Abstract
Urban community gardens provide affordable, locally grown, healthy foods and many other benefits. However, urban garden soils can contain lead (Pb) that may pose risks to human health. To help evaluate these risks, we measured Pb concentrations in soil, vegetables, and chicken eggs from New York City community gardens, and we asked gardeners about vegetable consumption and time spent in the garden. We then estimated Pb intakes deterministically and probabilistically for adult gardeners, children who spend time in the garden, and adult (non-gardener) household members. Most central-tendency Pb intakes were below provisional total tolerable intake (PTTI) levels. High-contact intakes generally exceeded PTTIs. Probabilistic estimates showed approximately 40% of children and 10% of gardeners exceeding PTTIs. Children’s exposure came primarily from dust ingestion and exposure to higher-Pb soil between beds. Gardeners’ Pb intakes were comparable to children’s (in µg/d) but were dominated by vegetable consumption. Adult household members ate less garden-grown produce than gardeners and had the lowest Pb intakes. Our results suggest that healthy gardening practices to reduce Pb exposure in urban community gardens should focus on encouraging cultivation of lower-Pb vegetables (i.e., fruits) for adult gardeners and on covering higher-Pb non-bed soils accessible to young children. However, the common practice of replacement of root-zone bed soil with clean soil (e.g., in raised beds) has many benefits and should also continue to be encouraged.
Keywords: Urban agriculture, community garden, urban soil, lead (Pb) exposure
Introduction
Community gardening is an increasingly popular activity in the US and elsewhere. It has been estimated that participation in community gardens in the US has tripled from one million households in 2008 to three million households in 2013 (NGA 2014). Most community gardens are located in urban centers, where they can provide affordable, locally grown, healthy foods and many other benefits associated with urban green space, opportunities for recreation and community building activities, and reduced environmental impacts of food transport and large-scale production (Alaimo et al. 2010; Groenewegen et al. 2006; Leake et al. 2009; Van Den Berg and Custers 2011). For these reasons, many federal, state, and local governmental and non-governmental organizations (NGOs) in the US and elsewhere encourage and fund development of urban community gardens.
However, it has been well established that most urban soils are contaminated with lead (Pb) (Datko-Williams et al. 2014; Mielke et al. 1984). A number of studies have documented soil Pb contamination in urban home gardens (Clark et al. 2008; Mielke et al. 1983; Szolnoki et al. 2013) and community gardens (Bugdalski et al. 2014; Chaney et al. 1984; Clarke et al. 2015; Mitchell et al. 2014; Stilwell et al. 2008) with central tendency soil Pb concentrations ranging from around 100 to 1000 mg/kg, considerably higher than natural background levels, and maxima reaching above 10,000 mg/kg. Garden vegetables can become contaminated by Pb in soil through soil adherence and limited direct uptake (Attanayake et al. 2014; Bacigalupo and Hale 2011; Bassuk 1986; Finster et al. 2004; McBride 2013; McBride et al. 2014; Moir and Thornton 1989), and both garden soil and vegetables can present opportunities for exposure to Pb. Any exposure to Pb is considered to be potentially harmful to human health, since no threshold for adverse effects has been identified (Miranda et al. 2007). Studies have shown that elevated blood Pb levels are associated with decreased performance in functions of the nervous system, increases in blood pressure, anemia, and reproductive effects (ATSDR 2007). Relatively recent studies (Jusko et al. 2008; Miranda et al. 2007) have reported effects at lower blood lead levels than earlier studies as acknowledged by the US Department of Health and Human Services (National Toxicology Program 2012).
Some previous studies have assessed Pb exposures from home gardens considering consumption of vegetables only (Abbasi et al. 2013; Beccaloni et al. 2013), in combination with ingestion of soil using estimated vegetable Pb levels (Bacigalupo and Hale 2012; Hough et al. 2004), or for specific situations such as flooding (Sipter et al. 2008). However, exposures may be different for urban community gardeners than for home gardeners, who are generally thought to be predominantly rural and suburban. Differences between community and home gardeners may include food consumption / medium contact rates, exposure frequencies, and commonly encountered exposure medium Pb levels. Although home grown vegetable consumption rates have been estimated (US EPA 2011a), little is known about vegetable consumption rates and the amount of time spent in gardens by urban community gardeners. Additionally, chickens are once again becoming increasingly common in urban home and community gardens (Beam et al. 2013; Pollock et al. 2012; Spliethoff, Mitchell, Ribaudo, et al. 2014). We are not aware of any studies that evaluated multipathway Pb exposures for urban community gardeners, nor any that considered garden-raised chicken-egg consumption as an exposure pathway. Although exposures may vary from garden to garden and city to city, there may be a range of exposures common to many urban community gardens across the US and elsewhere. However, in general, the relevance and direct applicability of previously published garden Pb exposure studies for current US urban community gardener populations may have some limitations that could be addressed through further study.
Although urban community gardener multipathway exposures are not well defined, various exposure reduction strategies targeting specific exposure pathways are known to exist (washing/peeling vegetables, mulching pathways, washing hands, etc.) (US EPA 2011b). Arguably, the strategy most commonly recommended to and used by gardeners is to add clean soil and amendments to garden beds (e.g., raised beds) (Connecticut Department of Health 2014; Stilwell et al. 2008; Wieland et al. 2010). Implicit in adoption of this as the sole exposure mitigation strategy is that exposures associated with the garden beds (vegetable consumption and contact with root zone soil) are the most significant garden-related exposures. However, since the relative importance of these exposures for urban community gardeners has not been well defined, the effectiveness of this strategy and others intended to reduce transfer of garden bed soil Pb to vegetables (e.g., amending and mulching bed soil) in reducing overall garden-related exposure is not known. Improved understanding of the relative importance of specific exposure pathways in urban community gardens could help inform, prioritize, and refine risk reduction messaging promoted by governmental and NGO organizations.
Understanding the relative importance of gardening exposure pathways requires information on exposure media contaminant levels, media contact rates, and the gardeners themselves, garden visitors and those with whom they share garden-produced food. This kind of quantitative exposure information is not readily available for community gardens. To help fulfill these needs, a community-research partnership of Cornell University, Cornell University Cooperative Extension, the New York State Department of Health, the community gardening organization New York City (NYC) Parks GreenThumb, and other stakeholders addressed community concerns through collaborative research to inform the development of education and public health action strategies.
There are more than a thousand community gardens in NYC with tens of thousands of gardeners. Many of these gardens were established decades ago, and most have raised beds throughout (Mitchell et al. 2014). In our previous work in NYC gardens, we measured concentrations of Pb in soil, vegetables, and chicken eggs and found that at least some samples in each of these media exceeded health-based guidance values (McBride et al. 2014; Mitchell et al. 2014; Spliethoff, Mitchell, Ribaudo, et al. 2014), suggesting the need for a more refined evaluation of exposure and health risk. We also found that garden-grown vegetable Pb levels have a poor association with soil Pb level but a strong association with crop type (McBride et al. 2014). In contrast, we found that chicken egg Pb was strongly associated with Pb content of garden soil (Spliethoff, Mitchell, Ribaudo, et al. 2014). In this study, we consider egg Pb to be dependent on soil Pb and vegetable Pb to be independent of soil Pb, and we distinguish vegetable Pb due to adhered soil from Pb taken up by roots. We have compiled exposure-related information we collected as part of previous studies on concentrations of Pb in NYC community garden soil, vegetables, and chicken eggs as well as vegetable consumption rate and time spent in NYC community gardens. Based on this information, we estimate Pb intakes using deterministic and probabilistic methods for gardeners and household members.
Methods
We assessed Pb intake deterministically and probabilistically by collecting data and identifying values for exposure media concentrations, media intake rates, exposure frequencies and duration, and averaging time (US EPA 1989) for three potentially exposed individuals/populations: an adult gardener; an adult household member who spends no time in the garden; and, because children visit and play in many NYC community gardens, a six-year-old child who spends time in the garden, playing in non-growing or “non-bed” areas. Intake via each pathway was calculated as follows (and later expressed in terms of µg/d for comparison to tolerable intakes):
Where
IPb = Intake of Pb (μg/kg-d)
IRM = Exposure medium intake rate (kg/d)
CPb = Exposure medium Pb concentration (μg/kg)
ED = Exposure duration (y)
EF = Exposure frequency (d/y)
BW = body weight (kg), and
AT = Averaging time (d).
All three populations (or a percentage thereof) were assumed to eat garden produce and eggs and to ingest house dust containing soil tracked in from non-growing areas. We assumed that direct incidental ingestion of garden soil occurred only in the garden, and therefore we evaluated this route for gardeners and children, but not for adult household members. We assumed gardeners would have most direct contact with (and therefore primarily incidentally ingest) soil from garden beds, while, based on our observations, young children would be more likely to ingest soil in non-bed areas where they tended to play while adults were gardening.
