Abstract
Oligodendrocyte progenitor cells (OPCs) are the often-overlooked fourth glial cell type in the central nervous system (CNS), comprising about 5% of the CNS. For a long time, our vision of OPC function was limited to the generation of mature oligodendrocytes. However, new studies have highlighted the multifaceted nature of the OPCs. During homeostatic and pathological conditions, OPCs are the most proliferative cell type in the CNS, a property not consistent with the need to generate new oligodendrocytes. Indeed, OPCs modulate neuronal activity and OPC depletion in the brain can trigger depressive-like behavior. More importantly, OPCs are actively recruited to injury sites, where they orchestrate glial scar formation and contribute to the immune response. The following is a comprehensive analysis of the literature on OPC function beyond myelination, in the context of the healthy and diseased adult CNS.
Keywords: Oligodendrocyte progenitor cells, myelin, glial scar, inflammation, multiple sclerosis
1. Introduction
The central nervous system (CNS) is home to three major classes of glia: Astrocytes, microglia, and oligodendrocytes. In the last two decades, a fourth class of glia has emerged, called the oligodendrocyte progenitor cells (OPCs). OPCs have a stellate morphology and are present in both the gray and white matter. They belong to the same population of progenitors that give rise to oligodendrocytes during CNS development. However, a large fraction of OPCs do not differentiate and remain in a cycling state throughout adulthood. OPCs represent the largest dividing population among neural cells and are uniformly distributed, making up, on average, 5% of total CNS cells (Dawson et al., 2003). The first described function of adult OPCs was differentiation into oligodendrocytes (Gensert and Goldman, 1997). When myelin repair is needed after CNS injury, local OPC proliferation occurs, followed by OPC differentiation into oligodendrocytes (Lytle et al., 2009). OPC differentiation is accompanied by expression of mature oligodendrocyte markers such as proteolipid protein and myelin basic protein. Oligodendrocytes then generate a large amount of plasma membrane and begin to wrap around neuronal axons to form the myelin sheath in a process called myelination (Baron and Hoekstra, 2010; Snaidero et al., 2014). The mammalian brain undergoes myelination beyond postnatal stages and well into adulthood, highlighting the importance of adult OPCs in generating oligodendrocytes (Young et al., 2013). However, the differentiation rate of OPCs into myelinating oligodendrocytes decreases with age, with only a small percentage of OPCs giving rise to oligodendrocytes, suggesting that OPCs may have important functions beyond myelination (Psachoulia et al., 2009; Rivers et al., 2008; Zhu et al., 2011).
Adult OPCs are evenly dispersed throughout the CNS and in vivo imaging studies have shown that they are poised to detect perturbations within the CNS, by sending numerous filopodia to survey their surroundings (Hughes et al., 2013). For example, OPCs rapidly respond during CNS injury and disease by proliferating extensively and quickly populating the lesion site (Kang et al., 2013a; Simon et al., 2011). Although some OPCs in the lesion differentiate into oligodendrocytes, there is a burgeoning interest in other possible roles of OPCs in response to CNS injury. Furthermore, OPCs can influence neuronal activity by altering the composition of the extracellular matrix (Sakry et al., 2014). Recently, loss of OPCs in the prefrontal cortex has been shown to alter glutamatergic signaling and promote depressive-like behavior in mice (Birey et al., 2015). These studies highlight a novel role of OPCs in modulating neuronal network activity adding to our understanding of glia-neuron interactions. The multifunctional role of OPCs during CNS homeostasis and pathologies will be presented in the following chapters, keeping in mind the gaps that still exist in our knowledge about these fascinating cells.
