Abstract
Gene delivery vectors based on adeno-associated virus (AAV) have been utilized in a large number of gene therapy clinical trials, which have demonstrated their strong safety profile and increasingly their therapeutic efficacy for treating monogenic diseases. For cancer applications, AAV vectors have been harnessed for delivery of an extensive repertoire of transgenes to preclinical models and, more recently, clinical trials involving certain cancers. This review describes the applications of AAV vectors to cancer models and presents developments in vector engineering and payload design aimed at tailoring AAV vectors for transduction and treatment of cancer cells. We also discuss the current status of AAV clinical development in oncology and future directions for AAV in this field.
Keywords: Gene therapy, gene delivery, adeno-associated virus, AAV, gene delivery vectors, cancer
Graphical abstract
INTRODUCTION
Cancer, a large group of diseases characterized by the unregulated proliferation and spread or metastasis of abnormal cells, collectively represents a major worldwide healthcare problem. In the U.S. alone more than 1.5 million cases are diagnosed each year, and cancer overall has a 5-year relative survival rate of 68%, making it the second leading cause of death after heart disease [1]. Standard treatments include surgery, chemotherapy, and radiotherapy; however, these are often incapable of completely eradicating a malignancy [2] and can be accompanied by serious side effects [3]. Thus, there is a strong unmet medical need for the development of novel therapies that offer improved clinical efficiency and longer survival times in patients afflicted with disease.
Gene therapy, defined as the introduction of genetic material into a target cell for therapeutic benefit, is a very promising treatment for many diseases, including monogenic diseases, cancer, cardiovascular disease, and neurodegenerative diseases. To date, more than 2,000 clinical trials employing gene transfer have taken place and in general have established that a number of vehicles or vectors are safe [4, 5]. Furthermore, the majority (64%, n=1,415 [6]) of gene therapy clinical trials to date have targeted cancer – including lung, skin, neurological, and gastrointestinal tumors – and have utilized a variety of therapeutic strategies such as anti-angiogenic factors, tumor suppressors, immunostimulation, and oncolytic viruses. In 2015, the first recombinant viral therapy – an oncolytic herpesvirus for the treatment of melanoma – received regulatory approval in the U.S. [7].
For cancer gene therapies to be increasingly successful, however, a major hurdle must be overcome: the development of gene delivery vectors that can safely, efficiently, and specifically deliver genetic material to the target cells. Non-viral vectors can be easily produced at a large scale and are readily amenable to engineering or enhancement of their functional properties via chemical modifications; however, they suffer from a low delivery efficiency and in some cases cell toxicity [8]. On the other hand, viral vectors harness the highly evolved mechanisms that the parental viruses have developed to efficiently recognize and infect cells and offer several advantages, which make them suitable for both therapeutic application and as tools for biological studies; however, their delivery properties can be challenging to engineer and improve. That said, viral vectors have been used in the majority (over 68% [6]) of gene therapy clinical trials, and the most frequently used have been based on adenovirus, retrovirus, vaccinia virus, herpesvirus, and AAV [9].
AAV vectors in particular have been increasingly successful due to their gene delivery efficacy, lack of pathogenicity, and strong safety profile [10]. As a result of these properties, AAV vectors have enabled clinical successes in a number of recent clinical trials that have established the promise of gene therapy in general, including for the treatment of diseases such as Leber’s congenital amaurosis (LCA) [11, 12], where over four Phase I and I/II clinical trials have demonstrated safety and long-term (over five years) improvement in retinal and visual function; hemophilia B, targeted in several Phase I and Phase I/II clinical trials that have shown long-term efficacy and no toxic effects [5, 13]; and the Sanfilippo B syndrome, where gene expression and consequently improved cognitive development have been sustained for at least a year and are still ongoing (Pasteur Institute Phase I/II trial, unpublished). Moreover, alipogene tiparvovec (Glybera; uniQure), a gene therapy for lipoprotein lipase deficiency (LPLD) that employs an AAV vector, received regulatory approval by the European Medicines Agency in 2012 [14]. AAV vectors may also offer a strong potential for the treatment of cancer, and as presented in this review, their excellent gene delivery properties have been harnessed for in vitro cancer studies, in vivo pre-clinical cancer models, and more recently cancer clinical trials under development.
ADENO-ASSOCIATED VIRUS (AAV) AND AAV VECTORS
AAV Biology
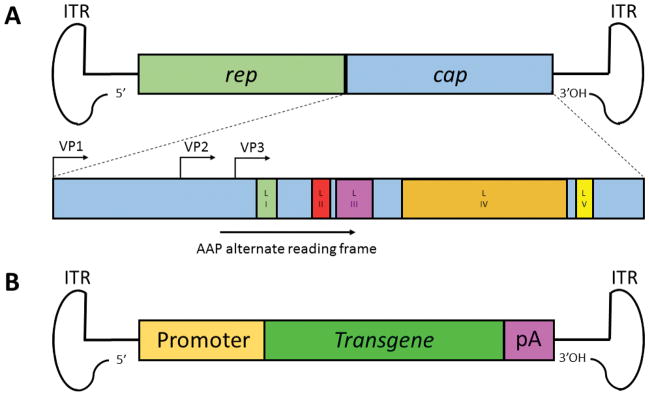
AAV is a single-stranded DNA parvovirus with a 4.7 kb genome (Figure 1A) composed of the rep and cap genes flanked by inverted terminal repeats (ITRs) [15]. The rep gene encodes non-structural proteins involved in viral replication, packaging, and genomic integration, whereas the cap gene codes for structural proteins (VP1, VP2, VP3) that assemble to form the viral capsid, which serves as the viral gene delivery vehicle. Additionally, an alternative open reading frame nested within the cap gene encodes the assembly activating protein (AAP), involved in the targeting and assembly of capsid proteins [16]. Following cellular entry through cell surface receptor-mediated endocytosis, endosomal escape, trafficking to the nucleus, uncoating, and second DNA strand synthesis, the virus can enter its replication cycle in the presence of a helper virus [17]. In the absence of a helper, however, AAV genomes can establish latency and persist as episomes [18] or in some cases integrate into host chromosomal DNA [19].
Figure 1. Genomic structure of AAV and AAV vectors.
(A) The 4.7kb AAV genome is composed of the rep and cap genes flanked by inverted terminal repeats (ITRs). The rep gene codes for non-structural proteins involved in viral replication, packaging, and genomic integration, while the cap gene encodes the structural proteins VP1, VP2, and VP3 that assemble to form the viral capsid in a ratio of 1:1:10, respectively, in a total of 60 protein subunits. The assembly-activating protein (AAP) is translated from an alternate open reading frame. Also depicted are capsid loop domains I through V (LI-LV), which contain variable regions that influence gene delivery properties. (B) Recombinant AAV vectors are generated by replacing the rep and cap genes with a gene expression cassette (e.g. promoter, transgene, poly(A) tail) flanked by the ITRs. Vectors are then packaged by supplying the rep and cap genes in trans as well as adenoviral helper genes required for AAV replication.
AAV-Based Vectors: Properties and Clinical Success
Recombinant AAV vectors can be generated by replacing the endogenous rep and cap genes with an expression cassette consisting of a promoter driving a transgene of interest and a poly(A) tail (Figure 1B). The rep and cap genes are then provided in trans as helper packaging plasmids together with adenoviral helper genes needed for AAV replication [10]. Over 100 natural AAV variants have been isolated, and variations in amino acid sequences result in somewhat different tropisms (the range of cells and tissues a virus can infect) [20], though none are pathogens [21]. Recombinant vectors have been generated from a number of these serotypes [10], though vectors based on AAV-serotype 2 (AAV2) have been the most widely studied and used in preclinical models and clinical trials to date. In general, vectors based on natural AAV variants have desirable gene delivery properties: a lack of pathogenicity and immunotoxicity, which grants them a strong safety profile [21]; the ability to infect dividing and non-dividing cells with reasonable efficiency [22]; the ability to mediate stable, long-term gene expression following delivery [20]; a ~5 kb genome that can carry a broad range of cargoes [23]; access to faster expression kinetics when using self-complementary, double stranded DNA forms of the vector genome [24]; and importantly the potential for engineering and optimizing the viral capsid and thus vector delivery properties [15]. Accordingly, AAV-based vectors have been harnessed in an increasing number of clinical trials (>130 to date) for tissue targets including liver, lung, brain, eye, and muscle [10, 25]. As a result of its properties, as mentioned above, AAV has enabled clinical efficacy in an increasing number of trials for monogenic diseases [5, 26–29].
For oncology applications, AAV vectors can transduce a wide variety of cancer primary cells and cell lines [30–32] and have the capacity to carry highly potent therapeutic payloads for cancer including anti-angiogenesis genes, suicide genes, immunostimulatory genes, and DNA encoding smaller nucleic acids (e.g. shRNAs, siRNAs) for post-transcriptional regulation of oncogenes [33]. AAVs therefore offer a strong potential as gene delivery vehicles for cancer gene therapy and have consequently been employed in numerous preclinical cancer models and in early stage clinical trials for cancer.
ENGINEERING AAV VECTORS FOR CANCER GENE THERAPY
Gene Delivery Challenges of AAV Vectors
Natural variants of AAV have enabled increasing success in human clinical trials, which have in turn provided strong momentum to the gene therapy field as a whole. That said, natural AAV serotypes have some shortcomings that render this success challenging to extend to the majority of human diseases, including cancer. As has been reviewed [10], barriers for AAV and other vectors include: prior exposure of most people to natural AAVs leading to anti-AAV neutralizing antibodies that can reduce vector delivery efficiency by orders of magnitude in vivo, poor vector biodistribution to important tissue targets, limited spread within those tissues, an inability to target specific cells, and limited efficiency for many therapeutically relevant target cells. These concerns have motivated the engineering of AAV capsids that can more efficiently traffic to and transduce cells, as well as the engineering of genetic cargos for higher potency and selective expression.
General Developments in AAV Vector Engineering
The amino acid sequence of the proteins that constitute the viral capsid (Figure 2A) determines an AAV vector’s delivery properties, including interactions with tissue and vasculature, humoral and cellular components of the immune system, specific receptors on the target cell surface, the endosomal network following receptor-mediated internalization, the cytosol after the viral phospholipase domain enables endosomal escape, and ultimately the nucleus. Thus, engineering the AAV capsid can generate novel AAV variants with novel and enhanced delivery properties [10]. Such vector engineering efforts can be grouped into two categories: rational design, where structure-function relationships are used to guide specific capsid modifications, and directed evolution, where libraries of AAV capsids are generated using a range of mutagenesis techniques and then subjected to a selective pressure for properties of interest.
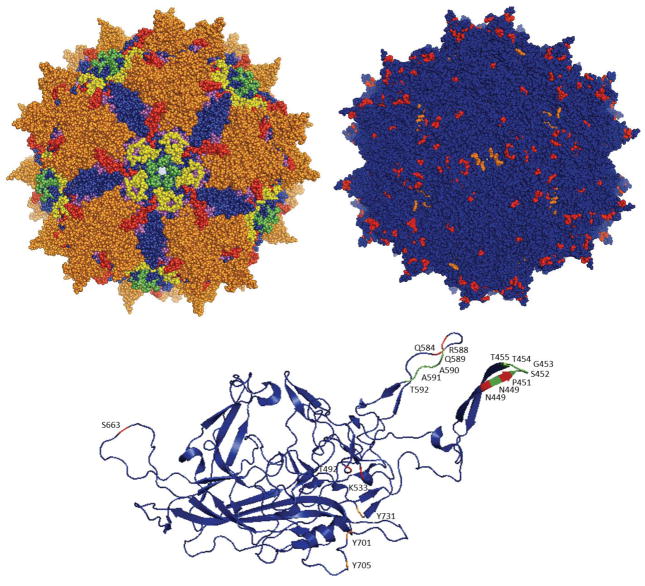
Figure 2. Representation of AAV2 capsid structure and individual monomeric protein.
(A) Crystal structure of the AAV2 capsid [183], the most widely used and studied AAV serotype. Loop domains I through V are depicted following the same color scheme as in Figure 1A. (B) Residues that have been mutated to engineer vectors for transduction of cancer cells are mapped onto the AAV2 crystal structure and depicted in orange (tyrosine to phenylalanine mutations) or red (other mutations). (C) Mutated residues are similarly depicted in the individual VP3 monomer structure. Additionally, residues that have been removed to insert protein-binding peptides [58] are depicted in green. Images were produced with Pymol [184].
The AAV capsid has been rationally engineered in several ways. For example, capsid surface-exposed tyrosine residues, whose phosphorylation targets the virion for ubiquitination and subsequent proteasomal degradation, have been modified via site-directed mutagenesis to phenylalanine residues to generate variants with reduced proteasomal degradation and subsequent higher gene expression [34]. Structural capsid information has also been used to generate variants with some resistance to pre-existing neutralizing antibodies by mutating surface residues that may interact with Immunoglobulin G antibodies (IgG’s) [35]. As reviewed below, rational design has also been employed to generate variants with enhanced transduction (infection and transgene expression) in tumor cells by using site-directed mutagenesis and inserting peptides with motifs that bind to receptors highly expressed in cancer cells.
In general, however, the AAV delivery pathway from point of administration until the particle arrives in the nuclei of target cells is extremely complex, and there is often insufficient knowledge of viral structure-function relationships to enable rational design efforts. Therefore, another approach is based on the idea that evolution can generate novel and useful biological function even in the absence of detailed mechanistic knowledge. Specifically, directed evolution has been developed and implemented to generate greatly enhanced AAV variants for a variety of applications. In this approach, the AAV cap gene is genetically diversified to create large libraries of novel AAV variants (~104 – 108) utilizing a range of molecular approaches including DNA shuffling, random point mutagenesis, insertional mutagenesis, random peptide insertions, and most recently ancestral reconstructions [36–45]. The libraries are then subjected to a selective pressure to acquire specific, advantageous delivery properties [10], and after a suitable number of selection rounds individual AAV variants are isolated, validated, and harnessed for therapeutic gene delivery in disease models. Directed evolution has been applied [15] to create novel, optimized AAV vectors with enhanced delivery to non-permissive cells such as human airway epithelium [46], neural stem cells [47], human pluripotent stem cells [48], retinal cells [49], and other tissues in vitro and in vivo [36, 37, 49–53]. AAV vectors have also been evolved for in vivo enhanced tissue spread and infection of non-permissive cell types [49, 50]. Thus, in vivo directed evolution strategies could potentially be extended to engineer novel AAV vectors for enhanced gene delivery to tumors.
Rational Design for the Engineering of Cancer-Specific Transduction
Changes in protein expression patterns [54] and subsequent presentation of tumor-specific antigens [55, 56] in cancer tissues may enable the preferential targeting of AAV gene delivery vehicles to tumor tissues [57, 58]. For example, Grifman et al. [59] targeted aminopeptidase N (or CD13), a membrane-bound enzyme that is highly expressed in cancerous tissue and vessels and has consequently been explored for targeted cancer therapies [60]. Specifically, they modified the AAV2 capsid, whose primary receptor for cellular entry is heparin sulfate proteoglycan (HSPG), by introducing a NGR peptide motif, which binds to CD13 [57], either in replacement of antigenic loops of AAV2 (residues T448-T455 and N587-A591) or after residues 449 and 588 of the capsid (Figures 2B, 2C). Mutant viruses with the NGR motif exhibited reduced affinity for heparin, suggesting a tropism different from AAV2, and transduced the sarcoma cell lines KS1767 and RD (which express CD13 at high levels) 10- to 20-fold better than wild-type AAV2, demonstrating the selectivity of these vectors.