We collected data in two separate surveys of NYC community gardeners to assess vegetable consumption rates and time spent in the garden, and we obtained rates of soil, dust, and egg consumption from the literature. Soil particle inhalation was not accounted for separately because rates of soil ingestion are generally considered to include inhalation and swallowing of particles (US EPA 2011a). Dermal absorption of lead was not accounted for because USEPA has not determined a dermal absorption fraction for this metal, and it is generally considered negligible (NYSDEC and NYSDOH 2006; US EPA 2004). Input data and exposure assumptions for probabilistic and deterministic assessments are provided in Table S1. Our deterministic exposure assessment considered central and high contact estimates for the ingestion/consumption rates to estimate central tendency and high contact exposures. The assessment followed a traditional risk assessment protocol, using primarily exposure parameter values “recommended” for exposure assessment by the USEPA (US EPA 2004, 2011a). For our probabilistic assessment, we determined that our input data could be best approximated by lognormal distributions, and we used a 10,000-trial Monte Carlo simulation with @Risk version 6 software (Palisade Corp, Ithaca, NY) in conjunction with Microsoft Excel 2013 to arrive at median and 95th percentile Pb intake estimates for each population. A sensitivity analysis was performed by assessing the correlation (Spearman rank-order) of 14 input parameter distributions (Table S1) with the simulated probability distributions of Pb intakes for gardeners, children and adult household members. SAS 9.4 and Minitab Release 14 were used for non-parametric statistical analyses, since most data were not normally distributed.
Exposure Media Concentrations
We extracted data from our previous studies of total Pb concentrations in NYC community garden soils (bed and non-bed areas, 0–12 cm), garden vegetables, and chicken eggs (McBride et al. 2014; Mitchell et al. 2014; Spliethoff, Mitchell, Ribaudo, et al. 2014), and we estimated levels in household dust containing tracked-in soil. For the deterministic assessments, the 95% upper confidence limit on the arithmetic mean was chosen as a conservative estimate of the exposure media (i.e., soil, dust, vegetables) concentrations likely to be contacted over time (US EPA 1989). Considering the default assumption of 70% soil in indoor dust in US EPA’s IEUBK model (US EPA 2010), estimates in the literature of approximately 50% soil in indoor dust (Allott et al. 1992; Chaney and Mielke 1986; NYSDEC and NYSDOH 2006; Trowbridge and Burmaster 1997), and professional judgment that not all soil in the homes of NYC gardeners would originate in community gardens, we assumed that the percentage of household dust consisting of garden soil was 40%. As previously reported for vegetables from NYC and Buffalo gardens (McBride et al. 2014), NYC garden vegetable Pb levels were not associated with root-zone soil Pb over a wide range of soil Pb concentrations, but they were related to vegetable type: significant differences were reported between fruiting vegetables (e.g., tomatoes, peppers); leafy vegetables (e.g., lettuce, callaloo); root vegetables (e.g., carrots, beets) and herbs (e.g., thyme, basil) (Figure S1 & S2). For this reason, vegetable Pb was not considered to be a function of soil Pb in our assessments, and our measured distributions of NYC vegetable Pb levels by vegetable type (fruit, leafy, root, herb) (McBride et al. 2014) were used as input data (Figure S3). Based on our previously reported medians of estimates of the amount of adhered soil (using Al as a tracer) on NYC and Buffalo garden vegetables (McBride et al. 2014) , we approximated the percentage of Pb concentration due to adhered soil (Pbadh), and we carried the median of those percentages by vegetable type through our calculations:
Where the following were measured:
[Pb]soil = mg Pb / kg soil
[Al]veg = mg Al / kg vegetable (wet weight)
[Pb]veg = mg Pb / kg vegetable (wet weight)
[Al]soil = mg Al / kg soil
Because Al was not measured in soil from the specific gardens and beds where the vegetables were grown, we used the median soil Al concentration from our earlier study of 564 soil samples from 54 NYC community gardens (Mitchell et al., 2014) for [Al]soil. Because there was no correlation between root zone soil Pb and vegetable Pb, we assumed that adhered soil would have a Pb concentration equal to the median garden bed soil Pb concentration from the garden in which a vegetable was grown.
We had previously shown that Pb levels in NYC community garden chicken eggs (specifically, maximum Pb level in eggs and percentage of eggs with detectable levels of Pb within a henhouse) were strongly associated with soil Pb concentrations ranging up to 558 mg/kg (Spliethoff, Mitchell, Ribaudo, et al. 2014). For the current study, the association with henhouse maximum Pb was adapted to model henhouse average Pb (by considering the average ratio of the henhouse maximum to mean egg Pb) over the full range of non-bed soil concentrations measured in 54 community gardens (Figure S4).
Soil ingestion
Because there are no published soil ingestion rates specific to community gardeners, we based soil ingestion rates on recommended values from the US EPA Exposure Factors Handbook (EFH) (US EPA 2011a). For the deterministic assessment, we used recommended “central tendency” soil ingestion rates for adults and children and the “upper percentile” rate for children. There was no recommended adult soil dust ingestion upper percentile estimate, so we assumed the ratio of upper percentile to central tendency ingestion rates for adults would be the same as that for children (4:1), and we calculated an upper percentile rate for adult gardeners from the adult central tendency rate using this ratio. For the probabilistic assessment, we assigned lognormal distributions to soil ingestion rates, with arithmetic means equal to recommended “central tendency” ingestion rates (US EPA 2011a) and geometric standard deviations (Williams et al. 2013) based on the work of others (Özkaynak et al. 2011; Van Holderbeke et al. 2008).
Dust ingestion
Similar to our approach for soil ingestion, we used recommended central tendency dust ingestion rates for children and adults, and upper percentile ingestion rate for children (US EPA 2011a). For adults, we assumed the ratio of upper percentile to central tendency ingestion rates would be the same as that for children (in this case, 5:3), and we calculated an upper percentile rate from the central tendency rate for adults accordingly. For the probabilistic assessment, we used the same approach for dust ingestion rates as for soil ingestion rates: lognormal distributions with arithmetic means based on recommended central tendency rates, and standard deviations used by Williams et al. (2013).
Produce consumption
A written survey instrument was created to collect information about garden-grown produce consumption from NYC community gardeners (Spliethoff, Mitchell, Marquez-Bravo, et al. 2014). The NYSDOH and Cornell Institutional Review Boards for the Protection of Human Subjects reviewed the instrument and administration protocol and found it to be exempt from further review. The survey was administered in the fall after harvest was completed through a mailing to contact gardeners at 76 NYC community gardens from which we had sampled soil (Mitchell et al. 2014; unpublished data, 2014) and to volunteers at NYC gardening workshops.
The survey asked gardeners to indicate each crop (e.g., tomatoes) they had grown in the past 12 months and to estimate the mass (in lb or kg) of each crop they had harvested during that time. The survey also asked gardeners to estimate the fractions of the harvest consumed by themselves and by each of their household members and the fraction of the harvest that had not been consumed within the household. Gardeners were also asked to provide age and body weight for themselves and each member of their households who ate produce from the garden. In order to allow us to gauge the validity of estimated vegetable consumption using this method, we also assessed total garden produce consumption by asking, for both the growing- and non-growing seasons, how many servings of fruits and vegetables the gardeners ate daily and what fraction of all fruits and vegetables they consumed came from their own community gardens. Data on mass harvested, percent consumed and body weight were used to calculate annualized consumption of each crop on a per-kg basis for each gardener and household member. Gardeners also provided the names and addresses of their community gardens and estimated the fractions of their own consumption of each vegetable over the past 12 months that had come from other urban gardens/farms. Of 58 responses we received, 46 responses (with information on a total of 93 adults and 13 children in their households) were deemed sufficiently complete for consumption rate calculations.
For the deterministic assessment, we used the survey responses to calculate median and 95th percentile consumption rates for four crop types (fruiting, leafy, root, and herb) for gardeners (n=46), and adult (18+ years; n=47) and child (< 18 years; n=13) household members. For the probabilistic assessments, we fit lognormal distributions to consumption rates for each crop type for consumers only (Figure S5); to account for the percentage of people who reported not consuming a particular crop type, we assigned a consumption rate of zero for that crop type to a percentage of randomly selected trials in each simulation. Consumption rates for some crop types were correlated with one another (Spearman correlation coefficients ranged from −0.07 to 0.63 for gardeners, −0.05 to 0.71 for adult household members, and 0.50 to 1.0 for children). Incorporation of correlations did not significantly affect the results of a preliminary Monte Carlo analysis, so, for simplicity, consumption rates of different crop types were not linked with one another in the final analysis.
Egg Consumption
For the deterministic calculations, we used recommended median and 95th percentile body-weight-normalized consumption rates for home-produced eggs (US EPA 2011a). The probabilistic assessment used a lognormal distribution fit to the distribution of recommended consumption rates, and we assumed that 4% of the NYC community gardening population would consume home-raised eggs, based on a survey in which 4% of NYC community gardens reported having henhouses (Gittleman et al. 2010).