2. OPCs in the adult CNS
OPC development has been extensively studied and is beyond the scope of this review (Gallo and Deneen, 2014; Takebayashi and Ikenaka, 2015). In adults, OPCs are present across the CNS but their quantity varies between the white and gray matter (Dawson et al., 2003). In the white matter, such as the dorsal column of the spinal cord, OPCs can account for up to 8% of all cells, while in the dorsal horn gray matter OPCs account for only 3% of the cellular content (Dawson et al., 2003). With this difference in mind, adult OPCs remain uniformly distributed across the brain and the spinal cord. Live-imaging studies performed in the cortex reveal that OPCs have elegantly ramified processes that constantly survey the environment and maintain an even distribution using self-repulsion mechanisms (Hughes et al., 2013). In the superficial layers of the cortex, each OPC covers its own territory, with clear borders and no apparent overlap (Hughes et al., 2013). An important characteristic of OPCs is that they represent the major dividing cell population in the CNS. Using BrdU labeling methods, multiple studies have shown that OPCs account for more than 70% of CNS dividing cells, potentially outpacing the need for replacement of mature oligodendrocytes (Dawson et al., 2003; Gensert and Goldman, 2001). Yet, the principal role of adult OPCs was believed to be the generation of new myelinating oligodendrocytes. Myelination in white matter tracts of the mouse CNS occurs continuously after birth, even after 8 months of age (Psachoulia et al., 2009; Rivers et al., 2008). However, the rate at which OPCs differentiate into oligodendrocytes decreases with age, suggesting that OPCs could have functions other than myelination. Given their uniform distribution and constant surveillance of the environment, OPCs are well positioned to sense changes in CNS homeostasis. Indeed, OPCs respond to primary demyelination and several types of CNS injury, with extensive proliferation, migration and morphological changes, but the utility of these actions in the context of CNS repair is not fully understood (Dimou and Gallo, 2015).
3. OPC heterogeneity
In development, OPCs have distinct spatial and temporal origins, raising the question of whether there is functional heterogeneity among the OPC populations (Takebayashi and Ikenaka, 2015). Ablation of any one OPC population during development results in compensation by neighboring OPCs of different origin, suggesting that spatial origin does not commit OPCs to a single function (Kessaris et al., 2006). Although no overt functional differences in OPCs across the CNS have yet been identified, region-specific environments can alter OPC behavior. In fact, fate-mapping studies have shown OPCs in white matter differentiate into oligodendrocytes more frequently than gray matter OPCs (Dimou et al., 2008). In addition, OPCs in white matter have a shorter cell division cycle (10 days in the corpus callosum at P60) compared to gray matter OPCs (36 days in the motor cortex at P60) (Young et al., 2013). Hill et al. also demonstrated that white matter perinatal OPCs have a greater proliferative response to PDGFα than gray matter OPCs and that this difference was not due to differential expression of the PDGFα receptor (PDGFRα) (Hill et al., 2013). This suggests that regional differences in environment might affect OPC properties and account for dissimilarities between OPC populations. Furthermore, transplantation of white matter OPCs into gray or white matter results in comparable amounts of oligodendrocyte differentiation (Vigano et al., 2013). On the other hand, gray matter OPCs only exhibited greater oligodendrocyte differentiation when transplanted into white matter. Therefore, it appears that the white matter environment supports oligodendrocyte maturation, and that this effect is preserved in OPCs even after they have been taken out of the white matter environment (Vigano et al., 2013). These differences highlight the long-term effects that the extracellular environment could have on OPC function. However, OPC functional heterogeneity remains a matter of debate, and identification of OPC subtypes characterized by expression of specific markers is warranted. It is possible that a subtype of OPCs specializes in remyelination and repair, while others have a role in CNS homeostasis. The recent improvement in single cell RNA sequencing methods could aid in answering these open questions about OPC biology.
4. CNS Myelination in Adulthood
When they were first phenotypically characterized, adult OPCs were shown to express the same markers as embryonic OPCs. However, questions were raised about whether these cells shared the same lineage and whether they also generated oligodendrocytes. With advances in transgenic reporter mice, it became possible to conduct fate-mapping studies using specific Cre reporter lines, and the functions of adult OPCs began to emerge. Zhu et al. used NG2-Cre stop-flox-EGFP reporter mice to examine the fate of postnatal OPCs. In this system, NG2-expressing OPCs express the Cre recombinase and excise the stop-flox sequence, and are labeled with EGFP. As expected, 78% of oligodendrocytes in the corpus callosum and striatum are EGFP+, indicating that OPCs gave rise to those oligodendrocytes (Zhu et al., 2008). This study, however, was conducted at the peak of myelination (P14) in the mouse CNS, and hence OPCs examined here likely belong to the last wave of the progenitors that occurs at birth. Therefore, a P14 OPC is likely not the same as an OPC from, for example, a three- or an eight-month old mouse. Surprisingly, EGFP+ astrocytes were also found in the ventral gray matter, implying that either OPCs can also generate astrocytes in vivo, or that astrocyte precursors also express NG2 (Zhu et al., 2008). Rivers et al. used an inducible PDGFRα-driven Cre YFP reporter line to label OPCs at P45 and examine their progeny 240 days later (Rivers et al., 2008). The authors found that an astonishing 29% of oligodendrocytes in the corpus callosum were generated by OPCs between P45 and P255 (Rivers et al., 2008). Interestingly, the authors found no evidence to support the fact that OPCs also generate astrocytes, but did report a small population of reporter positive neurons in the piriform cortex (Rivers et al., 2008). Subsequently, three independent fate-mapping studies concluded that adult OPCs produce oligodendrocytes exclusively throughout life (Dimou et al., 2008; Kang et al., 2010; Zhu et al., 2011). It is unclear whether imperfections in the reporter systems used contributed to the conclusions that OPCs are multipotent progenitors that can give rise to astrocytes and neurons, in addition to oligodendrocytes (Richardson et al., 2011).