Integrins, which contribute to tumor progression and metastasis, are also highly expressed in tumor cells and tumor vasculature [61] and have accordingly also been harnessed for selective tumor transduction. AAV2 vectors have been modified by inserting a 4C-RGD peptide [62], whose RGD motif selectively binds to αvβ3 and αvβ5 integrins [57], into different sites of the cap gene. Mutant vectors with the RGD insertion after residues 584 and 588 of VP3 (Figures 2B, 2C) retained infectivity, and the A5884C-RGD mutant was shown to bind to integrin and to mediate increased in vitro and in vivo gene delivery to integrin-expressing tumor cells. A5884C-RGD- mediated gene delivery was 40-fold higher on K562 human chronic myelogenous leukemia cells, 13-fold higher on Raji human lymphoblast-like cells, and 6-fold higher on SKOV-3 human ovarian adenocarcinoma cells, compared to wild-type AAV2.
Additionally, designed ankyrin repeat proteins (DARPins) targeting cancer-associated receptors have been fused to AAV2 capsid proteins for enhanced selectivity to cancer cells [63]. DARPin 9.29, which specifically binds to HER2/neu, a receptor overexpressed in cancer cells, was fused to VP2 and then used to package AAV2 particles whose affinity for HSPG had been ablated through site-directed mutagenesis. The resulting vectors (Her2-AAV) transduced cells in a HER2-dependent manner, showing selectivity for HER2-expressing cells and only weakly transducing cells not expressing the receptor. Moreover, systemically administered Her2-AAV vectors localized to subcutaneous tumors of HER2+ SK-OV-3 cells in mice, compared to a lack of tumor cell transduction of AAV2 vectors, which instead localized primarily to the liver.
AAV5 has also been engineered by inserting homing peptides for integrins, sialyl Lewis X (sLex), and tenascin C (TnC), which are overexpressed in many cancer tumors [64]. Mutants with RGD peptides infected integrin-expressing cells 5-fold better than AAV5, and both integrin- and TnC-targeting AAVs preferentially transduced cells presenting these molecules while showing very low transduction of cells negative for these antigens. Mutants with the sLex-targeting peptide did not transduce sLex-expressing cells.
Cheng et al. [65] studied the effects of mutating surface-exposed tyrosine residues to phenylalanines on AAV3. Three of the single-residue mutants – Y701F, Y705F, and Y731F (corresponding residues in AAV2 depicted in Figures 2B, 2C) – showed 1.5-, 2.2-, and 8.8-fold enhanced transduction, respectively, of Huh7 hepatocellular carcinoma (HCC) cells and 2.3-, 3.3-, and 9.1-fold enhanced transduction, respectively, of Hep293TT human hepatoblastoma tumor cells compared to wild-type AAV3. A double mutant, Y705F+Y731F, showed further increased transduction (11-fold higher) on Huh7 cells. This double mutant, when administered intra-tumorally or systemically to a mouse xenograft model of human liver tumors, also showed enhanced transduction, as reported by fluorescence microscopy, compared to AAV3. Additional studies with AAV3 have mutagenized serine, threonine, and lysine residues to valine, glutamate, and arginine residues, respectively, in addition to changing tyrosine residues to phenylalanines (corresponding residues in AAV2 depicted in Figures 2B, 2C) [66]. Mutants S663V+T492V+K533R, S663V+T492V+K533R, and S663V+T492V had transduction efficiencies over 10-fold higher than wild-type AAV3 on Huh7 cells. Some of these mutants also showed 2- and 8-fold enhanced transduction on HepG2 and Hep293TT cells, respectively, and the S663V+T492V mutant showed 2-fold higher transduction than the Y705+731F mutant in mouse xenografts of these cells lines.
There has also been a strong interest in AAV delivery to immune cells. In particular, given that cancers can develop resistance mechanisms against both drugs and the immune system, cancer research efforts have also focused on stimulating the adaptive immune system to mount a T cell-mediated anti-tumor response. Immunotherapy offers important potential advantages over traditional therapies, including selectivity for tumor cells and the generation of memory T cells that protect against recurring tumors [67]. This has motivated the engineering of viral vectors with enhanced infectivity for dendritic cells (DCs), antigen-presenting cells that can prime T cells and generate an anti-tumor cytotoxic T lymphocyte (CTL) immune response. Surface-exposed serine and threonine residues in AAV6 (corresponding residues in AAV2 depicted in Figures 2B, 2C) have been mutated to valine residues for enhanced in vitro transduction efficiency on monocyte-derived DCs (moDCs) [68]. Mutants T492V, S663V, and T492V+S663V showed enhanced infectivity, with the double mutant showing 5-fold higher transduction. This T492V+S663V mutant was used to transduce moDCs with human prostate-specific antigen (hPSA), which led to and a 3-fold higher hPSA expression in moDCs and a 1.3-fold stronger CTL response against human prostate adenocarcinoma cells compared to wild-type AAV6 gene delivery, underscoring the utility of enhanced AAV vectors that can be used for cancer immunotherapy, particularly if delivery can be achieved in vivo.
Additionally, AAV vectors have been modified by inserting protease recognition sequences on the capsid such that protease cleavage is required for complete viral transduction [69]. Specifically, short sequences encoding negatively charged amino acids, which serve as “locks” by interfering with virus-receptor interactions, were flanked by protease cleavage sites recognized by matrix metalloproteinases (MMPs) and genetically inserted into surface-exposed regions near the heparin binding domain of the AAV2 capsid. Inactivated or “locked” vectors had reduced heparin affinity and infectivity, and treatment with MMPs restored heparin binding and allowed efficient transduction. MMPs are highly expressed in most cancers compared to normal tissue [70], so such protease-responsive AAV vectors could provide enhanced selectivity towards cancerous tissues, as other studies that have exploited high levels of MMP expression for selective viral gene delivery have demonstrated [71].
Directed Evolution for the Engineering of Cancer-Specific Transduction
As described above, directed evolution and library selection are alternative approaches that can generate highly efficient vectors, even in the absence of mechanistic knowledge underlying a particular gene delivery barrier. Michelfelder et al. [72] employed an in vitro selection scheme to identify variants from an AAV2-based random peptide insertion library that had high infectivity on tumor cells. In this case, analogous to the rational approach, the selected AAV variants shared the RGDXXXX amino acid motif and exhibited over 15-fold higher transduction than wild-type AAV2 vectors on PymT breast cancer cells. The investigators also performed an in vivo selection, in which they administered the AAV library to tumor-bearing mice, harvested tumor tissue, and then recovered the peptide sequences of viral particles that had successfully infected tumor cells. Selected mutants had peptide sequences rich in serine and glycine residues. Dominant clones displayed 40- to 200-fold higher in vivo transduction of breast tumor tissue compared to wild-type vectors and interestingly also showed enhanced cardiac tropism; however, the liver tropism of the mutants was comparable or only moderately lower than that of wild-type vectors.
A DNA shuffled library of AAV serotypes 1, 2, 5, 9, rh8, and rh10 was selected for transduction of U87 human glioma cells and generated infectious mutants after seven rounds of in vitro selection [73]. One of the selected clones, AAV-U87R7-C5, had a chimeric cap gene with contributions from serotypes 1, 2, rh.8 and rh.10. This mutant transduced U87 cells and a panel of other glioma cells moderately better than AAV2.
Payload Engineering for Cancer-Specific Expression
In addition to tissue specificity and high transduction efficiency, AAV vectors for cancer gene delivery may also have regulatory elements that promote tissue-selective gene expression, a feature particularly important when off-target transduction with a cytotoxic or immunostimulatory factor is a potential risk. Promoters that are tissue-specific, tumor-specific, or tumor microenvironment-specific have been explored to restrict transcription of the delivered transgene to a cancerous tissue of interest [74]. For instance, the promoter for C-X-C chemokine receptor type 4 (CXCR4), which is overexpressed in many cancer tissues, was implemented to restrict AAV-mediated transgene expression to primary and metastatic breast cancer [75]. AAV2 vectors encoding firefly luciferase under the control of either the CMV promoter or the CXCR4 promoter were directly delivered to subcutaneous or liver-localized MCF-7 (a human breast cancer cell line) xenografts in mice, and the ratio of expression levels between tumor tissue and muscle was determined. Off-target luminescence in muscle from control CMV vectors was 4- to 21-fold higher than in tumor tissue. In contrast, expression from CXCR4 vectors was selective for tumor tissue, with off-target muscle luminescence levels of only 10%–50% relative to tumor luminescence. Systemic administration via a splenic port of CXCR4 vectors to tumor-bearing mice resulted in luminescence levels at the tumor site that were five-fold higher than in tumor-free mice, confirming selectivity for tumor tissue.
The human telomerase reverse transcriptase (hTERT) promoter has been widely used as a cancer-selective promoter, though its in vivo utility has sometimes been limited by its low strength [76]. In one study, it was combined with an advanced two-step transcriptional activation system (TSTA) to enhance cancer-specific gene expression in a panel of cancer cell lines in vitro and in an in vivo orthotopic liver tumor mouse model [77]. An intravenously delivered AAV2 vector carrying hTERT-driven firefly luciferase with the TSTA system led to liver tumor bioluminescence levels 18-fold higher than delivery of only hTERT-driven luciferase, and 16-fold higher than using the standard TSTA system.
As mentioned earlier, Pandya et al. generated an AAV6-based mutant with enhanced transduction of DCs [68]. They also developed a chimeric promoter sufficiently small to fit into AAV vectors by combining different functional modules of the CD11c promoter, which is specific to DCs. Gene expression using this chimeric promoter (chmCd11c) was restricted to DCs, and delivery of the hPSA gene under its control to DCs resulted in a CTL response against human prostate adenocarcinoma cells, albeit 50% lower than when using the stronger yet ubiquitous chicken beta-actin (CBA) promoter.
Recently AAV8’s strong murine liver tropism was combined with a liver-specific promoter and post-transcriptional regulation based on miR-122a to restrict gene expression to cancer cells in a murine model of metastatic hepatocarcinoma (HCC) [78]. MiR-122a, which is highly expressed in healthy liver tissue but downregulated in HCC, suppresses translation of mRNAs that harbor its target binding sequence. The HLP liver-specific promoter [79] was combined with tandem repeats of miR-122a-binding sequences inserted in the 3′ untranslated region of the expression cassette. Systemic delivery of the resulting construct encoding firefly luciferase led to 40-fold lower liver expression compared to control vector without miR-122a elements. Furthermore, vector administration to mice bearing a subcutaneous HCC xenograft of miR-122a-negative SK-Hep1 cells led to tumor-restricted bioluminescence, with no luminescence from surrounding healthy liver tissue, confirming the combinatorial tumor tissue specificity of this vector and expression cassette.
AAV DELIVERY OF THERAPEUTIC PAYLOADS IN PRECLINICAL MODELS OF CANCER
In addition to efficiently transducing a variety of cancer cells in vitro [30–32], AAV has been increasingly employed to deliver therapeutic genes to in vivo preclinical tumor models. Over the last decade, the arsenal of delivered transgenes has greatly expanded, as have the types of cancer for which AAV vectors have been used. These transgenes can be divided into several categories: anti-angiogenesis genes, cytotoxic or suicide genes, cytokines for stimulating the immune system, tumor suppression and anti-tumor genes, DNA encoding small RNA’s, antigens to stimulate antigen-presenting cells, and antibodies that block signaling.
Anti-Angiogenesis Therapy
Angiogenesis, the formation of new blood vessels from existing ones, is an important process for tumor nourishment, growth, and metastasis [80]. Consequently, inhibiting angiogenesis in tumors to reduce their progression and capacity to metastasize is a longstanding anti-cancer strategy. Multiple gene delivery approaches have been used to inhibit vascular endothelial growth factor (VEGF), a potent angiogenic growth factor, including using decoy receptors, monoclonal antibodies, and a combination of gene delivery with small molecule inhibitors. Mahendra et al. [81] used AAV2 to deliver a soluble splice variant of VEGF-Receptor-1 (sFlt1), a decoy that competitively inhibits VEGF-A binding to its endogenous receptor. Intramuscular AAV2-sFlt1 injection of vector to mice harboring a subcutaneous human ovarian cancer cell line (SKOV3.ip1) xenograft resulted in reduced tumor volume and in 83% survival rate, compared to no survival of untreated mice, six weeks post tumor implantation. In an analogous study, intramuscular administration of AAV1-sFlt1 suppressed tumor growth and enhanced survival in a subcutaneous SHIN-3 ovarian cancer cell line xenograft model [82].
Soluble VEGFR1/R2, a chimeric VEGF receptor comprising domains of both VEGFR-1 and VEGFR-2, was combined with irradiated granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting tumor cell immunotherapy in mouse models of melanoma and colon carcinoma [83]. AAV8-sFVEGFR1/R2 was intravenously delivered to mice, followed by subcutaneous implantation of B16F10 melanoma tumor cells and later immunization with irradiated GM-CSF – secreting B16F10 (B16.GM) tumor cells. VEGR1/R2 delivery resulted in a mean survival time (MST) of 63 days, compared to 31 days for no treatment. This effect was further enhanced by combining vector delivery with B16.GM vaccination (MST of 93 days), which also led to increased numbers of activated DCs and T cells.
VEGF-C, which can mediate angiogenesis and metastasis to lymphatic vessels, has been targeted by delivering a secreted soluble VEGF-C decoy receptor, sVEGFR3-Fc, to melanoma and renal cell carcinoma mouse models [84]. Systemic portal vein administration of AAV2-sVEGFR3-Fc before subcutaneous implantation of A375-mln1 metastatic tumor cells had no effect on primary tumor growth, but inhibited metastasis to the lymph nodes, with only two out of seven mice developing lymph node metastases compared to all of nine mice in the control group. However, vector-mediated inhibition of metastasis to lung was less effective. Additionally, intramuscular injection of AAV2-sVEGFR3-Fc to the quadriceps inhibited lymph node metastasis in renal cell carcinoma (Caki-2) and prostate cancer models (PC-3) by 70% and 75%, respectively. Finally, intramuscular injection of AAV1-sVEGFR3 to mice bearing orthotopic endometrial cancer tumors (HEC1A) led to complete ablation of detectable lymph node and lung metastases compared to untreated mice [85].
In addition to VEGF receptors, other native inhibitors of angiogenesis have also been employed in anti-angiogenic gene delivery studies. Pigment epithelium-derived growth factor (PEDF) is a very potent inhibitor of angiogenesis that prevents the formation of new vessels from endothelial cells by interacting with VEGFR-1, without affecting the existent vasculature [86]. Intratumoral delivery of AAV2-PEDF to a mouse model of Lewis lung carcinoma (LCC) led to a 58% reduction in tumor size, reduced tumor microvessel density, increased tumor cell necrosis, and a 75% increase in MST compared to no treatment [87]. In a subsequent study, combining AAV2-PEDF with the chemotherapeutic drug cisplatin [88] prolonged survival by 150% and 50% compared to no treatment and either treatment alone, respectively. Investigators also reported greater tumor size reduction, tumor apoptosis, and tumor angiogenesis suppression compared to untreated mice or those receiving either treatment alone.