Other Exposure Parameters
We used recommended mean body weights (US EPA 2011a) associated with the median survey-reported ages of gardeners and adult household members and the youngest child in deterministic calculations. The probabilistic estimates used lognormal distributions of body weight based on nationally representative percentiles (US EPA 2011a). Exposure frequencies were determined in consideration of a survey of time spent in the garden and the length of the growing season in NYC. NYC community gardeners reported spending an average of 6.5 hours per week gardening with maximum of >17.3 hours per week (M. Gregory and L. Drinkwater, unpublished, 2011) throughout the growing season adjusted for extra time reported for spring garden startup, or the equivalent of about 2 days and >6 days, respectively, of soil ingestion at the approximately three hours outdoors per day upon which soil ingestion rate estimates are based (NYSDEC and NYSDOH 2006). We assumed gardeners, children, and adult household members ingested household dust that contained garden soil 7 days per week. We assumed these days of soil and dust ingestion took place over the entire 31 frost-free week growing season (Ameroso and Mazza 2013), and we calculated corresponding annualized exposure frequencies. Vegetable consumption rates were annualized, and daily chicken egg consumption was conservatively assumed to take place over the entire year. Exposure duration and averaging time were both assumed to be one year.
Results and Discussion
NYC community garden soil Pb levels (mg/kg) in growing beds (median=96, 95th percentile =532) were lower by about half compared to non-bed areas (median=181, 95th percentile =1000) (Table 1), likely due to gardening practices of bringing in clean soil and amendments for beds. As we had previously reported, a substantial fraction of soil samples exceeded health-based guidance values for Pb although the bed central tendency across all gardens was comparable to or lower than those from other urban garden studies (Mitchell et al. 2014). Vegetable Pb levels from NYC gardens were consistent with data previously reported for NYC and Buffalo, NY, with most exceeding market-basket concentrations and a few (mostly root vegetables) above health-based guidance values (McBride et al. 2014). Fruits were the vegetable type with the lowest concentrations, followed by leafy and root vegetables, while herbs had the highest concentrations (Figure S2). There was no association with soil Pb (R2 = 0.04; Figure S1); this was true even after stratifying by vegetable type (e.g., leafy R2 = 0.10), although leafy median Pb was somewhat higher (0.09 mg/kg vegetable) in soils with Pb>median than in soils with Pb<median (0.05 mg/kg vegetable) (data not shown). Our previous estimates of the amount of adhered soil by vegetable type (McBride et al. 2014) allowed us to estimate that adhered Pb was the dominant contributor to vegetable Pb for fruiting (80% of total Pb) and leafy vegetables (55%), but not root vegetables (18%) or herbs (35%). Adhered soil contributed the highest percentage of Pb to fruiting vegetables, likely because very little Pb is taken up via plant roots into fruiting vegetables. Thus, while fruits have lower Pb concentrations and less adhered soil on an absolute basis than other vegetable types, the Pb that is present in fruits is primarily due to adhered soil rather than uptake through roots. The low Pb adherence estimate (greater uptake estimate) for root vegetables may reflect in part the predominance of carrots among root vegetables grown by NYC gardeners (Spliethoff, Mitchell, Marquez-Bravo, et al. 2014). Carrots have been shown to accumulate Pb in inner xylem tissue at higher concentrations than the outer peel (Codling et al. 2015); because of this greater uptake compared to other root vegetables, a higher percentage of the Pb in carrots may be within the tissue rather than associated with adhered soil.
Table 1.
Pb concentrations in NYC community garden soils and produce (Mitchell et al., 2014; McBride et al., 2014; Spliethoff et al., 2013), and modeled concentrations in dust and eggs based on non-bed soil concentrations. Values in bold Italic font used for exposure calculations. Abbreviations: pctl = percentile; UCL = upper confidence limit; Geo. Mean = geometric mean; Geo. SD= geometric standard deviation.
| Pb Concentrationa (mg/kg) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Type |
Number of Samples |
Number with Detected Pb |
% of Pb from adhered soil |
Median | 75th pctl |
95th pctl |
Max | Meanb | 95% UCLc |
Geo. Mean |
Geo. SD |
| Bed soil | 508 | 508 | - | 96 | 173 | 562 | 1531 | 163 | 202 | 101 | 2.6 |
| Non-bed soil |
54 | 54 | - | 181 | 375 | 1000 | 2455 | 334 | 444 | 207 | 2.6 |
| Fruit | 67 | 35 | 80% | 0.013 | 0.018 | 0.056 | 0.21 | 0.016 | 0.023 | 0.011 | 2.1 |
| Leafy | 53 | 48 | 55% | 0.068 | 0.11 | 0.38 | 0.59 | 0.10 | 0.17 | 0.068 | 2.4 |
| Root | 23 | 20 | 18% | 0.12 | 0.22 | 0.90 | 1.95 | 0.29 | 0.64 | 0.11 | 3.5 |
| Herb | 16 | 16 | 35% | 0.25 | 0.40 | 1.49 | 2.10 | 0.44 | 0.72 | 0.29 | 2.3 |
| Dust | Modeled concentrationsd | 72 | 150 | 400 | 982 | 133 | 178 | 83 | 2.6 | ||
| Eggs | Modeled concentrations based on NYC garden egg analysise |
0.018 | 0.035 | 0.089 | 0.209 | 0.031 | 0.041 | 0.020 | 2.6 | ||
Produce and egg Pb concentrations are on a fresh-weight basis
Mean calculated with ProUCL (USEPA, 2013) using imputed values for non-detects assuming lognormal distribution.
Recommended UCL calculated with ProUCL (USEPA, 2013)
Dust Pb concentrations modeled as 0.4 × non-bed soil Pb concentrations
Egg Pb concentrations modeled as log(PbEgg [µg/kg]) = [0.95 log (non-bed soil Pb [mg/kg]) - 0.90] based on previous work by Spliethoff et al. (2014)
Our estimated dust Pb concentrations were dictated by concentrations in non-bed soils, which were likely to be the soil that was tracked into the home. These estimated dust Pb concentrations due to soil tracking had a median of 72 mg/kg Pb and a 95th percentile of over 400 mg/kg. Modeled chicken-egg Pb concentrations (median=18, 95th percentile=92 µg/kg) based on chicken contact with non-bed soils ranging up to 2455 mg/kg Pb were somewhat higher than those we had previously measured (median=10, 95th percentile =42 µg/kg) in eggs of chickens that had foraged in soil with Pb concentrations up to 558 mg/kg.
Our vegetable consumption survey results (Table 2) indicated that all NYC gardeners and child household members, and nearly all adult household members (89%), ate at least some vegetables from their community gardens. NYC gardeners had total vegetable consumption rates that were somewhat lower (mean=1308 mg/kg-day) than nationally representative consumption rates for home-produced vegetables (mean=2020 mg/kg-day) recommended by USEPA. This is not unexpected, considering that there is typically less gardening space available to an individual urban community gardener than is available for the majority of gardening households in the US, which are likely to be located in rural and suburban areas. Total vegetable consumption rates for NYC gardeners calculated using our primary method were correlated with those calculated using our secondary method for validation (Spearman r = 0.49, p = 0.0008). Taken together, these comparisons suggest that, despite the small number of respondents, our consumption estimates based on our survey results are plausible estimates of annualized vegetable consumption for community gardeners in NYC. NYC gardeners consumed significantly more of the produce from their gardens on a per-kg-body-weight basis than their adult (Mann-Whitney, p=0.002) and child (P=0.05) household members. It has been previously reported that members of gardening households eat more produce than those from non-gardening households (Alaimo et al. 2008), and it may be that the primary gardeners tend to eat more of their garden produce than other members of their households. It is not clear why our rates for children and adult household members are considerably lower (562 and 662 mg/kg-day, respectively) than recommended rates (2020 and 2070 mg/kg-day, respectively). Reasons may include that these rates were estimated using different methodologies and that USEPA recommended rates do not consider the distinction between primary gardeners and other household members when estimating consumption rates for home-produced vegetables.
Table 2.
