Abstract
Background and Purpose
The computed tomography angiography (CTA) spot sign is a validated predictor of hematoma expansion and poor outcome in supratentorial intracerebral hemorrhage (ICH), but patients with brainstem ICH have typically been excluded from analyses. We investigated the frequency of spot sign and its relationship with hematoma expansion and outcome in patients with primary pontine hemorrhage (PPH).
Methods
We performed a retrospective analysis of PPH cases obtained from a prospectively collected cohort of consecutive ICH patients who underwent CTA. CTA first pass readings for spot sign presence were analyzed by two trained readers. Baseline and follow-up hematoma volumes on non-contrast CT scans were assessed by semi-automated computer-assisted volumetric analysis. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratio and accuracy of spot sign for prediction of in-hospital mortality were calculated.
Results
49 subjects met the inclusion criteria of whom 11 (22.4%) showed a spot sign. In-hospital mortality was higher in spot sign positive versus negative subjects (90.9% vs 47.4%, p=0.020). Spot sign showed excellent specificity (95%) and PPV (91%) in predicting in-hospital mortality. Absolute hematoma growth, defined as parenchymal and intraventricular hematoma expansion of any amount, was significantly higher in spot sign positive versus negative subjects (13.72 ± 20.93 vs 3.76 ± 8.55 mL, p=0.045).
Conclusions
As with supratentorial ICH, the CTA spot sign is a common finding and is associated with higher risk of hematoma expansion and mortality in PPH. This marker may assist clinicians in prognostic stratification.
Keywords: CT angiography, spot sign, brainstem, pontine, prognosis, intracerebral hemorrhage
Introduction
Intracerebral hemorrhage (ICH) accounts for 10 to 20% of all strokes, with one-month mortality up to 40% and severe disability in the majority of survivors (1). Primary pontine hemorrhage (PPH) represents the deadliest type of ICH, with in-hospital case fatality rate of 47.5% (2) and long term mortality over 60 % (3). Many studies of ICH, both observational and clinical trials, exclude patients with ICH in the pontine location because outcomes are so poor. However, the ability to predict which of these subjects in fact have potential for reasonable outcome would provide both clinicians and researchers with the opportunity to include them and provide valuable prognostic information.
Computerized tomography angiography (CTA) spot sign is an established, independent predictor of hematoma expansion, in-hospital mortality and poor long-term outcome in supratentorial ICH (4,5). However, most of the previous studies on spot sign excluded patients with PPH, and therefore the frequency of spot sign and its relation with hematoma growth and outcome in PPH is currently unknown (6,7). As this type of ICH may have a subtly different pathophysiology (and clearly worse outcomes), it may be that the likelihood of detecting a spot sign and any relationship with expansion and outcome will be different in this group. The purpose of the present study was therefore to investigate whether the presence of spot sign is associated with hematoma expansion and poor outcome in PPH.
Methods
Patient selection
We performed a retrospective analysis of data collected from an ongoing prospectively collected cohort of consecutive patients with primary ICH from a single center (8). Patients were included if they presented from January 2001 to May 2015 and met the following eligibility criteria: (1) diagnosis of PPH based on non-contrast CT scan (NCCT); 2) CTA performed within 24 h from presentation to the Emergency Department. Patient exclusion criteria were the prsence of (1) a vascular lesion or neoplastic lesion determined as the cause for the ICH; 2) surgical evacuation of the hematoma; 3) traumatic intracranial bleeding; 4) supratentorial or cerebellar location of the ICH; 5) absence of thin slices axial CTA images (0.625 to 1.25 mm slice thickness); 6) poor quality of the CTA images. All aspects of the study were approved by the Institutional Review Board.
