Abstract
Cancer is predominantly a somatic disease. A mutant allele present in a cancer cell genome is considered somatic when it’s absent in the paired normal genome along with public SNP databases. The current build of dbSNP, the most comprehensive public SNP database, however inadequately represents several non-European Caucasian populations, posing a limitation in cancer genomic analyses of data from these populations. We present the Tata Memorial Centre-SNP database (TMC-SNPdb), as the first open source, flexible, upgradable, and freely available SNP database (accessible through dbSNP build 149 and ANNOVAR)—representing 114 309 unique germline variants—generated from whole exome data of 62 normal samples derived from cancer patients of Indian origin. The TMC-SNPdb is presented with a companion subtraction tool that can be executed with command line option or using an easy-to-use graphical user interface with the ability to deplete additional Indian population specific SNPs over and above dbSNP and 1000 Genomes databases. Using an institutional generated whole exome data set of 132 samples of Indian origin, we demonstrate that TMC-SNPdb could deplete 42, 33 and 28% false positive somatic events post dbSNP depletion in Indian origin tongue, gallbladder, and cervical cancer samples, respectively. Beyond cancer somatic analyses, we anticipate utility of the TMC-SNPdb in several Mendelian germline diseases. In addition to dbSNP build 149 and ANNOVAR, the TMC-SNPdb along with the subtraction tool is available for download in the public domain at the following:
Database URL: http://www.actrec.gov.in/pi-webpages/AmitDutt/TMCSNP/TMCSNPdp.html
Introduction
Somatic mutations sequentially accumulate in cancer cell genomes. In addition, a typical cancer genome contains several polymorphic ‘normal’ germline variants (1–3). Subtracting the tumor DNA variants against matched normal DNA derived from the same individual and those polymorphic in the population is, therefore, essential to identify an exclusive somatic event (4). Apropos, a critical aspect of any tumor genome sequence analysis involves depletion of paired normal variants followed by depletion of residual variants from public databases of common single nucleotide polymorphism (SNP) such as dbSNP (5) and 1000 Genomes Project (6). A sequence variant not observed in matched normal derived genome sequence and absent from public SNP database is considered somatic in origin. Adopting such an analytical approach ensures filtering of paired-germline and population-specific polymorphic variants from dbSNP and 1000 Genomes Project for Caucasian population (7).
However, despite depletion against dbSNP, unknown SNPs especially those with lower minor allele frequency not represented in dbSNP, are likely to confound somatic mutation analyses in studies involving non- Caucasian and non-European Caucasian populations (5). Two exhaustive initiatives addressing this issue are the publicly available exome variation datasets: NHLBI Exome Sequencing Project (https://esp.gs.washington.edu/EVS/) and Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/) (8). Information gathered from these studies is an integral part of variant annotation tools like Annovar (9).
Multiple studies such as the Indian Genome Variation Consortium (10, 11) and HUGO Pan- Asian SNP Consortium (12) have described the genomic distinctiveness of Indian population based on varying allele frequency of known SNPs, complex origin, genetic diversity (13–16), and high variation of male lineages (Y-chromosome) within the population (17, 18). However, a concerted effort to comprehensively identify and catalogue novel SNPs present exclusively in Indian population is yet to be undertaken. Lack of Indian specific SNP database has been an important impediment in cancer research, especially in efforts to discover bona fide novel somatic mutations.
Here, we describe Tata Memorial Centre-SNP database ‘TMC-SNPdb’ as the first, open source, freely available database of unique germline variants obtained from whole exome data of 62 ‘normal’ samples from tongue, gallbladder, and cervical cancer patients of Indian origin. ‘TMC-SNPdb’ is presented with an easy-to-use graphic user interface feature to enable researchers to call true somatic mutations by depleting against Indian population specific SNPs, in addition to those already catalogued in dbSNP and 1000 Genomes databases. We demonstrate that ‘TMC-SNPdb’ effectively filters false positive somatic events across 75 tumor whole exome data.
Materials and methods
Ethical approval and informed consent
The sample set and study protocol was approved by Institutional Review Board (project no. 116 for cervical adenocarcinoma samples; project no. 88 for head and neck cancer samples, project 104 for gallbladder cancer samples). Cervical squamous carcinoma whole exome data have been described earlier in (19). Written informed consent was obtained from all patients.
