Abstract
Electron paramagnetic resonance (EPR), an established and powerful methodology for studying atomic-scale biomolecular structure and dynamics, typically requires in excess of 1012 labeled biomolecules. Single-molecule measurements provide improved insights into heterogeneous behaviors that can be masked in ensemble measurements and are often essential for illuminating the molecular mechanisms behind the function of a biomolecule. Here, we report EPR measurements of a single labeled biomolecule. We selectively label an individual double-stranded DNA molecule with a single nanodiamond containing nitrogen-vacancy centers, and optically detect the paramagnetic resonance of nitrogen-vacancy spins in the nanodiamond probe. Analysis of the spectrum reveals that the nanodiamond probe has complete rotational freedom and that the characteristic timescale for reorientation of the nanodiamond probe is slow compared with the transverse spin relaxation time. This demonstration of EPR spectroscopy of a single nanodiamond-labeled DNA provides the foundation for the development of single-molecule magnetic resonance studies of complex biomolecular systems.
Introduction
Single-molecule studies are essential for understanding the structural dynamics of biomolecules and the mechanisms responsible for their functions (1, 2, 3, 4, 5, 6, 7, 8, 9, 10). Many single-molecule techniques probe local biomolecular dynamics (1, 2, 3, 4, 5, 7, 8, 10) and their impact on the distribution of the structures and correlation times (1, 3, 4, 5, 8, 10) of these systems, providing insights into the mechanisms underlying biomolecular functionality (1, 2, 4, 6, 8, 9, 10). Single-molecule techniques typically rely on the attachment of a probe that can be detected and/or manipulated at the single-probe level, such as organic fluorophores, quantum dots, gold nanoparticles, superparamagnetic particles, and polystyrene beads (1, 2, 3, 4, 6, 7, 8, 9, 10, 11). An essential factor in using these probes for single-molecule detection is the development of the chemistry that enables site-specific attachment of the probe to the biomolecule of interest. A remaining key challenge for electron paramagnetic resonance (EPR) measurements is the development of electron spin probes that can be detected at the single-molecule level and site-specifically attached to biomolecules. Such a probe could enable EPR studies of single biomolecules.
EPR is a proven and versatile method for quantifying timescales and the degree of rotational freedom of molecular motion (12, 13, 14, 15, 16, 17, 18). Motion on subnanosecond to millisecond timescales can be explored using existing continuous-wave (CW) and pulsed EPR techniques (16, 17), offering a potentially powerful enhancement of the dynamic range available in studies of individual biomolecules. The power of EPR for measuring structural dynamics at widely varying rates arises from the sensitivity of the EPR spectral lineshape to motion. This has the advantage that fluctuations due to motion, characterized by a correlation time , will be evident through its impact on the linewidth and hence will be limited by the intrinsic spectral linewidth relative to the mechanism responsible for motion-dependent broadening (in this case, the anisotropic Zeeman interaction). Fluctuations arising from motion will average the dephasing induced by this interaction, leading to a linewidth proportional to . The ability to tune the anisotropy and hence the broadening via the Zeeman interaction makes the subnanosecond regime accessible. This approach complements imaging approaches such as fluorescence resonance energy transfer (FRET), optical tweezers, and magnetic tweezers that measure motion through sequential measurements in the time domain and thus are limited for short by their sampling rate. However, the severe sensitivity limitation imposed by conventional inductive detection of the EPR signals of site-specifically attached spin probes, such as a nitroxide, imposes the requirement that measured samples must contain 1010–1015 electron spin-labeled molecules (19, 20, 21). A single-molecule approach can also facilitate EPR studies of biomolecules that are difficult to prepare at high concentrations. More generally, crucial information regarding the molecular function of biomolecular complexes is masked by the averaging that is inherent to ensemble studies (1, 2, 3, 4, 5, 6, 7, 8, 9, 10).
Recent advances that take advantage of the optical properties of the nitrogen-vacancy (NV) center in diamond (see Fig. 1) to enable optically detected magnetic resonance (ODMR) can be used to sensitively measure dynamics of adjacent spin labeled proteins (22) and nanomagnets (23). In the complementary approach we present here, a nanodiamond is site-specifically attached to a single biomolecule, creating an electron spin resonance (ESR) active, in situ spin label that can be sensitively detected using NV ODMR to measure the molecular dynamics. This approach to performing EPR on an individual spin label offers an attractive tool that could enable application of these high-resolution spectral techniques to probe the biomolecular structure and dynamics of single molecules.
Figure 1.