Image acquisition
NCCT examinations were performed using an axial technique with 5-mm slice thickness reconstruction. CTA was performed by scanning from the base of the skull base to the vertex using an axial technique, 0.5 pitch, 1.25-mm collimation, 100 to 140 kVp, maximum tube current 350 mA. For CTA images acquisition, 65 to 85 mL of iodinated contrast material administered by a power injector at 4 to 5 mL/s into an antecubital vein with Smart-Prep, a semiautomatic contrast bolus triggering technique. Baseline NCCT, CTA and follow-up NCCT were acquired on the same hardware platform.
Image analysis
The baseline NCCT scans were reviewed to determine the presence of associated intraventricular hemorrhage (IVH) and hydrocephalus. Determination of the baseline and follow-up ICH and IVH volumes on NCCT was performed using Analyze Direct 11.0 software. For the identification of Spot Sign presence, CTA images were independently reviewed by two trained readers (AM, MJ). Differences in reader interpretation were adjudicated by consensus agreement, under the supervision of an expert neuroradiologist (JMR). 0.625 or 1.25 mm axial CTA source images were reviewed in “Spot Windows” (width 200, level 110) as previously described (7). Delayed CTA images were available only for a minority of patients so only first pass CTA acquired images were analyzed. Significant hematoma expansion was defined as as increase in hematoma volume of 6 mL or >30% from the baseline ICH volume on NCCT (9). The hematoma expansion analysis was performed in the subgroup of subjects that underwent a follow-up NCCT. Illustrative images of CTA spot sign positive pontine hemorrahages are shown in figure 1 and a representative case of CTA spot sign predicting hematoma expansion is shown in figure 2.
Figure 1.
A–C) Illustrative examples of spontaneous pontine hemorrhages on NCCT. B–D) Evidence of active contrast extravasation (Spot Sign) on CTA images (arrows)
Figure 2.
A) Pontomesencephalic ICH on NCCT, with baseline volume of 29 mL. B–C) CTA showing presence of multiple spot signs (arrows). D–E–F) Follow-up NCCT at 5 hours demonstrated significant hematoma growth to a volume of 66 mL with massive intraventricular extension and hydrocephalus. The patient passed away shortly after the follow-up scan.
Clinical Variables
Clinical data was captured prospectively including age, sex, medical history of hypertension, diabetes mellitus, hypercholesterolemia, pre-ICH treatment with warfarin or other anticoagulants, antiplatelet medications, statins; hospital records were reviewed for Glasgow Coma Scale score, systolic blood pressure and blood glucose on admission, and time from symptom onset to CTA. Outcome at discharge (modified Rankin Scale [mRS]) was captured from chart review. In-hospital and 30-day mortality was assessed in long term follow-up as described (10,11).
Statistical Analysis
Statistical analysis was performed using SPSS v. 21 (www.spss.com). Discrete variables were summarized as count (%) and continuous variables as mean (Standard Deviation [SD]) or median (Inter-Quartile Range [IQR]). Spot sign positive and spot sign negative subjects were compared, using Fisher exact test, Student t test and Mann – Whitney test, as appropriate. Patients’ outcome at discharge was stratified as absent or minor disability (mRS 0–2), moderate to severe disability (mRS 3–5) and in-hospital death (mRS 6). The distribution of mRS at discharge was analyzed with the Wilcoxon rank sum test. Interobserver agreement for the identification of any spot sign was determined using the kappa statistic. Subsequently, we calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio, negative likelihood ratio, and accuracy, using standard methods, to determine the performance of the spot sign in predicting in-hospital and 30-day mortality. The association between CTA spot sign presence, in-hospital and 30-day mortality was investigated with a multivariable logistic regression analysis.
Results
During the study period, 1931 patients presented with ICH, of whom 93 (4.8 %) had PPH. Of these, 43 were excluded for lack of CTA and 1 for surgical intervention, leaving 49 patients for analysis. The characteristics of the study population are summarized in Table 1.
Table 1.