Extraction of DNA
All ‘normal’ tissue samples under study were verified by an onco-pathologist to not harbor any cancer. A total of 62 samples ‘normal’ samples (16 peripheral venous blood and 46 adjacent normal tissue) were obtained for analysis: peripheral venous blood from patients with cervical squamous cell carcinoma (n = 10), cervical adenocarcinoma (n = 18) (adjacent normal tissue; n = 12 and peripheral venous blood; n = 6) and adjacent normal tissue from patient with tongue squamous cell carcinoma (n = 23) and gallbladder (n = 11) were obtained from Tata Memorial Hospital (TMH). Genomic DNA from tissues was extracted using DNeasy blood and tissue DNA extraction kit (Qiagen) according to manufacturer’s instructions. Quantification of DNA was assessed using Nanodrop 2000c Spectrophotometer (Thermo Fisher Scientific Inc.) and DNA integrity was determined by resolving on 0.8% Agarose gel. DNA was also quantified using Qubit ds DNA BR assay kit (Life Technologies, USA). DNA samples showing DNA concentration >50 ng/µl and intact DNA visualized on agarose gel were used for whole exome sequencing.
Exome capture, library preparation and sequencing
Three different library preparation kits were used to prepare libraries for different tumor types (Supplementary Table S1). First, TruSeq Exome Enrichment kit (v2 and v3, Illumina) was used to capture 62 Mb region (>3 40 000 probes) of human genome comprising 201 121 exons representing 20 974 gene sequences, including 5′UTR, 3′UTR, microRNAs and other non-coding RNA. For exome library preparation, two microgram genomic DNA was sheared using Covaris (Covaris Inc) for generating fragment sizes of 200–300 bp. Fragments end repairing, purification, A-tailing, adaptor ligation and quality control steps were carried out using TruSeq DNA Sample Prep Kit (Illumina) following manufacturer’s instructions. Qualitative and quantitative analysis of genomic DNA libraries were performed using High Sensitivity DNA chip on 2100 Bioanalyzer (Agilent) and qPCR with KAPA Library Quant Kit (Kapa Biosystems). Exome enrichment was done by incubation at 93 °C for 1 min (decreasing 2 °C per cycle for 18 cycles) followed by 58 °C for 19 h in ABI 9700 PCR system (Life Technologies) using 500 ng of genomic libraries.
Second, NimbleGen SeqCap EZ Exome Library (v3.0, Roche) targeting 64 Mb of the human genome was also used for library preparation. The protocol was adopted from the manufacture’s application note (http://www.nimblegen.com/products/lit/NimbleGen_SeqCap_EZ_SR_Pre-Captured_Multiplexing.pdf). Sequencing libraries were exome captured and later quality-controlled using a bioanalyzer (Agilent 2100) and libraries were qPCR quantified using KAPA Library Quant Kit (Kapa Biosystems) prior to cluster generation on an Illumina cBOT.
Third, SureSelect Human All Exon Kit, v5 (Agilent Technologies, Santa Clara, CA, USA) was also used to capture 50 Mb of the human genome using > 5 50 000 probes. One microgram of genomic DNA was utilized for library preparation and a similar protocol was followed as previously stated. Eluted exome-enriched library fragments were PCR amplified and purified.
qPCR quantified 7 pmol of 6-plex DNA library pool was loaded per lane on flow cell (Flow Cell v3) to generate clusters using TruSeq PE Cluster Kit v3-cBot-HS kit and clustered flow was sequenced for 201 and 301 cycles on HiSeq-1500 and NextSeq System (Illumina) using TruSeq SBS Kit v3 (Illumina), respectively.
Exome sequencing variant analysis for TMC-SNP database
Paired-end raw sequence reads were mapped to human reference genome (build hg19) using BWA v. 0.6.2 (20). Quality control analysis of bam files was carried out using qualimap (v0.7.1) (21). Mapped reads were then used to identify and remove PCR duplicates using Picard tools v.1.74 (http:broadinstitute.github.io/picard/). Base quality score recalibration and indel re-alignment were performed and variants were called from each sample separately using GATK Unified Genotyper (version 2.5-2) (22).
