Abstract
Cannabis misuse accounts for nearly all of the substance abuse treatment admissions among youth in the United States. Most youth do not experience sustained benefit from existing psychosocial treatments, however, and medication development research for treating adolescent cannabis misuse is almost nonexistent. We conducted a double-blind, placebo-controlled, pilot study to test the potential efficacy of topiramate plus motivational enhancement therapy (MET) for treating cannabis use among adolescents. Sixty-six heavy cannabis users, ages 15 to 24 years, were randomized to one of two 6-week treatment conditions: topiramate plus MET or placebo plus MET. Topiramate was titrated over 4-weeks then stabilized at 200 mg/day for two weeks. MET was delivered biweekly for a total of 3 sessions. Only 48% of youths randomized to topiramate completed the 6-week trial (n=19), compared to 77% of youths in the placebo condition (n=20). Adverse medication side effects were the most common reason for withdrawal among participants in the topiramate group. Latent growth models showed that topiramate was superior to placebo for reducing the number of grams smoked per use day, but it did not improve abstinence rates. The same pattern of results was found when values for missing outcomes were imputed. We show that topiramate combined with MET demonstrated efficacy for reducing how much cannabis adolescents smoked when they used but did not affect abstinence rates. The magnitude of this effect was modest, however, and topiramate was poorly tolerated by youths, which calls into question the clinical importance of these findings.
Keywords: adolescents, cannabis use disorder, motivational enhancement therapy, topiramate
Introduction
Cannabis is the most widely used illicit drug in the United States, with the highest prevalence of use among adolescents and young adults. One of every 17 high school seniors smokes marijuana on a near daily basis, a figure that has remained fairly stable for the past two decades despite declines in adolescent alcohol and cigarette use (Johnston et al., 2015). Although reasons for the disproportionately high prevalence of cannabis misuse among youth are poorly understood, the acute and long-term adverse effects of cannabis in youth are well documented (Volkow et al., 2014) and cannabis use accounts for the majority of all substance abuse treatment admissions among teenagers (SAMHSA, 2012), underscoring the need for effective treatment options for youth.
Several psychosocial interventions are moderately effective for reducing cannabis use among adolescents and young adults, including cognitive-behavioral and family therapies (Bender et al., 2011) and motivational interviewing (MI; (D’Amico et al., 2008). Motivational enhancement therapy (MET) combines MI techniques, such as exploring ambivalence about behavior change in a nonjudgmental manner, with personalized feedback. In controlled trials, MET was efficacious for reducing cannabis use among treatment-seeking and nontreatment-seeking adolescents (Dennis et al., 2004; Stephens et al., 2007; Walker et al., 2006). Yet, despite promising effects, outcomes are suboptimal for many youth. Meta-analyses indicate that while psychosocial interventions are efficacious for reducing cannabis use, especially when compared to inactive control conditions, the majority of individuals do not experience sustained benefit (Davis et al., 2015). These suboptimal outcomes highlight the need for research on ways to augment psychosocial treatment effects.
One potential method for improving treatment response rates is to augment behavioral interventions with adjunct pharmacotherapy. Pharmacotherapy trials for treating cannabis misuse are few, however, and most, but not all (Mason et al., 2012), have produced null findings (Carpenter et al., 2009; Levin et al., 2013; Levin et al., 2011; Levin et al., 2004; McRae-Clark et al., 2009; McRae-Clark et al., 2010). Moreover, only two controlled pharmacotherapy trials have studied adolescents. The first tested fluoxetine in youths with comorbid depression and found no effect (Cornelius et al., 2010). The second tested N-acetylcysteine, a prodrug of the amino acid cysteine thought to affect glutamate regulation, in combination with contingency management and weekly cessation counseling for cannabis dependent adolescents. N-acetylcysteine increased the odds of abstinence during the 8-week trial, but this effect was not sustained at the 4-week follow up (Gray et al., 2012).
Topiramate is a sulfamate-substituted fructopyranose derivative that reduced alcohol, cocaine, and nicotine use in clinical trials with adults (Johnson et al., 2003; Johnson et al., 2013; Kranzler et al., 2014; Miranda et al., 2008; Miranda et al., 2014; Oncken et al., 2014). It has multiple mechanisms of action, including blockade of voltage-sensitive sodium and calcium channels, potentiation of γ-aminobutyric acid (GABA), enhancement of GABAA receptor function, antagonism of AMPA/kainate glutamate receptors, and inhibition of carbonic anhydrase (Shank et al., 2000; Simeone et al., 2006). Although its effects on cannabis use have not been studied, its potentiation of GABA and antagonism of glutamate suggest it may be useful for reducing the acute reinforcing effects of cannabis. Cannabinoid type 1(CB1) receptors, the primary target of cannabis, are highly present on GABAergic interneurons, and modulation of these neurons is generally believed to mediate most of the subjective effects of cannabis (Moreira and Lutz, 2008). These receptors are also highly expressed on glutamatergic neurons, however, and animal data suggest that glutamatergic activity may mediate some of the effects of Δ9-tetrahydrocannabinol (THC) – the primary psychoactive constituent of cannabis.
This initial pilot study evaluated the feasibility of combining topiramate with MET for treating cannabis misuse among youth. We also evaluated several domains of neurocognitive functioning shown to be affected by topiramate in prior studies with adults (Salinsky et al., 2005) and to a lesser degree with adolescents (Pandina et al., 2010). Heavy cannabis use is associated with diminished memory and executive functioning (Bolla et al., 2002; Solowij et al., 2002) and difficulties sustaining attention and filtering irrelevant information (Solowij et al., 1995). It is therefore clinically important to investigate the effects of topiramate on neurocognitive functioning in this population in order to more fully evaluate the potential clinical utility of this adjunctive treatment. Finally, we tested the hypothesis that topiramate plus MET, compared to placebo plus MET, would reduce abstinence rates, as assessed by self-reported frequency of use (percent use days) and biochemical verification of abstinence (urine toxicology), as well as self-reported quantity of use (average number of grams smoked on cannabis use days) of cannabis use among youth.
