Head and Neck Surgery. 6th. Saunders; 2015. [Google Scholar]
- 24.Pasha R. Otolaryngology- Head and Neck Surgery: Clinical Reference Guide. 4th. Plural Publishing; 2013. [Google Scholar]
- 25.Meyer H, Stöver T, Fouchet F, Bastiat G, Saulnier P, Bäumer W, et al. Lipidic nanocapsule drug delivery: Neuronal protection for cochlear implant optimization. Int. J. Nanomedicine. 2012;7:2449–2464. doi: 10.2147/IJN.S29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy S, Johnston AH, Newman Ta, Glueckert R, Dudas J, Bitsche M, et al. Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: Potential tool for drug delivery. Int. J. Pharm. 2010;390:214–224. doi: 10.1016/j.ijpharm.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 28.Fritzsch B, Beisel KW, Hansen LA. The molecular basis of neurosensory cell formation in ear development: A blueprint for hair cell and sensory neuron regeneration? BioEssays. 2006;28:1181–1193. doi: 10.1002/bies.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salt AN, Ma Y. Quantification of solute entry into cochlear perilymph through the round window membrane. Hear. Res. 2001;154:88–97. doi: 10.1016/s0378-5955(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 30.Plontke SKR, Salt AN. Quantitative interpretation of corticosteroid pharmacokinetics in inner fluids using computer simulations. Hear. Res. 2003;182:34–42. doi: 10.1016/s0378-5955(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 31.Salt AN, Plontke SKR. Local inner-ear drug delivery and pharmacokinetics. Drug Discov. Today. 2005;10:1299–1306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goycoolea MV. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–447. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekhar SSSSS, Rubinstein RY, Kwartler Ja, Gatz M, Connelly PE, Huang E, et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol. -- Head Neck Surg. 2000;122:521–528. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- 34.Schachern PA, Paparella MM, Goycoolea MV, Duvuall AJ, Choo Y-B. The permeability of the round window membrane during otitis media. Otolaryngol. - Head Neck Surg. 1987;113:625–629. doi: 10.1001/archotol.1987.01860060051014. http://www.ncbi.nlm.nih.gov/pubmed/3566945. [DOI] [PubMed] [Google Scholar]
- 35.Nordang L, Linder B, Anniko M. Morphologic changes in round window membrane after topical hydrocortisone and dexamethasone treatment. Otol. Neurotol. 2003;24:339–343. doi: 10.1097/00129492-200303000-00034. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Zhang X, Yang F, Mu L. Disposition of nanoparticle-based delivery system via inner ear administration. Curr. Drug Metab. 2010;11:886–897. doi: 10.2174/138920010794479673. [DOI] [PubMed] [Google Scholar]
- 37.Buckiova D, Ranjan S, Newman Ta, Johnston AH, Sood R, Kinnunen PKJ, et al. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. 2012:1339–1354. doi: 10.2217/nnm.12.5. [DOI] [PubMed] [Google Scholar]
- 38.Tamura T, Kita T, Nakagawa T, Endo T, Kim T-S, Ishihara T, et al. Drug delivery to the cochlea using PLGA nanoparticles. Laryngoscope. 2005;115:2000–2005. doi: 10.1097/01.mlg.0000180174.81036.5a. [DOI] [PubMed] [Google Scholar]
- 39.Ge X, Jackson RL, Liu J, Harper Ea, Hoffer ME, Wassel Ra, et al. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol. - Head Neck Surg. 2007;137:619–623. doi: 10.1016/j.otohns.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Zou J, Saulnier P, Perrier T, Zhang Y, Manninen T, Toppila E, et al. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J. Biomed. Mater. Res. - Part B Appl. Biomater. 2008;87:10–18. doi: 10.1002/jbm.b.31058. [DOI] [PubMed] [Google Scholar]
- 41.Praetorius M, Brunner C, Lehnert B, Klingmann C, Schmidt H, Staecker H, et al. Transsynaptic delivery of nanoparticles to the central auditory nervous system. Acta Otolaryngol. 2007;127:486–490. doi: 10.1080/00016480600895102. [DOI] [PubMed] [Google Scholar]