Development of TMC-SNP database
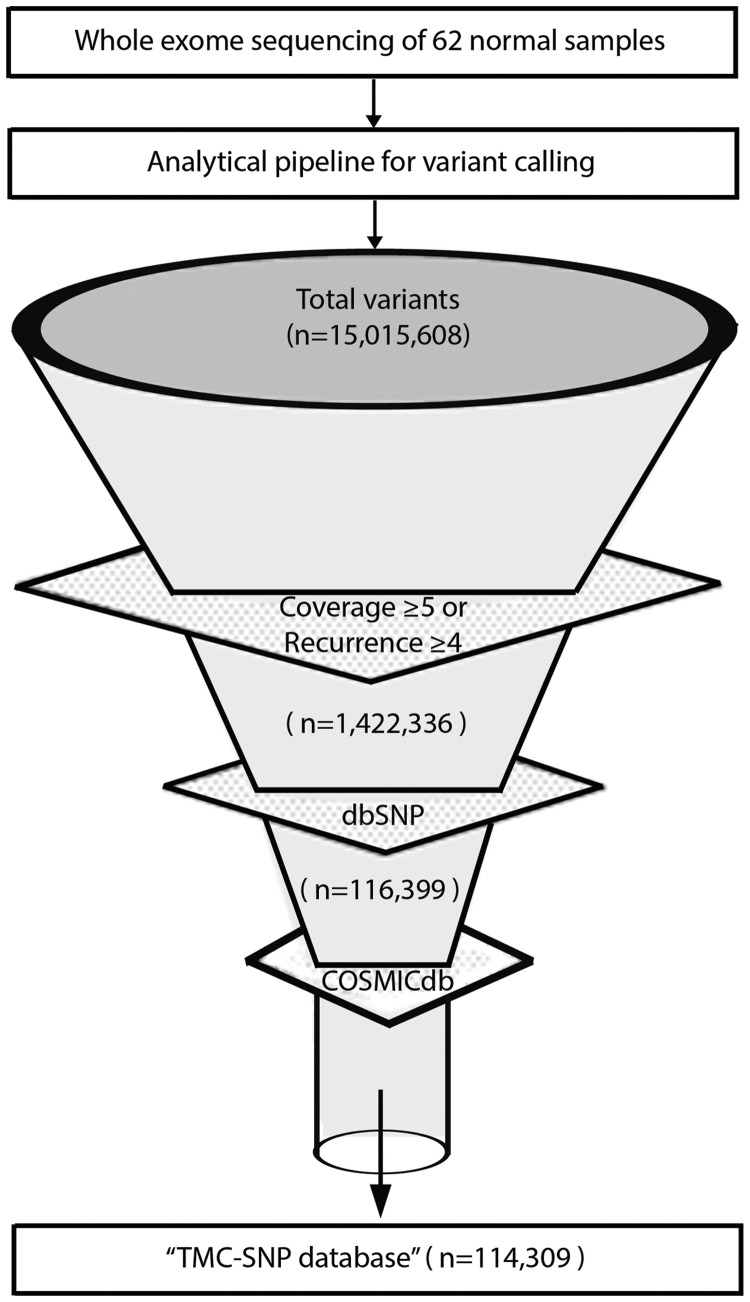
To restrict our analysis to high quality germline variants we applied filters of minimal base coverage and recurrence in cohort. In house developed scripts (Awk and Perl) were used to merge all 62 VCF files from normal tissues and mutational recurrence was calculated. We applied a standard filter of coverage ≥5 reads for altered alleles. Additionally, we included variants with coverage ≤5 but recurrent in ≥4 normal samples. Using these filters, we identified high quality variants in the dataset. High quality variants were further annotated using COSMICdb (version 68) (23) and dbSNP (version 142) (5). Remaining variants were further depleted against dbSNP and COSMICdb to remove all known somatic and germline variants. Finally, all remaining variants constitute the TMC-SNP database. A detailed schema of resource and data representation is provided in Supplementary Figure 3.