Oligodendrocyte generation in gray matter occurs less frequently than in white matter (Dimou et al., 2008). Studies using in vivo two-photon imaging of superficial cortical layers in adult mice reported that less than 1% of OPCs mature into oligodendrocytes, while 96% remain stable over a 40-day timespan (Hughes et al., 2013). Though one might expect higher OPC turnover in highly myelinated regions, the overall oligodendrocyte production does not differ between adult structures with varying degrees of myelination (Young et al., 2013). Young et al. compared the robustly myelinated optic nerve to the partially myelinated corpus callosum in adult mice and found no long-term change in the number of new oligodendrocytes integrating in those tracts (Young et al., 2013). Whether newly differentiated OPCs replace dying oligodendrocytes to stabilize a circuit or integrate to improve network function is still not known. Evidence to support the latter comes from observations that newly generated oligodendrocytes have significantly shorter internodes compared to neonatal oligodendrocytes, suggesting integration into already myelinated axons (Young et al., 2013). At odds with this explanation is the fact that myelinated axons are not encapsulated with myelin uniformly. For example, myelinated axons from pyramidal neurons in the neocortex have lengthy unmyelinated tracts in which newly generated oligodendrocytes can integrate without restrictions on internode length (Tomassy et al., 2014).
The factors responsible for inducing continuous oligodendrocyte generation in adulthood are also incompletely understood. In mice, physical exercise can cause cortical OPCs to exit the cell cycle and generate oligodendrocytes (Simon et al., 2011). In humans, structural changes associated with white matter can be detected by magnetic resonance imaging after performing complex tasks such as practicing piano (Bengtsson et al., 2005). In rodents, it has been demonstrated that motor skill learning induces myelination (Sampaio-Baptista et al., 2013). More importantly, blocking the differentiation of OPCs into myelinating oligodendrocytes impairs motor skill learning (McKenzie et al., 2014). These findings are consistent with the role of myelin in improving the stability and function of neuronal circuits that are involved in learning. As discussed in more detail below, neuronal activity is implicated in OPC differentiation and proliferation, but myelination is not exclusively activity-dependent (Gibson et al., 2014; Hines et al., 2015). Elucidation of the specific molecular and behavioral trigger mechanisms that control myelination is a fascinating area of future research.
Another intriguing question is whether the myelination dynamics observed in rodent models are also a feature of human myelination. While in mice new oligodendrocytes appear to be generated throughout adulthood, emerging studies challenge our rodent-based perception of oligodendrocyte dynamics in the adult CNS. In the human corpus callosum, the oligodendrocyte number is established early in childhood and remains stable throughout life, such that an annual oligodendrocyte generation rate is estimated at 0.3% (Yeung et al., 2014). This number is strikingly low even when compared to a conservatively calculated rate in mice of 36% (Yeung et al., 2014). Of note, however, is that although oligodendrocytes are established early in life, the myelin sheath is continuously renewed, suggesting that it is a highly dynamic structure (Yeung et al., 2014). An attractive hypothesis is that human oligodendrocytes have evolved to remodel the myelin sheath without requiring generation of a new oligodendrocyte from an OPC (Yeung et al., 2014). Thereby, oligodendrocyte turnover could result in segments of axons temporarily demyelinated, not conducive for efficient neuronal activity. The discrepancies in adult myelination and OPC function between species remain to be reconciled. It is an essential problem warranting further studies, as much of our understanding of human demyelinating diseases comes from non-human vertebrate models.