Baseline Characteristics of the Population (n=49)
| Parameters | |
|---|---|
| Age, mean ± SD (median), y | 65.5 ± 14.4 (65.2) |
| Sex, male, n (%) | 27 (55.1) |
| GCS on admission, median (IQR) | 4 (3 – 15) |
| History of hypertension, n (%) | 40 (83.3) |
| Admission SBP, mean ± SD, mm Hg | 187.3 ± 40.1 |
| History of diabetes, n (%) | 18 (38.3) |
| Admission blood glucose, mean ± SD, mg/dL | 173.0 ± 73.2 |
| History of hypercholesterolemia, n (%) | 17 (37.0) |
| Statin treatment, n (%) | 15 (32.6) |
| Antiplatelet treatment, n (%) | 21 (42.9) |
| Anticoagulant treatment, n (%) | 13 (26.5) |
| Baseline ICH volume, mean ± SD, mL | 8.1 ± 8.0 |
| Baseline IVH volume, mean ± SD, mL | 2.4 ± 4.9 |
| Time from symptom onset to CTA, mean ± SD, h | 8.7 ± 10.5 |
| Presence of IVH, n (%) | 25 (51.0) |
| Hydrocephalus, n (%) | 14 (28.6) |
| In-hospital mortality, n (%) | 28 (57.1) |
| 30-day mortality, n (%) | 30 (61.2) |
| Spot sign presence, n (%) | 11 (22.4) |
CT indicates computed tomography; GCS, Glasgow Coma Scale CTA indicates computed tomography angiography, ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; SBP, systolic blood pressure; IQR, interquartile range.
The subjects excluded from the analysis because of unavailable CTA had larger baseline hematoma volumes (mean volume: 15.1 ± 13.9 versus 8.1 ± 8.0 mL, p = 0.011). The remaining demographic, clinical and imaging characteristics were similar between the two groups (all p values > 0.05).
Spot sign was present in 11 patients (22.4 %) and hematoma growth of 6 mL or 30% occurred in 10 of the 38 subjects with a follow-up NCCT available (26.3 %). Inter-rater reliability for spot sign detection was excellent (k 0.82, 95% CI 0.62 – 1.00). A total of 18 (36.7 %) subjects were found in coma with unknown time of symptom onset. For patients with clear symptom onset, mean time from stroke to CTA was 8.7 ± 10.5 hours. Overall in-hospital mortality rate was 57.1% and 30 day mortality was 61.2%.
Spot sign correlation with baseline ICH volume and hematoma expansion
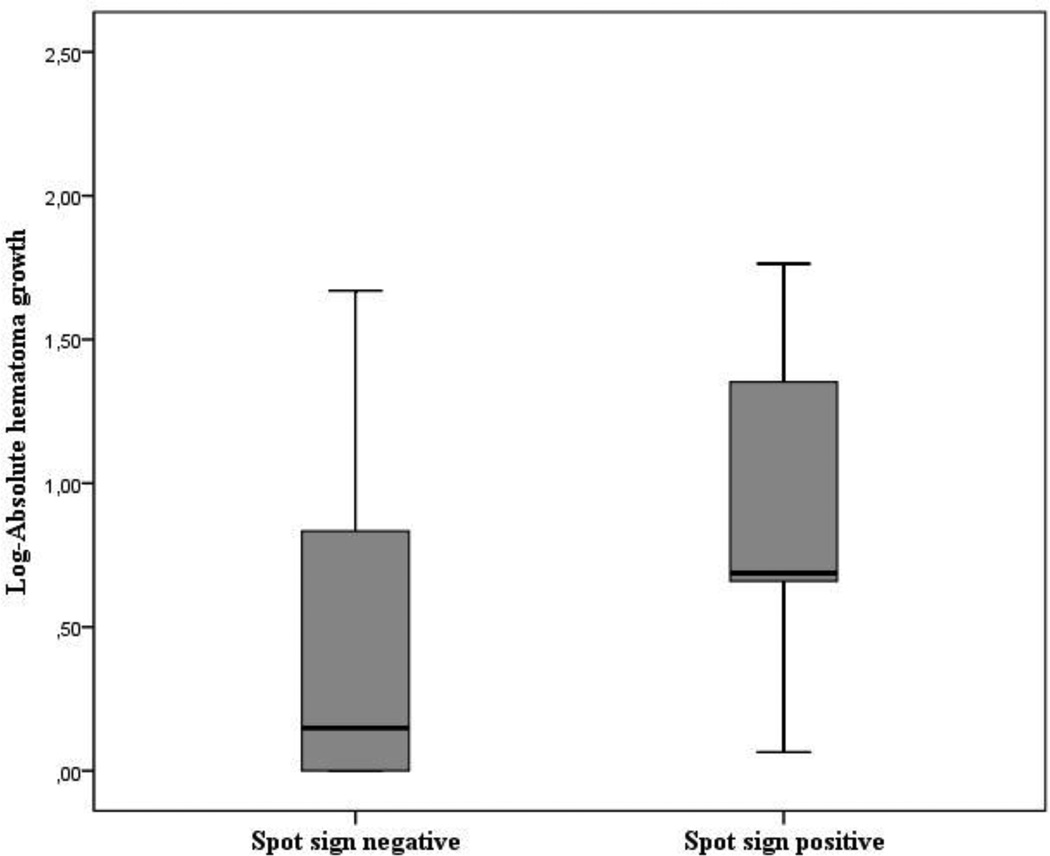