Application of TMC-SNP database in analyzing tumor samples
GATK (version 2.5-2) and MuTect (version 1.0.2) (24) were utilized to generate raw variants of tumor samples and filtered against its matched normal . Variants obtained from GATK and MuTect were merged and variants having ≥5 reads for altered allele were kept for further downstream analysis. Similar analysis was carried out for three cancer types. Comparison with dbSNP(version142) and COSMICdb(version 68) was performed using in-house developed scripts in Perl and Awk which were later used to calculate the percentage changes in variants in different cancer type post filtration with dbSNP and TMC-SNP database. Functional annotation of variants was performed using Oncotator (variant annotation tool) (25).
Germline variant subtraction program
TMC-SNPdb is distributed as a SQLite file containing variant information table. A companion tool for subtraction of germline variants from tumor sample has been developed in python (version 3.4). It depends on PyVCF (version ≥ 1.6) and sqlite3 python packages. The variants in TMC-SNPdb are characterized by a unique combination of chromosome number, genomic position, reference allele, altered allele for each variant and subtraction was carried out based on these unique fields for each variant in VCF file. The tool is an executable compatible with Linux operating system and has been tested on several Linux platform such Red Hat (version 6.5), Fedora (version 22) and Ubuntu (version 14.04). It can be executed using a command line interface (‘TMC-SNP’) or a graphical user interface (GUI) (‘TMC-SNP_GUI’). The GUI mode additionally depends on TKinter python library (version ≥ 2.4). Moreover, the tool has a feature which lets users create their own germline variant database from VCF format files of normal samples. The output obtained from the tool is in VCF format. Detailed user manual with snapshots of the GUI and schematic representation of overall usages are provided in Supplementary file 1 and Supplementary Figure S2.
Availability of supporting data
The raw sequence data has been deposited at the ArrayExpress (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-4618), hosted by the European Bioinformatics Institute (EBI). The ‘TMC-SNPdb’ has been submitted to Annovar (http://annovar.openbioinformatics.org/en/latest/user-guide/download/) and dbSNP (http://www.ncbi.nlm.nih.gov/SNP/snp_viewTable.cgi?handle=TMC_SNPDB) for public access.
Results
Development of TMC-SNP database
We analyzed whole exome sequencing at a median of 88x coverage for 62 normal samples derived from cancer patients, comparable with similar reports (26) as detailed in Supplementary Table S1. Of note, coverage among 4 of 62 samples were <30× due to high duplication reads and low yield in these samples. Germline mutations were called using GATK (22): a total of 15 015 608 germline variants were identified across the complete dataset. As shown in Figure 1, standard quality filters of minimal 5× coverage or recurrence in at least four samples for each variant led to about 90% reduction in raw variants (see Materials and Methods section for details). The remaining 1 422 336 variants of higher confidence were further depleted against dbSNP v142. 1 305 937 of 1 422 336 variants, constituting 92% SNPs were depleted. To remove variants known to be somatically associated with cancer in literature but figured as a germline event in our study (most likely due to inadequate or non-uniform coverage of their paired normal samples), we further depleted 2090 variants (2%) overlapping with COSMICdb with an assumption of these variants to be false somatic events in our data set. Finally, a total of 114 309 variants were identified after filtering with dbSNP and COSMICdb as a pool of previously unknown germline variants of high confidence recurring in the Indian population to constitute the ‘TMC-SNPdb’.
Figure 1.
Development of TMC-SNPdb using whole exome sequencing. Schematic flow representation of steps followed during development of TMC-SNP database. The whole exome sequencing of 62 normal tissue obtained from three different tissues of cancer patients was performed and analysed using GATK (Genome Analysis Tool Kit) to generate VCF files. Raw variants obtained were further filtered using mentioned criteria to find a list of variants absent in dbSNP v142 and COSMICdb v68. Remaining variants constitutes the ‘TMC-SNPdb’ shown at the end of the funnel.