5. Neuron-OPC bidirectional crosstalk
Recently, neuronal activity has been studied as a regulator of myelination in the CNS (Gibson et al., 2014; Hines et al., 2015). OPCs were first reported to receive excitatory glutamatergic synaptic input via OPC-expressed AMPA receptors (Bergles et al., 2000). Subsequently, Lin and Bergles showed that OPC also express GABAA receptors and are responsive to GABAergic input (Lin and Bergles, 2004). These seminal works established the notion of a bona fide neuron-OPC synapse, but little is known about its significance. Gibson et al. recently used optogenetic technology to stimulate the mouse premotor cortex and showed that neuronal activity promotes OPC proliferation, differentiation and myelination (Gibson et al., 2014). OPC proliferation in the premotor cortex was detectable with three hours of optogenetic stimulation. Four weeks following a seven-day optogenetic stimulation paradigm, there was also an increase in newly generated oligodendrocytes. The authors also noted a mild but significant increase in myelin thickness in the premotor cortex, which correlated with increased limb swing speed (Gibson et al., 2014). Follow up studies demonstrated that activity-dependent synaptic vesicle release is necessary to induce myelination during zebrafish development, confirming the hypothesis that endogenous neuronal activity can affect myelination in the CNS (Hines et al., 2015; Mensch et al., 2015). There are many questions left unanswered regarding the effects of neuronal activity on OPCs. It is important to note that suppressing neuronal activity by blocking voltage-gated sodium channels did not completely prevent myelination during zebrafish development (Hines et al., 2015). Specific deletion of NMDA receptor subunit NR1 in OPCs also does not affect myelination in the developing rodent CNS (De Biase et al., 2011). Yet, NR1 deletion in OPCs results in their upregulation of calcium-permeable AMPA receptors, another avenue by which glutamate signaling might modulate OPC biology. Activation of AMPA receptors has been shown to inhibit OPC differentiation in vitro and stimulate OPC migration in vivo, but OPC-specific deletion of AMPA receptor signaling is needed to fully elucidate their contribution to OPC biology (Gallo et al., 1996; Harlow et al., 2015). In addition, the magnitude of OPC proliferation and the newly derived oligodendrocyte number after optogenetic stimulation did not translate into a robust increase in myelin thickness. In fact, only a surprisingly low increase in g-ratio was observed between stimulated and unstimulated mice, suggesting a potential unconventional fate for these newly generated cells (Gibson et al., 2014).
OPCs are well positioned in the CNS to directly modulate neuronal function. At the synapse, OPCs have been shown to make contact with pre and post-synaptic terminals (Bergles et al., 2000; Ong and Levine, 1999). Furthermore, OPCs are known to contact axons at the nodes of Ranvier, suggesting that OPCs could maintain node function (Butt et al., 1999). Because OPCs receive GABAergic and glutamatergic synaptic input, they could be capable of sensing and modulating network activity. Birey et al. have now demonstrated that OPC deletion in the prefrontal cortex (PFC) compromises glutamatergic signaling in pyramidal neurons (Birey et al., 2015). This perturbation in neurotransmission is accompanied by a decrease in astrocytic gluatamate uptake following OPC ablation. Aberrations in glutamatergic signaling translate into a behavioral phenotype, as loss of OPCs triggered depressive-like behavior, which was rescued after endogenous OPC repopulation. Likewise, mice susceptible to a social defeat stress paradigm had reduced numbers of OPCs in the PFC, a feature shared by patients with major depressive disorder (Birey et al., 2015). This study is the first to demonstrate a robust physiological and behavioral change after OPC ablation, elucidating a novel role of OPCs in regulating CNS homeostasis. However, as this OPC ablation model does not delete OPCs uniformly across the CNS, novel roles for OPCs outside the PFC remain unknown.