Materials and Methods
Study Design
This double-blind study used a randomized, parallel group design to test the feasibility and efficacy of topiramate versus placebo for reducing cannabis use in youth. Topiramate was titrated to 200 mg daily over four weeks then stabilized for two weeks (see Table 1). Participants in both conditions received a three-session manual-driven MET intervention designed to enhance motivation and build skills to reduce cannabis use. Procedures were identical across conditions except for the medication administered. The Brown University Institutional Review Board approved this study, and before participation written informed consent was obtained from youths ≥18 years and from the parents of minors; assent was obtained from minors.
Table 1.
Daily Total Topiramate Dose (mg): Titration and Taper Schedules
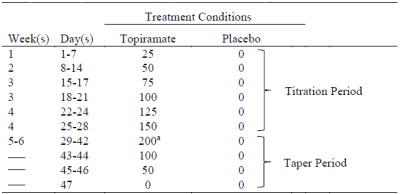
|
Note.
a Target dose
Participants
Participants were youths, ages 15 to 24 years, recruited from the community (e.g., posting advertisements in recreational settings, high schools, and other settings frequented by youth) with an interest in receiving a psychosocial intervention combined with a novel medication (or placebo) that may help them reduce their cannabis use. Youths who were mandated to treatment by the court system or by their parents were ineligible. All participants used cannabis use at least twice weekly in the past 30 days and experienced some clinically significant problems associated with their cannabis use (i.e., ≥one symptom of cannabis abuse or dependence) and 80% of youths met criteria for cannabis abuse or dependence (Diagnostic and Statistical Manual-Fourth Edition; DSM-IV-TR; Association (2000). Participants were not required to meet diagnostic threshold for a cannabis use disorder because DSM-IV-TR criteria do not adequately capture substance use disorders in youth (Degenhardt et al., 2002). Exclusion criteria were cannabis treatment in the past 30 days; current Axis I psychopathology other than cannabis, alcohol, nicotine, or disruptive behavior disorders, as defined by DSM-IV-TR; actively suicidal or psychotic; and medical conditions or medications that contraindicated taking topiramate. Females were ineligible if they were pregnant, nursing, or unwilling to use birth control. Medical eligibility was assessed by completing a comprehensive medical history, physical exam, and laboratory tests.
Diagnostic Assessment and Outcome Measures
Demographic and clinical characteristics
Demographic and clinical information was collected at baseline. Although we did not require youths to meet diagnostic criteria for a cannabis use disorder to be eligible for the study, we did assess for abuse and dependence for descriptive purposes and to ensure medication conditions were similar in terms of the severity of cannabis misuse. Psychiatric diagnoses, including cannabis use disorders, were derived using the Kiddie Schedule for Affective Disorders for School-Age Children, a semi-structured interview based on DSM-IV-TR criteria (Kaufman et al., 1997). Interviewers received systematic training and achieved a high level of inter-rater reliability (kappa > 0.90). Diagnostic decisions were based on participants’ reports, made by case consensus, and used for randomization (see below) and descriptive purposes. To further describe the sample, participants completed the Rutgers Cannabis Problem Index (Johnson and White, 1995), a continuous measure of cannabis-related problems.
Cannabis use
Cannabis use prior to the study was assessed using the 90-day timeline follow-back interview (TLFB) (Sobell and Sobell, 1992), which correlates highly with plasma THC levels (Hjorthoj et al., 2012). Assessment of cannabis use during the trial included urinalysis (Redwood Toxicology Reditest®; cutoff = 50ng/mL) and TLFB. To facilitate accurate reporting of the quantity of cannabis use on a specific day, participants estimated how much cannabis they used by weighing a surrogate substance (oregano). The total weight was divided by the number of users when participants shared cannabis. This method of estimating daily quantities of cannabis use produces reliable outcome data (Norberg et al., 2012).
Adverse events
Included in the consent/assent forms was a list of topiramate’s known side effects. The Systematic Assessment for Treatment Emergent Effects interview of side effects (Levine and Schooler, 1986) was adapted to assess side effects of topiramate at weekly appointments. To ensure expected and unexpected events were reported, side effects were collected in an open-ended way first, and then participants were queried about known topiramate effects. In addition, given that topiramate can potentiate negative affect, we administered the Beck Depression Inventory at baseline and at weekly visits to capture mood changes across the trial.
Neurocognitive functioning
Participants completed the Kaufman Brief Intelligence Test, Second Edition (KBIT) (Kaufman and Kaufman, 1990) prior to randomization for randomization purposes (see below). In addition, a neurocognitive test battery was selected to measure domains of functioning shown to be sensitive to topiramate. Eight standardized tests comprised this battery. All tests were administered by trained research staff at baseline (prior to randomization) and again during study weeks 3 (100 mg/day) and 6 (200 mg/day) except for the Story Recall subtest of the Woodcock Johnson® III Tests of Cognitive Abilities (WJTCA), which was only completed at baseline and study week 6 due to the limited number of available test administrations. Descriptive information for neurocognitive tests by medication group and tests of differences by study week is reported online in supporting information (Table S1 and S2).
Study Treatments
Randomization to treatment conditions
After completion of baseline assessments, participants were randomized to one of two 6-week treatment conditions: topiramate plus MET or placebo plus MET. An investigator with no direct participant contact used a computer-generated random allocation sequence to assign participants to treatment conditions on a 2:1 (topiramate to placebo) ratio. A randomized block design, with block sizes of 8 participants, stratified participants by sex, cannabis dependence, and baseline working memory function to ensure treatment conditions were balanced on these variables. We stratified by working memory using a cutoff score of 18 on the Memory for Words subtest of the WJTCA due to topiramate’s known adverse cognitive effects. The imbalanced randomization schedule ensured that sufficient numbers of participants in the topiramate condition reached the target dose given the anticipated attrition rate (Johnson et al., 2007).
MET
Three 50-min manual-driven MET sessions were individually administered across both medication conditions during study weeks 1, 3, and 5 of the intervention period. The MET protocol was based on prior work (Walker et al., 2006) and incorporated the principles of MI (Miller and Rollnick, 2002). The first session focused on enhancing motivation to reduce or quit cannabis use by establishing rapport, assessing motivation for change, and discussion of perceived pros and cons of cannabis use. During the second session, participants received a personalized cannabis use feedback packet, which was reviewed in the context of an MI-style discussion about the content. During the third session, the counselor reviewed the prior sessions, facilitated a discussion about progress toward goals set during the trial, queried about barriers to change, and helped problem solve and set new goals for behavior change.