Characteristic features of TMC-SNP database
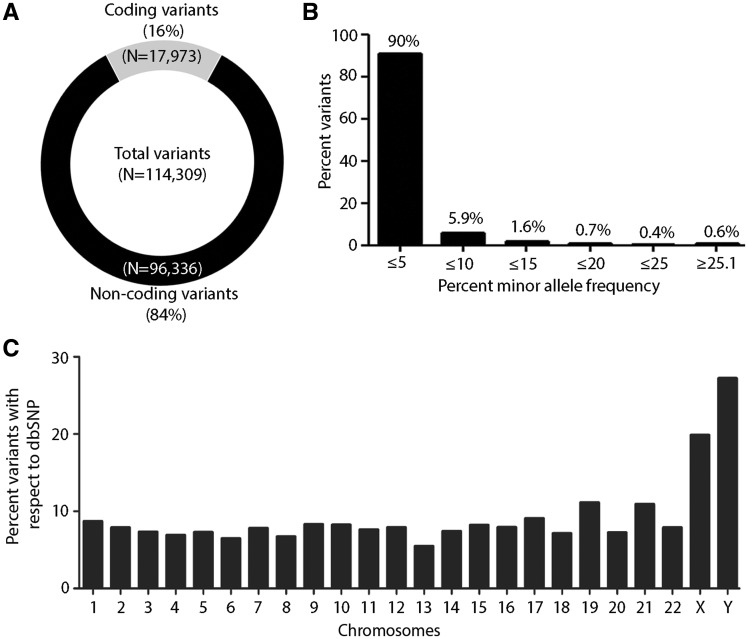
A total 114 309 variants were annotated using Oncotator for functional features (25). A distribution pattern of coding (∼17 973) and non-coding variants germline variants (∼96 336) is shown in Figure 2A. Of 17 973 coding variants, 11 466 were of non-synonymous (NS) (∼63%) and 6507 were synonymous variants (S) (∼36%) with NS/S ratio 1.76, consistent with previous reports for exome data from normal samples (27, 28). Furthermore, we observed a high frequency of missense (∼58%) and silent variants (∼30%) as compared with indel (∼3%), nonsense (∼2%) and splice site (∼6%) region (Supplementary Figure 1A). Of all the SNPs present in TMC-SNPdb, distribution varied across the genome as follows: protein-coding exon (15.7%), intron (40%), IGR (25.8%), 3′UTR (9.5%), 5′UTR (2.37%), RNA (3.74%) and lincRNA (1.7%), consistent with earlier report from exome sequencing data (Supplementary Figure S1B) (29, 30). Next, we computed the allele frequency of all 114 309 variants present in the TMC-SNPdb, across 62 samples. Given that TMC-SNPdb predominantly enlists low frequency germline variants prevalent among Indian population, similar to 1000 genomes and ExAC wherein about 99% of SNPs are estimated to have a minor allele frequency over 1% (8, 26), Similarly, in TMC-SNPdb >90% of variants present exist at a minor allele frequency≤5% (Figure 2B).
Figure 2.
Overall overview of characteristic features of TMC-SNP database. (A) Circle plot of coding and non-coding variants obtained in the dataset. (B) Percent minor allele frequency distribution of variants in ‘TMC-SNPdb’ across 62 normal samples. Percentage frequencies are presented on the top of each bar. (C) Genome-wide distribution of percent frequency of variants obtained in each chromosome as compared with dbSNP database.
Furthermore, a comparative measure of variability added by the TMC-SNPdb variants to the known pool of SNPs per chromosome was reckoned following comparison with dbSNP variants across the genome. Interestingly, we found maximal variability at the Y-chromosome among 2418 of 8885 SNPs (27%), while the distribution of the variants across the autosomal chromosomes was found to be uniformly distributed among 106 184 of 1 346 256 SNPs (7.6%) similar to the dbSNP (Figure 2C). Of note, variants at Y-chromosome tend to be more localized geographically than those of mitochondrial DNA (mtDNA) and autosomes, which is reflective of the degree of inter-population genetic differences (31–33). Y-chromosomes have been shown to harbor population specific unique haplotype in Indian population and have frequently been used as a marker for studying human demographic history (34, 35). The higher variability at the Y-chromosome found in TMC-SNPdb is thus consistent with several earlier reports describing a high variation of male lineages within Indian population (17, 18) that further emphasizes the Indian specific characteristics of the TMC-SNPdb germline variants, and a need for distinct Indian specific germline database .
Finally, a significant characteristic feature of TMC-SNPdb is the companion subtraction tool with command line and GUI based interface. The user can deplete their data set against TMC-SNPdb or create a customized normal variant database. The program has been tested to run on various Linux platforms such Fedora, Ubuntu and Red Hat operating systems. (Detailed user manual and snapshot of different steps have been provided in Supplementary Materials S1, S2 and Supplementary Figure S2). Using the companion tool on an 8GB machine, it takes 56 and 72 min to filter standard VCFs containing 115 884 and 227 779 raw variants (provided as example file with tool) against the TMC-SNPdb variants, respectively.