Sakry et al. have demonstrated that ectodomain cleavage of NG2, a proteoglycan highly expressed by OPCs, can also modulate neuronal networks (Sakry et al., 2014). Cleavage of NG2 in OPCs was activity dependent, and its blockade with protease inhibitors resulted in the impairment of NMDAR-dependent long-term potentiation (Sakry et al., 2014). This loss of long-term potentiation was recapitulated using the global NG2 knockout mouse, which also had diminished AMPAR- and NMDAR-mediated neuronal currents but did not present with learning and memory deficits (Sakry et al., 2014). Finally, bath application of the NG2 ectodomain onto brain slices results in more c-Fos positive neurons compared to controls after glutamate stimulation (Sakry et al., 2014). The lack of an OPC-specific NG2 knockout model questions whether these findings are mediated exclusively by OPCs. A follow up study found that OPCs express neuromodulatory factor neuronal Pentraxin 2 (Nptx2), which affects AMPAR stability and trafficking at the cell surface (Sakry and Trotter, 2015). Taken together, the data suggests that OPC-derived factors can affect neuronal network activity, but the importance of this bidirectional OPC-neuron communication requires further investigation.
Oligodendrocytes also participate in a novel mode of bidirectional crosstalk using the transfer of exosomes from oligodendrocytes to neurons (Fruhbeis et al., 2013). Neuronal activity mediates exosome secretion from oligodendrocytes, which are then endocytosed by the neurons and promote survival under oxidative stress or nutrient deprivation conditions (Fruhbeis et al., 2013). Neurons can also endocytose exosomes from oligodendrocytes during the resting state, but the contents of the exosome cargo and how they impact neurons under these conditions is not known. Exosome secretion is further triggered by the Ca2+ influx that results from the glutamate binding NMDA and AMPA receptors on oligodendrocytes (Fruhbeis et al., 2013). Given that OPCs express NMDAR and AMPAR and are part of the oligodendroglial lineage, it is possible that similar modes of communication exist in OPCs (Sakry et al., 2015).
6. OPCs respond to CNS Injury
One of the most unique qualities of OPCs is their ability to respond to several types of CNS injury. OPCs respond to secondary demyelinating insults, even when oligodendrocytes are not primarily targeted, for example in mechanical lesions, spinal cord injury, ischemia, and neurodegeneration (Kang et al., 2010; Levine, 1994; McTigue et al., 2001; Zhang et al., 2013). Increased levels of OPCs have also been documented in the prefrontal cortex after acute methamphetamine exposure, perhaps as a part of the alterations to addiction circuitry that needs to be examined further (Somkuwar et al., 2014). OPCs, along with microglia, respond within a day after traumatic brain injury by migrating to and occupying the lesion site, where they become hypertrophic and highly proliferative (Dimou and Gallo, 2015). Surprisingly, in vivo imaging of CNS stab wound injuries showed that at 5 days post-injury astrocytes have a low proliferation rate, and do not exhibit migration to the lesion site (Bardehle et al., 2013). Reactive OPCs are also found in models of CNS neurodegeneration, such as the SOD1 (G93A) mutant mouse that recapitulates features of human amyotrophic lateral sclerosis (ALS) (Kang et al., 2010). Progressive motor neuron death in the spinal cord of these mice is accompanied by a robust increase in OPC proliferation and differentiation (Kang et al., 2010). This suggests that OPCs and microglia, but not astrocytes, are playing an immediate role in the CNS injury response that remains to be fully explored.
7. OPCs contribute to glial scar formation
After any kind of CNS injury, glial cells become activated and orchestrate the formation of the glial scar. The glial scar is characterized by a high content of ECM molecules forming an environment known to block axonal regeneration and remyelination (Cregg et al., 2014; Silver and Miller, 2004). Chondroitin sulfate proteoglycans (CSPGs) are a key component of the inhibitory glial scar (Davies et al., 1997; Lau et al., 2012). While reactive astrocytes are the principal cells present within the glial scar, it is now clear that OPC are not bystanders during the scar formation. Following spinal cord injury, OPCs proliferate and upregulate NG2 expression, thereby inhibiting axonal regeneration (Tan et al., 2005). Rhodes et al. reported that treatment with antimitotic drugs, aimed at diminishing glial scar formation by eliminating OPC proliferation in knife wound injuries, results in a slight improvement in axonal regeneration (Rhodes et al., 2003). This result suggests that OPC proliferation and upregulation of NG2 can be detrimental to axon regrowth. Conversely, OPC expression of NG2 has also been shown to support axon growth in vitro, even when NG2 was overexpressed in OPCs (Yang et al., 2006). The contribution of the OPC to the glial scar is not limited to only one type of CSPG (Jones et al., 2003). OPC have been show to also produce keratan sulfate proteoglycan after injury, and can also produce neurocan and versican, CSPGs known to impair CNS repair (Asher et al., 2000; Asher et al., 2002; Jones and Tuszynski, 2002). In conclusion, OPC clearly participate in the formation of the glial scar and produce CSPGs that impair CNS repair and their differentiation into mature myelinating oligodendrocytes. This apparent duality in function clearly highlights the multi-functionality of OPC in the CNS.