Two masters- and three doctoral-level counselors provided MET across both medication conditions to ensure that differences between conditions were not confounded with counselor personality, experience, or individual style. All counselors reviewed the MET manual, completed at least 20 hours of training in MET, and conducted a minimum of two mock cases. The same counselor conducted all 3 sessions in order to enhance rapport, provide continuity of care, and minimize attrition. Counselors were blind to participants’ medication condition and did not conduct any research assessments with participants. All sessions were audio taped and rated by trained clinicians with respect to intervention integrity/adherence using Motivational Interviewing Treatment Integrity (MITI) scale (Moyers et al., 2007). Five domains were rated for each session (evocation, collaboration, autonomy/support, direction, and empathy) using a 5-point scale (1 = low, 5 = high) then averaged across sessions to yield an overall score for each dimension.
Pharmacological treatment with topiramate
Participants and study personnel in direct contact with participants were blind to treatment assignments. An independent compounding pharmacy provided topiramate and placebo capsules, which were identical in appearance. Capsules were prepackaged in 7-day blister packaging cards consecutively numbered according to a computer-generated randomization schedule to ensure the researchers who enrolled and assessed participants were blind to treatment assignments. Participants were given one morning and one evening dose. We did not provide participants with the option to reduce or delay the maximum dosage.
Topiramate capsules contained appropriate unit dosages of the active study medication (see Table 1) and placebo capsules contained pharmacologically inert filler. All capsules also contained 50mg of riboflavin to assess medication compliance in both treatment conditions; riboflavin concentrations in urine were assessed at weekly visits. Two raters, blind to randomization, independently evaluated urine samples under ultraviolet light to determine the presence or absence of riboflavin (Del Boca et al., 1996). A third rater resolved discrepancies. Participants also provided blood samples at study weeks 3 and 6 for topiramate quantification in serum, which was dichotomized as present or absent for compliance purposes.
Data Analysis
Pretreatment differences between conditions were evaluated using independent sample t-tests and chi-squared analyses conducted in SPSS 22.0 (IBM, Armonk, NY). Comparisons between conditions on MET treatment attendance and adherence were examined using independent sample t-tests. Chi-squared analyses compared the frequency of reported side effects between treatment conditions. Changes in performance on neuropsychological tests from baseline to study weeks 3 and 6 were analyzed using generalized estimating equations (GEE), which allows for varying numbers of observations per participant while controlling for autocorrelation (Zeger et al., 1988). An independent structure best fit the data and all models assumed a normal link function. To test for changes relative to baseline, study weeks were coded as binary variables and the baseline assessment served as a reference category (Table S2).
Multiple-domain latent growth modeling (MDLGM) in Mplus 7.0 (Muthén and Muthén, 1998-2013) was used to simultaneously estimate the influence of topiramate on change in urine toxicology (binary outcome), percent use days, and grams per use day (continuous outcomes) over the course of the 6-week trial. MDLGM is increasingly recognized as a useful modeling approach for intervention studies because of its flexibility to model change in multiple treatment outcomes and also mixed response types (Whittaker, Pituch, & McDougall, 2014). Additionally, MDLGM increases power to test the influence of explanatory variables on outcomes by reducing the standard error associated with these tests, which is particularly useful when missing outcome data are likely (Whittaker et al., 2014). Data were analyzed in two ways. First, all available data were included in “intent-to-treat” analyses. Next, data for missing outcomes were imputed by averaging over 10 datasets with Bayesian estimation using baseline variables as missing-data correlates to improve the estimates (see Table 2 for descriptors). Both sets of analyses utilized full information maximum likelihood estimation procedures with numerical integration.
Table 2.
Summary of Participant Characteristics at Baseline Stratified by Treatment Condition and Completer Status
| Variable | Placebo
|
Topiramate
|
||||
|---|---|---|---|---|---|---|
| Full | Completers | Attriters | Full | Completers | Attriters | |
| N | 26 (39.4) | 20 (76.9) | 6 (23.1) | 40 (60.6) | 19 (47.5) | 21 (52.5) |
| Age | 18.81 ± 2.08* | 18.70 ± 2.27 | 19.17 ± 1.33 | 20.30 ± 2.03* | 20.16 ± 2.27 | 20.43 ± 1.83 |
| Male | 12 (46.2) | 9 (45.0) | 3 (50.0) | 20 (50.0) | 9 (47.4) | 11 (52.4) |
| Minority | 13 (50.0) | 9 (45.0) | 4 (66.7) | 19 (47.5) | 12 (63.2) | 7 (33.3) |
| Overall IQ | 96.85 ± 13.60 | 98.15 ± 13.12 | 92.50 ± 15.53 | 102.80 ± 17.18 | 97.84 ± 15.07 | 107.29 ± 18.08 |
| BDI score | 6.08 ± 8.50 | 5.25 ± 6.54 | 8.83 ± 13.69 | 4.55 ± 5.08 | 5.68 ± 5.23 | 3.52 ± 4.83 |
| Baseline cannabis problems | 25.46 ± 23.30 | 20.05 ± 13.64 | 43.50 ± 38.73 | 20.08 ±16.86 | 24.74 ± 20.07 | 15.86 ± 12.35 |
| Baseline percent use days | 70.94 ± 28.60 | 66.78 ± 30.00 | 84.81 ± 19.02 | 70.22 ± 26.89 | 70.29 ± 27.11 | 70.16 ± 27.46 |
| Baseline grams per use day | 0.88 ± 0.73 | 0.86 ± 0.69 | 0.93 ± 0.91 | 0.53 ± 0.37 | 0.62 ± 0.46 | 0.45 ± 0.25 |
Note. BDI = Beck Depression Inventory. All variables were measured at baseline. Tabled values for categorical variables are count (%), whereas values for continuous variables are mean ± standard deviation. Differences in categorical variables were calculated via chi-square tests, whereas differences in continuous variables were calculated via independent sample t-tests. Significant column differences based on bias-corrected 95% confidence intervals with 1000 bootstrap draws and noted by superscript letters.