Application of ‘TMC-SNPdb’ in depleting germline variants predominant among indian population
With the flexibility of using GUI interface or through the command line (refer to Supplementary Material S1), we tested the robustness and practical utility of ‘TMC-SNPdb’ across various cancer types to infer the extent of depletion of population specific variants over and above the dbSNP. We analyzed 132 samples of three cancer types: head and neck cancer (n = 43), cervical cancer (n = 62) and gallbladder cancer (n = 27). Significant fold reduction of variants was observed following TMC-SNPdb subtraction in addition to depletion by dbSNP in all cancer types studied. Of 613 055 variants found across 24 head and neck cancer tumor samples about 92% SNPs were depleted post dbSNP subtraction with 84 001 candidate somatic variants. Subsequent depletion using TMC-SNPdb identified 35 819 additional variants as Indian specific germline variants existing at varying frequency in normal Indian population. In overall, TMC-SNPdb allowed us to filter an additional 42.6% of post dbSNPs depleted SNPs. in 24 tongue cancer samples (Table 1). Similarly, TMC-SNPdb significantly reduced about 33.3% and 27.7% SNPs in 17 gallbladder and 34 cervical tumor whole exome data, as tabulated in detail in Table 1.
Table 1.
Application of TMC-SNPdb across cancer types to filter germline variants in Indian population
| S.No. | Cancer type | Total variants | Number of samples | Number and percentage (along row) of novel variants |
Overall reduction by TMC-SNPdb post dbSNP depletion | |
|---|---|---|---|---|---|---|
| Post dbSNP depletion | Post TMC-SNPdb depletion | |||||
| 1 | Tongue cancer | 613 055 | 24 | 84 001 (13.7%) | 48 182 (7.8%) | 42.6% |
| 2 | Cervical cancer | 923 547 | 34 | 99 032 (10.7%) | 71 594 (7.7%) | 27.7% |
| 3 | Gall-bladder | 328 245 | 17 | 26 530 (8%) | 17 682 (5.3%) | 33.3% |
Total number of variants observed for each cancer types and reduction in number and percent variants post dbSNP and post TMC-SNPdb subtraction is tabulated for three cancer types. Number of samples analysed across tumor is also denoted.
Discussion
TMC-SNPdb is a freely available open access Indian population specific germline variant database consisting of 114 309 germline variants using whole exome sequencing of 62 normal tissues from patients with different types of cancer. Its usage is analogous to depletion against pooled normal variants from unrelated normal samples of Indian origin for paired or orphan tumor samples. The utility of subtraction against pooled normal variants has been well described as a reference for depletion, especially for orphan tumor samples wherein paired normal variant data for the tumor samples are not available (36–39). Our dataset and companion tool can be used, along with other public databases, as ‘normal’ counterpart to identify disease specific somatic mutations, especially in cancer exome studies. Using TMC-SNPdb across 132 whole exome data of 3 tumor types, we show that it can significantly deplete false positive somatic variants.
TMC-SNPdb is presented with a companion program with command line or user-friendly GUI interface for non-computational biologists. It has two built-in features: first, a user can input tumor VCF to subtract against TMC-SNPdb and second, create a custom database of germ line mutation with the availability of multiple normal VCF files and then subtract with tumor VCF to deplete germ line variants. The subtraction program has been tested on several Linux platforms such as Fedora, Ubuntu and Red Hat system. Because it is an open source tool, it could be further modified to alter filtering parameters for analysis indicative of its expandability and universal applicability on Linux platforms.