Vegetable consumption rates (mg/kg-day) based on community gardener survey responses (estimates of harvest weight and % consumed by crop), compared to US EPA recommended consumption rates
| Children |
Adults |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (< 18 years) n = 13 |
Gardeners (survey respondents) n= 46 |
Household members (18+) n = 47 |
||||||||||||||
| Data Source |
Value | Total | Fruit | Leafy | Root | Herb | Total | Fruit | Leafy | Root | Herb | Total | Fruit | Leafy | Root | Herb |
| Survey, consumption from own garden | ||||||||||||||||
| All responses | ||||||||||||||||
| Median | 241 | 110 | 62 | 3 | 1 | 702 | 348 | 165 | 39 | 10 | 282 | 196 | 68 | 7 | 0 | |
| Mean | 562 | 340 | 151 | 53 | 18 | 1308 | 808 | 344 | 114 | 42 | 591 | 381 | 145 | 53 | 13 | |
| 95th Pctl | 1481 | 1024 | 440 | 233 | 103 | 3777 | 2678 | 923 | 470 | 186 | 1918 | 1483 | 596 | 297 | 57 | |
| Consumers only | ||||||||||||||||
| Geometric mean | 134 | 195 | 21 | 4 | 353 | 220 | 85 | 39 | 226 | 127 | 45 | 11 | ||||
| Geometric standard deviation |
4.9 | 2.4 | 8.6 | 7.8 | 4.8 | 3.2 | 3.1 | 3.4 | 3.2 | 2.8 | 4.0 | 4.2 | ||||
| Consumer only mean | 562 | 1308 | 662 | |||||||||||||
| Percent consuming | 100 % | 100 % | 62% | 62 % | 69 % | 100 % | 100 % | 83% | 67 % | 61% | 89% | 89 % | 68% | 55 % | 51 % | |
| Survey, consumption including other urban gardens |
||||||||||||||||
| Median | 603 | 329 | 62 | 3 | 1 | 1229 | 508 | 236 | 59 | 15 | 346 | 274 | 75 | 7 | 0 | |
| Mean | 628 | 393 | 161 | 53 | 21 | 1870 | 1045 | 526 | 249 | 50 | 794 | 485 | 212 | 83 | 14 | |
| 95th Pctl | 1593 | 1053 | 491 | 233 | 125 | 5469 | 3170 | 1562 | 748 | 197 | 2723 | 1490 | 828 | 466 | 76 | |
| US EPA (2011), recommended consumption, consumers only, homegrown vegetables (unadjusted)a | ||||||||||||||||
| Mean | 2020 | 2070 | 2070 | |||||||||||||
| 95th Pctl | 6160 | 6940 | 6940 | |||||||||||||
Table 13–10, values for child 6–11 years of age (n = 171), adults (gardeners and household members) 40 – 69 years (n = 700). US EPA defines “unadjusted” as “not adjusted to account for preparation or post-cooking losses”
Anecdotal field observations indicated that root vegetables were not commonly grown in NYC gardens, perhaps for cultural reasons, or because soil sometimes contained shards of brick and debris (Mitchell et al. 2014), which could inhibit root vegetable growth. For gardeners and adult household members, root and herb consumption was significantly lower (p<0.0001) than leafy vegetable consumption (collards were the most consumed leafy vegetable), which in turn was significantly lower (p<0.0001) than fruit consumption (tomatoes were the dominant contributor). Children’s consumption (n = 13) followed a similar trend, though fruit and leafy consumption rates did not differ significantly. The percentage of those consuming various crop types also followed a similar trend (fruit>leafy>root>herbs).
Gardeners also reported eating substantial amounts of produce from urban gardens other than their primary garden. Forty-three percent of gardeners’ median total consumption of NYC-grown vegetables (1229 mg/kg-day) was due to eating vegetables from gardens other than their primary community gardens. This related potential source of Pb exposure is noted, but it was not quantified nor added into this assessment because evaluating exposure due to all urban agriculture sources was not the intended focus of this study.
Based on our deterministic assessment, intake of Pb due to vegetable consumption (Table 3) was highest for gardeners (central tendency = 5.4 micrograms per day (µg/day)) followed by adult (1.7 µg/day) and child (0.49 µg/day) household members. Intake of adhered Pb tended to be about half of total Pb intake associated with vegetable consumption (e.g., central tendency of 46% for gardeners). Consumption of leafy vegetables was the largest contributor to central tendency vegetable Pb intake for all exposed individuals due to both relatively high Pb concentrations and high consumption rates. Because of low consumption rates, herbs, despite high Pb concentrations, were the smallest contributor to total central tendency vegetable Pb intake. High contact intakes based on the 95th percentile of consumption for total, fruiting, and leafy vegetables were approximately 10–20 times higher than central tendency intakes. High contact intakes due to root and herb consumption, however, were about 100 times higher than central tendency estimates due to highly skewed consumption distributions. The skewed consumption distribution for root vegetables was responsible for root vegetable consumption being the predominant source of high contact Pb intake for all individuals in the deterministic assessment. Fruit consumption was the lowest contributor to all high contact Pb intakes due to low concentrations and less skewing of the consumption distribution.
Table 3.
Estimated Pb intakes (µg/day) from community garden produce consumption - deterministic assessments
| Child | Adult |
|||||
|---|---|---|---|---|---|---|
| Gardener | Household member | |||||
| Consumption Scenario | Central Tendency |
High Contacta |
Central Tendency |
High Contacta | Central Tendency |
High Contacta |
| All produce - total intake | 0.49 | 10.2 | 5.4 | 52.2 | 1.7 | 29.3 |
| Produce - from adhered soil | 0.27 | 3.6 | 2.29 | 18.8 | 0.87 | 10.4 |
| Produce - from uptake into produce | 0.22 | 6.7 | 3.15 | 33.4 | 0.79 | 18.9 |
| Fruits (80% adhered soil; 20% uptake) | 0.08 | 0.7 | 0.63 | 4.8 | 0.35 | 2.7 |
| Leafy (55% adhered soil, 45% uptake) | 0.33 | 2.4 | 2.23 | 12.5 | 0.93 | 8.1 |
| Roots (18% adhered soil, 82% uptake) | 0.05 | 4.8 | 2.01 | 24.2 | 0.35 | 15.3 |
| Herbs (35% adhered soil, 65% uptake) | 0.02 | 2.4 | 0.57 | 10.7 | 0.03 | 3.3 |
High contact consumption = 95th percentile survey response
Total Pb intakes including exposures from ingestion of soil and dust and consumption of produce and eggs calculated using the deterministic method were considerably higher than those calculated probabilistically (Figure 1). Differences in the central tendencies of the deterministic and probabilistic intakes are due to the more conservative nature of the deterministic assessment, which follows US EPA recommendations (95th percentile UCL of the mean exposure medium concentrations and some default medium contact rates which were higher than the corresponding geometric means). Lower high-contact intake rates for the probabilistic assessment may also reflect a more reasonable representation of the compounded variability of the input parameter values. Finally, the probabilistic estimates considered that not all individuals consumed every type of produce and that consumption of garden-raised chicken eggs was relatively rare (only 4% of gardens were reported to have chickens (Gittleman et al. 2010)).
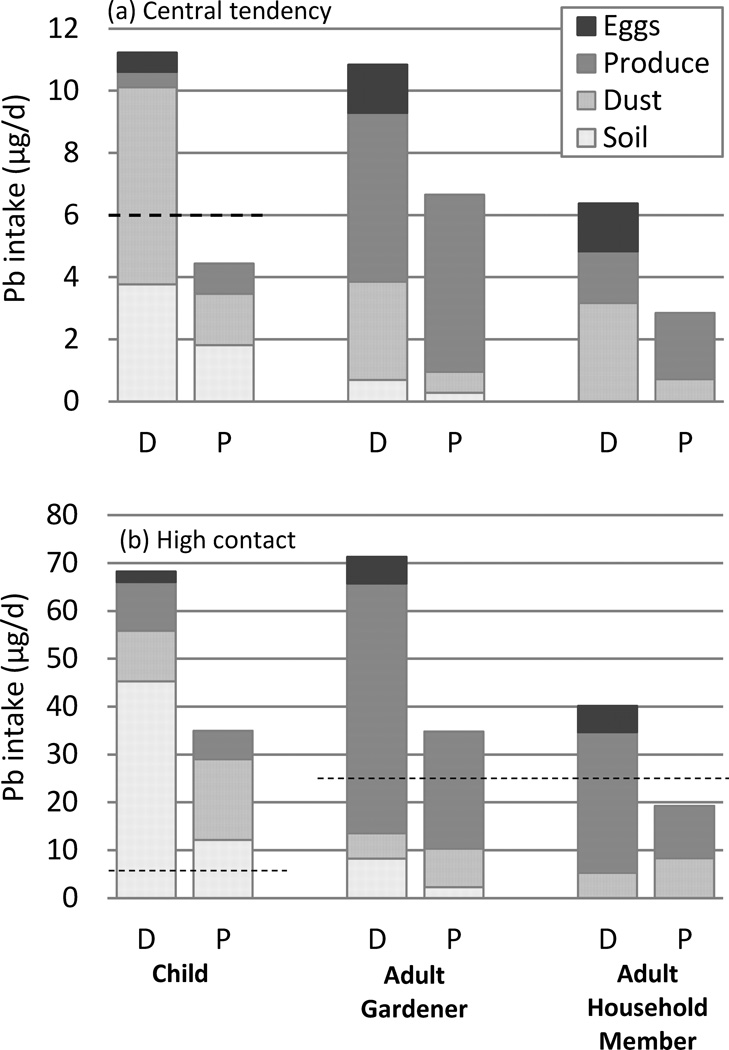
Figure 1.