8. OPC as innate immune cells
Microglia and astrocytes are considered to be the classic innate immune cells of the CNS because of their response to pathogens or tissue damage and their ability to recruit peripheral immune cells (Ransohoff and Brown, 2012). However, the literature contains evidence that OPCs are not simple spectators of the CNS immune response. In a mouse model of cerebral prolonged hypoperfusion, OPCs are the initial producers of MMP9, an enzyme necessary for degradation of the extracellular matrix, prior to the appearance of white matter damage (Seo et al., 2013). Furthermore, authors demonstrated that OPC derived MMP9 could mediate the opening of the BBB and the infiltration of neutrophils that ultimately damage the myelin sheath in this model (Seo et al., 2013). Moyon et al. have recently demonstrated that OPCs isolated from the brain of mice undergoing cuprizone-induced demyelination express high levels of CCL-2 and IL-1β (Moyon et al., 2015). CCL-2 has a critical role in recruiting monocytes (Deshmane et al., 2009) while IL-1β is a powerful inflammatory cytokine involved in many aspects of the immune response (Sims and Smith, 2010). While authors did not explore the role of these mediators on immune cell recruitment/activation, they discovered that CCL-2 promotes OPC migration in vivo and is also expressed by OPCs present in active multiple sclerosis (MS) lesions (Moyon et al., 2015). Recent work by Gadani et al. also highlights the role that myelinating glia play in repair after spinal cord injury. Oligodendrocytes release IL-33, a nuclear alarmin implicated in orchestrating the recruitment of peripheral immune cells necessary for CNS repair (Gadani et al., 2015b). Although predominantly expressed by oligodendrocytes, OPCs also express IL-33 and participate in promoting CNS repair (Gadani et al., 2015b). Beyond producing inflammatory mediators, OPC can respond to cytokines and chemokines, such as TNF-α, IL-1β and IFN-γ (Arnett et al., 2001; Vela et al., 2002). The impact of these molecules on OPCs remains controversial, with reports suggesting both a beneficial outcome on myelination and induction of OPC death. For example, TNFα is highly expressed in demyelinating MS lesions and has been shown to potentiate IFN-γ-induced cell death in vitro (Andrews et al., 1998; Watzlawik et al., 2010). Arnett et al. showed a modest delay in cuprizone-induced oligodendrocyte death in TNF-α knockout mice, implicating it as a potentially harmful cytokine (Arnett et al., 2001). Unexpectedly, the study also showed that TNF-α knockout mice suffered from impaired remyelination (Arnett et al., 2001). Mice lacking TNF-α showed decreased OPC proliferation and, as a result, less oligodendrocytes were ultimately generated, accounting for decreased remyelination (Arnett et al., 2001). This data implies that TNF-α has a dual role in both demyelination and remyelination. Furthermore, a recent study demonstrated that OPCs respond to the T cell produced cytokine, IL-17, and actively participate in the amplification of pathology in experimental autoimmune encephalomyelitis, an animal model of MS (Kang et al., 2013b). Finally, OPCs have been shown to be phagocytic in vitro, as isolated cultures of OPCs can efficiently engulf myelin debris (Gaultier et al., 2009). More work needs to be performed in vivo to determine if OPC could participate in the clearance of cellular debris in the context of CNS pathology, especially considering their rapid recruitment at the injury site. In conclusion, OPCs contribute to the immune response in the CNS by producing and responding to inflammatory mediators in a multitude of pathologies.