There are two major limitations of TMC-SNPdb database. First, it is presumed that a sample derived from cancer patients represents ‘normal’ genome variation. However, because of their diseased status, a fraction of such individuals are likely to harbor cancer predisposing variants in their germline. Any such germline variant that is novel in Indian population (not yet included in Caucasian databases) and which predisposes to cancer (e.g. in BRCA 1 gene) would be characterized as ‘normal’ population variation in TMC-SNPdb. Thus, this database will be limited in application to analyses that seek to evaluate germline predisposition to cancer. Second, majority of ‘normal’ samples were obtained from sites adjacent to a tumor with histopathological based inspection for the absence of tumor cells. However, it is possible that these tissues harbors some bona fide somatic mutations due to effect of field cancerization (40, 41). Thus, depleting against TMC-SNPdb could potentially ‘over-subtract’ mutations that are bona fide somatic. To minimize this possibility, we have filtered TMC-SNPdb variants against COSMIC database to remove any known cancer related somatic variants. However, there remains a residual potential for missing ‘somatic’ mutations that are novel in tumors of Indian patients and present in adjacent ‘normal’ tissue. With these caveats, we believe that TMC-SNPdb with its companion tool is a step towards fulfilling a significant unmet need for an Indian population ‘normal’ variant database, especially in somatic mutation analyses in tumors from Indian patients.
In summary, TMC-SNPdb is an open source database of ‘normal’ germline variants derived from Indian—non-European Caucasian—population, not yet included in the public databases with predominant Caucasian representations. It comes along with a companion tool that can apply this information for somatic cancer genome analyses by depleting against the TMC-SNPdb. This database is flexible to accommodate the need for customization by allowing inclusion of similar datasets from additional individuals.
Supplementary data
Supplementary data are available at Database Online.
Acknowledgements
Sandor Lifesciences Pvt. Ltd., Genotypic Pvt. Ltd, and Scigenome Labs, as commercial vendors for generating the whole exome library preparation and sequencing services. Rita Mulherkar for cervical squamous sample exome data.
Funding
A.D. is supported by an Intermediate Fellowship from the Wellcome Trust/DBT India Alliance (IA/I/11/2500278), by a grant from DBT (BT/PR2372/AGR/36/696/2011), and intramural grants (IRB project 92 and 55). P.U is supported by Senior Research Fellowship from CSIR, N.G is supported by Junior Research Fellowship from TMH, P.C and P.I. are supported by Senior Research Fellowship from ACTREC and T.T. and S.D. is supported by Junior Research Fellowship from DBT and ACTREC, respectively. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the article.
Conflict of interest. None declared.
References
- 1.Martincorena I., Campbell P.J. (2015) Somatic mutation in cancer and normal cells. Science, 349, 1483–1489. [DOI] [PubMed] [Google Scholar]
- 2.Stratton M.R., Campbell P.J., Futreal P.A. (2009) The cancer genome. Nature, 458, 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelstein B., Papadopoulos N., Velculescu V.E. et al. (2013) Cancer genome landscapes. Science, 339, 1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones S., Anagnostou V., Lytle K. et al. (2015) Personalized genomic analyses for cancer mutation discovery and interpretation. Sci. Transl. Med., 7, 283ra253.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherry S.T., Ward M.H., Kholodov M. et al. (2001) dbSNP: the NCBI database of genetic variation. Nucleic Acids Res., 29, 308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genomes Project C., Abecasis G.R., Auton A. et al. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature, 491, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L., Wheeler D.A. (2014) Genomic sequencing for cancer diagnosis and therapy. Annu. Rev. Med., 65, 33–48. [DOI] [PubMed] [Google Scholar]
- 8.Lek M., Karczewski K., Minikel E., et al. (2016) Analysis of protein-coding genetic variation in 60,706 humans. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H., Wang K. (2015) Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc., 10, 1556–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narang A., Roy R.D., Chaurasia A. et al. (2010) IGVBrowser–a genomic variation resource from diverse Indian populations. Database (Oxford), 2010, baq022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indian Genome Variation C. (2005) The Indian Genome Variation database (IGVdb): a project overview. Hum. Genet., 118, 1–11. [DOI] [PubMed] [Google Scholar]
- 12.Consortium H.P.A.S., Abdulla M.A., Ahmed I. et al. (2009) Mapping human genetic diversity in Asia. Science, 326, 1541–1545. [DOI] [PubMed] [Google Scholar]
- 13.Tamang R., Singh L., Thangaraj K. (2012) Complex genetic origin of Indian populations and its implications. J. Biosci., 37, 911–919. [DOI] [PubMed] [Google Scholar]