Table 2 shows that the presence of spot sign was associated with higher baseline ICH volume (16.12 ± 10.38 versus 5.71 ± 5.36 mL, p<0.001) and baseline IVH volume (5.80 ± 6.1 versus 0.96 ± 2.43 mL, p<0.001). The frequency of intraventricular extension was higher in the spot sign positive group (81.8 % versus 42.1, p = 0.037). A total of 11 (22.4%) subjects did not receive a follow up NCCT scan and 4 (36.4%) of these were spot sign positive. Hematoma expansion was therefore analyzed in the remaining 38 patients who underwent a follow-up NCCT. While hematoma expansion was observed in 42.8% of the spot sign positive patients compared to 22.6% in the spot sign negative group, the difference was not statistically significant (p=0.351). However, patients with spot sign experienced higher absolute hematoma growth (total parenchymal and intraventricular hemorrhage growth) compared to spot sign negative patients (absolute hematoma expansion 13.72 ± 20.93 versus 3.76 ± 8.55 mL, p=0.045) as shown in figure 3.
Table 2.
Comparison between spot sign positive and spot sign negative subjects
| Spot sign negative (n=38) |
Spot sign positive (n=11) |
p value | |
|---|---|---|---|
| Age, mean ± SD, y | 65.04 ± 14.7 | 67.3 ± 13.7 | 0.651 |
| Sex, male, n (%) | 20/38 (52.6) | 7/11 (63.6) | 0.732 |
| GCS on admission, median (IQR) | 8 (3 – 15) | 4 (3 – 8) | 0.156 |
| History of hypertension, n (%) | 33/38 (86.8) | 7/10 (70.0) | 0.336 |
| Admission SBP, mean ± SD, mm Hg | 186.3 ± 41.9 | 190.7 ± 35.1 | 0.779 |
| History of diabetes, n (%) | 13/37 (35.1) | 5/10 (50.0) | 0.473 |
| Admission blood glucose, mean ± SD, mg/dL | 170.5 ± 78.1 | 182.3 ± 53.6 | 0.655 |
| History of hypercholesterolemia, n (%) | 14/36 (38.9) | 3/10 (30.0) | 0.723 |
| Statin treatment, n (%) | 11/36 (30.6%) | 4/10 (40.0%) | 0.418 |
| Antiplatelet treatment, n (%) | 17/38 (44.7) | 4/11 (36.4) | 0.737 |
| Anticoagulant treatment, n (%) | 10/38 (26.3) | 3/11 (27.3) | 0.614 |
| Baseline ICH volume, mean ± SD, mL | 5.71 ± 5.36 | 16.12 ± 10.38 | <0.001 |
| Baseline IVH volume, mean ± SD, mL | 0.96 ± 2.43 | 5.8 ± 6.1 | <0.001 |
| Time from symptom onset to CTA, mean ± SD, h | 9.0 ± 11.4 | 7.5 ± 6.7 | 0.767 |
| Presence of IVH, n (%) | 16/38 (42.1) | 9/11 (81.8) | 0.037 |
| Hydrocephalus, n (%) | 7/38 (18.4%) | 7/11 (63.6) | 0.007 |
| In-hospital mortality | 18/38 (47.4) | 10/11 (90.9) | 0.020 |
| 30-day mortality | 20/38 (52.6) | 10/11 (90.9) | 0.033 |
GCS indicates Glasgow Coma Scale; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; SBP, systolic blood pressure; CTA indicates computed tomography angiography.