(a) Schematic of the NV center atomic structure (left), composed of a nitrogen impurity adjacent to a vacant site in the diamond tetrahedral lattice. The NV center axis can adopt any of four allowed orientations in the diamond lattice (middle). Our NV nanodiamonds are single-crystalline and range from 10 to 200 nm in diameter (right). (b) Simplified schematic of the electronic structure of the NV center. (c) Optically detected magnetic resonance spectrum of a collection of NV centers in a diamond crystal, exhibiting the Zeeman splitting of the magnetic resonance peaks by an applied magnetic field (shown by dark blue arrows) and the dependence of the splitting on the orientation of the field relative to the NV axis. To see this figure in color, go online.
Here, we take advantage of the NV defect center in diamond that offers an extraordinarily sensitive method for optically detecting EPR (24, 25, 26, 27). We demonstrate optical detection of EPR from the NV centers in a single-crystal nanodiamond attached to a single biomolecule. In particular, we report site-specific labeling of double-stranded DNA molecules with individual nanodiamonds, the experimental design for optically detecting magnetic resonance from nanodiamond-labeled DNA, and experimental measurements of the EPR spectra of these nanodiamond-labeled DNA molecules. The Zeeman shift of the magnetic resonance frequency is sensitive to the orientation of the applied magnetic field relative to the symmetry axis of the NV center, which makes the EPR spectrum sensitive to fluctuations in the orientation of the diamond crystal. Our single-molecule EPR (smEPR) spectra demonstrate that the nanodiamond probe, while attached to a single DNA molecule, explores all available orientations on timescales slower than the spin relaxation time, , of the nanodiamond. In this slow motional limit, the spectrum of the single nanodiamond is equivalent to that obtained from a large collection of nanodiamonds because all crystalline orientations will be represented in this static collection (the spectrum in this case is a powder pattern (12, 13)). This approach should ultimately enable measurement of the local flexibility of the biomolecule to which the spin probe is attached, such as is done in EPR studies of nitroxide-labeled biomolecules (15, 16, 17, 18). This study demonstrates the feasibility of using NV-containing nanodiamonds to probe biomolecular dynamics with smEPR, and provides a foundation for combining the ability to spectroscopically measure many decades of motional timescales provided by EPR with the power of single-molecule methodologies.
Materials and Methods
Nanodiamond sample preparation
The NV nanodiamonds (Van Moppes SYP0.09, Geneva, Switzerland) are synthesized from a high pressure, high temperature (HPHT) diamond with a nitrogen impurity content of ∼200 ppm. The diamond is milled down via microfracturing into monocrystalline nanodiamonds with a size distribution of 10–200 nm in diameter. The nanodiamond is then irradiated (Prism Gem, New York, NY) with a 1.5 MeV, 3.48 × 1018/cm2/h electron beam for 3 h to create a vacancy density nV ∼59 ppm. The nanodiamond is then annealed at 900°C for 3 h in 96% Ar and 4% H2 to bring the nitrogen impurities and vacancies adjacent to each other to create NV centers, which typically leads to an NV center density of ∼30 ppm (28, 29, 30, 31).
Next, the nanodiamonds are cleaned to remove any graphitic residue from the surface. The removal of the graphite both prepares the surface of the diamond for biochemical functionalization (32, 33) and also reduces damping of the fluorescence of the NV centers near the surface of the nanocrystal due to surface effects (32, 34, 35). The nanodiamond powder is acid-cleaned under reflux. First, it is boiled in a 9:1 mixture of H2SO4 (98%) and HNO3 (70%) at 90°C for 3 days. Next, it is washed in deionized water and then resuspended in a new 9:1 acid mixture and boiled for 1 day. Then, it is washed and resuspended in 0.1 M NaOH and boiled at 90°C under reflux for 2 h. Finally, it is washed and resuspended in 0.1 M HCl and boiled at 90°C for another 2 h. The first two acid-washing steps in H2SO4 and HNO3 are then repeated. Lastly, it is triple rinsed with deionized water and ready for surface functionalization (32, 33, 34, 35, 36, 37, 38, 39).