- 14.Tamang R., Thangaraj K. (2012) Genomic view on the peopling of India. Investig. Genet., 3, 20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reich D., Thangaraj K., Patterson N. et al. (2009) Reconstructing Indian population history. Nature, 461, 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder P.P., Basu A. (2015) A genomic view of the peopling and population structure of India. Cold Spring Harb Perspect Biol, 7, a008540.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basu A., Mukherjee N., Roy S. et al. (2003) Ethnic India: a genomic view, with special reference to peopling and structure. Genome Res., 13, 2277–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta S., Zhivotovsky L.A., King R. et al. (2006) Polarity and temporality of high-resolution y-chromosome distributions in India identify both indigenous and exogenous expansions and reveal minor genetic influence of Central Asian pastoralists. Am. J. Hum. Genet., 78, 202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrani P., Kulkarni V., Iyer P. et al. (2015) NGS-based approach to determine the presence of HPV and their sites of integration in human cancer genome. Br. J. Cancer, 112, 1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Durbin R. (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics, 25, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Alcalde F., Okonechnikov K., Carbonell J. et al. (2012) Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics, 28, 2678–2679. [DOI] [PubMed] [Google Scholar]
- 22.McKenna A., Hanna M., Banks E. et al. (2010) The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res., 20, 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamford S., Dawson E., Forbes S. et al. (2004) The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer, 91, 355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cibulskis K., Lawrence M.S., Carter S.L. et al. (2013) Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol., 31, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos A.H., Lichtenstein L., Gupta M. et al. (2015) Oncotator: cancer variant annotation tool. Hum. Mutat., 36, E2423. (2429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genomes Project C., Auton A., Brooks L.D. et al. (2015) A global reference for human genetic variation. Nature, 526, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu B., Roos J.L., Dexheimer P. et al. (2011) Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat. Genet., 43, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Genomes Project C., Abecasis G.R., Altshuler D. et al. (2010) A map of human genome variation from population-scale sequencing. Nature, 467, 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y., Long J., He J. et al. (2012) Exome sequencing generates high quality data in non-target regions. BMC Genomics, 13, 194.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuels D.C., Han L., Li J. et al. (2013) Finding the lost treasures in exome sequencing data. Trends Genet., 29, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez-Lezaun A., Calafell F., Comas D. et al. (1999) Sex-specific migration patterns in Central Asian populations, revealed by analysis of Y-chromosome short tandem repeats and mtDNA. Am. J. Hum. Genet., 65, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oota H., Kitano T., Jin F. et al. (2002) Extreme mtDNA homogeneity in continental Asian populations. Am. J. Phys. Anthropol., 118, 146–153. [DOI] [PubMed] [Google Scholar]
- 33.Kumar V., Langstieh B.T., Madhavi K.V. et al. (2006) Global patterns in human mitochondrial DNA and Y-chromosome variation caused by spatial instability of the local cultural processes. PLoS Genet., 2, e53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jobling M.A., Tyler-Smith C. (2003) The human Y chromosome: an evolutionary marker comes of age. Nat. Rev. Genet., 4, 598–612. [DOI] [PubMed] [Google Scholar]
- 35.Shrivastava P., Gupta U., Jain T. et al. (2015) Y STR haplotype diversity in central Indian population. Ann. Hum. Biol., 1–8. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A., White T.A., MacKenzie A.P. et al. (2011) Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc. Natl. Acad. Sci. U S A, 108, 17087–17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki A., Mimaki S., Yamane Y. et al. (2013) Identification and characterization of cancer mutations in Japanese lung adenocarcinoma without sequencing of normal tissue counterparts. PLoS One, 8, e73484.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raymond V.M., Gray S.W., Roychowdhury S. et al. (2016) Germline findings in tumor-only sequencing: points to consider for clinicians and laboratories. J. Natl. Cancer Inst., 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy M. (2015) Genomic sequencing of only tumor tissue could be misleading in nearly half of patients, study shows. Bmj, 350, h2036.. [DOI] [PubMed] [Google Scholar]
- 40.Dakubo G.D., Jakupciak J.P., Birch-Machin M.A. et al. (2007) Clinical implications and utility of field cancerization. Cancer Cell Int., 7, 2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohan M., Jagannathan N. (2014) Oral field cancerization: an update on current concepts. Oncol. Rev., 8, 244.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.