Figure 3.
Log-mean absolute hematoma growth (parenchymal and intraventricular hematoma expansion). Results are presented stratified by spot sign status.
Clinical outcome
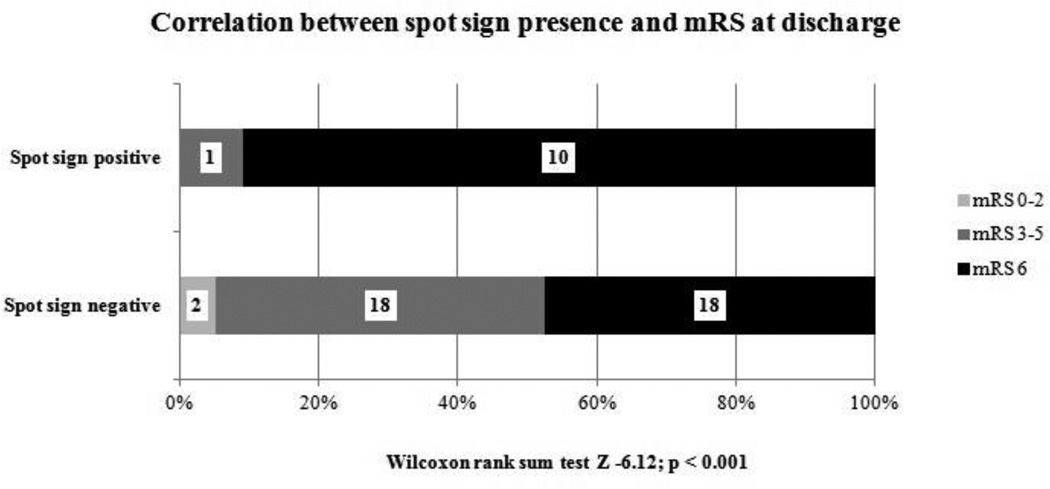
The overall distribution of the mRS at discharge was significantly different between spot sign positive and negative subjects (Figure 4, Wilcoxon Rank Sum Test Z – 6.12, p < 0.001). Spot sign was also significantly associated with 30-day mortality (90.9% in the spot sign positive group compared to 52.6% for the patients without spot sign, p=0.033). Presence of any spot sign showed very good specificity (95%) and PPV (91%) in predicting both in-hospital and one month mortality. The overall accuracy of spot sign in predicting mortality is shown in Table 3.
Figure 4.
Correlation between spot sign presence and outcome (mRS) at discharge.
Table 3.
Accuracy of Spot Sign for prediction of mortality in PPH
| In-hospital mortality (95% CI) |
30-days mortality (95% CI) |
|
|---|---|---|
| Sensitivity | 0.36 (0.19–0.56) | 0.33 (0.18 – 0.53) |
| Specificity | 0.95 (0.74–1.00) | 0.95 (0.72 – 1.00) |
| Positive predictive value | 0.91 (0.57–1.00) | 0.91 (0.57 – 1.00) |
| Negative predictive value | 0.53 (0.36–0.69) | 0.47 (0.31 – 0.64) |
| Positive likelihood ratio | 7.50 (1.04–54.12) | 6.33 (0.88 – 45.58) |
| Negative likelihood ratio | 0.68 (0.51–0.89) | 0.70 (0.54–0.91) |
| Accuracy | 0.61 | 0.57 |
Multivariable logistic regression model found that spot sign was not an independent predictor of in-hospital of 30-day mortality when we accounted for other known predictors of ICH outcome such as baseline hematoma volume, age and admission GCS (12) (Table 4).