Monoclonal antibodies that block angiogenesis, widely used as a front-line treatment for many types of cancer, have been encoded into AAV vectors for sustained expression and therapeutic effects. Intravenous administration of AAV8-DC101 – encoding a neutralizing mAb against VEGFR2 – resulted in high antibody expression levels in serum, conferred protection against subcutaneous B16F10 melanoma and U87 glioblastoma tumors, and led to reduced tumor size (65% and 82% reduction, respectively) and long-term survival (10% and 80%, respectively) in both models [89]. Tumor angiogenesis was targeted in a DU 145 metastatic lung cancer model by delivering AAVrh.10 encoding a murine mAb with a VEGF-A antigen recognition site equivalent to that of the humanized VEGF-A antibody bevacizumab. Intrapleural vector administration led to high levels of anti-VEGF-A mAb expression, which resulted in reduced growth (76%), vascularization (63%), and proliferation (74%) of metastatic lung tumors and subsequent over 2-fold longer mean survival of treated mice [90]. In another study, the same group intraperitoneally delivered AAVrh.10 packaged with bevacizumab to intraperitoneal models of ovarian carcinomatosis based on A2780 or SK-OV3 cells, eliciting 90% reduced A2780 tumor growth, 82% lower A2780 tumor angiogenesis, and prolonged survival (1.6-fold and 1.2-fold higher mean survival time in A2780 and SK-OV3 tumors, respectively) compared to untreated mice [91]. Additionally, the combination of vector delivery with chemotherapy drugs topotecan or paclitaxel generated further enhanced anti-tumor effects on A2780 xenografts (3.2-fold and 1.9-fold higher mean survival time, respectively, compared to untreated mice).
Endostatin and angiostatin, endogenous inhibitors of angiogenesis that prevent pro-angiogenic factors from interacting with endothelial cells [92], have been employed as protein therapies in clinical trials, motivating their use in gene therapies. Intratumoral delivery of AAV2-endostatin to mice carrying a subcutaneous human bladder cancer tumor (T24) yielded a 40% reduction in tumor volume and a 60% reduction in tumor angiogenesis, as well as enhanced tumor cell apoptosis [93]. The same group later combined endostatin with herpes simplex virus thymidine kinase (HSV-TK), a suicide gene that converts the pro-drug ganciclovir into a thymidine analog that is incorporated into and subsequently fragments DNA undergoing synthesis, for intratumor AAV2-mediated delivery to bladder cancer tumors [94]. This combination therapy led to threefold slower tumor progression and a 60% reduction in tumor size compared to untreated mice, with either therapy individually producing a 40% reduction in size. Another group [95] delivered AAV2-angiostatin to a mouse liver cancer model based on intraportally injected EL-4 tumor cells previously transduced in vitro with AAV2-B7.1, a molecule that stimulates T-cells. Delivery of AAV2-angiostatin to mice vaccinated with B7.1-transduced cells suppressed tumor growth by 87% and greatly increased survival rates, with six of ten treated, vaccinated mice surviving for over 100 days, compared to median survival rates of 33, 42, and 25 days in untreated vaccinated mice, angiostatin-treated unvaccinated mice, and untreated, unvaccinated mice.
Isayeva et al. [96] studied the effect of co-delivering endostatin and angiostatin in an intraperitoneal mouse model of ovarian cancer (SKOV3.ip1). Intramuscular injection of bicistronic AAV2-angiostatin-endostatin (AAV2-E+A) resulted in a 50% reduction in tumor size, increased tumor cell apoptosis, decreased tumor angiogenesis, and 30% of mice surviving for over 150 days compared to control mice surviving an average of 45 days. In a subsequent study [97], combining intraperitoneal AAV2-E+A delivery with the chemotherapy drug taxol led to complete survival of 90% of dually-treated mice and a 90% reduction in tumor size compared to untreated mice. The same group extended this combinatorial approach to the transgenic adenocarcinoma of mouse prostate (TRAMP) prostate cancer model by intramuscularly delivering bicistronic AAV6 encoding endostatin and angiostatin [98]. Mice receiving AAV6-E+A at an early age (5 or 10 weeks old) had low grade, smaller tumors, and 60% of them survived longer than 60 weeks, compared to median survival times of 30–35 weeks for untreated mice or those receiving AAV6-E+A at older ages (>18 weeks), respectively. Endostatin has also been used in conjunction with another angiogenic inhibitor, thrombospodin-1 (TSP-1), in a mouse orthotopic pancreatic cancer model (AsPC-1) [99]. Intramuscular delivery of either AAV2-endostatin or AAV2-3TSR (the antiangiogenic domain of TSP-1) prior to xenografting resulted in similar levels of protection, causing 45% lower tumor microvessel density and a 43% reduction in tumor size. Co-delivery of both vectors led to more marked effects in those parameters (65% and 62%, respectively) compared to either treatment alone.
Intramuscular delivery of AAV2-P125A-endostatin, an endostatin mutant with enhanced binding to endothelial cells and stronger anti-angiogenic effects, in an ovarian carcinoma mouse model (MA148) led to 72% smaller tumors and decreased angiogenesis, with a 46% reduction in the mean number of vessel nodes [100]. In a subsequent study [101], the same group delivered AAV2-P125A-endostatin in combination with the chemotherapy drug carboplatin to an orthotopic ovarian cancer model (MA148). This combination led to 25% higher median survival and 52% less vessel nodes compared to untreated mice.
A range of other antiangiogenic transgenes have been delivered with AAV vectors. AAV2 was used to deliver tissue factor pathway inhibitor (TFPI-2) – a suppressor of angiogenesis, tumor growth, and tumor cell invasiveness – to a glioblastoma mouse model (SNB19) [102]; kringle 5, a fragment of plasminogen with potent antiangiogenic properties, to a mouse model of ovarian cancer (MA148) [103]; and self-complementary cargoes encoding siRNAs against the unfolded protein response (UPR) proteins IRE1α, XBP-1, or ATF6 in a mouse breast cancer model (NeuT) with an AAV2 mutant with seven surface tyrosine to phenylalanine mutations [104]. Cai et al. [105] used AAV5 to deliver vasostatin – an endogenous inhibitor of angiogenesis – to a subcutaneous, orthotopic xenograft, and a spontaneous metastasis model of lung cancer (A549, LLC Lewis lung carcinoma, respectively). Finally, AAV8 encoding human plasminogen kringle 1–5, an inhibitor of angiogenesis, was administered to murine models of mouse melanoma (B16F1), mouse lung cancer (LLC), and human melanoma (A2058) [106]. Delivery of the described transgenes resulted in suppression of both angiogenesis and tumor growth.
Delivery of Cytotoxic or Suicide Genes
Suicide gene therapy has been broadly investigated as an anti-cancer therapy [107]. The most utilized system is the herpes simplex virus type 1 thymidine kinase (HSV-TK), which converts ganciclovir (GCV) into the toxic metabolite GCV-triphosphate within cells expressing the enzyme and also induces bystander toxicity to neighboring tumor cells [108]. Intratumoral administration of AAV2-HSV-TK under Dox-inducible Tet-On regulation to a mouse model of breast cancer (MCF-7) resulted in 75% suppression of tumor growth with only moderate toxicity [109]. In a subsequent study, the same group confirmed these results and elaborated on the therapeutic mechanism of the HSV-TK system in MCF-7 cells [110]. AAV2-mediated delivery of sc39TK, a hyperactive variant of HSV-TK with enhanced affinity for GCV, to HeLa cells that were later implanted into mice to generate subcutaneous tumors enabled 70% tumor growth suppression upon GCV administration [111].
Other cytotoxic genes have also been employed in AAV-mediated delivery to cancer cells. Kohlschütter et al. [32] used AAV2 to deliver diphtheria toxin A (DTA) and p53 upregulated modulator of apoptosis (PUMA) to HeLa cells, SiHa cervical carcinoma cells, and RPMI 8226 myeloma cells in vitro. DTA exerted a cytotoxic effect on all cell lines, whereas PUMA delivery cause cytotoxicity in HeLa and RPMI cells. They also used an AAV2 mutant they had previously developed, RGDLGLS [72], to deliver DTA to mammary tumor cells from mice carrying the polyomavirus middle T antigen (PymT cells) and induced a 40% cytotoxicity.
Immunomodulation through Delivery of Cytokines
The delivery of stimulatory molecules such as cytokines can elicit an enhanced immune response against tumors. A widely used cancer therapeutic is tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), which elicits a strong apoptosis effect primarily in tumor cells, but not in normal tissue, by binding to death receptors [112]. Intraportal administration of AAV2-TRAIL in an orthotopic mouse model of lymphoma (EL-4) suppressed tumor growth by 95% and enhanced median survival by 92%, an effect of induced apoptosis in the tumor cells metastasizing to the liver [113]. The same group subsequently delivered AAV2-sTRAIL (soluble TRAIL) to a subcutaneous mouse model of human liver cancer (SMMC-7721) [114]. Oral or intraperitoneal administration of AAV2-sTRAIL suppressed tumor growth by 88% and 82%, respectively. This group also observed 82% tumor size suppression and 52% enhanced median survival when administering sTRAIL, packaged in AAV5, to mice bearing subcutaneous or orthotopic A549 lung adenocarcinoma tumors [115]. Another group [116] also delivered sTRAIL, packaged in AAV2, to an A549-based subcutaneous lung adenocarcinoma tumor model. Intratumor vector delivery led to 62% reduced tumor size, and systemic vector delivery before implantation of A549 cells lowered the frequency of tumor occurrence to 43% compared to 100% in untreated mice.
Intratumoral delivery of TRAIL, packaged in AAV2 under the control of the cancer-specific hTERT promoter, to a subcutaneous SMMC7721 HCC xenograft led to a 70% suppression of tumor growth and long-term survival, compared to a survival of 78–105 days in untreated mice [117]. They subsequently combined AAV2-hTERT-TRAIL and cisplatin in a subcutaneous BEL7404 HCC model, which resulted in 94% reduction in tumor size and complete survival [118]. In another study, intratumoral AAV2-hTERT-TRAIL delivery to an HCC SMMC7221 mouse model was combined with administration of the chemotherapeutic 5-fluorouracil (5-FU) [119]. Combination therapy led to a strong anti-tumor effect, with an 83% reduction in tumor growth compared to treatment with vector or 5-FU only (60% and 16% reduction, respectively). The same group [120] extended intratumoral delivery of AAV2-TRAIL, combined with cisplatin, to a subcutaneous mouse model of head and neck squamous cell carcinoma (HNSCC), resulting in 40% smaller tumors. In a different study, intracranial delivery of AAV2-interleukin-12(IL-12), a potent immunostimulatory cytokine, in a RG2 rat model of glioblastoma led to enhanced TRAIL expression and microglia activation, 3.5-fold higher median survival time, and 30% reduced tumor volume [121].
Interferons (IFNs) are cytokines that induce antitumor effects that include interfering with cancer cell division and slowing tumor growth progression. Systemic administration of AAV8-hIFN-β to a mouse retroperitoneal xenograft of NB-1691 human neuroblastoma cells, by itself or combined with trichostatin, resulted in similar (90%) suppression of tumor growth relative to untreated or trichostatin-treated mice [122]. Maguire et al. [123] studied the protective effects of IFN-β expression against intracranial, orthotopic U87 xenografts of glioblastoma multiforme. Stereotactic delivery of AAVrh.8-IFN-β followed by cancer cell implantation prevented growth of glioma tumors, and even led to 100% survival against glioma challenge. Additionally, vector delivery into tumor-bearing mice led to regression of established tumors and subsequent complete survival. In another study, AAV2-IFN-β under the control of the hTERT promoter reduced tumor growth by over 90% and enhanced survival in mouse models of colorectal cancer (SW620) and lung cancer (A549), with 87% and 83% long-term survival in treated mice, respectively [124]. Other interferons have been used in AAV-mediated delivery to cancer models, including intravenously administered AAV6-IFN-α to a B16F10 mouse model of metastatic melanoma, leading to a 60% reduction in the number of metastatic colonies and a modest enhancement in the survival of treated mice [125].
CD40-ligand (CD40L) is an immunostimulatory protein implicated in the activation of dendritic cells and induction of tumor cell apoptosis. Intratumor delivery of self-complementary AAV5-CD40L to subcutaneous A549 lung cancer tumors led to a 67% reduction in tumor size and 2.7-fold higher level of tumor cell apoptotic death [126]. Recently, self-complementary AAV5 vectors expressing a non-cleavable CD40L mutant were used to treat subcutaneous A549 lung cancer xenografts [127]. Intratumoral delivery of AAV5-CD40L and AAV5-CD40LM (i.e. mutant) reduced tumor size and increased apoptosis, with the CD40L mutant exhibiting greater effects than wild-type ligand (33% vs. 28% tumor reduction for wtCD40L).
Interleukins, another class of immunostimulatory molecules, have been employed in cancer gene therapies. Systemic delivery of AAV1 carrying melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL24), a cytokine capable of inducing tumor apoptosis and inhibiting tumor growth and metastasis, to a subcutaneous Ehrlich ascites tumor mouse model suppressed tumor growth by 63%, increased tumor cell apoptotic death, reduced tumor angiogenesis, and enhanced mean survival time by over 80% [128]. Likewise, intratumoral delivery of AAV2-IL24 to an orthotopic MHCC97-H HCC model increased apoptotic tumor cell death and inhibited tumor recurrence and metastasis in the liver and lung [129]. In another study, intramuscular delivery of AAV8-IL24 to a transgenic mouse model of mixed-lineage leukemia/AF4-positive acute lymphoblastic leukemia (MLL/AF4-positive ALL) reduced tumor angiogenesis by 57% [130].
Chang et al. [131] studied the delivery of IL-15, a cytokine capable of stimulating an immune response by inducing proliferation and activation of natural killer cells and T cells, to a BNL-h1 mouse model of metastatic HCC. Prophylactic intravenous delivery of AAV8 encoding an IL-15 superagonist followed by cancer cell implantation led to 82% lower tumor metastasis. Analogously, therapeutic delivery (vector delivered to tumor-bearing mice) led to both 80% lower tumor metastasis and enhanced mean survival by 41% with no apparent liver toxicity observed. Another group intramuscularly administered AAV2-IL15 to a transgenic model of SV40 T/t antigen-induced breast cancer prior to induction of breast cancer, which stimulated lymphokine-activated killer (LAK) cells, slowed tumor growth (tumor size reached 2500 mm3 after 33 days vs. 20 days for control) and reduced final tumor size by 30% [132].
Other AAV-delivered immunostimulatory transgenes that have elicited anti-tumor effects include secondary lymphoid tissue chemokine (SLC), delivered preventatively and therapeutically by AAV2 to a Hepal-6 mouse liver cancer tumor model [133]; Nk4, delivered by AAV2 to a metastatic Lewis lung carcinoma (LLC) mouse model [134]; the cytokine LIGHT, delivered by AAV2 to a TC-1 mouse cervical cancer model [135]; the granulocyte-macrophage colony stimulation factor (GM-CSF) cytokine, delivered by AAV1 to a 9L tumor model [136]; and TNF-α, delivered by AAV2 to a U251 human glioma xenograft mouse model [137].
Delivery of Tumor Suppression and Repair Genes
Another anti-cancer gene therapy strategy is the delivery of transgenes and nucleic acids that can elicit tumor suppression or down-regulation of tumorigenic proteins (i.e. those overexpressed in tumor cells). Several groups have delivered dominant negative mutants of survivin, an anti-apoptotic protein overexpressed in most types of cancer. Tu et al. [138] used AAV2 to deliver the C84A survivin mutant, capable of inducing apoptosis, to SW1116 and Colo205 colon cancer cells that were then subcutaneously injected in nude mice, and they observed suppressed tumorigenesis and reduced tumor growth by 80% and 55%, respectively. Alternatively, intratumor therapeutic delivery inhibited the growth of previously established tumors by 81% and prolonged mean survival of treated mice by 75%. The same group [139] subsequently delivered another dominant-negative survivin mutant capable of inducing apoptosis and reducing tumor growth, T34A, to an HCT-116 human colon cancer mouse model using AAV2 and obtained similar results, with further enhanced therapeutic effects when combining AAV with oxaliplatin (62% of mice showing complete survival). Two other groups [140, 141] also used AAV-mediated delivery of the C84A and T34A survivin mutants, respectively, for in vivo models of gastric cancer and observed reduced tumor growth, increased tumor cell apoptosis, and increased tumor sensitivity to 5-fluoracil.