Nanodiamond surface biotinylation
The acid-reflux cleaning of the nanodiamond prepares the surface with terminal carboxyl groups (32, 33, 37, 38, 39). The nanodiamond is then reacted with glycidol (Sigma-Aldrich, St. Louis, MO) to create a hydroxyl terminated surface (40, 41). The diamonds are then rinsed three times with dimethylacetamide and resuspended in 100 of the same solvent. This is diluted into 900 of dimethylformamide containing 100 mM N′-disuccinimidyl carbonate (DSC; EMD Millipore, Billerica, MA) and allowed to react for 2 h at room temperature. Unreacted DSC is removed by washing three times in dimethylacetamide and then quickly rinsing once in cold phosphate-buffered saline containing 0.05% Tween 20 (PBST). The N-hydroxysuccinimide (NHS)-activated diamonds are resuspended in PBST with a 1% solution of 4,7,10-Trioxa-1,13-tridecanediamine (TTDD; Sigma-Aldrich). The reaction is incubated for 2 h at room temperature, and unreacted TTDD is removed by exhaustive rinsing with PBST. The resulting amine-PEG3-functionalized diamonds are finally reacted with 0.22 M/mL NHS-dPEG-biotin (Quanta Biodesign, Powell, OH) for 2 h, triple rinsed in PBST, and finally suspended in PBST for the experiment. This process is illustrated in Fig. 2.
Figure 2.
Workflow of the NVND biotinylation chemistry protocol. To see this figure in color, go online.
λ DNA preparation
λ DNA functionalized with biotin and digoxigenin is prepared using commercially available linear λ DNA (New England Biolabs, Ipswich, MA), which in its linear form contains 48,490 bp of double-stranded DNA with a 12 nt overhang at both 5′ ends. The biotin is attached by ligating a synthetic oligonucleotide (Operon) containing a 3′ biotin (5′-AGGTCGCCGCCC-3′-biotin), and the digoxigenin is attached by ligating a synthetic oligonucleotide (Eurofins MWG Operon, Louisville, KY) containing a 3′ digoxigenin (5′-GGGCGGCGACCT-3′-Dig) to the λ DNA. The ligations are done simultaneously with a 100-fold molar excess of each oligonucleotide in 1× T4 Ligase buffer, 1× BSA, and 400 units of T4 Ligase (New England Biolabs). This reaction is allowed to proceed for 4 h at room temperature and then incubated at 65°C for 20 min to heat-kill the ligase enzyme. The functionalized λ DNA, which is now 48,514 bp (16.5 μm) in length, is purified from excess oligonucleotide ends via a Microspin G-50 column (GE Healthcare Lifesciences, Pittsburgh, PA).
DNA-nanodiamond attachment chemistry
In preparation for the attachment of DNA to the flow-cell surface, the flow-cell coverslip is Piranha cleaned (2:1, H2SO4 to H2O2) and then treated with 3-aminopropyl-triethoxysilane (MP Biomedicals 154766, Santa Ana, CA) to terminate the surface with amine groups. Next, the surface is coated with glutaraldehyde (OCHC3H6HCO; Electron Microscopy Sciences 16320) and then terminated with antidigoxigenin antibody (Roche Diagnostics 11333089001, Indianapolis, IN) (42). The DNA molecule is bound to the glass surface of our flow cell using an antidigoxigenin-digoxigenin antibody attachment. A schematic of this chemistry is given in Fig. 3 a.
Figure 3.
Attachment of a single nanodiamond to a single 16.5-μm-long DNA molecule. (a) Schematic representation showing the binding between streptavidin, antidigoxigenin antibodies, individual dual-labeled (digoxigenin and biotin) λ DNA molecules, and biotinylated fluorescent nanodiamonds. The assembly occurs on the surface of the glass coverslip. (b) Epifluorescence microscope image of the attached nanodiamond. On the top is an image of the surface through a 570 nm bandpass filter. On the bottom is the same surface as seen through a 670 nm bandpass filter. The NV diamond fluorescence will pass through the 670 nm filter and the SYBR Gold-dyed DNA molecules will emit through the 570 nm bandpass filter only. To see this figure in color, go online.
To create the single DNA-nanodiamond attachments on the flow-cell surface, first the biotinylated NV nanodiamond is pipetted into 1 mg/mL streptavidin (Sigma S4762-1MG). After a 10 min incubation period, the excess streptavidin is removed with two washes in PBS. The streptavidin-coated NV nanodiamond is resuspended in PBST and 0.2 mg/mL bovine serum albumin (BSA). The λ DNA is gently pipetted into the sample and allowed to incubate for 15 min. The sample is finally pumped into the prepared flow cell and allowed to incubate for 20 min, allowing ample time for the digoxigenin tag on the DNA molecule to attach to the flow-cell surface. The cell is washed with PBST-BSA to remove any excess DNA.