Mdifference = − 1.49, t(64) = −2.89, p = .005, SE = .52, 95%CI [− 2.52, − 0.46]
Linear growth (slope) parameters were coded from 0 to 1 (i.e., 0, .2, .4, .6, .8, 1, see Supporting Information, Figure S1) to allow the intercept to be interpreted as week-1 outcome levels, which were not expected to differ between medication conditions, and the slope to be interpreted as the change in outcomes from week 1 to week 6. Initial, unconditional MDLGMs provided average intercept and slope parameters for all outcomes. Next, intercepts and slopes were regressed on Medication Condition to test whether topiramate influenced week 1 outcomes (intercept) or change in outcomes from week 1 to week 6 (slope). Final models included baseline levels of outcomes assessed via TLFB, age, and cannabis dependence as covariates.
Results
Randomization, Pretreatment Comparison of Conditions, and Completion Rates
Sixty-six participants were randomized (see Figure 1). The majority met criteria for cannabis abuse or dependence, with similar rates of these disorders in the topiramate (abuse = 22.5%; dependence = 62.5%) and placebo-control (abuse = 19%; dependence = 76.9%) conditions. As shown in Table 2, treatment groups did not differ significantly on pretreatment clinical characteristics, pretreatment study outcome variables, and other demographic characteristics except for age. The topiramate group was older, on average, relative to the placebo group, Mdifference = −1.49, 95%CI = [−2.52, −0.46], p = .005.
Figure 1.
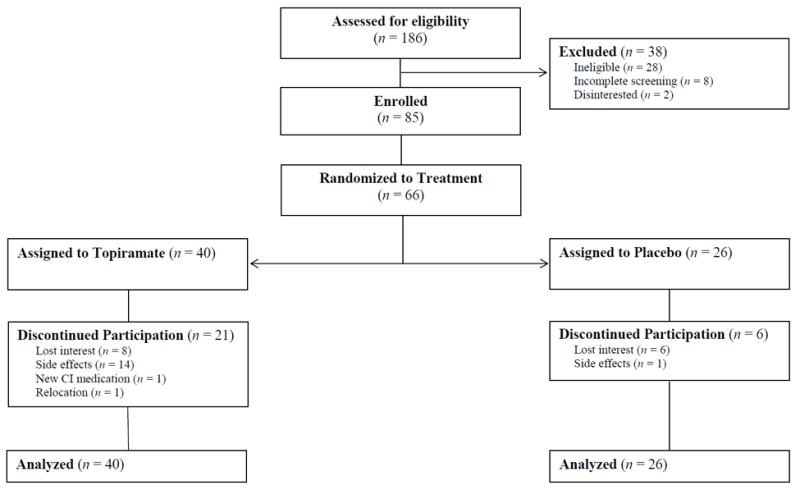
Of the 66 participants, 39 youth (59.1%) completed the trial. As expected, attrition occurred disproportionally in the topiramate condition (52.5%) compared to the placebo condition (23.1%), χ2 = 5.64, df = 1, p = .018. Participants assigned to placebo completed a mean of 34.77 days (SD = 12.50), whereas those assigned to topiramate completed a mean of 28.26 days (SD = 13.96); Mdifference = 6.51, p = .057, 95%CI [−0.92, 12.91]. Table 2 presents descriptive characteristics of the full sample, completers, and those participants who withdrew, stratified by medication group. No differences were found between medication groups within completers or within those who withdrew (see Table 2).
Due to their higher attrition, participants randomized to topiramate attended fewer sessions of MET (M = 2.00, SD = 1.07) than those randomized to placebo (M = 2.65, SD = 0.75; p = .004). Ninety percent of participants randomized to topiramate completed Session 1, 60% completed Session 2, and 47.5% completed Session 3. By comparison, 100% of the placebo group completed Session 1, 84.6% completed Session 2, and 80.8% completed Session 3. Comparisons between treatment conditions on MET fidelity revealed no differences between conditions on any of the five domains of MITI scales.
Medication Compliance and Tolerability
Weekly urine samples indicated a high rate of adherence in both conditions throughout the trial. Participants randomized to placebo were compliant on an average of 89.7% (SD = 18.9) of their urine assays compared to 93.4% (SD = 18.4) in the topiramate condition (p = .44). Among participants randomized to topiramate, comparisons between urine assays under ultraviolet light and serum topiramate quantification showed 86.4% concordance (kappa = 0.40) between the two measures at study weeks 3 and 6.
Of those participants who withdrew from the topiramate condition, 67% attributed their withdrawal to adverse medication side effects. Table 3 summarizes side effects reported by ≥10% of participants in either condition at any time during the study. Although no serious adverse events occurred in this trial, participants randomized to topiramate were significantly more likely to report depression, anxiety, difficulty with coordination or balance, weight loss, and paresthesias. Participants randomized to placebo were more likely to report rash. Otherwise, there were no statistically significant differences between conditions.
Table 3.
Frequency (%) of Adverse Events Reported by ≥ 10% of Participants in Either Arm of the Study
| Adverse Event | Topiramate (N = 40) | Placebo (N = 26) | χ2 |
|---|---|---|---|
| Neurocognitive | |||
| Drowsiness or sleepiness | 12 (30.0) | 13 (50.0) | 2.68 |
| Slow thinking or reactions | 12 (30.0) | 4 (15.4) | 1.83 |
| Word finding difficulties | 11 (27.5) | 4 (15.4) | 1.32 |
| Difficulty with concentrating or attention | 11 (27.5) | 2 (7.7) | 3.91 † |
| Dizziness | 10 (25.0) | 2 (7.7) | 3.17 † |
| Depression | 10 (25.0) | 1 (3.8) | 5.01 * |
| Anxiety | 10 (25.0) | 0 | 7.66 ** |
| Fatigue | 9 (22.5) | 9 (34.6) | 1.17 |
| Difficulty with memory | 8 (20.0) | 5 (19.2) | 0.01 |
| Difficulty with coordination or balance | 6 (15.0) | 0 | 4.29 * |
| Confusion | 5 (12.5) | 0 | 3.52 † |
| Nervousness | 4 (10.0) | 0 | 2.77 |
| Irritability | 2 (5.0) | 3 (11.5) | 0.96 |
| Gastrointestinal | |||
| Weight loss | 20 (50.0) | 6 (23.1) | 4.78 * |
| Decrease in appetite | 19 (47.5) | 9 (34.6) | 1.07 |
| Nausea | 12 (30.0) | 8 (30.8) | 0.00 |
| Changes in vision | 4 (10.0) | 0 | 2.77 |
| Stomach pain, cramps | 2 (5.0) | 3 (11.5) | 0.96 |
| Otolaryngolic | |||
| Runny nose, sinus problems, sneezing | 14 (35.0) | 12 (46.2) | 0.82 |
| Sore throat | 4 (10.0) | 2 (7.7) | 0.96 |
| Other | |||
| Paresthesias | 15 (37.5) | 2 (7.7) | 7.32 ** |
| Injuries | 8 (20.0) | 5 (19.0) | 0.01 |
| Headache | 8 (20.0) | 6 (23.1) | 0.09 |
| Cough, cold symptoms | 5 (12.5) | 5 (19.2) | 0.56 |
| Rash | 0 | 3 (11.5) | 4.84 * |
Note.