The C-terminal fragment of the human telomerase reverse transcriptase (hTERTC27), which exerts an anti-tumor activity by inducing telomere dysfunction, was packaged in AAV2 and intratumorally delivered to a human glioblastoma multiforme U87-MG tumor xenograft mouse model [142]. The results were increased levels of tumor necrosis and apoptosis, increased infiltration of polymorphonuclear neutrophils into the tumor, reduced angiogenesis, and consequently an 83% reduction in tumor growth and two-fold higher median survival of treated mice. The same group then combined AAV2 and adenoviral vector delivery of hTERTC27 and obtained a synergistic therapeutic effect of 2.56-fold higher median survival compared to untreated mice [143].
A wide array of other anti-tumor transgenes have been delivered to preclinical cancer models using AAV vectors. These include AAV2-maspin to LNCaP and DU145 prostate cancer tumors [144]; AAV2-nm23H1 to SW626-M4 metastatic ovarian cancer tumors [145]; AAV2-HGFK1 (kringle 1 domain of human hepatocyte growth factor) to CT26 and Lovo colorectal carcinoma tumors [146]; AAV2-encoded anti-calcitonin ribozymes to an orthotopic implantation model and a transgenic model of prostate cancer [147]; AAV2-4EBP1 (eukaryotic translation initiation factor 4E-binding protein 1) to a K-rasLA1 lung cancer model [148]; AAV2-mediated delivery of the chemokine receptor CXC chemokine receptor 2 (CXCR2) C-tail sequence to an HPAC pancreatic tumor model [149]; AAV1 delivery of IL-24 and apoptotin to a HepG2 liver cancer model [150]; AAV2-TAP (alpha-tocopherol-associated protein) to PC-3 and LNCaP prostate cancer tumors [151]; delivery of trichosanthin, packaged in AAV3-S663V+T492V vectors, to HuH7 hepatocellular carcinoma (HCC) tumors [66]; AAV2-decorin to a U87MG glioblastoma multiforme model [152]; AAV2-cathelicidin to HT-29 colon cancer tumors [153]; AAV8-mediated delivery of Niemann-Pick type C2 (NPC2) to the N-methyltransferase knockout (Gnmt−/−) transgenic spontaneous HCC model [154]; and AAV9-mediated delivery of human Mullerian inhibiting substance (MIS, albumin leader Q425R MIS (LRMIS)) in a xenograft model of ovarian cancer [155]. Additionally, groups have delivered the p53 tumor suppressor gene, commonly mutated in cancerous cells, using AAV2 vectors to bladder cancer cells [156] and non-small cell lung cancer cells [157] in vitro and to an H358 bronchioalveolar carcinoma tumor model in vivo [158].
AAV Vectors Encoding Small RNAs
AAVs encoding RNA cargoes are another anti-cancer modality that has been employed in a variety of tumor models. Prophylactic administration of AAV2 encoding small hairpin RNA (shRNA) targeting Epstein-Barr virus (EBV) latent membrane protein-1 (LMP-1) to a C666-1 nasopharyngeal carcinoma (NPC) mouse model led to a 47% inhibition of tumor metastasis, though no effects on tumor growth were observed [159]. AAV2 encoding antisense RNA targeting the E7 oncogene from human papilloma virus 16 (HPV16) present in CaSki cervical cancer cells and subsequent implantation of these cells reduced tumor formation by 80% and inhibited the size of formed tumors by 79% compared to transplantation of uninfected cells [160]. Sun et al. [161] delivered AAV2 encoding shRNA targeting the androgen receptor (AR) gene to 22Rv1 prostate cancer xenografts. Among the group of AR-shRNAs that they screened, AAV2-ARHP8 suppressed tumor growth by 88% when delivered intratumorally, and systemic delivery of AAV2-ARHP8 caused elimination of xenografts. Intratumoral AAV2 encoding siRNA against Snail, a transcription factor involved in anti-apoptotic and chemoresistance upregulated in pancreatic cancer, to a PANC-1 xenograft model of pancreatic cancer suppressed tumor growth by 76% [162]. Subsequently, the same group delivered AAV encoding siRNA targeting the Slug gene, a suppressor of apoptosis, to an orthotopic QBC939 model of cholangiocarcinoma, a type of liver cancer [163]. Intratumoral injection of AAV2-SlugsiRNA led to 51% reduced tumor growth alone and complete tumor regression when combined with radiation treatment. Delivery of AAV2 encoding shRNA against FHL2 (four and a half LIM-only protein 2), a putative oncogene involved in various cellular processes including proliferation and migration, to a LoVo colon cancer xenograft led to 66% reduced tumor volume, an effect that was enhanced to a 95% reduction with co-administration of 5-FU [164]
Other AAV serotypes have also been employed for delivery of small RNA-encoding payloads. Delivery of AAV1 encoding shRNA against Hec1 (Highly Expressed in Cancer 1) to a U251 glioma xenograft mouse model increased tumor cell apoptosis but did not ultimately reduce tumor growth [165]. Systemic delivery of self-complementary AAV8 vectors encoding microRNA miR-26a, which becomes downregulated in HCC cells, to the tet-o-MYC, LAP-tTA bi-transgenic HCC mouse model resulted in high expression levels of miR-26a in the liver, reduced tumor occurrence, and 65% smaller average tumor size without any observed toxicity [166].
Delivery of Antigens for Stimulating Antigen-Presenting Cells (APCs)
Adeno-associated virus vectors have also been employed to deliver antigens to antigen-presenting cells and thereby elicit an immune response against tumor cells expressing that antigen, i.e. a tumor vaccine. An excellent example of AAV-mediated vaccination focused on antigens from human papilloma virus 16 (HPV16), which is associated with the development of cervical and anogenital cancer. Several groups have used AAV to express the HPV16 structural protein L1 and thereby induce anti-HPV neutralizing antibodies [167]. L1-based virus-like particles (VLPs), which are non-infectious and morphologically identical to HPV virions but do not carry any oncogenes, are safe vaccine agents that can elicit high titers of neutralizing antibodies. Liu et al. used intramuscularly delivered AAV2-HPV16L1 to elicit anti-L1 antibodies, then compared the resulting titers with those generated by either an AAV control vector, HPV16 VLPs composed of L1, naked DNA encoding L1, adenovirus coding for mGM-CSF, or a combination of AAV2 and adenovirus [167]. The antibody titer induced by AAV2-L1 delivery was 20% lower than that generated by VLPs; however, a single dose of combined AAV and adenovirus led to titers as high three doses of VLPs. AAV2-L1 delivery also led to accumulation of macrophages and DCs at levels comparable to VLP delivery.
Interestingly, a single dose of intranasal AAV5-mediated delivery of HPV16-L1 was sufficient to generate long-lasting, high titers of serum anti-L1 antibodies in mice, comparable to those generated by VLP delivery [168]. Additionally, vector delivery led to generation of mucosal antibodies in vaginal washes and to a long-term cellular immune response against HPV16. The same group subsequently investigated intranasal vaccination of mice by delivering an HPV16 L1/E7 fusion gene using AAV5, AAV8, or AAV9 vectors and showed serotypes 5 and 9 were most effective at generating neutralizing antibodies and a CTL response against HPV16 [169]. Moreover, the murine study was followed by successful intranasal vaccination of rhesus macaques using HP16 L1 delivered by AAV5 and AAV9 vectors, where the latter induced long lasting immunization [170]. Building upon their previous study of AAV-mediated vaccination against HPV16 [171], Liao et al. [172] developed vaccines against three HPV16 oncogenes – E5, E6, and E7 – that conferred immune protection against cervical cancer tumor growth. They used AAV2 to deliver a long peptide targeting HPV16 E5, E6, and E7 that induced an immune response against TC-1 cells, which express HPV16 proteins; vaccinated mice were protected from tumor growth for 300 days.
Various other antigens have been delivered using AAV vectors to preclinical cancer models for APC stimulation and antibody generation. AAV2 encoding a B-cell leukemia/lymphoma 1 (BCL1) idiotype led to the generation of anti-Id antibodies and protection against BCL1 cell-based tumors [173]. Intramuscular AAV2 packaged with the LMP2/1-hsp fusion gene, consisting of the Epstein-Barr virus latent membrane proteins 1 and 2 fused to heat shock protein as an adjuvant, to a tumor model based on SP2/0 cells expressing LMP2 led to a humoral and CTL response against LMP2-expressing tumor cells, 83% reduction in tumor growth, and long-term survival of 90% of treated animals [174]. In addition, intramuscular AAV6 encoding melanoma antigen Trp2 generated an antitumor response against B16.F10 tumors when combined with Toll-like receptor (TLR) agonists [175] The same group subsequently co-administered TLR agonists and AAV2 encoding carcinoembryonic antigen (CEA) to vaccinate mice against colon cancer cells, resulting in antitumor response against MC38 cells expressing CEA [176]. In another study investigating neu-expressing TUBO breast cancer tumors [177], intramuscular vaccination with AAV5-neu or AAV6-neu resulted in humoral and cell-mediated immune response, leading to 50% and 100% long-term survival, respectively. Similarly, oral vaccination led to 80% and 100% long-term survival with AAV5 and AAV6, respectively. Additionally, oral AAV6-neu vaccination also protected against re-challenge with TUBO tumor cells 320 days after original tumor cell implantation. Han et al. [178] used AAV2 to systemically deliver a soluble form of B and T lymphocyte attenuator (BTLA) in combination with a heat shock protein (HSP70) vaccine to a B16F1 mouse melanoma pulmonary metastasis model, which initially reduced metastatic foci but did not prevent late-stage metastatic melanoma. Conversely, prophylactic treatment caused enhanced innate and adaptive immune responses against tumor cells, leading to 80% inhibition of tumor formation and long-term survival of 83% of treated mice. One general challenge with prophylactic tumor vaccination with AAV, however, is that a single administration can lead to long-term neutralizing antibodies that cross-react against multiple AAV serotypes, complicating subsequent AAV administrations to treat other conditions.
Delivery of Antibodies to Block Signaling
A number of anti-cancer monoclonal antibodies (mAbs) therapeutics have been developed to target cancer cells for immune system processing and presentation, to inhibit cancer cell growth and tumor progression by blocking antigens involved in tumor cellular processes such as migration, and to inhibit immunosuppressive signaling molecules and thereby boost anti-tumor immune responses. Adeno-associated virus vectors have been employed to deliver genes encoding such monoclonal antibodies for long-term expression in preclinical models.
Ho et al. [179] delivered 14E1, a murine antihuman epidermal growth factor (EGFR) antibody, by intramuscular administration of AAV1 vectors in the A431 human vulvar carcinoma xenograft model. Administration of vector prior to tumor cell xenografting completely inhibited or reduced tumor growth by 93% when administered 28 days before and 1 day after tumor cell implantation, respectively. Gene delivery also led to long-term survival, with a majority of mice showing complete tumor regression. Intratumorally injected AAV2 encoding adximab, a mouse-human chimeric antibody against death-receptor 5 (DR5), to mouse models of human liver cancer (SMMC7221) and colon cancer (HCT116) reduced tumor growth (58% and 40% reduction, respectively) and increased tumor cell apoptotic death (2-fold and 2.6-fold higher, respectively) in both models [180]. Finally, administration of an AAV9 vector encoding a monoclonal antibody against the glycolytic enzyme alpha-enolase (ENO1) prior to xenografting orthotopic CFPAC-1 pancreatic ductal adenocarcinoma (PDAC) tumors led to high concentrations of anti-ENO1 antibody in serum and a 95% reduction in lung metastases [181].
AAV VECTORS IN CANCER CLINICAL TRIALS
The promising results of adeno-associated virus vectors in preclinical models of cancer, coupled with their clinical successes for monogenic diseases [10], have motivated the translation of AAV vectors into oncology clinical trials.
A phase I trial performed between the Peking University School of Oncology and the Beijing Cancer Hospital and Institute [182] administered cytotoxic T lymphocytes (CTLs) that had been activated by dendritic cells (DCs) previously transduced with AAV2 vectors carrying carcinoembryonic antigen (CEA) to cancer patients who had failed to respond to standard treatments. From the 25 patients evaluated after treatment, 2 showed partial remission, 10 showed stable diseases, and 13 had progressive disease, with a resulting mean progression-free survival of 3.1 months. Importantly, no treatment-related serious adverse events were reported for any patients. Larger, randomized studies may follow.
A clinical trial (ClinicalTrials.gov Identifier:NCT02496273), led by Wu Changping, M.D. and Jiang Jingting, Ph.D. at The First People’s Hospital of Changzhou in China, is investigating the clinical safety and efficacy of administering CEA-specific CTLs activated by DCs previously loaded with CEA via AAV2 transduction. This phase I clinical trial is focused on stage IV gastric cancer patients, will monitor T cell populations and tumor progression, and is projected to initiate in January, 2016.
Another clinical trial (ClinicalTrials.gov Identifier: NCT02602249), developed by Beijing Doing Biomedical Co., Ltd in China, is similarly investigating the clinical safety and efficacy of administering CTLs specific for the Mucin 1 (MUC1) antigen, whose overexpression can be associated with cancer, to stage IV gastric cancer patients. MUC1-specific CTLs will be activated by DCs either loaded with MUC1 via AAV2 transduction or directly pulsed with a MUC1 peptide. This study is projected to be completed in June, 2018.
Finally, although not targeting cancer cells per se, another phase I clinical trial (ClinicalTrials.gov Identifier: NCT02446249) led by John A Chiorini, Ph.D. at the NIH is investigating the safety of using an AAV2-aquaporin (AAV-hAQP1) gene therapy for patients with irradiation-induced parotid salivary hypofunction (xerostomia), a condition that can develop in patients with a history of radiation therapy for head and neck cancer. The investigators have already completed a separate phase I clinical trial (06-D-0206) using adenovirus, where they showed safety and therapeutic efficacy of hAQP1 delivery to a single parotid gland. The study initiated in April, 2015, and the investigators will monitor therapeutic efficacy of the treatment by measuring parotid salivary gland output as well as safety through traditional clinical and immunological measures.
FUTURE PROSPECTS AND CONCLUSIONS
AAV vectors have enjoyed increasing clinical success as a result of their excellent safety profile and high gene delivery efficacy. To date, over 130 clinical trials [6] have employed AAV vectors to treat conditions in a wide range of tissues, including muscle, eye, liver, central nervous system, heart, and lung diseases [10]. The approval of Glybera in the European Union and recent reports on clinical trials, including those for Sanfilippo B syndrome (Pasteur Institute, Phase I/II) and Leber’s congenital amaurosis (Spark Therapeutics, Phase III), underscore the strong promise of AAV-mediated therapeutic gene delivery and foreshadow the development and approval of AAV gene therapies in the United States in the near future.
As presented in this review and summarized in Table I, AAV vectors, particularly AAV2, have been extensively used in a variety of preclinical models of cancer to deliver a wide array of transgenes, including anti-angiogenic factors, suicide genes, immunostimulatory genes and antigens, tumor suppressors, payloads encoding small interfering nucleic acids, and monoclonal antibodies. Despite the high prevalence of gene therapy clinical trials directed at cancer, AAV vectors have only recently entered this field, which has so far been focused on oncolytic viruses – including adenovirus, herpes simplex virus, and reovirus – and to a lesser extent, non-viral methods [183]. However, AAV vectors offer several complementary advantages that can be harnessed for anti-cancer therapies, including the potential for high efficiency transduction, the promise of vector engineering for targeted delivery, and gene expression in post-mitotic cells.
Table 1.