Table 4.
Multivariate Analysis of Predictors of In-Hospital and 30-Day mortality
| In-Hospital Mortality | 30-Day Mortality | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p value | OR | (95% CI) | p value | |
| Age | 1.09 | (0.99 – 1.21) | 0.081 | 1.15 | (1.03 – 1.28) | 0.015 |
| Admission GCS | 0.70 | (0.53 – 0.93) | 0.012 | 0.71 | (0.53 – 0.95) | 0.021 |
| Baseline ICH volume | 1.35 | (1.00 – 1.83) | 0.054 | 1.44 | (1.03 – 2.02) | 0.034 |
| Baseline IVH volume | 1.14 | (0.79 – 1.65) | 0.483 | 1.22 | (0.83 – 1.79) | 0.306 |
| CTA Spot Sign | 2.70 | (0.07 – 103.62) | 0.594 | 1.14 | (0.03 – 49.70) | 0.543 |
GCS indicates Glasgow Coma Scale; OR, Odds Ratio, CI, confidence interval; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; CTA, computed tomography angiography.
Discussion
We found that in patients with PPH, as in supratentorial ICH, spot sign has good accuracy for the prediction of mortality and poor outcome and is associated with higher total hematoma growth. The frequency of spot sign in our study population was similar to what we have observed in supratentorial ICH (13,14). While we did not find a statistically significant association with hematoma expansion, the direction of the association is similar to findings in supratentorial ICH, and the lack of significance may simply be due to the small numbers in this cohort. Those studies that have included brainstem ICH in their cohorts found that less than 5% are in this location(13,15). We noted a relatively high frequency of intraventricular extension of ICH (overall frequency 57.1 %), compared to previous results (38.1% – 37 %)(13,15). Furthermore, IVH presence was significantly more frequent in the spot positive group. As such, we analyzed total ICH growth including both parenchymal and ventricular blood, demonstrating a statistically significant effect. This result again suggests the lack of significance in “hematoma expansion” is simply due to sample size constraints.
From a pathophysiological standpoint, our findings confirm that spot sign is an important radiological marker of active bleeding (16,17). Furthermore, we found a higher frequency of IVH and hydrocephalus in the spot sign positive population, both of which are also strong predictors of mortality (18,19). The proportion of patients with brainstem ICH included in major clinical trials targeting hematoma growth was either not specified (20) or extremely low (less than 1%) (21). Our findings suggest that spot sign may be valuable in identifying patients suffering PPH that have an opportunity to benefit from anti-expansion therapies. We do note that the effect of spot sign on mortality became non-significant after multivariable analysis controlling for ICH and IVH volume, suggesting that hematoma volume is still the most powerful predictor of outcome. It is likely that our sample size was too small to confirm an additional independent effect on mortality that has been seen in larger studies of non-pontine ICH (6,7).
Despite progress in prevention and treatment, ICH is still a disease with high morbidity and mortality, and almost half of patients with PPH die in hospital. Accurate prediction of outcome is important not only in guiding therapeutic options but also in discussions regarding withdrawal of support and palliative care (22). CTA is increasingly used in the diagnostic workup of ICH (23) and CTA findings may provide valuable additional prognostic information for clinical providers managing patients with PPH.