< .10,
p < .05,
p < .01,
p < .001
We also examined the effects of topiramate on depressive symptoms as measured by the BDI. Medication conditions scored similarly on the BDI at baseline (β = −0.06, 95%CI [−0.56, 0.45], p = .827), and the main effect of medication condition on BDI scores across the trial was not significant (β = 0.10, 95%CI [−0.34, 0.53], p = .666). The main effect of study week was significant, however, such that overall participants reported lower levels of depressive symptoms across the trial (β = −0.47, 95%CI [−0.76, 0.18], p = .001). Examination of the effects of medication condition on BDI scores at each study week with baseline BDI scores coded as the reference category indicated that the topiramate group, as compared to the placebo-control, had significantly less attenuation of BDI scores from baseline in study week 6 (β = 0.66, 95%CI [0.10, 1.23], p = .022). No other differences were found.
Neurocognitive Test Performance
Medication conditions were equivalent at baseline on neurocognitive tests. Table S1 shows descriptive statistics for the effects of topiramate on neurocognitive function across the trial. Youths randomized to topiramate, as compared to placebo, showed decreased performance on retrieval fluency from baseline at study weeks 3 and 6 and decreased memory for words at week 6 (see Table S2). As shown in Table S2, no other differences between the medication conditions emerged in terms of neurocognitive test performance.
Cannabis Use Outcomes
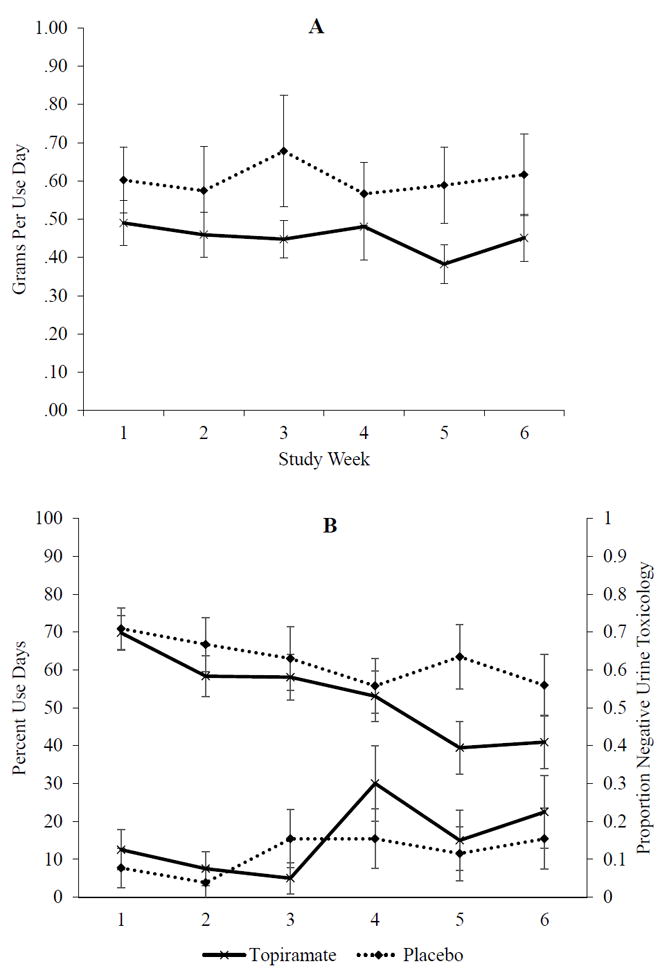
Results using all available data (intent-to-treat analyses) were consistent with those based on imputation analyses. Statistics reported in text are based on imputed data for both unconditional and conditional MDLGMs. Results of tests for medication effects based on all available data are provided in Table 4. Standardized effect estimates for the conditional MDLGM with imputed data are included in Figure S1. Raw data and standard errors for grams per use day are shown in Figure 2, Panel A. Raw data and standard errors for percent use days and proportion negative urine toxicology outcomes are shown in Figure 2, Panel B, on the left and right y axes, respectively.
Table 4.