AAV vectors employed in gene delivery to preclinical models of cancer.
| Therapeutic Modality | AAV Vector | Type of Cancer Model | Delivered Transgene | References |
|---|---|---|---|---|
|
| ||||
| Anti-angiogenesis | AAV1 | Ovarian cancer | VEGFR-1 (sFlt1) | 82 |
| Endometrial cancer | Soluble VEGFR3 | 85 | ||
|
| ||||
| AAV2 | Ovarian cancer | VEGFR-1 (sFlt1) | 81 | |
| Melanoma | Soluble VEGFR3-Fc | 84 | ||
| Renal cell carcinoma | Soluble VEGFR3-Fc | 84 | ||
| Prostate cancer | Soluble VEGFR3-Fc | 84 | ||
| Lewis lung carcinoma | PEDF | 87, 88 | ||
| Bladder cancer | Endostatin | 93, 94 | ||
| Liver cancer | Angiostatin | 95 | ||
| Ovarian cancer | Endostatin + angiostatin | 96, 97 | ||
| Pancreatic cancer | Endostatin | 99 | ||
| Pancreatic cancer | 3TSR | 99 | ||
| Ovarian carcinoma | P125A endostatin | 100, 101 | ||
| Glioblastoma | TFPI-2 | 102 | ||
| Ovarian cancer | Kringle 5 | 103 | ||
|
| ||||
| AAV2 5YF mutant | Breast cancer | siRNA against IRE1α, XBP-1, or ATF6 | 104 | |
|
| ||||
| AAV5 | Lung cancer | Vasostatin | 105 | |
| Lung cancer | Vasostatin | 105 | ||
|
| ||||
| AAV6 | Prostate cancer | Endostatin + angiostatin | 98 | |
|
| ||||
| AAV8 | Mouse melanoma | Plasminogen kringle 1–5 | 106 | |
| Lung cancer | Plasminogen kringle 1–5 | 106 | ||
| Melanoma | Plasminogen kringle 1–5 | 106 | ||
| Melanoma | Soluble VEGFR1/R2 | 83 | ||
| Colon cancer | Soluble VEGFR1/R2 | 83 | ||
|
| ||||
| Cytotoxic or suicide genes | AAV2 | Breast cancer | HSV-TK | 109, 110 |
| HeLa cells tumor | sc39TK | 111 | ||
|
| ||||
| Immunomodulation through cytokines | AAV1 | Ehrlich ascites tumor | mda-7/IL24 | 128 |
| Flank tumor in rats | GM-CSF | 136 | ||
|
| ||||
| AAV2 | Lymphoma | TRAIL | 113 | |
| Liver cancer | Soluble TRAIL | 114 | ||
| Lung adenocarcinoma | Soluble TRAIL | 116 | ||
| Hepatocellular carcinoma | TRAIL | 117, 119 | ||
| Hepatocellular carcinoma | TRAIL | 118 | ||
| Head and neck squamous cell carcinoma | TRAIL | 120 | ||
| Glioblastoma | TRAIL | 121 | ||
| Colorectal cancer | IFN-β | 124 | ||
| Lung cancer | IFN-β | 124 | ||
| Hepatocellular carcinoma | IL24 | 129 | ||
| Breast cancer | IL15 | 132 | ||
| Liver cancer | SLC | 133 | ||
| Metastatic Lewis lung carcinoma | Nk4 | 134 | ||
| Cervical cancer | LIGHT | 135 | ||
| Glioma | TNF-α | 137 | ||
|
| ||||
| AAV5 | Lung adenocarcinoma | Soluble TRAIL | 115 | |
| Lung cancer | CD40L | 128 | ||
| Lung cancer | CD40L mutant | 127 | ||
|
| ||||
| AAV6 | Metastatic melanoma | IFN-α | 125 | |
|
| ||||
| AAV8 | MLL/AF4-positive ALL | IL24 | 130 | |
| Metastatic hepatocellular carcinoma | IL15 | 131 | ||
| Neuroblastoma | IFN-β | 122 | ||
|
| ||||
| AAVrh.8 | Glioblastoma multiforme | IFN-β | 123 | |
|
| ||||
| Tumor suppression and repair | AAV1 | Liver cancer | IL24 + apoptotin | 150 |
|
| ||||
| AAV2 | Colon cancer | C84A survivin | 138 | |
| Colon cancer | C84A survivin | 138 | ||
| Colon cancer | T34A survivin | 139 | ||
| Gastric cancer | C84A survivin | 140 | ||
| Gastric cancer | T34A survivin | 141 | ||
| Glioblastoma multiforme | Htertc27 | 142, 143 | ||
| Prostate cancer | Maspin | 144 | ||
| Prostate cancer | Maspin | 144 | ||
| Metastatic ovarian cancer | nm23H1 | 145 | ||
| Colorectal carcinoma | HGFK1 | 148 | ||
| Colorectal carcinoma | HGFK1 | 148 | ||
| Prostate cancer | Anti-calcitonin rybozymes | 147 | ||
| Lung cancer | 4EBP1 | 148 | ||
| Pancreatic cancer | CXCR2 C-tail | 149 | ||
| Prostate cancer | TAP | 151 | ||
| Prostate cancer | TAP | 151 | ||
| Glioblastoma multiforme | Decorin | 152 | ||
| Colon cancer | Cathelicidin | 153 | ||
| Bronchioalveolar carcinoma | p53 | 158 | ||
|
| ||||
| AAV3-S663V+T492V | Hepatocellular carcinoma | Trichosanthin | 66 | |
|
| ||||
| AAV8 | Hepatocellular carcinoma | NPC2 | 154 | |
|
| ||||
| AAV9 | Ovarian cancer | LRMIS | 155 | |
|
| ||||
| RNA interference | AAV1 | Glioma | shRNA against Hec1 | 165 |
|
| ||||
| AAV2 | Nasopharyngeal carcinoma | shRNA against EBV LMP-1 | 159 | |
| Cervical cancer | Anti-sense RNA against HPV16-E7 | 160 | ||
| Prostate cancer | shRNA against AR | 161 | ||
| Pancreatic cancer | siRNA against Snail | 162 | ||
| Cholangiocarcinoma (liver cancer) | siRNA against Slug | 163 | ||
| Colon cancer | shRNA against FHL2 | 164 | ||
|
| ||||
| AAV8 | Hepatocellular carcinoma | miR-26a | 166 | |
|
| ||||
| Stimulation of APCs | AAV2 | Cervical cancer | Vaccination with HPV16-L1 | 167 |
| Cervical cancer | Vaccination against HPV16 E5/E6/E7 | 172 | ||
| B cell leukemia/lymphoma 1 | Vaccination with BLC1 idiotype | 173 | ||
| Nasopharyngeal carcinoma | Vaccination with EBV LMP2/1-hsp | 174 | ||
| Colon cancer | Vaccination with CEA | 176 | ||
| Melanoma pulmonary metastasis | Vaccination with BTLA and HSP70 | 178 | ||
|
| ||||
| AAV5 | Cervical cancer | Vaccination with HPV16-L1 | 168 | |
| Cervical cancer | Vaccination with HPV16-L1 /E7 | 169 | ||
| Breast cancer | Vaccination with neu | 177 | ||
| Cervical cancer in rhesus macaques | Vaccination with HPV16-L1 | 170 | ||
|
| ||||
| AAV6 | Melanoma | Vaccination with Trp2 | 175 | |
| Breast cancer | Vaccination with neu | 177 | ||
|
| ||||
| AAV8 | Cervical cancer | Vaccination with HPV16-L1 /E7 | 169 | |
|
| ||||
| AAV9 | Cervical cancer | Vaccination with HPV16-L1 /E7 | 169 | |
| Cervical cancer in rhesus macaques | Vaccination with HPV16-L1 | 170 | ||
|
| ||||
| Antibodies | AAV1 | Vulvar carcinoma | 14D1, anti-EGFR mAb | 179 |
|
| ||||
| AAV2 | Liver cancer | adximab | 180 | |
| Colon cancer | adximab | 180 | ||
|
| ||||
| AAV8 | Melanoma | DC101, anti-VEGFR2 mAb | 89 | |
| Glioblastoma | DC101, anti-VEGFR2 mAb | 89 | ||
|
| ||||
| AAV9 | Pancreatic ductal adenocarcinoma | anti-ENOI1 mAb | 181 | |
|
| ||||
| AAVrh.10 | Metastatic lung cancer | Murine anti-VEGFA mAb | 90 | |
| Ovarian carcinomatosis | bevacizumab | 91 | ||
| Ovarian carcinomatosis | bevacizumab | 91 | ||
Due to their inability to replicate or efficiently integrate in transduced tumor cells, AAV vectors may have a limited potential for sustained oncolytic or pro-apoptotic effects, as evidenced by some in vitro and in vivo studies [184]. Instead, AAV vectors may be an excellent platform for eliciting protective anti-tumor effects via tumor suppression and immunostimulation – a strategy that ongoing AAV cancer clinical trials are currently employing. Delivery of tumor-suppressive or stimulatory payloads – such as anti-angiogenic factors, monoclonal antibodies, cytokines, antigens for loading APCs, and immunomodulatory factors - would harness AAV’s excellent safety and delivery properties, and, more importantly, would not require transduction of all tumor cells to induce an efficient therapeutic effect.
In particular, AAV vectors can deliver tumor-specific antigens to generate tumor-specific humoral and T cell-mediated responses – in efforts to vaccinate against the tumor – and this approach has been explored in both murine and non-human primate preclinical models that have strongly suggested the vector’s potential against conditions like cervical cancer (HPV-L1) and prostate cancer (hPSA). Antigen delivery can also be employed in the ex vivo transduction of antigen-presenting cells like dendritic cells (DCs), which after being stimulated, loaded with the antigen, and reinjected into the patient, can elicit a CTL response against the tumor. Dendritic cell vaccines are safe and effective, and as explained above, mark some of the first AAV clinical trials directed at treating a type of cancer; this approach could potentially be extended to other types of cancer previously treated with DC vaccines, including melanoma, colon cancer, and prostate cancer [185].
Another important immunostimulatory strategy consists of augmenting anti-tumor cytotoxic T lymphocyte (CTL) responses by inhibiting negative immunoregulators such as CTLA-4 and PD-1 [186]. Blocking antibodies against CTLA-4, PD-1, and PD-1 Ligand 1 (PD-L1) have led to significant improvements in the treatment of various cancers (e.g. melanoma, renal cell carcinoma, lung cancer), and are either approved by the FDA (ipilimumab, anti-CTLA-4 approved for melanoma) or in advanced clinical trials [187]. AAV’s gene transfer properties have already been exploited for the delivery of recombinant anti-angiogenesis monoclonal antibodies, including bevacizumab, to preclinical cancer models. AAV vectors could therefore be used to stimulate a CTL response by local delivery of blocking antibodies against CTLA-4 or PD-1. Long-term, sustained expression of these or other therapeutic antibodies could reduce overall dosage while increasing local expression levels, and consequently improve treatments for multiple indications such as non-Hodgkin’s lymphoma, breast cancer, and colorectal cancer.
As another future direction, AAV vectors can benefit from further developments that would make them even more suitable gene delivery vehicles for cancer therapies. Novel vectors with selective tropism towards the tissue of interest, low off-target transduction, and the capacity to evade pre-existing neutralizing antibodies would promote high levels of gene expression, a requirement for a strong therapeutic effect. Furthermore, AAV vectors would strongly benefit from engineering for localization to primary and secondary tumors as well as to tumor initiating cells (sometimes regarded as “cancer stem cells”), which are resistant to traditional therapies and greatly contribute to the poor prognosis and post-therapy relapse of many cancers [2]. Vectors may also be engineered for specific transduction following different routes of administration – for example, systemic delivery, localized injection to post-mitotic non-tumor tissue for sustained transgene expression and secretion, or intratumoral administration – which can in turn influence gene delivery efficacy [188]. As previously discussed, directed evolution – which has successfully been applied to enhance existing vector properties or engineer entirely new and optimized properties for delivery to normal tissues – similarly offers strong potential for engineering novel AAV vectors for cancer therapies. Capsid engineering efforts can additionally be combined with the development of innovative payloads that can provide tissue selectivity, strong expression, and maximization of genomic space for transgene delivery.
Finally, numerous studies presented in this review combined therapeutic gene delivery with traditional chemotherapy drugs or other therapeutic strategies to yield therapeutic effects greater than those elicited by either treatment alone. Thus, integrating AAV-mediated gene delivery with standard therapies (e.g. surgery, chemotherapy, radiotherapy) to develop novel anti-tumor treatment strategies offers strong promise in future cancer gene therapy studies.
Acknowledgments
FUNDING
JLSO has been supported by a Ford Foundation Fellowship, a National Science Foundation Graduate Fellowship, and two UC Berkeley’s Graduate Division Fellowships. This work was also funded by R01EY022975.
Footnotes
Conflict of interest statement. DVS and JLSO are inventors on patents involving AAV directed evolution, and DS is the co-founder of an AAV gene therapy company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.A.C. Society. Cancer Facts & Figures 2015. American Cancer Society; Atlanta, GA: 2015. [Google Scholar]
- 2.Venere M, Fine HA, Dirks PB, Rich JN. Cancer stem cells in gliomas: identifying and understanding the apex cell in cancer’s hierarchy. Glia. 2011;59:1148–1154. doi: 10.1002/glia.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clinical medicine & research. 2006;4:218–227. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. The New England journal of medicine. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Indications Addressed by Gene Therapy Clinical Trials. The Journal of Gene Medicine. www.wiley.co.uk/genmed/clinical.