- 42.Mondalek FG, Zhang YY, Kropp B, Kopke RD, Ge X, Jackson RL, et al. The permeability of SPION over an artificial three-layer membrane is enhanced by external magnetic field. J. Nanobiotechnology. 2006;4:4. doi: 10.1186/1477-3155-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnes AL, Wassel Ra, Mondalek F, Chen K, Dormer KJ, Kopke RD. Magnetic characterization of superparamagnetic nanoparticles pulled through model membranes. Biomagn. Res. Technol. 2007;5:1. doi: 10.1186/1477-044X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett. 2005;5:829–834. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 45.Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells, Nanomedicine, Biotechnol. 2014:1–13. doi: 10.3109/21691401.2014.955107. [DOI] [PubMed] [Google Scholar]
- 46.Nitin N, Javier DJ, Richards-Kortum R. Oligonucleotide-coated metallic nanoparticles as a flexible platform for molecular imaging agents. Bioconjug. Chem. 2007;18:2090–2096. doi: 10.1021/bc0701242. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro B, Kulkarni S, Nacev a, Sarwar a, Preciado D, Depireux Da. Shaping magnetic fields to direct therapy to ears and eyes. Annu. Rev. Biomed. Eng. 2014;16:455–481. doi: 10.1146/annurev-bioeng-071813-105206. [DOI] [PubMed] [Google Scholar]
- 48.Kopke RD, Wassel Ra, Mondalek F, Grady B, Chen K, Liu J, et al. Magnetic nanoparticles: Inner ear targeted molecule delivery and middle ear implant. Audiol. Neurotol. 2005;11:123–133. doi: 10.1159/000090685. [DOI] [PubMed] [Google Scholar]
- 49.Du X, Chen K, Kuriyavar S, Kopke RD, Grady BP, Bourne DH, et al. Magnetic targeted delivery of dexamethasone acetate across the round window membrane in guinea pigs. Otol. Neurotol. 2013;34:41–47. doi: 10.1097/MAO.0b013e318277a40e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan BT, Foong KH, Lee MM, Ruan R. Polyethylenimine-mediated cochlear gene transfer in guinea pigs. Arch Otolaryngol Head Neck Surg. 2008;134:884–891. doi: 10.1001/archotol.134.8.884. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18711065. [DOI] [PubMed] [Google Scholar]
- 51.Wareing M, Mhatre AN, Pettis R, Han JJ, Haut T, Pfister MHF, et al. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear. Res. 1999;128:61–69. doi: 10.1016/s0378-5955(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 52.Jero J, Mhatre aN, Tseng CJ, Stern RE, Coling DE, Goldstein Ja, et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum. Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- 53.Maeda Y, Fukushima K, Kawasaki A, Nishizaki K, Smith RJH. Cochlear expression of a dominant-negative GJB2R75W construct delivered through the round window membrane in mice. Neurosci. Res. 2007;58:250–254. doi: 10.1016/j.neures.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Zuris Ja, Thompson DB, Shu Y, Guilinger JP, Bessen JL, Hu JH, et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 2014;33:73–80. doi: 10.1038/nbt.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neuberg P, Kichler A. Recent developments in nucleic Acid delivery with polyethylenimines. Elsevier; 2014. [DOI] [PubMed] [Google Scholar]
- 56.Wagner E. Polymers for Nucleic Acid Transfer — An Overview. Elsevier; 2014. [DOI] [PubMed] [Google Scholar]
- 57.Elias DR, Cheng Z, Tsourkas A. An intein-mediated site-specific click conjugation strategy for improved tumor targeting of nanoparticle systems. Small. 2010;6:2460–2468. doi: 10.1002/smll.201001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warden-Rothman R, Caturegli I, Popik V, Tsourkas A. Sortase-tag expressed protein ligation: Combining protein purification and site-specific bioconjugation into a single step. Anal. Chem. 2013;85:11090–11097. doi: 10.1021/ac402871k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman AD, Claypool SE, Liu R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013;19:6315–6329. doi: 10.2174/13816128113199990375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berrade L, Camarero Ja. Expressed protein ligation: A resourceful tool to study protein structure and function. Cell. Mol. Life Sci. 2009;66:3909–3922. doi: 10.1007/s00018-009-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flavell RR, Muir TW. Expressed protein ligation (EPL) in the study of signal transduction, ion conduction, and chromatin biology. Acc. Chem. Res. 2009;42:107–116. doi: 10.1021/ar800129c. [DOI] [PubMed] [Google Scholar]