Results from Multiple-domain Latent Growth Models of Urine Toxicology, Percent Use Days, and Grams per Use Day for Imputed and All Available Data
| Parameter | Imputed data
|
All available data
|
||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Slope | Intercept | Slope | |||||
| Urine toxicology
|
||||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
|
|
|
|||||||
| Model 1a | ||||||||
| Medication | − 1.26 | [− 3.81, 1.30] | − 0.42 | [− 2.96, 2.12] | − 1.41 | [− 4.59, 1.76] | − 0.30 | [− 3.39, 2.80] |
| Model 2b | ||||||||
| Medication | 0.09 | [− 2.46, 2.63] | 0.15 | [− 2.69, 2.99] | 0.46 | [− 2.73, 3.66] | 0.33 | [− 3.25, 3.91] |
| Age | − 0.39 | [− 0.95, 0.16] | − 0.09 | [− 0.70, 0.52] | − 0.31 | [− 1.03, 0.41] | − 0.34 | [− 1.14, 0.47] |
| Dependence | 0.85 | [− 1.64, 3.33] | 2.62 | [− 0.23, 5.48] | 1.26 | [− 1.71, 4.22] | 3.57 | [− 0.04, 7.18] |
| Percent use daysc
|
||||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
|
|
|
|||||||
| Model 1a | ||||||||
| Medication | − 0.01 | [− 0.16, 0.13] | − 0.10 | [− 0.27, 0.07] | − 0.02 | [− 0.16, 0.13] | − 0.12 | [− 0.28, 0.04] |
| Model 2b | ||||||||
| Medication | 0.03 | [− 0.08, 0.14] | − 0.08 | [− 0.25, 0.09] | 0.03 | [− 0.08, 0.14] | − 0.08 | [− 0.24, 0.09] |
| Age | − 0.01 | [− 0.04, 0.01] | − 0.02 | [− 0.06, 0.03] | − 0.01 | [− 0.04, 0.01] | − 0.01 | [− 0.04, 0.03] |
| Dependence | 0.09 | [− 0.02, 0.21] | 0.07 | [− 0.12, 0.26] | 0.06 | [− 0.06, 0.17] | 0.15 | [− 0.03, 0.33] |
| TLFB | 0.01 *** | [0.00, 0.01] | − 0.01 * | [− 0.01, 0.00] | 0.01 *** | [0.00, 0.01] | 0.00 | [− 0.01, 0.00] |
| Grams per use day
|
||||||||
| Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | Est. | 95% CI | |
|
|
|
|||||||
| Model 1a | ||||||||
| Medication | − 0.14 | [− 0.35, 0.08] | − 0.17 † | [− 0.35, 0.00] | − 0.10 | [− 0.29, 0.10] | − 0.24 * | [− 0.42, − 0.05] |
| Model 2b | ||||||||
| Medication | 0.07 | [− 0.09, 0.24] | − 0.26 ** | [− 0.43, − 0.09] | 0.10 | [− 0.05, 0.24] | − 0.28** | [− 0.48, − 0.08] |
| Age | − 0.02 | [− 0.06, 0.01] | 0.02 | [− 0.02, 0.06] | − 0.02 | [− 0.05, 0.01] | 0.04 | [0.00, 0.09] |
| Dependence | − 0.02 | [− 0.19, 0.15] | 0.01 | [− 0.17, 0.20] | − 0.03 | [− 0.17, 0.12] | 0.08 | [− 0.16, 0.32] |
| TLFB | 0.53 *** | [0.39, 0.67] | − 0.20 * | [− 0.39, −0.01] | 0.54 *** | [0.42, 0.66] | − 0.07 | [− 0.29, 0.16] |
Note. Medication = medication condition (topiramate = 1, placebo = 0); TLFB = 90-day timeline follow back measure of outcome variable collected at baseline; Dependence = cannabis dependence.
Conditional model including only medication condition
Covariate model
Percent use days were divided by 100 to facilitate model convergence
p = .05,
p < .05,
p < .01,
p < .001
Figure 2.
Unconditional MDLGM
An initial, unconditional MDLGM estimated general trends, i.e., average week 1 outcomes (intercepts) and average change in outcomes from week 1 to week 6 (slopes). On the whole, youth used cannabis on 68.4% of days in week 1, Mintercept = 0.68, 95%CI [0.61, 0.58], p < .001, and the average growth rate for percent use days decreased over the course of the study, Mslope = −0.18, 95%CI [−0.27, −0.08], p < .001. Results of urine analysis also showed that the probability of positive urine screens decreased over time, Mslope = −1.82, 95%CI [−3.13. −0.51], p = .006. In terms of the quantity, participants used 0.51 grams of cannabis on use days, on average, during week 1, Mintercept = 0.51, 95%CI [0.40, 0.61], p < .001. On average, youths’ quantity of cannabis use (grams per use day) did not change significantly over the course of the study, Mslope = −0.02, 95%CI [−0.12, 0.07], p = .638.
Conditional MDLGM
Results of conditional tests of medication condition on week 1 outcomes (intercepts) and change over time (slopes) are shown in Table 4 for both imputed data and all available data. Percent use days. The effect of Medication Condition on the percent-use-days intercept was not significant, suggesting that the frequency of cannabis use did not differ between medication groups at week 1, b = −0.01, 95%CI [−0.16, −0.13], p = .849. The slope effect was also not significant, suggesting that medication treatment did not significantly affect change in percent use days from weeks 1 to 6 beyond average trends for this sample, b = −0.10, 95%CI [−0.27, −0.07], p = .238. Urine toxicology. Consistent with findings based on self-report, medication did not significantly influence the probability of positive urine screens at week 1, b = −1.26, 95%CI [−3.81, −1.30], p = .335, or produce change in positive urine screens from week 1 to week 6, b = −0.42, 95%CI [−2.96, −2.12], p = .746. Grams per use day. The effect of Medication Condition on the grams-per-use-day intercept was not significant, b = −0.14, 95%CI [−0.35, −0.08], p = .213, suggesting that the quantity of cannabis use did not differ between medication groups at week 1. However, a trend emerged for the effect of medication condition on grams-per-use-day slope, suggesting that youth in the topiramate group smoked fewer grams of cannabis when they used during the final week of the trial (week 6), relative to the placebo group, b = −0.17, 95%CI [−0.35, 0.00], p = .052. The effect size (r) was equivalent to the standardized estimate, β = − 0.36, 95%CI [−.73, 0.02], p = .066. The effect was strengthened when accounting for age, cannabis dependence, and use prior to the trial, b = −0.26, 95%CI [−0.43, −0.09], p = .003.
Discussion
This study is the first to examine the feasibility and efficacy of topiramate as an adjunctive therapy for treating cannabis misuse. Adolescents and young adults in both conditions smoked less frequently as the trial progressed, but there was no advantage of adjunctive topiramate treatment on this effect. However, topiramate, as compared to placebo, produced modest reductions in how much cannabis participants smoked on use days. Topiramate was not well tolerated, however, and the clinical significance of this reduction is uncertain.