- 7.Ledford H. Cancer-fighting viruses win approval. Nature. 2015;526:622–623. doi: 10.1038/526622a. [DOI] [PubMed] [Google Scholar]
- 8.Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. The AAPS journal. 2007;9:E92–104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. Journal of controlled release : official journal of the Controlled Release Society. 2012;161:377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nature reviews Genetics. 2014;15:445–451. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalkara D, Sahel JA. Gene therapy for inherited retinal degenerations. Comptes rendus biologies. 2014;337:185–192. doi: 10.1016/j.crvi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Ohmori T, Mizukami H, Ozawa K, Sakata Y, Nishimura S. New approaches to gene and cell therapy for hemophilia. Journal of thrombosis and haemostasis : JTH. 2015;13(Suppl 1):S133–142. doi: 10.1111/jth.12926. [DOI] [PubMed] [Google Scholar]
- 14.Carpentier AC, Frisch F, Labbe SM, Gagnon R, de Wal J, Greentree S, Petry H, Twisk J, Brisson D, Gaudet D. Effect of alipogene tiparvovec (AAV1-LPL(S447X)) on postprandial chylomicron metabolism in lipoprotein lipase-deficient patients. The Journal of clinical endocrinology and metabolism. 2012;97:1635–1644. doi: 10.1210/jc.2011-3002. [DOI] [PubMed] [Google Scholar]
- 15.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene therapy. 2012;19:694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 16.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10220–10225. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartlett JS, Samulski RJ, McCown TJ. Selective and rapid uptake of adeno-associated virus type 2 in brain. Human gene therapy. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 18.Duan D, Sharma P, Yang J, Yue Y, Dudus L, Zhang Y, Fisher KJ, Engelhardt JF. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. Journal of virology. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, McLaughlin S, Muzyczka N, Rocchi M, Berns KI. Site-specific integration by adeno-associated virus. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis BL, Hirsch ML, Barker JC, Connelly JP, Steininger RJ, 3rd, Porteus MH. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virology journal. 2013;10:74. doi: 10.1186/1743-422X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berns KI, Linden RM. The cryptic life style of adeno-associated virus. BioEssays : news and reviews in molecular cellular and developmental biology. 1995;17:237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- 22.Flotte TR, Afione SA, Zeitlin PL. Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. American journal of respiratory cell and molecular biology. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 23.Mancheno-Corvo P, Martin-Duque P. Viral gene therapy. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2006;8:858–867. doi: 10.1007/s12094-006-0149-y. [DOI] [PubMed] [Google Scholar]
- 24.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene therapy. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 25.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A, Conlon TJ, Calcedo R, Pang JJ, Erger KE, Olivares MB, Mullins CL, Swider M, Kaushal S, Feuer WJ, Iannaccone A, Fishman GA, Stone EM, Byrne BJ, Hauswirth WW. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Archives of ophthalmology. 2012;130:9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, Maas MM, Zwinderman AH, Ross C, Aronica E, High KA, Levi MM, Hayden MR, Kastelein JJ, Kuivenhoven JA. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arteriosclerosis, thrombosis and vascular biology. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- 29.Gaudet D, Methot J, Dery S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene therapy. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicklin SA, Dishart KL, Buening H, Reynolds PN, Hallek M, Nemerow GR, Von Seggern DJ, Baker AH. Transductional and transcriptional targeting of cancer cells using genetically engineered viral vectors. Cancer letters. 2003;201:165–173. doi: 10.1016/j.canlet.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Teschendorf C, Emons B, Muzyczka N, Graeven U, Schmiegel W. Efficacy of recombinant adeno-associated viral vectors serotypes 1, 2, and 5 for the transduction of pancreatic and colon carcinoma cells. Anticancer research. 2010;30:1931–1935. [PubMed] [Google Scholar]
- 32.Kohlschutter J, Michelfelder S, Trepel M. Novel cytotoxic vectors based on adeno-associated virus. Toxins. 2010;2:2754–2768. doi: 10.3390/toxins2122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer gene therapy. 2005;12:913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lochrie MA, Tatsuno GP, Christie B, McDonnell JW, Zhou S, Surosky R, Pierce GF, Colosi P. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. Journal of virology. 2006;80:821–834. doi: 10.1128/JVI.80.2.821-834.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koerber JT, Maheshri N, Kaspar BK, Schaffer DV. Construction of diverse adeno-associated viral libraries for directed evolution of enhanced gene delivery vehicles. Nature protocols. 2006;1:701–706. doi: 10.1038/nprot.2006.93. [DOI] [PubMed] [Google Scholar]
- 37.Koerber JT, Jang JH, Schaffer DV. DNA shuffling of adeno-associated virus yields functionally diverse viral progeny. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:1703–1709. doi: 10.1038/mt.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koerber JT, Schaffer DV. Transposon-based mutagenesis generates diverse adeno-associated viral libraries with novel gene delivery properties. Methods in molecular biology. 2008;434:161–170. doi: 10.1007/978-1-60327-248-3_10. [DOI] [PubMed] [Google Scholar]
- 39.Santiago-Ortiz J, Ojala DS, Westesson O, Weinstein JR, Wong SY, Steinsapir A, Kumar S, Holmes I, Schaffer DV. AAV ancestral reconstruction library enables selection of broadly infectious viral variants. Gene therapy. 2015 doi: 10.1038/gt.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perabo L, Endell J, King S, Lux K, Goldnau D, Hallek M, Buning H. Combinatorial engineering of a gene therapy vector: directed evolution of adeno-associated virus. The journal of gene medicine. 2006;8:155–162. doi: 10.1002/jgm.849. [DOI] [PubMed] [Google Scholar]
- 41.Zinn E, Pacouret S, Khaychuk V, Turunen HT, Carvalho LS, Andres-Mateos E, Shah S, Shelke R, Maurer AC, Plovie E, Xiao R, Vandenberghe LH. In Silico Reconstruction of the Viral Evolutionary Lineage Yields a Potent Gene Therapy Vector. Cell reports. 2015;12:1056–1068. doi: 10.1016/j.celrep.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, Kay MA. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. Journal of virology. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, Asokan A, Wu Z, Van Dyke T, DiPrimio N, Johnson JS, Govindaswamy L, Agbandje-McKenna M, Leichtle S, Redmond DE, Jr, McCown TJ, Petermann KB, Sharpless NE, Samulski RJ. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- 44.Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, Trepel M. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nature biotechnology. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 45.Varadi K, Michelfelder S, Korff T, Hecker M, Trepel M, Katus HA, Kleinschmidt JA, Muller OJ. Novel random peptide libraries displayed on AAV serotype 9 for selection of endothelial cell-directed gene transfer vectors. Gene therapy. 2012;19:800–809. doi: 10.1038/gt.2011.143. [DOI] [PubMed] [Google Scholar]
- 46.Excoffon KJ, Koerber JT, Dickey DD, Murtha M, Keshavjee S, Kaspar BK, Zabner J, Schaffer DV. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang JH, Koerber JT, Kim JS, Asuri P, Vazin T, Bartel M, Keung A, Kwon I, Park KI, Schaffer DV. An evolved adeno-associated viral variant enhances gene delivery and gene targeting in neural stem cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:667–675. doi: 10.1038/mt.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Asuri P, Bartel MA, Vazin T, Jang JH, Wong TB, Schaffer DV. Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:329–338. doi: 10.1038/mt.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Science translational medicine. 2013;5:189ra176. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 50.Klimczak RR, Koerber JT, Dalkara D, Flannery JG, Schaffer DV. A novel adeno-associated viral variant for efficient and selective intravitreal transduction of rat Muller cells. PloS one. 2009;4:e7467. doi: 10.1371/journal.pone.0007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dalkara D, Kolstad KD, Guerin KI, Hoffmann NV, Visel M, Klimczak RR, Schaffer DV, Flannery JG. AAV mediated GDNF secretion from retinal glia slows down retinal degeneration in a rat model of retinitis pigmentosa. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19:1602–1608. doi: 10.1038/mt.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koerber JT, Klimczak R, Jang JH, Dalkara D, Flannery JG, Schaffer DV. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:2088–2095. doi: 10.1038/mt.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nature biotechnology. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 54.Shin BK, Wang H, Yim AM, Le Naour F, Brichory F, Jang JH, Zhao R, Puravs E, Tra J, Michael CW, Misek DE, Hanash SM. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. The Journal of biological chemistry. 2003;278:7607–7616. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 55.Buonaguro L, Petrizzo A, Tornesello ML, Buonaguro FM. Translating tumor antigens into cancer vaccines. Clinical and vaccine immunology : CVI. 2011;18:23–34. doi: 10.1128/CVI.00286-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer immunology immunotherapy : CII. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 58.Hajitou A. Targeted systemic gene therapy and molecular imaging of cancer contribution of the vascular-targeted AAVP vector. Advances in genetics. 2010;69:65–82. doi: 10.1016/S0065-2660(10)69008-6. [DOI] [PubMed] [Google Scholar]
- 59.Grifman M, Trepel M, Speece P, Gilbert LB, Arap W, Pasqualini R, Weitzman MD. Incorporation of tumor-targeting peptides into recombinant adeno-associated virus capsids. Molecular therapy : the journal of the American Society of Gene Therapy. 2001;3:964–975. doi: 10.1006/mthe.2001.0345. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Xu W. Aminopeptidase N (APN/CD13) as a target for anti-cancer agent design. Current medicinal chemistry. 2008;15:2850–2865. doi: 10.2174/092986708786242840. [DOI] [PubMed] [Google Scholar]
- 61.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature reviews Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi W, Bartlett JS. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Molecular therapy : the journal of the American Society of Gene Therapy. 2003;7:515–525. doi: 10.1016/s1525-0016(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 63.Munch RC, Janicki H, Volker I, Rasbach A, Hallek M, Buning H, Buchholz CJ. Displaying high-affinity ligands on adeno-associated viral vectors enables tumor cell-specific and safe gene transfer. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:109–118. doi: 10.1038/mt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee HS, Kim JY, Lee WI, Kim SJ, Ko MJ, Jeong S, Park K, Choe H, Lee H. Acquisition of selective antitumoral effects of recombinant adeno-associated virus by genetically inserting tumor-targeting peptides into capsid proteins. Oncology letters. 2011;2:1113–1119. doi: 10.3892/ol.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng B, Ling C, Dai Y, Lu Y, Glushakova LG, Gee SW, McGoogan KE, Aslanidi GV, Park M, Stacpoole PW, Siemann D, Liu C, Srivastava A, Ling C. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene therapy. 2012;19:375–384. doi: 10.1038/gt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ling C, Wang Y, Zhang Y, Ejjigani A, Yin Z, Lu Y, Wang L, Wang M, Li J, Hu Z, Aslanidi GV, Zhong L, Gao G, Srivastava A, Ling C. Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Human gene therapy. 2014;25:1023–1034. doi: 10.1089/hum.2014.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandya J, Ortiz L, Ling C, Rivers AE, Aslanidi G. Rationally designed capsid and transgene cassette of AAV6 vectors for dendritic cell-based cancer immunotherapy. Immunology and cell biology. 2014;92:116–123. doi: 10.1038/icb.2013.74. [DOI] [PubMed] [Google Scholar]
- 69.Judd J, Ho ML, Tiwari A, Gomez EJ, Dempsey C, Van Vliet K, Igoshin OA, Silberg JJ, Agbandje-McKenna M, Suh J. Tunable protease-activatable virus nanonodes. ACS nano. 2014;8:4740–4746. doi: 10.1021/nn500550q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nature reviews Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 71.Peng KW, Morling FJ, Cosset FL, Murphy G, Russell SJ. A gene delivery system activatable by disease-associated matrix metalloproteinases. Human gene therapy. 1997;8:729–738. doi: 10.1089/hum.1997.8.6-729. [DOI] [PubMed] [Google Scholar]
- 72.Michelfelder S, Kohlschutter J, Skorupa A, Pfennings S, Muller O, Kleinschmidt JA, Trepel M. Successful expansion but not complete restriction of tropism of adeno-associated virus by in vivo biopanning of random virus display peptide libraries. PloS one. 2009;4:e5122. doi: 10.1371/journal.pone.0005122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maguire CA, Gianni D, Meijer DH, Shaket LA, Wakimoto H, Rabkin SD, Gao G, Sena-Esteves M. Directed evolution of adeno-associated virus for glioma cell transduction. Journal of neuro-oncology. 2010;96:337–347. doi: 10.1007/s11060-009-9972-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robson T, Hirst DG. Transcriptional Targeting in Cancer Gene Therapy. Journal of biomedicine & biotechnology. 2003;2003:110–137. doi: 10.1155/S1110724303209074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rajendran S, Collins S, van Pijkeren JP, O’Hanlon D, O’Sullivan GC, Tangney M. Targeting of breast metastases using a viral gene vector with tumour-selective transcription. Anticancer research. 2011;31:1627–1635. [PubMed] [Google Scholar]
- 76.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer science. 2008;99:1528–1538. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watanabe M, Ueki H, Ochiai K, Huang P, Kobayashi Y, Nasu Y, Sasaki K, Kaku H, Kashiwakura Y, Kumon H. Advanced two-step transcriptional amplification as a novel method for cancer-specific gene expression and imaging. Oncology reports. 2011;26:769–775. doi: 10.3892/or.2011.1371. [DOI] [PubMed] [Google Scholar]
- 78.Della Peruta M, Badar A, Rosales C, Chokshi S, Kia A, Nathwani D, Galante E, Yan R, Arstad E, Davidoff AM, Williams R, Lythgoe MF, Nathwani AC. Preferential targeting of disseminated liver tumors using a recombinant adeno-associated viral vector. Human gene therapy. 2015;26:94–103. doi: 10.1089/hum.2014.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McIntosh J, Lenting PJ, Rosales C, Lee D, Rabbanian S, Raj D, Patel N, Tuddenham EG, Christophe OD, McVey JH, Waddington S, Nienhuis AW, Gray JT, Fagone P, Mingozzi F, Zhou SZ, High KA, Cancio M, Ng CY, Zhou J, Morton CL, Davidoff AM, Nathwani AC. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood. 2013;121:3335–3344. doi: 10.1182/blood-2012-10-462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Al-Husein B, Abdalla M, Trepte M, Deremer DL, Somanath PR. Antiangiogenic therapy for cancer: an update. Pharmacotherapy. 2012;32:1095–1111. doi: 10.1002/phar.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mahendra G, Kumar S, Isayeva T, Mahasreshti PJ, Curiel DT, Stockardt CR, Grizzle WE, Alapati V, Singh R, Siegal GP, Meleth S, Ponnazhagan S. Antiangiogenic cancer gene therapy by adeno-associated virus 2-mediated stable expression of the soluble FMS-like tyrosine kinase-1 receptor. Cancer gene therapy. 2005;12:26–34. doi: 10.1038/sj.cgt.7700754. [DOI] [PubMed] [Google Scholar]