Deterministic (D) and probabilistic (P) total lead intake estimates for children, adult gardeners, and adult household members under (a) central tendency and (b) high contact exposure scenarios. Dashed lines indicate provisional tolerable total intake (PTTI) levels of 6 µg/day for children and 25 µg/day for pregnant women (US FDA, 1992). Total height of probabilistic columns represents median (a) and 95th percentile (b) total lead intake estimates.
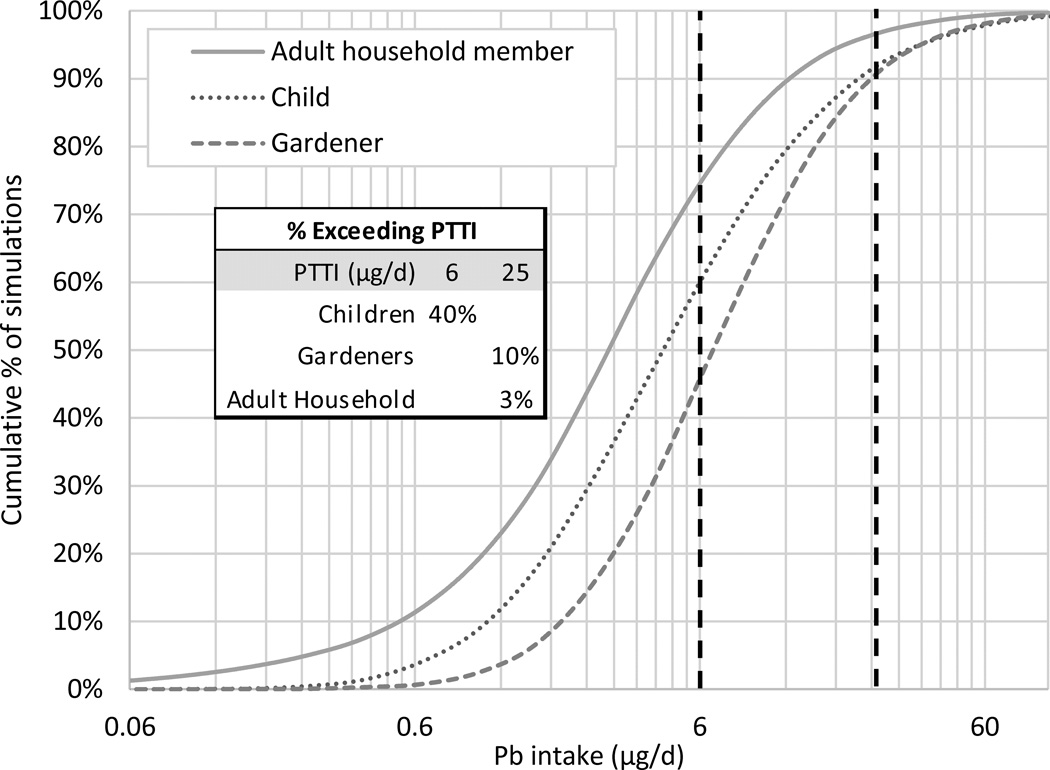
In general, children’s total Pb intake was similar to that of gardeners, while adult household members’ intakes were lower. Because a reference dose for Pb has never been determined by the US EPA and because the World Health Organization has withdrawn its reference values (WHO 2011), we compared our intake estimates with older but relatively conservative Provisional Tolerable Total Intakes (PTTIs) for oral exposures established by the US Food and Drug Administration: 6 µg/day for children, 75 µg/day for adults, and 25 µg/day for pregnant women (Carrington and Bolger 1992). Central tendency total intakes for gardeners and adult household members were well below PTTIs. The child’s probabilistically determined intake was also below the PTTI, but that calculated using the deterministic method exceeded the PTTI. All high contact intake estimates exceeded the PTTIs except the probabilistic estimate for household members. Cumulative distributions of simulated total Pb intakes from our probabilistic assessment (Figure 2) indicate that essentially no gardeners or adult household members exceeded the PTTI of 75 µg/day, and only 10% of gardeners and 3% of adult household members exceeded the PTTI for pregnant women of 25 µg/day. However, our simulations suggest that 40% of children visitors could exceed the PTTI for children of 6 µg/day.
Figure 2.
Cumulative distributions of simulated total Pb intakes from probabilistic exposure assessment for adult household members, children, and gardeners. Dark vertical lines indicate PTTIs of 6 µg/d for children and 25 µg/d for pregnant women (US FDA, 1992).
The exposure pathways that resulted in by far the highest Pb intakes for children were soil and dust ingestion (Figure 1). Intakes via these pathways exceeded those of adults because children are in contact with the higher-Pb concentration soils between the beds, and because they have higher incidental dust and soil ingestion rates than adults. In contrast, gardeners’ exposure was dominated by produce consumption. For adult household members, produce consumption was the biggest contributor for the high contact scenarios and the probabilistic central tendency intake; dust ingestion was more significant only for the deterministic central tendency scenario.
A sensitivity analysis (Figure S6) confirmed the importance of vegetable consumption rates for gardeners and those related to the soil and dust ingestion for children. Besides the non-bed soil Pb concentration for children (Spearman rho=0.59), no one parameter was particularly influential over the outcome. The importance of the dust ingestion rate in this assessment, not only for children, but also for gardeners and adult household members apparently reflects the high variability of this rate (geometric standard deviation = 4.3) (Özkaynak et al. 2011; Williams et al. 2013). The high degree of influence the dust ingestion distribution has over Pb intake suggests that reducing dust ingestion rates (e.g., by good housekeeping practices and frequent handwashing) could be important in reducing exposure via garden soil tracked into the home.
In summary, despite some exceedances of Pb guidance values for soil and produce (McBride et al. 2014; Mitchell et al. 2014), the majority of estimated intakes were below the relevant PTTIs. Considering the many benefits of gardening and local food production for individual health and community well-being (Alaimo et al. 2010; Groenewegen et al. 2006; Leake et al. 2009; Van Den Berg and Custers 2011), this outcome suggests that urban community gardens and gardening do not pose unacceptable risks related to Pb exposure for typical gardeners and their household members. On the contrary, the benefits of gardening are likely to far outweigh the risks. However, the fractions of gardeners (10%) and child garden visitors (40%) that exceed PTTIs are far from negligible. Furthermore, the PTTIs were derived at a time when blood Pb levels over 10 µg/dL were considered to be elevated; however, the current reference level for children is 5 µg/dL (CDC 2012), and any lead exposure can be considered potentially harmful (WHO 2011). In addition, many gardens are located in neighborhoods vulnerable to additional Pb exposure (Mitchell et al. 2014). Therefore, further exposure mitigation, particularly for more contaminated gardens and more exposed individuals, is warranted.
The importance of the vegetable consumption Pb exposure pathway for gardeners and household members suggests that exposure mitigation for these populations should focus on reducing the lead content of the variety of garden vegetables grown and eaten. However, our study found a lack of correlation between total soil Pb and vegetable Pb concentrations, as have other studies (Chojnacka et al. 2005; Hough et al. 2004; Murray et al. 2011; Nabulo et al. 2012; Warming et al. 2015), and therefore does not provide evidence that reducing bed soil Pb levels alone by soil replacement or amendment will measurably reduce vegetable Pb levels across different vegetables and gardens. Numerous factors other than soil Pb concentration may influence vegetable Pb levels, including soil Pb speciation and bioaccessibility, soil organic matter and pH, vegetable type and variety, adjacent and nearby soil Pb concentrations, local sources of atmospheric deposition, and gardening and food preparation practices (Attanayake et al. 2014; Dalenberg and Driel 1990; Sauvé et al. 1998, 2000). We are not controlling for those factors, and therefore may not be able to discern a reduction in vegetable Pb that could be associated with lower bed soil Pb as has been shown for single vegetable varieties in some more controlled studies (McBride et al. 2015). However, data presented here do strongly suggest that healthy practices focused on growing fruiting vegetables rather than leafy and root vegetables would be effective in reducing exposure via this pathway across a variety of conditions. Our adhered soil results also suggest that washing and /or peeling vegetables may be effective in lowering exposure. These practices could be particularly important for sensitive subgroups of gardeners, such as pregnant women. In contrast to the lack of association between soil and vegetable Pb, the strong association we found between soil Pb and chicken egg Pb (Spliethoff, Mitchell, Ribaudo, et al. 2014) suggests that reducing chickens’ contact with high-Pb soil would be an effective means of reducing Pb in eggs, although the benefit in terms of reduced human Pb intake may not be large.