9. Conclusion
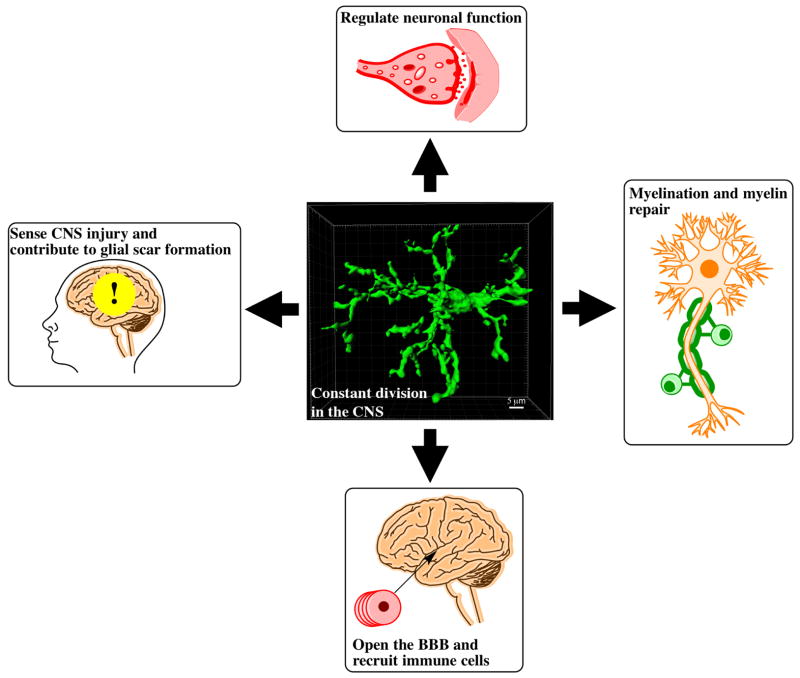
Over the past decade, we have greatly expanded our knowledge of OPC biology and function within the CNS. We now appreciate that adult OPCs are responsible for generating myelinating oligodendrocytes throughout life. Yet, myelination is not their only role within the CNS, as presented in this review (Figure 1). Recent studies have provided compelling evidence that OPCs are capable of modulating neuronal and astrocytic functions that can ultimately affect behavior (Birey et al., 2015; Sakry et al., 2014), providing a new perspective on adult OPC function. After all, adult OPCs are uniformly distributed across the CNS, giving them the ability to survey widespread network activity. However, questions remain regarding adult OPC function. Despite their unique developmental origins within the CNS, no functional differences have been found regarding their myelination potential. It is possible that differences in function might arise in their ability to regulate neuronal activity in distinct regions of the CNS. As the field continues to examine the OPC-neuron synapse, other roles of adult OPCs might become clearer.
Figure 1.
The multiple functions of oligodendrocyte progenitor cells in the central nervous system. Central panel represents a 3D rendering of an OPC in the cortex.
OPCs also respond to several types of CNS injury. As microglia and astrocytes have traditionally held the spotlight in CNS injury, the contribution of OPCs to tissue repair would be interesting to explore. Recent work has shown that OPCs upregulate expression of immune signaling molecules CCL-2 and IL-1β to promote their migration into demyelinating lesions (Moyon et al., 2015). These immune molecules were shown to be important for OPC migration, but they could also be involved in orchestrating an inflammatory response. CCL-2 produced by astrocytes and IL-1β produced by microglia has been shown to amplify the immune response following CNS injury, which can be beneficial to tissue repair (Gadani et al., 2015a). Although it is clear that OPCs can produce immune molecules during CNS injury, their contribution to the inflammatory response needs to be further elucidated.
The need to target OPCs to promote remyelination in demyelinating diseases such as MS, is an important therapeutic goal. Although much of the research has been focused on identifying the factors implicated in inhibition of OPC differentiation and remyelination, acknowledgement that OPC can exert a multitude of function might accelerate the discovery of novel treatment option for demyelinating disorders. Given the evidence presented in this review, it is highly possible that the multi-functionality of OPCs during CNS disease might steer OPCs away from repair and towards the immune response and/or the glial scar formation. A deeper understanding of OPC function in the CNS is warranted before we can truly begin to explore their therapeutic potential.
Highlights.
Oligodendrocyte progenitor cells comprise about 5% of the CNS cellular content.
OPC are continuously renewed, with rates increasing during pathologies.
Beyond myelination, oligodendrocyte precursor cells can modulate neuronal activity.
Oligodendrocyte precursor cells are recruited to injury sites
OPC contribute to the immune response and the formation of the glial scar.