- 82.Takei Y, Mizukami H, Saga Y, Yoshimura I, Hasumi Y, Takayama T, Kohno T, Matsushita T, Okada T, Kume A, Suzuki M, Ozawa K. Suppression of ovarian cancer by muscle-mediated expression of soluble VEGFR-1/Flt-1 using adeno-associated virus serotype 1-derived vector. International journal of cancer Journal international du cancer. 2007;120:278–284. doi: 10.1002/ijc.22307. [DOI] [PubMed] [Google Scholar]
- 83.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, Prell R, VanRoey MJ, Simmons AD, Jooss K. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 84.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer research. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi K, Mizukami H, Saga Y, Takei Y, Urabe M, Kume A, Machida S, Fujiwara H, Suzuki M, Ozawa K. Suppression of lymph node and lung metastases of endometrial cancer by muscle-mediated expression of soluble vascular endothelial growth factor receptor-3. Cancer science. 2013;104:1107–1111. doi: 10.1111/cas.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. The Journal of biological chemistry. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 87.He SS, Shi HS, Yin T, Li YX, Luo ST, Wu QJ, Lu L, Wei YQ, Yang L. AAV-mediated gene transfer of human pigment epithelium-derived factor inhibits Lewis lung carcinoma growth in mice. Oncology reports. 2012;27:1142–1148. doi: 10.3892/or.2012.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He SS, Wu QJ, Gong CY, Luo ST, Zhang S, Li M, Lu L, Wei YQ, Yang L. Enhanced efficacy of combination therapy with adenoassociated virus-delivered pigment epithelium-derived factor and cisplatin in a mouse model of Lewis lung carcinoma. Molecular medicine reports. 2014;9:2069–2076. doi: 10.3892/mmr.2014.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M, Jooss K. Stable antibody expression at therapeutic levels using the 2A peptide. Nature biotechnology. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe M, Boyer JL, Crystal RG. AAVrh.10-mediated genetic delivery of bevacizumab to the pleura to provide local anti-VEGF to suppress growth of metastatic lung tumors. Gene therapy. 2010;17:1042–1051. doi: 10.1038/gt.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie Y, Hicks MJ, Kaminsky SM, Moore MA, Crystal RG, Rafii A. AAV-mediated persistent bevacizumab therapy suppresses tumor growth of ovarian cancer. Gynecologic oncology. 2014;135:325–332. doi: 10.1016/j.ygyno.2014.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Folkman J. Angiogenesis inhibitors: a new class of drugs. Cancer biology & therapy. 2003;2:S127–133. [PubMed] [Google Scholar]
- 93.Pan JG, Zhou X, Zeng GW, Han RF. Suppression of bladder cancer growth in mice by adeno-associated virus vector-mediated endostatin expression. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2011;32:301–310. doi: 10.1007/s13277-010-0122-9. [DOI] [PubMed] [Google Scholar]
- 94.Pan JG, Zhou X, Zeng GW, Han RF. Potent antitumour activity of the combination of HSV-TK and endostatin armed oncolytic adeno-associated virus for bladder cancer in vitro and in vivo. Journal of surgical oncology. 2012;105:249–257. doi: 10.1002/jso.22107. [DOI] [PubMed] [Google Scholar]
- 95.Sun X, Krissansen GW, Fung PW, Xu S, Shi J, Man K, Fan ST, Xu R. Anti-angiogenic therapy subsequent to adeno-associated-virus-mediated immunotherapy eradicates lymphomas that disseminate to the liver. International journal of cancer. Journal international du cancer. 2005;113:670–677. doi: 10.1002/ijc.20624. [DOI] [PubMed] [Google Scholar]
- 96.Isayeva T, Ren C, Ponnazhagan S. Recombinant adeno-associated virus 2-mediated antiangiogenic prevention in a mouse model of intraperitoneal ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:1342–1347. [PubMed] [Google Scholar]
- 97.Isayeva T, Ren C, Ponnazhagan S. Intraperitoneal gene therapy by rAAV provides long-term survival against epithelial ovarian cancer independently of survivin pathway. Gene therapy. 2007;14:138–146. doi: 10.1038/sj.gt.3302853. [DOI] [PubMed] [Google Scholar]
- 98.Isayeva T, Chanda D, Kallman L, Eltoum IE, Ponnazhagan S. Effects of sustained antiangiogenic therapy in multistage prostate cancer in TRAMP model. Cancer research. 2007;67:5789–5797. doi: 10.1158/0008-5472.CAN-06-3637. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Xu J, Lawler J, Terwilliger E, Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:3968–3976. doi: 10.1158/1078-0432.CCR-07-0245. [DOI] [PubMed] [Google Scholar]
- 100.Subramanian IV, Ghebre R, Ramakrishnan S. Adeno-associated virus-mediated delivery of a mutant endostatin suppresses ovarian carcinoma growth in mice. Gene therapy. 2005;12:30–38. doi: 10.1038/sj.gt.3302352. [DOI] [PubMed] [Google Scholar]
- 101.Subramanian IV, Bui Nguyen TM, Truskinovsky AM, Tolar J, Blazar BR, Ramakrishnan S. Adeno-associated virus-mediated delivery of a mutant endostatin in combination with carboplatin treatment inhibits orthotopic growth of ovarian cancer and improves long-term survival. Cancer research. 2006;66:4319–4328. doi: 10.1158/0008-5472.CAN-05-3297. [DOI] [PubMed] [Google Scholar]
- 102.Yanamandra N, Kondraganti S, Gondi CS, Gujrati M, Olivero WC, Dinh DH, Rao JS. Recombinant adeno-associated virus (rAAV) expressing TFPI-2 inhibits invasion, angiogenesis and tumor growth in a human glioblastoma cell line. International journal of cancer. Journal international du cancer. 2005;115:998–1005. doi: 10.1002/ijc.20965. [DOI] [PubMed] [Google Scholar]
- 103.Bui Nguyen TM, Subramanian IV, Xiao X, Nguyen P, Ramakrishnan S. Adeno-associated virus-mediated delivery of kringle 5 of human plasminogen inhibits orthotopic growth of ovarian cancer. Gene therapy. 2010;17:606–615. doi: 10.1038/gt.2010.15. [DOI] [PubMed] [Google Scholar]
- 104.Ruan Q, Xi L, Boye SL, Han S, Chen ZJ, Hauswirth WW, Lewin AS, Boulton ME, Law BK, Jiang WG, Jiang H, Cai J. Development of an anti-angiogenic therapeutic model combining scAAV2-delivered siRNAs and noninvasive photoacoustic imaging of tumor vasculature development. Cancer letters. 2013;332:120–129. doi: 10.1016/j.canlet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai KX, Tse LY, Leung C, Tam PK, Xu R, Sham MH. Suppression of lung tumor growth and metastasis in mice by adeno-associated virus-mediated expression of vasostatin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:939–949. doi: 10.1158/1078-0432.CCR-07-1930. [DOI] [PubMed] [Google Scholar]
- 106.Kuo CH, Chang BI, Lee FT, Chen PK, Lee JS, Shi GY, Wu HL. Development of recombinant AAV2/8 carrying kringle domains of human plasminogen for sustained expression and cancer therapy. Human gene therapy. 2015 doi: 10.1089/hum.2013.220. [DOI] [PubMed] [Google Scholar]
- 107.Kroeger KM, Muhammad AK, Baker GJ, Assi H, Wibowo MK, Xiong W, Yagiz K, Candolfi M, Lowenstein PR, Castro MG. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discovery medicine. 2010;10:293–304. [PMC free article] [PubMed] [Google Scholar]
- 108.Chen CY, Chang YN, Ryan P, Linscott M, McGarrity GJ, Chiang YL. Effect of herpes simplex virus thymidine kinase expression levels on ganciclovir-mediated cytotoxicity and the “bystander effect”. Human gene therapy. 1995;6:1467–1476. doi: 10.1089/hum.1995.6.11-1467. [DOI] [PubMed] [Google Scholar]
- 109.Li ZB, Zeng ZJ, Chen Q, Luo SQ, Hu WX. Recombinant AAV-mediated HSVtk gene transfer with direct intratumoral injections and Tet-On regulation for implanted human breast cancer. BMC cancer. 2006;6:66. doi: 10.1186/1471-2407-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeng ZJ, Xiang SG, Xue WW, Li HD, Ma N, Ren ZJ, Xu ZJ, Jiao CH, Wang CY, Hu WX. The cell death and DNA damages caused by the Tet-On regulating HSV-tk/GCV suicide gene system in MCF-7 cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014;68:887–892. doi: 10.1016/j.biopha.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 111.Kim JY, Kim JH, Khim M, Lee HS, Jung JH, Moon DH, Jeong S, Lee H. Persistent anti-tumor effects via recombinant adeno-associated virus encoding herpes thymidine kinase gene monitored by PET-imaging. Oncology reports. 2011;25:1263–1269. doi: 10.3892/or.2011.1190. [DOI] [PubMed] [Google Scholar]
- 112.Griffith TS, Stokes B, Kucaba TA, Earel JK, Jr, VanOosten RL, Brincks EL, Norian LA. TRAIL gene therapy: from preclinical development to clinical application. Current gene therapy. 2009;9:9–19. doi: 10.2174/156652309787354612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma H, Liu Y, Liu S, Kung HF, Sun X, Zheng D, Xu R. Recombinant adeno-associated virus-mediated TRAIL gene therapy suppresses liver metastatic tumors. International journal of cancer. Journal international du cancer. 2005;116:314–321. doi: 10.1002/ijc.20982. [DOI] [PubMed] [Google Scholar]
- 114.Ma H, Liu Y, Liu S, Xu R, Zheng D. Oral adeno-associated virus-sTRAIL gene therapy suppresses human hepatocellular carcinoma growth in mice. Hepatology. 2005;42:1355–1363. doi: 10.1002/hep.20918. [DOI] [PubMed] [Google Scholar]
- 115.Shi J, Zheng D, Liu Y, Sham MH, Tam P, Farzaneh F, Xu R. Overexpression of soluble TRAIL induces apoptosis in human lung adenocarcinoma and inhibits growth of tumor xenografts in nude mice. Cancer research. 2005;65:1687–1692. doi: 10.1158/0008-5472.CAN-04-2749. [DOI] [PubMed] [Google Scholar]
- 116.Yoo J, Choi S, Hwang KS, Cho WK, Jung CR, Kwon ST, Im DS. Adeno-associated virus-mediated gene transfer of a secreted form of TRAIL inhibits tumor growth and occurrence in an experimental tumor model. The journal of gene medicine. 2006;8:163–174. doi: 10.1002/jgm.832. [DOI] [PubMed] [Google Scholar]
- 117.Wang Y, Huang F, Cai H, Zhong S, Liu X, Tan WS. Potent antitumor effect of TRAIL mediated by a novel adeno-associated viral vector targeting to telomerase activity for human hepatocellular carcinoma. The journal of gene medicine. 2008;10:518–526. doi: 10.1002/jgm.1177. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Huang F, Cai H, Wu Y, He G, Tan WS. The efficacy of combination therapy using adeno-associated virus-TRAIL targeting to telomerase activity and cisplatin in a mice model of hepatocellular carcinoma. Journal of cancer research and clinical oncology. 2010;136:1827–1837. doi: 10.1007/s00432-010-0841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, Ma H, Zhang J, Liu S, Liu Y, Zheng D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life sciences. 2008;82:1154–1161. doi: 10.1016/j.lfs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 120.Jiang M, Liu Z, Xiang Y, Ma H, Liu S, Liu Y, Zheng D. Synergistic antitumor effect of AAV-mediated TRAIL expression combined with cisplatin on head and neck squamous cell carcinoma. BMC cancer. 2011;11:54. doi: 10.1186/1471-2407-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chiu TL, Wang MJ, Su CC. The treatment of glioblastoma multiforme through activation of microglia and TRAIL induced by rAAV2-mediated IL-12 in a syngeneic rat model. Journal of biomedical science. 2012;19:45. doi: 10.1186/1423-0127-19-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hamner JB, Sims TL, Cutshaw A, Dickson PV, Rosati S, McGee M, Ng CY, Davidoff AM. The efficacy of combination therapy using adeno-associated virus--interferon beta and trichostatin A in vitro and in a murine model of neuroblastoma. Journal of pediatric surgery. 2008;43:177–182. doi: 10.1016/j.jpedsurg.2007.09.048. discussion 182–173. [DOI] [PubMed] [Google Scholar]
- 123.Maguire CA, Meijer DH, LeRoy SG, Tierney LA, Broekman ML, Costa FF, Breakefield XO, Stemmer-Rachamimov A, Sena-Esteves M. Preventing growth of brain tumors by creating a zone of resistance. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16:1695–1702. doi: 10.1038/mt.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He LF, Wang YG, Xiao T, Zhang KJ, Li GC, Gu JF, Chu L, Tang WH, Tan WS, Liu XY. Suppression of cancer growth in mice by adeno-associated virus vector-mediated IFN-beta expression driven by hTERT promoter. Cancer letters. 2009;286:196–205. doi: 10.1016/j.canlet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 125.Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu JQ, Zhao WH, Li Y, Zhu B, Yin KS. Adeno-associated virus mediated gene transfer into lung cancer cells promoting CD40 ligand-based immunotherapy. Virology. 2007;368:309–316. doi: 10.1016/j.virol.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 127.Xu W, Xu Y, Wei Y, Tan Y, Zhao H, Zhao W, Wu J. Self-complementary adeno-associated virus 5-mediated gene transduction of a novel CD40L mutant confers direct antitumor effects in lung carcinoma. Molecular medicine reports. 2015;11:482–488. doi: 10.3892/mmr.2014.2765. [DOI] [PubMed] [Google Scholar]
- 128.Tahara I, Miyake K, Hanawa H, Kurai T, Hirai Y, Ishizaki M, Uchida E, Tajiri T, Shimada T. Systemic cancer gene therapy using adeno-associated virus type 1 vector expressing MDA-7/IL24. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15:1805–1811. doi: 10.1038/sj.mt.6300225. [DOI] [PubMed] [Google Scholar]
- 129.Yang YJ, Chen DZ, Li LX, Sheng QS, Jin ZK, Zhao DF. Targeted IL-24 gene therapy inhibits cancer recurrence after liver tumor resection by inducing tumor cell apoptosis in nude mice. Hepatobiliary & pancreatic diseases international : HBPD INT. 2009;8:174–178. [PubMed] [Google Scholar]
- 130.Tamai H, Miyake K, Yamaguchi H, Takatori M, Dan K, Inokuchi K, Shimada T. AAV8 vector expressing IL24 efficiently suppresses tumor growth mediated by specific mechanisms in MLL/AF4-positive ALL model mice. Blood. 2012;119:64–71. doi: 10.1182/blood-2011-05-354050. [DOI] [PubMed] [Google Scholar]
- 131.Chang CM, Lo CH, Shih YM, Chen Y, Wu PY, Tsuneyama K, Roffler SR, Tao MH. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Human gene therapy. 2010;21:611–621. doi: 10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- 132.Yiang GT, Chou PL, Tsai HF, Chen LA, Chang WJ, Yu YL, Wei CW. Immunotherapy for SV40 T/t antigen-induced breast cancer by recombinant adeno-associated virus serotype 2 carrying interleukin-15 in mice. International journal of molecular medicine. 2012;29:809–814. doi: 10.3892/ijmm.2012.921. [DOI] [PubMed] [Google Scholar]
- 133.Liang CM, Zhong CP, Sun RX, Liu BB, Huang C, Qin J, Zhou S, Shan J, Liu YK, Ye SL. Local expression of secondary lymphoid tissue chemokine delivered by adeno-associated virus within the tumor bed stimulates strong anti-liver tumor immunity. Journal of virology. 2007;81:9502–9511. doi: 10.1128/JVI.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Buhles A, Collins SA, van Pijkeren JP, Rajendran S, Miles M, O’Sullivan GC, O’Hanlon DM, Tangney M. Anti-metastatic effects of viral and non-viral mediated Nk4 delivery to tumours. Genetic vaccines and therapy. 2009;7:5. doi: 10.1186/1479-0556-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maitituoheti M, Li Y, Wang W, Wang W, Han L, Yang R, Wang T, Wu Z, Ma D, Wang S. Adeno-associated virus-mediated local delivery of LIGHT suppresses tumorigenesis in a murine cervical cancer model. Journal of immunotherapy. 2011;34:581–587. doi: 10.1097/CJI.0b013e31822b9fe0. [DOI] [PubMed] [Google Scholar]