The importance of the soil and dust ingestion Pb exposure pathways for children suggests that exposure mitigation efforts should focus on reducing children’s direct contact with soil and the tracking home of soil. The common practice of bringing in clean soil and amendments for beds may have limited benefits in reducing children’s exposure via either of these pathways if soil between the beds, rather than garden bed soil, is the primary point of children’s direct contact and the primary source of soil tracked into the home. While improving soil quality in garden beds is one important way to reduce Pb exposure, particularly in the case in which more contaminated beds are a source of contamination for less contaminated adjacent beds and pathways, our study suggests that practices such as covering higher-Pb concentration soil between beds (with geotextile, grass cover, mulch, etc.) to reduce direct exposure to and tracking home of those soils could be more beneficial for children. A number of studies have shown that soil remediation in residential yards or neighborhoods can result in reduced children’s blood Pb levels (Aschengrau et al. 1994; Maisonet et al. 1997; Mielke and Reagan 1998; Sheldrake and Stifelman 2003; Weitzman et al. 1993; Zahran et al. 2010), and it seems reasonable the same would be true for the more highly contaminated gardens, especially considering that, in more recent years, background blood Pb levels have dropped due to reduction or elimination of Pb from other sources (such as lead-soldered food cans, leaded gasoline and lead paint). Overall, our results suggest that, while healthy gardening practices to reduce Pb concentrations in raised beds or other growing areas (e.g., importing clean soil and amendments) are important and should be encouraged, these practices need to be supplemented by other strategies to reduce exposures for this population.
Limitations and Uncertainty
There are a number of uncertainties in our assessment. The gardens from which we collected our data were not all the same gardens for every parameter (soil, vegetables, chicken eggs, survey results). We are assuming that much of the data collected is representative of the entire group of NYC gardens and gardeners from which we collected data, and that may not be the case. However, our vegetable data from 7 gardens was collected over the same range of bed soil Pb concentrations we found in our soil study of 54 gardens, so conclusions about the lack of association of vegetable Pb with root zone Pb should be relevant to all of the 54 soil study gardens. We analyzed bulk soil when finer fractions may have been more relevant for estimating soil ingestion exposure, and we used the USEPA method for total Pb when other measures (e.g., based on ammonium nitrate or Mehlich 3 extractions) may have assessed a more phytoavailable fraction that might have correlated better with vegetable concentrations (Pinto et al. 2015). Because no chicken eggs were collected from gardens with over 600 mg/kg soil Pb, we extrapolated the association we found to higher soil concentrations to improve our estimates of Pb intake due to chicken egg consumption. For our probabilistic assessment, we assumed that 4% of gardeners eat garden-raised eggs based on a survey indicating that 4% of NYC community gardens have chickens. This is likely an overestimate, because not all gardeners within a garden that has chickens will eat the eggs. We did not collect site-specific consumption data for garden-raised chicken eggs. Our use of nationally representative rates and our assumption that consumption took place every day of the year may have biased (likely higher) our intake estimates associated with this pathway, although this bias would not have a large effect on total gardening exposure estimates since the pathway is a relatively small contributor to total exposure. Our vegetable consumption survey was very small and relied on recall. Although our consumption estimates seemed reasonable, particularly for gardeners, consumption rates based on a larger nationally representative sample of home gardening households (US EPA 2011a) are higher, and using those estimates would have resulted in higher Pb intakes. However, our primary method for calculating vegetable consumption was based on estimates of the mass harvested and the fraction of the harvest consumed (fresh and preserved). This accounts for variable time frames of availability of different crops, in contrast to other methods of assessing homegrown vegetable consumption, for which this is a limitation.
Our estimate of the contribution of adhered soil Pb based on the median for each vegetable type is highly uncertain. Estimates for individual vegetable samples varied considerably, with some negative values and some values exceeding 100%. Depending upon the assumption regarding the source of the adhered soil and the corresponding soil Pb concentration (root zone, garden median, or citywide garden average) used in the calculation, the estimated contribution of adhered soil also varied significantly, particularly for our limited number of herb samples. However, regardless of the assumption, the percent of Pb due to soil adherence was always substantial and always higher in fruits than in leafy vegetables, and lowest in root vegetables.
We had relatively few non-bed soil samples (1 from each of 54 gardens), and we did not consider the relative size non-bed areas in our study gardens nor the extent to which they were covered with materials that might reduce contact with and tracking of soil. Adequate cover with grass, paving stones, or frequently renewed mulch may be reducing direct contact and tracking in some NYC gardens. We collected no data on NYC community gardeners’ dust Pb levels; more research is need to better assess the importance of this pathway.
Our decision to model exposure for a 6-year-old resulted in somewhat higher estimates of intake for the vegetable and chicken-egg consumption pathways (due to differences in body weight) than if we had chosen a younger child (e.g., 2–3 year old) who might be more vulnerable to Pb exposure. However, scenarios involving a 2-year-old in the garden for 3 hours per day, from 2–6 days per week, seemed less reasonable. Further, our consumption rates for children were based on survey results for older children (median=10 years old, range: 3–18), and older children eat less on a per-kg-body-weight basis than younger children, so assessing intake for a younger child would have introduced more error into our intake estimates. The time gardeners spent in the garden was based on a separate collaborative survey of 66 gardeners from many of the same gardens (unpublished data, 2011).
Despite the uncertainties associated with some input data and associated effects on intake estimates, it is likely that our conclusions regarding the most effective exposure mitigation strategies would remain the same. The extent to which our results may be applicable to other urban community gardening populations in the US and elsewhere is not clear. Soil Pb levels in NYC garden beds tended to be similar to or lower than those in other urban gardens (Mitchell et al. 2014), so exposure in other large cities could be similar or greater. Our deterministic results were extrapolated to higher soil Pb concentrations assuming that egg Pb, but not vegetable Pb, is a function of soil Pb (Table S2). For example, at non-bed concentrations of 1000 and 2000 mg/kg, a child’s central tendency Pb intake would be 25 and 49 µg/day, respectively, and a gardener’s intake would be 17 and 27 µg/day, respectively. With the same non-bed Pb concentration (444 mg/kg), even with a garden bed Pb concentration as high as 2000 mg/kg, a gardener’s intake (17 µg/day) would be well below the PTTI for pregnant women. However, implicit in this extrapolation is the assumption that vegetable Pb concentrations would not be associated with soil Pb even at these higher soil concentrations, and we did not evaluate that in this study.
The distinction we made between bed and non-bed soils may be less relevant for other urban areas where raised beds may be less prevalent. For gardens where there are no raised beds, soil from paths and beds may mix, and strategies like bringing in clean soil for beds may improve non-bed areas and reduce tracking and children’s contact with high-Pb soil. The assumption that children are in contact primarily with non-bed soils may not be true in some gardens; children who play or work in garden beds may be more exposed to garden bed soils.
There are few studies reporting chicken-egg Pb levels in relation to soil Pb levels, but one study from rural parts of Belgium reported egg Pb levels considerably higher than ours with lower Pb soil contaminated by industrial sources (Waegeneers et al. 2009). More research is needed to determine whether our findings are relevant for urban chicken keepers throughout the US and whether soil Pb bioaccessibility is an important factor that can influence egg Pb levels. Finally, we did not consider potential variability in bioavailability of Pb in soil (e.g., based on speciation) and potentially large bioavailability differences between soil and food (vegetables and eggs) (James et al. 1985). While consideration of this variability and differences is not necessary for a study of contaminant intake and comparison with tolerable intake levels, these differences would have to be better understood in order to model absorbed dose and blood Pb impacts.
In summary, central tendency estimates of Pb intakes for urban community gardeners and adult and child household members were generally below provisional total tolerable intake (PTTI) levels, except for deterministic central tendency intakes for children. High contact intakes generally exceeded the PTTIs, and probabilistic modeling indicated that approximately 40% of visiting children and 10% of gardeners would exceed the PTTI. Children visiting the garden had exposures driven by higher soil and dust ingestion rates and exposure to soil between the beds. Adult gardeners’ Pb intakes were comparable to children’s (in µg/d) but were dominated by vegetable consumption. Neither of these exposures would likely be significantly reduced through further implementation of the common exposure mitigation strategy of importing clean soil for garden beds. Household members, who did not visit and ate less produce from the garden, had the lowest exposures. Consumption of garden-raised chicken eggs generally accounted for less exposure than any of the other pathways.
Our results suggest that, while reducing Pb concentrations in garden bed soils is an important exposure reduction strategy, it should not be the only exposure reduction strategy for urban community gardeners. Healthy gardening practices for reducing Pb exposure in urban community gardens should focus more on encouraging cultivation of lower-Pb vegetables (i.e., fruits) for adult gardeners and covering or replacing all higher-Pb soils – even in non-growing areas of the garden – to reduce young children’s exposure.
Supplementary Material
Acknowledgments
Funding for this research was provided by the National Institute of Environmental Health Sciences, Award Number R21ES017921. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIEHS or the National Institutes of Health. We greatly appreciate the contributions of Megan Gregory, other Healthy Soils, Healthy Communities collaborators, and NYC community gardeners.