Acknowledgments
The authors are supported by NIH grants R01 NS083542 (A.G.), R43 NS092182 (A.G.) and T32 GM008328 (A.F.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews T, Zhang P, Bhat NR. TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res. 1998;54:574–583. doi: 10.1002/(SICI)1097-4547(19981201)54:5<574::AID-JNR2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22:2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Gotz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci. 2013;16:580–586. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- Baron W, Hoekstra D. On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett. 2010;584:1760–1770. doi: 10.1016/j.febslet.2009.10.085. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Birey F, Kloc M, Chavali M, Hussein I, Wilson M, Christoffel DJ, Chen T, Frohman MA, Robinson JK, Russo SJ, Maffei A, Aguirre A. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron. 2015;88:941–956. doi: 10.1016/j.neuron.2015.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63:1429–1451. doi: 10.1002/glia.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS biology. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron. 2015a;87:47–62. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015b;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, Campana WM, Gonias SL. Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J Cell Sci. 2009;122:1155–1162. doi: 10.1242/jcs.040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. Journal of neurobiology. 2001;48:75–86. [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, Saul KE, Komuro H, Macklin WB. Myelin Proteolipid Protein Complexes with alphav Integrin and AMPA Receptors In Vivo and Regulates AMPA-Dependent Oligodendrocyte Progenitor Cell Migration through the Modulation of Cell-Surface GluR2 Expression. J Neurosci. 2015;35:12018–12032. doi: 10.1523/JNEUROSCI.5151-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci. 2013;33:14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci. 2002;22:4611–4624. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Li Y, Fukaya M, Lorenzini I, Cleveland DW, Ostrow LW, Rothstein JD, Bergles DE. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013a;16:571–579. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Wang C, Zepp J, Wu L, Sun K, Zhao J, Chandrasekharan U, DiCorleto PE, Trapp BD, Ransohoff RM, Li X. Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+ glial cells. Nat Neurosci. 2013b;16:1401–1408. doi: 10.1038/nn.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Doring A, Sloka S, Stirling DP, Rivest S, Yong VW. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol. 2012;72:419–432. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57:270–285. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci. 2015;18:628–630. doi: 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Melik Parsadaniantz S, Franklin RJ, Lubetzki C. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J Neurosci. 2015;35:4–20. doi: 10.1523/JNEUROSCI.0849-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron glia biology. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122:1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Moon LD, Fawcett JW. Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience. 2003;120:41–56. doi: 10.1016/s0306-4522(03)00285-9. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Neitz A, Singh J, Frischknecht R, Marongiu D, Biname F, Perera SS, Endres K, Lutz B, Radyushkin K, Trotter J, Mittmann T. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS biology. 2014;12:e1001993. doi: 10.1371/journal.pbio.1001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakry D, Trotter J. The role of the NG2 proteoglycan in OPC and CNS network function. Brain Res. 2015 doi: 10.1016/j.brainres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Sakry D, Yigit H, Dimou L, Trotter J. Oligodendrocyte precursor cells synthesize neuromodulatory factors. PLoS One. 2015;10:e0127222. doi: 10.1371/journal.pone.0127222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C, Khrapitchev AA, Foxley S, Schlagheck T, Scholz J, Jbabdi S, DeLuca GC, Miller KL, Taylor A, Thomas N, Kleim J, Sibson NR, Bannerman D, Johansen-Berg H. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci. 2013;33:19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59:869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- Sims JE, Smith DE. The IL–1 family: regulators of immunity. Nature reviews Immunology. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, Simons M. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD. Role of NG2 expressing cells in addiction: a new approach for an old problem. Frontiers in pharmacology. 2014;5:279. doi: 10.3389/fphar.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Ikenaka K. Oligodendrocyte generation during mouse development. Glia. 2015;63:1350–1356. doi: 10.1002/glia.22863. [DOI] [PubMed] [Google Scholar]
- Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. Journal of anatomy. 2005;207:717–725. doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20:489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- Vigano F, Mobius W, Gotz M, Dimou L. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16:1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- Watzlawik J, Holicky E, Edberg DD, Marks DL, Warrington AE, Wright BR, Pagano RE, Rodriguez M. Human remyelination promoting antibody inhibits apoptotic signaling and differentiation through Lyn kinase in primary rat oligodendrocytes. Glia. 2010;58:1782–1793. doi: 10.1002/glia.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung MS, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisen J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Frontiers in cellular neuroscience. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]