- 136.Driessens G, Nuttin L, Gras A, Maetens J, Mievis S, Schoore M, Velu T, Tenenbaum L, Preat V, Bruyns C. Development of a successful antitumor therapeutic model combining in vivo dendritic cell vaccination with tumor irradiation and intratumoral GM-CSF delivery. Cancer immunology, immunotherapy : CII. 2011;60:273–281. doi: 10.1007/s00262-010-0941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ma H, Zhang Y, Wang H, Han C, Lei R, Zhang L, Yang Z, Rao L, Qing H, Xiang J, Deng Y. Effect and mechanism of Mitomycin C combined with recombinant adeno-associated virus type II against glioma. International journal of molecular sciences. 2014;15:1–14. doi: 10.3390/ijms15010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tu SP, Cui JT, Liston P, Huajiang X, Xu R, Lin MC, Zhu YB, Zou B, Ng SS, Jiang SH, Xia HH, Wong WM, Chan AO, Yuen MF, Lam SK, Kung HF, Wong BC. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology. 2005;128:361–375. doi: 10.1053/j.gastro.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 139.Xue Z, Sun PH, Zhu LM, Jiang SH, Qiao MM, Chi AL, Tu SP. Adeno-associated virus-mediated survivin mutant Thr34Ala cooperates with oxaliplatin to inhibit tumor growth and angiogenesis in colon cancer. Oncology reports. 2011;25:1039–1046. doi: 10.3892/or.2011.1166. [DOI] [PubMed] [Google Scholar]
- 140.Weng Y, Fei B, Chi AL, Cai M. Inhibition of gastric cancer cell growth in vivo by overexpression of adeno-associated virus-mediated survivin mutant C84A. Oncology research. 2013;20:411–417. doi: 10.3727/096504013X13657689383094. [DOI] [PubMed] [Google Scholar]
- 141.Dang SC, Feng S, Wang PJ, Cui L, Qu JG, Zhang JX. Overexpression of Survivin mutant Thr34Ala induces apoptosis and inhibits gastric cancer growth. Neoplasma. 2015;62:81–87. doi: 10.4149/neo_2015_010. [DOI] [PubMed] [Google Scholar]
- 142.Ng SS, Gao Y, Chau DH, Li GH, Lai LH, Huang PT, Huang CF, Huang JJ, Chen YC, Kung HF, Lin MC. A novel glioblastoma cancer gene therapy using AAV-mediated long-term expression of human TERT C-terminal polypeptide. Cancer gene therapy. 2007;14:561–572. doi: 10.1038/sj.cgt.7701038. [DOI] [PubMed] [Google Scholar]
- 143.Gao Y, Ng SS, Chau DH, Yao H, Yang C, Man K, Huang PT, Huang C, Huang JJ, Kung HF, Lin MC. Development of recombinant adeno-associated virus and adenovirus cocktail system for efficient hTERTC27 polypeptide-mediated cancer gene therapy. Cancer gene therapy. 2008;15:723–732. doi: 10.1038/cgt.2008.33. [DOI] [PubMed] [Google Scholar]
- 144.Watanabe M, Nasu Y, Kashiwakura Y, Kusumi N, Tamayose K, Nagai A, Sasano T, Shimada T, Daida H, Kumon H. Adeno-associated virus 2-mediated intratumoral prostate cancer gene therapy: long-term maspin expression efficiently suppresses tumor growth. Human gene therapy. 2005;16:699–710. doi: 10.1089/hum.2005.16.699. [DOI] [PubMed] [Google Scholar]
- 145.Li J, Zhou J, Chen G, Wang H, Wang S, Xing H, Gao Q, Lu Y, He Y, Ma D. Inhibition of ovarian cancer metastasis by adeno-associated virus-mediated gene transfer of nm23H1 in an orthotopic implantation model. Cancer gene therapy. 2006;13:266–272. doi: 10.1038/sj.cgt.7700899. [DOI] [PubMed] [Google Scholar]
- 146.Nie B, Shen Z, Wen JB, Wong OG, Hsueh WD, Huo LF, Kung HF, Jiang B, Lin MC. AAV-HGFK1 and Ad-p53 cocktail therapy prolongs survival of mice with colon cancer. Molecular cancer therapeutics. 2008;7:2855–2865. doi: 10.1158/1535-7163.MCT-08-0366. [DOI] [PubMed] [Google Scholar]
- 147.Shah GV, Thomas S, Muralidharan A, Liu Y, Hermonat PL, Williams J, Chaudhary J. Calcitonin promotes in vivo metastasis of prostate cancer cells by altering cell signaling, adhesion, and inflammatory pathways. Endocrine-related cancer. 2008;15:953–964. doi: 10.1677/ERC-08-0136. [DOI] [PubMed] [Google Scholar]
- 148.Chang SH, Kim JE, Lee JH, Minai-Tehrani A, Han K, Chae C, Cho YH, Yun JH, Park K, Kim YS, Cho MH. Aerosol delivery of eukaryotic translation initiation factor 4E-binding protein 1 effectively suppresses lung tumorigenesis in K-rasLA1 mice. Cancer gene therapy. 2013;20:331–335. doi: 10.1038/cgt.2013.24. [DOI] [PubMed] [Google Scholar]
- 149.Wang S, Wu Y, Hou Y, Guan X, Castelvetere MP, Oblak JJ, Banerjee S, Filtz TM, Sarkar FH, Chen X, Jena BP, Li C. CXCR2 macromolecular complex in pancreatic cancer: a potential therapeutic target in tumor growth. Translational oncology. 2013;6:216–225. doi: 10.1593/tlo.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan L, Zhao H, Zhang L, Liu X. The efficacy of combination therapy using adeno-associated virus-mediated co-expression of apoptin and interleukin-24 on hepatocellular carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34:3027–3034. doi: 10.1007/s13277-013-0867-z. [DOI] [PubMed] [Google Scholar]
- 151.Zhu B, Li X, Zhang Y, Ye C, Wang Y, Cai S, Huang H, Cai Y, Yeh S, Huang Z, Chen R, Tao Y, Wen X. Cross-talk of alpha tocopherol-associated protein and JNK controls the oxidative stress-induced apoptosis in prostate cancer cells. International journal of cancer. Journal international du cancer. 2013;132:2270–2282. doi: 10.1002/ijc.27927. [DOI] [PubMed] [Google Scholar]
- 152.Ma HI, Hueng DY, Shui HA, Han JM, Wang CH, Lai YH, Cheng SY, Xiao X, Chen MT, Yang YP. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. International journal of molecular sciences. 2014;15:4393–4414. doi: 10.3390/ijms15034393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng M, Ho S, Yoo JH, Tran DH, Bakirtzi K, Su B, Tran DH, Kubota Y, Ichikawa R, Koon HW. Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clinical and experimental gastroenterology. 2015;8:13–29. doi: 10.2147/CEG.S70906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liao YJ, Fang CC, Yen CH, Hsu SM, Wang CK, Huang SF, Liang YC, Lin YY, Chu YT, Arthur Chen YM. Niemann-Pick Type C2 protein regulates liver cancer progression via modulating ERK1/2 pathway: Clinicopathological correlations and therapeutical implications. International journal of cancer. Journal international du cancer. 2015 doi: 10.1002/ijc.29507. [DOI] [PubMed] [Google Scholar]
- 155.Pepin D, Sosulski A, Zhang L, Wang D, Vathipadiekal V, Hendren K, Coletti CM, Yu A, Castro CM, Birrer MJ, Gao G, Donahoe PK. AAV9 delivering a modified human Mullerian inhibiting substance as a gene therapy in patient-derived xenografts of ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E4418–4427. doi: 10.1073/pnas.1510604112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ruifa H, Liwei L, Binxin L, Ximing L. Additional gene therapy with rAAV-wt-p53 enhanced the efficacy of cisplatin in human bladder cancer cells. Urologia internationalis. 2006;77:355–361. doi: 10.1159/000096341. [DOI] [PubMed] [Google Scholar]
- 157.Neukirchen J, Meier A, Rohrbeck A, Garcia-Pardillos G, Steidl U, Fenk R, Haas R, Kronenwett R, Rohr UP. The proteasome inhibitor bortezomib acts differently in combination with p53 gene transfer or cytotoxic chemotherapy on NSCLC cells. Cancer gene therapy. 2007;14:431–439. doi: 10.1038/sj.cgt.7701029. [DOI] [PubMed] [Google Scholar]
- 158.Qazilbash MH, Xiao X, Seth P, Cowan KH, Walsh CE. Cancer gene therapy using a novel adeno-associated virus vector expressing human wild-type p53. Gene therapy. 1997;4:675–682. doi: 10.1038/sj.gt.3300444. [DOI] [PubMed] [Google Scholar]
- 159.Li X, Liu X, Li CY, Ding Y, Chau D, Li G, Kung HF, Lin MC, Peng Y. Recombinant adeno-associated virus mediated RNA interference inhibits metastasis of nasopharyngeal cancer cells in vivo and in vitro by suppression of Epstein-Barr virus encoded LMP-1. International journal of oncology. 2006;29:595–603. [PubMed] [Google Scholar]
- 160.Wu S, Meng L, Wang S, Wang W, Xi L, Tian X, Chen G, Wu Y, Zhou J, Xu G, Lu Y, Ma D. Reversal of the malignant phenotype of cervical cancer CaSki cells through adeno-associated virus-mediated delivery of HPV16 E7 antisense RNA. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:2032–2037. doi: 10.1158/1078-0432.CCR-05-2567. [DOI] [PubMed] [Google Scholar]
- 161.Sun A, Tang J, Terranova PF, Zhang X, Thrasher JB, Li B. Adeno-associated virus-delivered short hairpin-structured RNA for androgen receptor gene silencing induces tumor eradication of prostate cancer xenografts in nude mice: a preclinical study. International journal of cancer. Journal international du cancer. 2010;126:764–774. doi: 10.1002/ijc.24778. [DOI] [PubMed] [Google Scholar]
- 162.Zhang K, Jiao X, Liu X, Zhang B, Wang J, Wang Q, Tao Y, Zhang D. Knockdown of snail sensitizes pancreatic cancer cells to chemotherapeutic agents and irradiation. International journal of molecular sciences. 2010;11:4891–4892. doi: 10.3390/ijms11124891. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 163.Zhang K, Zhang B, Lu Y, Sun C, Zhao W, Jiao X, Hu J, Mu P, Lu H, Zhou C. Slug inhibition upregulates radiation-induced PUMA activity leading to apoptosis in cholangiocarcinomas. Medical oncology. 2011;28(Suppl 1):S301–309. doi: 10.1007/s12032-010-9759-x. [DOI] [PubMed] [Google Scholar]
- 164.Wu Y, Guo Z, Zhang D, Zhang W, Yan Q, Shi X, Zhang M, Zhao Y, Zhang Y, Jiang B, Cheng T, Bai Y, Wang J. A novel colon cancer gene therapy using rAAVmediated expression of human shRNA-FHL2. International journal of oncology. 2013;43:1618–1626. doi: 10.3892/ijo.2013.2090. [DOI] [PubMed] [Google Scholar]
- 165.Li L, Yang L, Scudiero DA, Miller SA, Yu ZX, Stukenberg PT, Shoemaker RH, Kotin RM. Development of recombinant adeno-associated virus vectors carrying small interfering RNA (shHec1)-mediated depletion of kinetochore Hec1 protein in tumor cells. Gene therapy. 2007;14:814–827. doi: 10.1038/sj.gt.3302933. [DOI] [PubMed] [Google Scholar]
- 166.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu DW, Chang JL, Tsao YP, Huang CW, Kuo SW, Chen SL. Co-vaccination with adeno-associated virus vectors encoding human papillomavirus 16 L1 proteins and adenovirus encoding murine GM-CSF can elicit strong and prolonged neutralizing antibody. International journal of cancer. Journal international du cancer. 2005;113:93–100. doi: 10.1002/ijc.20530. [DOI] [PubMed] [Google Scholar]
- 168.Kuck D, Lau T, Leuchs B, Kern A, Muller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. Journal of virology. 2006;80:2621–2630. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nieto K, Kern A, Leuchs B, Gissmann L, Muller M, Kleinschmidt JA. Combined prophylactic and therapeutic intranasal vaccination against human papillomavirus type-16 using different adeno-associated virus serotype vectors. Antiviral therapy. 2009;14:1125–1137. doi: 10.3851/IMP1469. [DOI] [PubMed] [Google Scholar]
- 170.Nieto K, Stahl-Hennig C, Leuchs B, Muller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with AAV5 and 9 vectors against human papillomavirus type 16 in rhesus macaques. Human gene therapy. 2012;23:733–741. doi: 10.1089/hum.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou L, Zhu T, Ye X, Yang L, Wang B, Liang X, Lu L, Tsao YP, Chen SL, Li J, Xiao X. Long-term protection against human papillomavirus e7-positive tumor by a single vaccination of adeno-associated virus vectors encoding a fusion protein of inactivated e7 of human papillomavirus 16/18 and heat shock protein 70. Human gene therapy. 2010;21:109–119. doi: 10.1089/hum.2009.139. [DOI] [PubMed] [Google Scholar]
- 172.Liao S, Zhang W, Hu X, Wang W, Deng D, Wang H, Wang C, Zhou J, Wang S, Zhang H, Ma D. A novel “priming-boosting” strategy for immune interventions in cervical cancer. Molecular immunology. 2015;64:295–305. doi: 10.1016/j.molimm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 173.Cesco-Gaspere M, Zentilin L, Giacca M, Burrone OR. Boosting anti-idiotype immune response with recombinant AAV enhances tumour protection induced by gene gun vaccination. Scandinavian journal of immunology. 2008;68:58–66. doi: 10.1111/j.1365-3083.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 174.Pan J, Zhang Q, Zhou J, Ma D, Xiao X, Wang DW. Recombinant adeno-associated virus encoding Epstein-Barr virus latent membrane proteins fused with heat shock protein as a potential vaccine for nasopharyngeal carcinoma. Molecular cancer therapeutics. 2009;8:2754–2761. doi: 10.1158/1535-7163.MCT-08-1176. [DOI] [PubMed] [Google Scholar]
- 175.Triozzi PL, Aldrich W, Ponnazhagan S. Regulation of the activity of an adeno-associated virus vector cancer vaccine administered with synthetic Toll-like receptor agonists. Vaccine. 2010;28:7837–7843. doi: 10.1016/j.vaccine.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 176.Triozzi PL, Aldrich W, Ponnazhagan S. Inhibition and promotion of tumor growth with adeno-associated virus carcinoembryonic antigen vaccine and Toll-like receptor agonists. Cancer gene therapy. 2011;18:850–858. doi: 10.1038/cgt.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Steel JC, Di Pasquale G, Ramlogan CA, Patel V, Chiorini JA, Morris JC. Oral vaccination with adeno-associated virus vectors expressing the Neu oncogene inhibits the growth of murine breast cancer. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:680–687. doi: 10.1038/mt.2012.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Han L, Wang W, Lu J, Kong F, Ma G, Zhu Y, Zhao D, Zhu J, Shuai W, Zhou Q, Chen P, Ye L, Tao J, Ahmad S, Li F, Sun J. AAV-sBTLA facilitates HSP70 vaccine-triggered prophylactic antitumor immunity against a murine melanoma pulmonary metastasis model in vivo. Cancer letters. 2014;354:398–406. doi: 10.1016/j.canlet.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 179.Ho DT, Wykoff-Clary S, Gross CS, Schneider D, Jin F, Kretschmer PJ, Hermiston TW. Growth inhibition of an established A431 xenograft tumor by a full-length anti-EGFR antibody following gene delivery by AAV. Cancer gene therapy. 2009;16:184–194. doi: 10.1038/cgt.2008.68. [DOI] [PubMed] [Google Scholar]
- 180.Lv F, Qiu Y, Zhang Y, Liu S, Shi J, Liu Y, Zheng D. Adeno-associated virus-mediated anti-DR5 chimeric antibody expression suppresses human tumor growth in nude mice. Cancer letters. 2011;302:119–127. doi: 10.1016/j.canlet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 181.Principe M, Ceruti P, Shih NY, Chattaragada MS, Rolla S, Conti L, Bestagno M, Zentilin L, Yang SH, Migliorini P, Cappello P, Burrone O, Novelli F. Targeting of surface alpha-enolase inhibits the invasiveness of pancreatic cancer cells. Oncotarget. 2015;6:11098–11113. doi: 10.18632/oncotarget.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Di L, Zhu Y, Jia J, Yu J, Song G, Zhang J, Che L, Yang H, Han Y, Ma B, Zhang C, Yuan Y, You M, Wan F, Wang X, Zhou X, Ren J. Clinical safety of induced CTL infusion through recombinant adeno-associated virus-transfected dendritic cell vaccination in Chinese cancer patients. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2012;14:675–681. doi: 10.1007/s12094-012-0854-7. [DOI] [PubMed] [Google Scholar]
- 183.Amer MH. Gene therapy for cancer: present status and future perspective. Molecular and cellular therapies. 2014;2:27. doi: 10.1186/2052-8426-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hadaczek P, Mirek H, Berger MS, Bankiewicz K. Limited efficacy of gene transfer in herpes simplex virus-thymidine kinase/ganciclovir gene therapy for brain tumors. Journal of neurosurgery. 2005;102:328–335. doi: 10.3171/jns.2005.102.2.0328. [DOI] [PubMed] [Google Scholar]
- 185.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dougan M, Dranoff G. Immune therapy for cancer. Annual review of immunology. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 187.Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS letters. 2014;588:368–376. doi: 10.1016/j.febslet.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 188.Fumoto S, Kawakami S, Hashida M, Nishida K. Targeted Gene Delivery: Importance of Administration Routes. 2013. [Google Scholar]
- 189.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.3r1. 2010. [Google Scholar]