Only 48% of youths randomized to topiramate completed the 6-week trial, compared to 77% of youths in the placebo condition. Of those who withdrew from topiramate, 67% attributed their withdrawal to adverse medication side effects. We also examined the effects of topiramate on several domains of neurocognitive functioning, given its known adverse cognitive effects. Results of neurocognitive testing showed that topiramate compromised retrieval fluency and the ability to form and verbalize echoic memories. In addition, self-report data showed significantly higher rates of neurocognitive adverse events associated with topiramate relative to placebo, including difficulty with concentration and attention, dizziness, depression, anxiety, difficulty with coordination or balance, confusion, and approximately twice the rate of slow thinking or reactions. It is difficult to compare these findings with other research given differences across studies in terms of the characteristics of patients who are typically prescribed topiramate (e.g., epilepsy) and the doses administered. Even so, our findings converge with research on adolescents prescribed topiramate for treating migraines (Pandina et al., 2010) or seizures and with an adult study that tested the effects of topiramate (300 mg/day) for treating alcohol misuse (Knapp et al., 2015).
Our finding that topiramate reduced the quantity but not the frequency of cannabis use may have several explanations. First, it is possible that topiramate alters subjective effects of cannabis use, thereby reducing how much additional cannabis individuals use on a given day. This notion is consistent with theoretical models and recent findings regarding how topiramate affects other drugs of abuse (Johnson et al., 2003; Miranda, In Press). Using addictive substances, including cannabis, produces a host of pharmacological effects that cause acute changes in affect and cognition. These subjective experiences, which are central in nearly all contemporary models of addiction, predict future use and are important targets for clinical interventions. Second, the behavioral platform for testing medication effects targeted harm reduction as the primary treatment goal. It is possible that topiramate enhanced the efficacy of this intervention approach. Finally, we tested whether topiramate affected cannabis use beyond the influence of an established psychosocial intervention. Although adjunctive topiramate treatment did not reduce the frequency of cannabis use, we found that across conditions participants significantly reduced how often they smoked. It is plausible that the behavioral intervention increased abstinence rates while topiramate reduced how much cannabis youth smoked on days they used. This possibility remains speculative, however, and cannot be determined based on our current study. Future research is needed to understand the ideal ways to combine pharmacological and psychosocial therapies.
Findings of this initial study should be considered in the context of its limitations. First, this pilot study had a small sample size and the duration of treatment was short, especially in terms of the length of topiramate treatment at the target dose (two weeks). As such, our findings are preliminary. Second, participants randomized to topiramate attended significantly fewer MET sessions than those in the placebo condition because of their higher attrition rates. Concerns about differential exposure to the behavioral intervention are somewhat mitigated, however, by the fact that effects of topiramate on cannabis use were observed. Third, participants were 15 to 24 years old and youths randomized to topiramate were older than those randomized to placebo. It is possible that developmental changes that occur across this age span (e.g., living arrangement, work/school changes, parental monitoring) influence how youths responded to treatment. This concern is somewhat lessened by the fact that our findings were upheld when age was statistically controlled. Nonetheless, larger studies are needed to adequately examine whether adolescents and young adults respond differently to combined interventions.
On balance, our study design tested the effects of topiramate on cannabis use in the context of an active behavioral treatment that served as a psychosocial intervention platform administered across both medication conditions. Advantages of this approach include the ability to evaluate whether topiramate is superior to an effective treatment that presents less potential risk. It also maximizes a favorable risk-benefit ratio for participants by delivering an active intervention to youths randomized to placebo. Importantly, the behavioral intervention was delivered in the same offices by the same counselors and overseen by the same expert supervisors in both conditions. MET adherence ratings indicated that counselors adhered to MI treatment strategies similarly across both medication conditions. Thus, our findings are not likely attributable to a weak control condition. In addition, objective measures indicated that participants were highly compliant with the medication regimen. Finally, the MDLGM approach for analyzing our outcomes allowed for a comprehensive evaluation of the impact of topiramate with the inclusion of multiple growth processes. Advantages of this method include better treatment of missing data, increased statistical power, and improved Type I error protection (Whittaker et al., 2014).
In this pilot study, we intervened with youths during a critical period in the development of cannabis dependence; early intervention can alter trajectories of continued substance misuse in adulthood. The potential significance of our findings is mitigated, however, by the small sample size and high attrition rates in the topiramate condition. On the whole, these preliminary findings which suggest a small reduction of cannabis use by a poorly tolerated medication – indicate limited promise of topiramate for treating cannabis misuse in youths.
Supplementary Material
Acknowledgments
The National Institute on Drug Abuse at the National Institutes of Health supported this work (DA026778). PM’s effort was supported by the National Institute on Alcohol Abuse and Alcoholism (AA019681). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The funding sources had no role in this research other than financial support.
Footnotes
Authors Contribution
All authors were responsible for the study concept and design. RM, AJ, and AB were responsible for the acquisition of study data. TC and RS conducted medical exams on study applicants and provided medical coverage for this study. RM provided clinical coverage during alcohol cue reactivity assessments. RM, HT, and AB were responsible for data analysis. All authors contributed to the interpretation of findings. RM and HT drafted the manuscript and all authors critically reviewed content and approved the final version for publication.
Disclosure/Conflict of Interest
RS is on the advisory board of D&A Pharma and received consultant fees from D&A Pharma, Farmaceutico CT and Lundbeck. Otherwise, none of the authors report any biomedical financial interests or potential conflicts of interest.
Contributor Information
Robert Miranda, Jr., Center for Alcohol and Addiction Studies, Brown University
Hayley Treloar, Center for Alcohol and Addiction Studies, Brown University.
Alexander Blanchard, Center for Alcohol and Addiction Studies, Brown University.
Alicia Justus, Center for Alcohol and Addiction Studies, Brown University.
Peter M. Monti, Center for Alcohol and Addiction Studies, Brown University
Thomas Chun, Center for Alcohol and Addiction Studies, Brown University.
Robert Swift, Providence Veterans Affairs Medical Center and also at the Center for Alcohol and Addiction Studies, Brown University.