References
- Abbasi AM, Iqbal J, Khan MA, Shah MH. Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicology and Environmental Safety. 2013;92:237–244. doi: 10.1016/j.ecoenv.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Alaimo K, Packnett E, Miles RA, Kruger DJ. Fruit and Vegetable Intake among Urban Community Gardeners. Journal of Nutrition Education and Behavior. 2008;40(2):94–101. doi: 10.1016/j.jneb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Alaimo K, Reischl TM, Allen JO. Community gardening, neighborhood meetings, and social capital. Journal of Community Psychology. 2010;38(4):497–514. [Google Scholar]
- Allott RW, Kelly M, Hewitt CN. Behavior of urban dust contaminated by Chernobyl fallout: environmental half-lives and transfer coefficients. Environmental Science & Technology. 1992;26(11):2142–2147. [Google Scholar]
- Ameroso LM, Mazza CP. Gardening Resources. Cornell University; 2013. Jun 14, [Accessed 18 July 2014]. Children, Gardens, and Lead. http://www.gardening.cornell.edu/factsheets/misc/cgandlead.html. [Google Scholar]
- Aschengrau A, Beiser A, Bellinger D, Copenhafer D, Weitzman M. The Impact of Soil Lead Abatement on Urban Children′s Blood Lead Levels: Phase II Results from the Boston Lead-In-Soil Demonstration Project. Environmental Research. 1994;67(2):125–148. doi: 10.1006/enrs.1994.1069. [DOI] [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Lead. Atlanta, GA: Agency for Toxic Substances and Disease Registry, Public Health Service, US Department of Health and Human Services; 2007. [Google Scholar]
- Attanayake CP, Hettiarachchi GM, Harms A, Presley D, Martin S, Pierzynski GM. Field Evaluations on Soil Plant Transfer of Lead from an Urban Garden Soil. Journal of Environment Quality. 2014;43(2):475. doi: 10.2134/jeq2013.07.0273. [DOI] [PubMed] [Google Scholar]
- Bacigalupo C, Hale B. Soil-Plant Transfer Factors for Garden Produce from Contaminated Soils: Site Specific versus Generic Estimates for As and Pb. Human and Ecological Risk Assessment. 2011;17(2):394–413. [Google Scholar]
- Bacigalupo C, Hale B. Human health risks of Pb and As exposure via consumption of home garden vegetables and incidental soil and dust ingestion: A probabilistic screening tool. Science of The Total Environment. 2012;423:27–38. doi: 10.1016/j.scitotenv.2012.01.057. [DOI] [PubMed] [Google Scholar]
- Bassuk NL. Reducing lead uptake in lettuce. HortScience. 1986;21(4):993–995. [Google Scholar]
- Beam A, Garber L, Sakugawa J, Kopral C. Salmonella awareness and related management practices in U.S. urban backyard chicken flocks. Preventive Veterinary Medicine. 2013 doi: 10.1016/j.prevetmed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Beccaloni E, Vanni F, Beccaloni M, Carere M. Concentrations of arsenic, cadmium, lead and zinc in homegrown vegetables and fruits: Estimated intake by population in an industrialized area of Sardinia, Italy. Microchemical Journal. 2013;107:190–195. [Google Scholar]
- Bugdalski L, Lemke LD, McElmurry SP. Spatial Variation of Soil Lead in an Urban Community Garden: Implications for Risk-Based Sampling. Risk Analysis. 2014;34(1):17–27. doi: 10.1111/risa.12053. [DOI] [PubMed] [Google Scholar]
- Carrington CD, Bolger PM. An assessment of the hazards of lead in food. Regulatory toxicology and pharmacology. 1992;16(3):265–272. doi: 10.1016/0273-2300(92)90006-u. [DOI] [PubMed] [Google Scholar]
- CDC. CDC Response to Advisory Committee on Childhood Lead Poisoning Prevention Recommendations in “Low Level Lead Exposure Harms Children: A Renewed Call of Primary Prevention.”. US Department of Health and Human Services, CDC; 2012. [Accessed 25 January 2013]. http://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf. [Google Scholar]
- Chaney RL, Mielke HW. Standards for Soil Lead Limitations in the United States. Trace Substances in Environmental Health. 1986:20. [Google Scholar]
- Chaney RL, Sterrett SB, Mielke HW. Proceedings of the symposium on heavy metals in urban gardens. Washington: Agricultural Experiment Station, University of the District of Columbia; 1984. [Accessed 24 July 2013]. The potential for heavy metal exposure from urban gardens and soils; pp. 37–84. http://www.indytilth.org/Links/Chaney_Exposure.pdf. [Google Scholar]
- Chojnacka K, Chojnacki A, Górecka H, Górecki H. Bioavailability of heavy metals from polluted soils to plants. The Science of the Total Environment. 2005;337(1–3):175–182. doi: 10.1016/j.scitotenv.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Clarke LW, Jenerette GD, Bain DJ. Urban legacies and soil management affect the concentration and speciation of trace metals in Los Angeles community garden soils. Environmental Pollution. 2015;197:1–12. doi: 10.1016/j.envpol.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Clark HF, Hausladen DM, Brabander DJ. Urban gardens: Lead exposure, recontamination mechanisms, and implications for remediation design. Environmental Research. 2008;107(3):312–319. doi: 10.1016/j.envres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Codling EE, Chaney RL, Green CE. Accumulation of Lead and Arsenic by Carrots Grown on Lead-Arsenate Contaminated Orchard Soils. Journal of Plant Nutrition. 2015;38(4):509–525. [Google Scholar]
- Connecticut Department of Health. What You Need to Know about Growing and Eating Fruits and Vegetables Safely. [Accessed 17 March 2015];Connecticut Department of Health. 2014 Jun; http://www.ct.gov/dph/lib/dph/environmental_health/eoha/pdf/safe_gardening_fact_sheet_2014rev.pdf.
- Dalenberg JW, Driel Wvan. Contribution of atmospheric deposition to heavy-metal concentrations in field crops. Netherlands Journal of Agricultural Science. 1990;38(3A):369–379. [Google Scholar]
- Datko-Williams L, Wilkie A, Richmond-Bryant J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Science of The Total Environment. 2014;468–469:854–863. doi: 10.1016/j.scitotenv.2013.08.089. [DOI] [PubMed] [Google Scholar]
- Finster ME, Gray KA, Binns HJ. Lead levels of edibles grown in contaminated residential soils: a field survey. Science of The Total Environment. 2004;320(2–3):245–257. doi: 10.1016/j.scitotenv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Gittleman M, Librizzi L, Stone E. Community Garden Survey New York City Results 2009/2010. GrowNYC; 2010. [Accessed 25 January 2013]. http://www.greenthumbnyc.org/pdf/GrowNYC_community_garden_report.pdf. [Google Scholar]
- Groenewegen PP, van den Berg AE, de Vries S, Verheij RA. Vitamin G: effects of green space on health, well-being, and social safety. BMC public health. 2006;6:149. doi: 10.1186/1471-2458-6-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough RL, Breward N, Young SD, Crout NMJ, Tye AM, Moir AM, Thornton I. Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environmental health perspectives. 2004;112(2):215–221. doi: 10.1289/ehp.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James HM, Hilburn ME, Blair JA. Effects of Meals and Meal Times on Uptake of Lead from the Gastrointestinal Tract in Humans. Human & Experimental Toxicology. 1985;4(4):401–407. doi: 10.1177/096032718500400406. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Jr, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentration <10 µg/dL and child intelligence at 6 years of age. Environmental Health Perspectives. 2008;116(2):243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake JR, Adam-Bradford A, Rigby JE. Health benefits of “grow your own” food in urban areas: implications for contaminated land risk assessment and risk management? Environmental Health. 2009;8(Suppl 1):S6. doi: 10.1186/1476-069X-8-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonet M, Bove FJ, Kaye WE. A case-control study to determine risk factors for elevated blood lead levels in children, Idaho. Toxicology and industrial health. 1997;13(1):67–72. doi: 10.1177/074823379701300106. [DOI] [PubMed] [Google Scholar]