- 62.Severinov K, Muir TW. Expressed protein ligation, a novel method for studying protein-protein interactions in transcription. J. Biol. Chem. 1998;273:16205–16209. doi: 10.1074/jbc.273.26.16205. [DOI] [PubMed] [Google Scholar]
- 63.Muralidharan V, Muir TW. Protein ligation: an enabling technology for the biophysical analysis of proteins. Nat. Methods. 2006;3:429–438. doi: 10.1038/nmeth886. [DOI] [PubMed] [Google Scholar]
- 64.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie - Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. doi:10.1002/1521-3773(20010601)40-11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 65.Thorek DLJ, Elias DR, Tsourkas A. Comparative analysis of nanoparticle-antibody conjugations: Carbodiimide versus click chemistry. Mol. Imaging. 2009;8:221–229. [PubMed] [Google Scholar]
- 66.Brennan JL, Hatzakis NS, Tshikhudo TR, Dirvianskyte N, Razumas V, Patkar S, et al. Bionanoconjugation via click chemistry: The creation of functional hybrids of lipases and gold nanoparticles. Bioconjug. Chem. 2006;17:1373–1375. doi: 10.1021/bc0601018. [DOI] [PubMed] [Google Scholar]
- 67.Hui JZ, Al Zaki A, Cheng Z, Popik V, Zhang H, Luning Prak ET, et al. Facile Method for the Site-Specific, Covalent Attachment of Full-Length IgG onto Nanoparticles. Small. 2014:1–10. doi: 10.1002/smll.201303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun J, Gao G, Liu Y, Zhou C, Jiang P. Solid Lipid Nanoparticles Loaded with Edaravone for Inner Ear Protection After Noise Exposure. Chin. Med. J. (Engl) 2015;128:203. doi: 10.4103/0366-6999.149202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito KI, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–242. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 70.Takemoto T, Sugahara K, Okuda T, Shimogori H, Yamashita H. The clinical free radical scavenger, edaravone, protects cochlear hair cells from acoustic trauma. Eur. J. Pharmacol. 2004;487:113–116. doi: 10.1016/j.ejphar.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Surovtseva EV, Johnston AH, Zhang W, Zhang Y, Kim A, Murakoshi M, et al. Prestin binding peptides as ligands for targeted polymersome mediated drug delivery to outer hair cells in the inner ear. Int. J. Pharm. 2012;424:121–127. doi: 10.1016/j.ijpharm.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 72.Roy S, Glueckert R, Johnston AH, Perrier T, Newman Ta, Saulnier P, et al. Strategies for drug delivery to the human inner ear by multifunctional nanoparticles. 2012;7:55–63. doi: 10.2217/nnm.11.84. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Zhang W, Johnston AH, Newman Ta, Pyykkö I, Zou J. Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. Int. J. Nanomedicine. 2012;7:1015–1022. doi: 10.2147/IJN.S28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lajud Sa, Nagda Da, Qiao P, Tanaka N, Civantos A, Gu R, et al. A Novel Chitosan-Hydrogel-Based Nanoparticle Delivery System for Local Inner Ear Application. Otol. Neurotol. 2014:341–347. doi: 10.1097/MAO.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, Bagby GC. High-frequency monitoring for early detection of cisplatin ototoxicity. Arch. Otolaryngol. Head. Neck Surg. 1993;119:661–666. doi: 10.1001/archotol.1993.01880180081015. [DOI] [PubMed] [Google Scholar]
- 76.Tropitzsch A, Arnold H, Bassiouni M, Müller A, Eckhard A, Müller M, et al. Assessing cisplatin-induced ototoxicity and otoprotection in whole organ culture of the mouse inner ear in simulated microgravity. Toxicol. Lett. 2014;227:203–212. doi: 10.1016/j.toxlet.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 77.Berglin CE, Pierre PV, Bramer T, Edsman K, Ehrsson H, Eksborg S, et al. Prevention of cisplatin-induced hearing loss by administration of a thiosulfate-containing gel to the middle ear in a guinea pig model. Cancer Chemother. Pharmacol. 2011;68:1547–1556. doi: 10.1007/s00280-011-1656-2. [DOI] [PubMed] [Google Scholar]