Some limitations of the present study should be considered. First we performed a single center, retrospective analysis, with a relatively small sample size. While, given the relative rarity of PPH, this represents one of the largest published cohorts, the numbers remain small. Second, given the high in-hospital mortality rate, follow-up NCCT scans were not available in several cases. A high proportion of the subjects with missing follow-up imaging (36.4%) had a spot sign, which may have led to an underestimation of the association between spot sign and ICH expansion. Finally, a significant number of patients had an unwitnessed symptom onset and therefore it was not possible to accurately estimate the time from symptom onset to CTA imaging and account for this variable in our analyses.
Conclusion
The presence of CTA spot sign is associated with increase in total ICH and IVH volume and poor clinical outcome in patients with PPH. Patients with PPH are often excluded from clinical trials and observational trials, and it may be that clinical providers are often quite pessimistic regarding outcome in this population. However, our findings suggest that some PPH patients do in fact have good outcomes. Given the high rate of expansion in these patients, it is reasonable to hypothesize that PPH patients may benefit from acute therapies. As CTA is a commonly available tool in the acute setting, it may be that this tool can help providers in identifying which PPH patients have the greatest opportunity for the best outcomes.
Acknowledgments
Sources of funding
This study was supported by the following awards from the NINDS: 5R01NS073344, K23AG02872605, K23 NS086873, R01NS059727.
None of the funding entities had any involvement in study design; data collection, analysis, and interpretation; writing of the manuscript; or decision to submit the study for publication.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Murata Y, Yamaguchi S, Kajikawa H, et al. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. J Neurol Sci. 1999;167:107–111. doi: 10.1016/s0022-510x(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi S, Suzuki G, Takasato Y, et al. Prognostic factors in patients with primary brainstem hemorrhage. Clin Neurol Neurosurg. 2012;115:732–735. doi: 10.1016/j.clineuro.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Brouwers HB, Goldstein JN, Romero JM, et al. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage a review. Stroke. 2012;43:3427–3432. doi: 10.1161/STROKEAHA.112.664003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): A prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 6.Almandoz JED, Yoo AJ, Stone MJ, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero JM, Bart Brouwers H, Lu J, et al. Prospective validation of the computed tomographic angiography spot sign score for Intracerebral hemorrhage. Stroke. 2013;44:3097–3102. doi: 10.1161/STROKEAHA.113.002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouwers HB, Falcone GJ, McNamara KA, et al. CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care. 2012;17:421–428. doi: 10.1007/s12028-012-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada R, Aviv RI, Fox AJ, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 10.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke. 2008;39:2304–2309. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 12.Hemphill JC, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers HB, Chang Y, Falcone GJ, et al. Predicting Hematoma Expansion After Primary Intracerebral Hemorrhage. JAMA Neurol. 2014;71:158–164. doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado Almandoz JE, Yoo AJ, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huynh TJ, Demchuk AM, Dowlatshahi D, et al. Spot sign number is the most important spot sign characteristic for predicting hematoma expansion using first-pass computed tomography angiography: analysis from the PREDICT study. Stroke. 2013;44:972–977. doi: 10.1161/STROKEAHA.111.000410. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers HB, Greenberg SM. Hematoma expansion following acute intracerebral hemorrhage. Cerebrovasc Dis. 2013;35:195–201. doi: 10.1159/000346599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowlatshahi D, Wasserman JK, Momoli F, et al. Evolution of computed tomography angiography spot sign is consistent with a site of active hemorrhage in acute intracerebral hemorrhage. Stroke. 2014;45:277–280. doi: 10.1161/STROKEAHA.113.003387. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 20.Anderson CS, Heeley E, Huang Y, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 21.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 22.Holloway RG, Arnold RM, Creutzfeldt CJ, et al. Palliative and End-of-Life Care in Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2014;45:1887–1916. doi: 10.1161/STR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 23.Khosravani H, Mayer SA, Demchuk A, et al. Emergency noninvasive angiography for acute intracerebral hemorrhage. Am J Neuroradiol. 2013;34:1481–1487. doi: 10.3174/ajnr.A3296. [DOI] [PMC free article] [PubMed] [Google Scholar]