- McBride MB. Arsenic and lead uptake by vegetable crops grown on historically contaminated orchard soils. Applied and Environmental Soil Science. 2013 doi: 10.1155/2013/283472. (ID 283742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MB, Shayler HA, Russell-Anelli JM, Spliethoff HM, Marquez-Bravo LG. Arsenic and Lead Uptake by Vegetable Crops Grown on an Old Orchard Site Amended with Compost. Water, Air, & Soil Pollution. 2015;226(8) doi: 10.1007/s11270-015-2529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MB, Shayler HA, Spliethoff HM, Mitchell RG, Marquez-Bravo LG, Ferenz GS, et al. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environmental Pollution. 2014;194:254–261. doi: 10.1016/j.envpol.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke HW, Anderson JC, Berry KJ, Mielke PW, Chaney RL, Leech M. Lead concentrations in inner-city soils as a factor in the child lead problem. American Journal of Public Health. 1983;73(12):1366–1369. doi: 10.2105/ajph.73.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke HW, Blake B, Burroughs S, Hassinger N. Urban lead levels in Minneapolis: the case of the Hmong children. Environmental Research. 1984;34(1):64–76. doi: 10.1016/0013-9351(84)90076-8. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Reagan PL. Soil is an important pathway of human lead exposure. Environmental health perspectives. 1998;106(Suppl 1):217–229. doi: 10.1289/ehp.98106s1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda ML, Kim D, Galeano MAO, Paul CJ, Hull AP, Morgan SP. The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environmental Health Perspectives. 2007;115(8):1242–1247. doi: 10.1289/ehp.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RG, Spliethoff HM, Ribaudo LN, Lopp DM, Shayler HA, Marquez-Bravo LG, et al. Lead (Pb) and other metals in New York City community garden soils: Factors influencing contaminant distributions. Environmental Pollution. 2014;187:162–169. doi: 10.1016/j.envpol.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir AM, Thornton I. Lead and cadmium in urban allotment and garden soils and vegetables in the United Kingdom. Environmental Geochemistry and Health. 1989;11(3–4):113–119. doi: 10.1007/BF01758660. [DOI] [PubMed] [Google Scholar]
- Murray H, Pinchin TA, Macfie SM. Compost application affects metal uptake in plants grown in urban garden soils and potential human health risk. Journal of Soils and Sediments. 2011;11(5):815–829. [Google Scholar]
- Nabulo G, Black CR, Craigon J, Young SD. Does consumption of leafy vegetables grown in peri-urban agriculture pose a risk to human health? Environmental Pollution. 2012;162:389–398. doi: 10.1016/j.envpol.2011.11.040. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. NTP Monograph: Health Effects of Low-Level Lead. NTP monograph. 2012;(1) i–148. [PubMed] [Google Scholar]
- NGA. Garden to Table: A 5-Year Look at Food Gardening in America. South Burlington, VT: National Gardening Association; 2014. http://www.hagstromreport.com/assets/2014/2014_0402_NGA-Garden-to-Table.pdf. [Google Scholar]
- NYSDEC, & NYSDOH. New York State Brownfield Cleanup Program Development of Soil Cleanup Objectives Technical Support Document. Appendix D. New York State Department of Environmental Conservation and New York State Department of Health; 2006. [Accessed 15 April 2013]. http://www.dec.ny.gov/docs/remediation_hudson_pdf/appendixde.pdf. [Google Scholar]
- Özkaynak H, Xue J, Zartarian VG, Glen G, Smith L. Modeled Estimates of Soil and Dust Ingestion Rates for Children. Risk Analysis. 2011;31(4):592–608. doi: 10.1111/j.1539-6924.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- Pinto E, Almeida AA, Ferreira IMPLVO. Assessment of metal(loid)s phytoavailability in intensive agricultural soils by the application of single extractions to rhizosphere soil. Ecotoxicology and Environmental Safety. 2015;113:418–424. doi: 10.1016/j.ecoenv.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Pollock SL, Stephen C, Skuridina N, Kosatsky T. Raising chickens in city backyards: The public health role. Journal of Community Health. 2012;37(3):734–742. doi: 10.1007/s10900-011-9504-1. [DOI] [PubMed] [Google Scholar]
- Sauvé S, Hendershot W, Allen HE. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environmental Science and Technology. 2000;34(7):1125–1131. [Google Scholar]
- Sauvé S, McBride M, Hendershot W. Soil Solution Speciation of Lead(II): Effects of Organic Matter and pH. Soil Science Society of America Journal. 1998;62(3):618–621. [Google Scholar]
- Sheldrake S, Stifelman M. A case study of lead contamination cleanup effectiveness at Bunker Hill. Science of The Total Environment. 2003;303(1–2):105–123. doi: 10.1016/s0048-9697(02)00354-6. [DOI] [PubMed] [Google Scholar]
- Sipter E, Rózsa E, Gruiz K, Tátrai E, Morvai V. Site-specific risk assessment in contaminated vegetable gardens. Chemosphere. 2008;71(7):1301–1307. doi: 10.1016/j.chemosphere.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Spliethoff HM, Mitchell RG, Marquez-Bravo LG, Shayler HA, McBride MB. Homegrown produce consumption among urban community gardeners and household members; Cincinnati, OH. jPresented at the 24th annual meeting of the International Society of Exposure Science.2014. [Google Scholar]
- Spliethoff HM, Mitchell RG, Ribaudo LN, Taylor O, Shayler HA, Greene V, Oglesby D. Lead in New York City community garden chicken eggs: influential factors and health implications. Environmental Geochemistry and Health. 2014;36(4):633–649. doi: 10.1007/s10653-013-9586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilwell DE, Rathier TM, Musante CL, Ranciato JF. Lead and Other Heavy Metals in Community Garden Soils in Connecticut (No. Bulletin 1019) The Connecticut Agricultural Experiment Station. 2008 http://www.ct.gov/caes/lib/caes/documents/publications/bulletins/b1019.pdf.
- Szolnoki Z, Farsang A, Puskás I. Cumulative impacts of human activities on urban garden soils: Origin and accumulation of metals. Environmental Pollution. 2013;177:106–115. doi: 10.1016/j.envpol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Trowbridge PR, Burmaster DE. A parametric distribution for the fraction of outdoor soil in indoor dust. Journal of Soil Contamination. 1997;6(2):161–168. [Google Scholar]
- US EPA. Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A) (No. EPA/540/1-89/002) Washington, D.C. 20450: Office of Emergency and Remedial Response U.S. Environmental Protection Agency; 1989. [Google Scholar]
- US EPA. Risk Assessment Guidance for Superfund Volume 1: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment) Washington, DC: Office of Emergency and Remedial Response; 2004. [Google Scholar]
- US EPA. Integrated Exposure-Uptake-Biokinetic Model for lead in children, Windows 32-bit version (IEUBKwin v1.1 build 11) Washington, DC: US Environmental Protection Agency; 2010. [Google Scholar]
- US EPA. Exposure Factors Handbook: 2011 Edition (No. EPA/600/R-09/052F) Washington, DC: National Center for Environmental Assessment, US Environmental Protection Agency; 2011a. [Accessed 27 May 2013]. http://www.epa.gov/ncea/efh/report.html. [Google Scholar]
- US EPA. [Accessed 19 March 2015];Brownfields and Urban Agriculture: Interim Guidelines for Safe Gardening Practices - bf_urban_ag.pdf. 2011b http://www.epa.gov/brownfields/urbanag/pdf/bf_urban_ag.pdf.
- Van Den Berg AE, Custers MHG. Gardening promotes neuroendocrine and affective restoration from stress. Journal of Health Psychology. 2011;16(1):3–11. doi: 10.1177/1359105310365577. [DOI] [PubMed] [Google Scholar]
- Van Holderbeke M, Cornelis C, Bierkens J, Torfs R. Review of the soil ingestion pathway in human exposure assessment. Flanders, Belgium: VITO/RIVM; 2008. [Google Scholar]
- Waegeneers N, Hoenig M, Goeyens L, De Temmerman L. Trace elements in home-produced eggs in Belgium: Levels and spatiotemporal distribution. Science of the Total Environment. 2009;407(15):4397–4402. doi: 10.1016/j.scitotenv.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Warming M, Hansen MG, Holm PE, Magid J, Hansen TH, Trapp S. Does intake of trace elements through urban gardening in Copenhagen pose a risk to human health? Environmental Pollution. 2015;202:17–23. doi: 10.1016/j.envpol.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Aschengrau A, Bellinger D, Jones R, Hamlin J, Beiser A. Lead-contaminated soil abatement and urban children’s blood lead levels. JAMA. 1993;269(13):1647–1654. [PubMed] [Google Scholar]
- WHO. Evaluation of certain food additives and contaminants (Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives) WHO Technical Report Series, No. 960. 2011 [Google Scholar]
- Wieland B, Leith A, Rosen C. Urban Gardens and Soil Contaminants. [Accessed 17 March 2015];University of Minnesota Extension & Hennepin County Department of Environmental Services. 2010 http://www.misa.umn.edu/prod/groups/cfans/@pub/@cfans/@misa/documents/asset/cfans_asset_287228.pdf.
- Williams ES, Mahler BJ, Van Metre PC. Cancer Risk from Incidental Ingestion Exposures to PAHs Associated with Coal-Tar-Sealed Pavement. Environmental Science & Technology. 2013;47(2):1101–1109. doi: 10.1021/es303371t. [DOI] [PubMed] [Google Scholar]
- Zahran S, Mielke HW, Gonzales CR, Powell ET, Weiler S. New Orleans before and after Hurricanes Katrina/Rita: a quasi-experiment of the association between soil lead and children’s blood lead. Environmental Science & Technology. 2010;44(12):4433–4440. doi: 10.1021/es100572s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.