- 78.Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 79.Campbell KCM, Rybak LP, Meech RP, Hughes L. D-Methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear. Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 80.Hagrman D, Goodisman J, Dabrowiak JC, Souid AK. Kinetic study on the reaction of cisplatin with metallothionein. Drug Metab. Dispos. 2003;31:916–923. doi: 10.1124/dmd.31.7.916. [DOI] [PubMed] [Google Scholar]
- 81.Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells: Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- 82.Davis RL. Gradients of neurotrophins, ion channels, and tuning in the cochlea. Neuroscientist. 2003;9:311–316. doi: 10.1177/1073858403251986. [DOI] [PubMed] [Google Scholar]
- 83.Pritz CO, Dudás J, Rask-Andersen H, Schrott-Fischer A, Glueckert R. Nanomedicine strategies for drug delivery to the ear. Nanomedicine (Lond) 2013;8:1155–1172. doi: 10.2217/nnm.13.104. [DOI] [PubMed] [Google Scholar]
- 84.Ishihara T, Izumo N, Higaki M, Shimada E, Hagi T, Mine L, et al. Role of zinc in formulation of PLGA. J. Control. Release. 2005;105:68–76. doi: 10.1016/j.jconrel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 85.Panyam J, William D, Dash A, Leslie-Pelecky D, Labhasetwar V. Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J. Pharm. Sci. 2004;93:1804–1814. doi: 10.1002/jps.20094. [DOI] [PubMed] [Google Scholar]
- 86.Wen X, Wen L, Tirelli N, Su H, Chen G. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. 2014:5591–5601. doi: 10.2147/IJN.S72555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scheper V, Wolf M, Scholl M, Kadlecova Z, Perrier T, Klok H-A, et al. Potential novel drug carriers for inner ear treatment: hyperbranched polylysine and lipid nanocapsules. Nanomedicine (Lond) 2009;4:623–635. doi: 10.2217/nnm.09.41. [DOI] [PubMed] [Google Scholar]
- 88.Chen G, Hou S-X, Hu P, Hu Q-H, Guo D-D, Xiao Y. In vitro dexamethasone release from nanoparticles and its pharmacokinetics in the inner ear after administration of the drug-loaded nanoparticles via the round window. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1022–1024. [PubMed] [Google Scholar]
- 89.Lee JS, Feijen J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release. 2012;161:473–483. doi: 10.1016/j.jconrel.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Jin L, Zeng X, Liu M, Deng Y, He N. Current Progress in Gene Delivery Technology Based on Chemical Methods and Nano-carriers. Theranostics. 2014;4:240–255. doi: 10.7150/thno.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Praetorius M, Pfannenstiel S, Klingmann C, Baumann I, Plinkert PK, Staecker H. Expressionsmuster für nichtviral transfiziertes gfp in der Cochlea der Maus in vitro und in vivo. HNO. 2008;56:524–529. doi: 10.1007/s00106-008-1738-6. [DOI] [PubMed] [Google Scholar]
- 92.Balazs Da, Godbey W. Liposomes for use in gene delivery. J. Drug Deliv. 2011;2011:326497. doi: 10.1155/2011/326497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lalwani AK, Jero J, Mhatre AN. Current issues in cochlear gene transfer. Audiol. Neuro-Otology. 2002;7:146–151. doi: 10.1159/000058300. [DOI] [PubMed] [Google Scholar]
- 94.Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 95.Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, et al. Prelingual deafness: High prevalence of a 30delG mutation in the connexin 26 gene. Hum. Mol. Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- 96.Kudo T, Kure S, Ikeda K, Xia AP, Katori Y, Suzuki M, et al. Transgenic expression of a dominant-negative connexin 26 causes degeneration of the organ of Corti and non-syndromic deafness. Hum. Mol. Genet. 2003;12:995–1004. doi: 10.1093/hmg/ddg116. [DOI] [PubMed] [Google Scholar]
- 97.Sokolov K, Follen M, Aaron J, Pavlova I, Malpica A, Lotan R, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]