Jennifer W. Tidey, Center for Alcohol and Addiction Studies, Brown University
Chad J. Gwaltney, Center for Alcohol and Addiction Studies, Brown University
References
- Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- Bender K, Tripodi SJ, Sarteschi C, Vaughn MG. A meta-analysis of interventions to reduce adolescent cannabis use. Research on Social Work Practice. 2011;21:153–164. [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Wood DS, Brown SJ. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug and alcohol dependence. 2010;112:39–45. doi: 10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico EJ, Miles JN, Stern SA, Meredith LS. Brief motivational interviewing for teens at risk of substance use consequences: a randomized pilot study in a primary care clinic. Journal of substance abuse treatment. 2008;35:53–61. doi: 10.1016/j.jsat.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Davis ML, Powers MB, Handelsman P, Medina JL, Zvolensky M, Smits JA. Behavioral therapies for treatment-seeking cannabis users: a meta-analysis of randomized controlled trials. Evaluation & the health professions. 2015;38:94–114. doi: 10.1177/0163278714529970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Lynskey M, Coffey C, Patton G. ‘Diagnostic orphans’ among young adult cannabis users: persons who report dependence symptoms but do not meet diagnostic criteria. Drug and alcohol dependence. 2002;67:205–212. doi: 10.1016/s0376-8716(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcoholism, clinical and experimental research. 1996;20:1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) Study: main findings from two randomized trials. Journal of substance abuse treatment. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. The American journal of psychiatry. 2012;169:805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorthoj CR, Fohlmann A, Larsen AM, Arendt M, Nordentoft M. Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clinical trial. Addiction. 2012;107:1123–1131. doi: 10.1111/j.1360-0443.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Wang XQ, Penberthy JK, Javors MA, Seneviratne C, Liu L. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA psychiatry. 2013;70:1338–1346. doi: 10.1001/jamapsychiatry.2013.2295. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM Topiramate for Alcoholism Advisory B, Topiramate for Alcoholism Study G. Topiramate for treating alcohol dependence: a randomized controlled trial. Jama. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Johnson V, White HR. The relationship between work-specific and generalized stress and alcohol and marijuana use. Journal of Drug Issues. 1995;25:237–251. [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2014 2015 [Google Scholar]
- Mich. Institute for Social Research, the University of Michigan; Ann Arbor: [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. American Guidance Service; Circle Pines, MN: 1990. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Ciraulo DA, Sarid-Segal O, Richardson MA, Devine E, Streeter CC, Oscar-Berman M, Surprise C, Colaneri L, Putnam M, Waters M, Richambault C. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. Journal of clinical psychopharmacology. 2015;35:34–42. doi: 10.1097/JCP.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. The American journal of psychiatry. 2014;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani J, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, Bisaga A, Sullivan MA, Carpenter KM. A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction. 2013;108:1084–1094. doi: 10.1111/add.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug and alcohol dependence. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, Vosburg SK. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology bulletin. 1986;22:343–381. [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Buffkins K, Kyle M, Adusumalli M, Begovic A, Rao S. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, Brady KT. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug and alcohol dependence. 2009;105:132–138. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2010;19:481–489. doi: 10.1111/j.1521-0391.2010.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2. Guilford Press; New York: 2002. [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcoholism, clinical and experimental research. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Treloar H, Blanchard A, Tidey JW, Swift RM, Chun T, Rohsenow DJ, Monti PM. Biobehavioral mechanisms of topiramate’s effects on alcohol use: an investigation pairing laboratory and ecological momentary assessments. Addiction biology. 2014 doi: 10.1111/adb.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Treloar H, Blanchard A, Tidey J, Swift RM, Chun T, Rohsenow D, Gwaltney CJ, Monti PM. Biobehavioral mechanisms of topiramate’s effects on alcohol use: An investigation pairing laboratory and ecological momentary assessments. Addiction biology. doi: 10.1111/adb.12192. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addiction biology. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Miller WR, Ernst D. Revised Global Scales: Motivational Interviewing Treatment Integrity Manual 3.0 (MITI 3.0) University of New Mexico Center on Alcoholism, Substance Abuse, and Addictions; 2007. [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 7. Muthén & Muthén; Los Angeles, CA: 1998-2013. [Google Scholar]
- Norberg MM, Mackenzie J, Copeland J. Quantifying cannabis use with the timeline followback approach: a psychometric evaluation. Drug and alcohol dependence. 2012;121:247–252. doi: 10.1016/j.drugalcdep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Oncken C, Arias AJ, Feinn R, Litt M, Covault J, Sofuoglu M, Kranzler HR. Topiramate for smoking cessation: a randomized, placebo-controlled pilot study. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2014;16:288–296. doi: 10.1093/ntr/ntt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandina GJ, Ness S, Polverejan E, Yuen E, Eerdekens M, Bilder RM, Ford L. Cognitive effects of topiramate in migraine patients aged 12 through 17 years. Pediatric neurology. 2010;42:187–195. doi: 10.1016/j.pediatrneurol.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64:792–798. doi: 10.1212/01.WNL.0000152877.08088.87. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Treatment Episode Data Set (TEDS): 2000-2010 National Admissions to Substance Abuse Treatment Services. DASIS Series S-61. Administration SAaMHS (ed) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl 1):S3–9. [PubMed] [Google Scholar]
- Simeone TA, Wilcox KS, White HS. Subunit selectivity of topiramate modulation of heteromeric GABA(A) receptors. Neuropharmacology. 2006;50:845–857. doi: 10.1016/j.neuropharm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Sobell LD, Sobell MD. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen RLJ, editor. Measuring Alcohol Consumption. The Humana Press Inc; 1992. [Google Scholar]
- Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biological psychiatry. 1995;37:731–739. doi: 10.1016/0006-3223(94)00178-6. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens R, Roffman RA, Babor T. Does marijuana use cause long-term cognitive deficits? Jama. 2002;287:2653–2654. [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Fearer SA, Williams C, Burke RS. The Marijuana Check-up: promoting change in ambivalent marijuana users. Addiction. 2007;102:947–957. doi: 10.1111/j.1360-0443.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. The New England journal of medicine. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DD, Roffman RA, Stephens RS, Wakana K, Berghuis J, Kim W. Motivational enhancement therapy for adolescent marijuana users: a preliminary randomized controlled trial. Journal of consulting and clinical psychology. 2006;74:628–632. doi: 10.1037/0022-006X.74.3.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker TA, Pituch KA, McDougall GJ., Jr Latent growth modeling with domain-specific outcomes comprised of mixed response types in intervention studies. Journal of consulting and clinical psychology. 2014;82:746–759. doi: 10.1037/a0036664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