Custom-designed confocal microscope
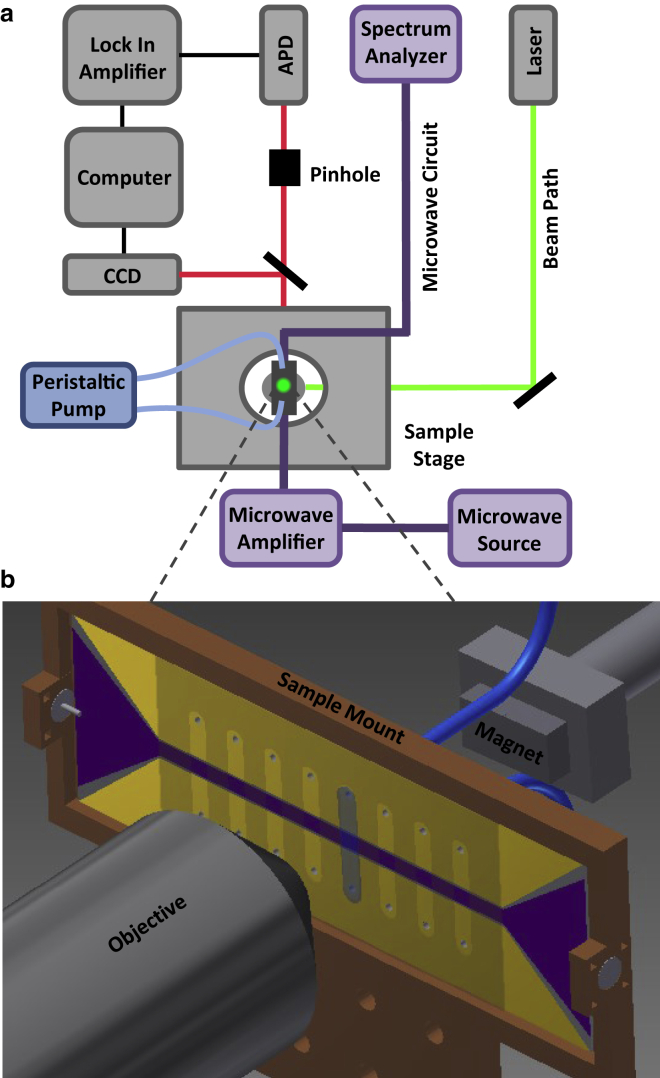
As shown in Fig. 4 a, the sample is pumped with a CW laser (532 nm, 300 mW DPSS laser; SNOC Electronics, Guangdong, China) through the objective (Nikon Plan Fluor 100× oil-immersion, 1.3 NA), and the subsequent photoluminescence from the NV centers is collected back through the objective and directed onto an avalanche photodiode (APD, Pacific Silicon Sensor AD500-8-S1BL, Westlake Village, CA). The APD signal is processed through a lock-in amplifier (model 7265 DSP; Signal Recovery, Oak Ridge, TN) at 400 Hz, which matches the amplitude modulation frequency of the microwave source (Agilent 8648C, Santa Clara, CA). The continuous microwave radiation is delivered to the sample through coaxial cables connected to the sample mount. The microwave and laser radiation powers are kept low to prevent sample heating and preserve sample integrity. (Our DNA-nanodiamond samples demonstrated stability over many hours of continuous excitation during our measurements.) The external magnetic field is applied via a set of rare-earth magnets that can be rotated 360° in the sample plane.
Figure 4.
(a) Simplified schematic of the custom-built confocal microscope. The optical components (in gray) are tightly integrated with the microwave circuit (components in purple) and flow-cell circuit (in blue). (b) Zoomed-in view of the integrated microwave and fluid circuits at the sample, illustrating eight flow channels for housing the in vitro single-molecule experiment, running perpendicular across the microwave coplanar waveguide. The microwave circuit path is highlighted in purple and the fluid circuit is in blue. To see this figure in color, go online.
Powder spectrum modeling and fitting
To analyze the smEPR spectrum, we simulate the spectrum and fit the simulation to the data (shown as solid lines in Figs. 1 c and 5, b and c). From these fits we can extract the applied field, strain, and resonance linewidth of the NV centers. The NV center is well described by the Hamiltonian (26)
| (1) |
where D = 2.87 GHz is the zero-field splitting, ge is the electron g-factor, is the Bohr magneton, B is an externally applied magnetic field, E is the strain, and Sx, Sy, Sz are the spin-1 Pauli matrices. Since carbon-13 is 1.1% abundant, some NV centers will also experience a strong hyperfine interaction with the carbon-13 nuclear spin, which adds an additional term to the Hamiltonian, , where A is the hyperfine tensor and I is the carbon-13 nuclear spin.
Figure 5.
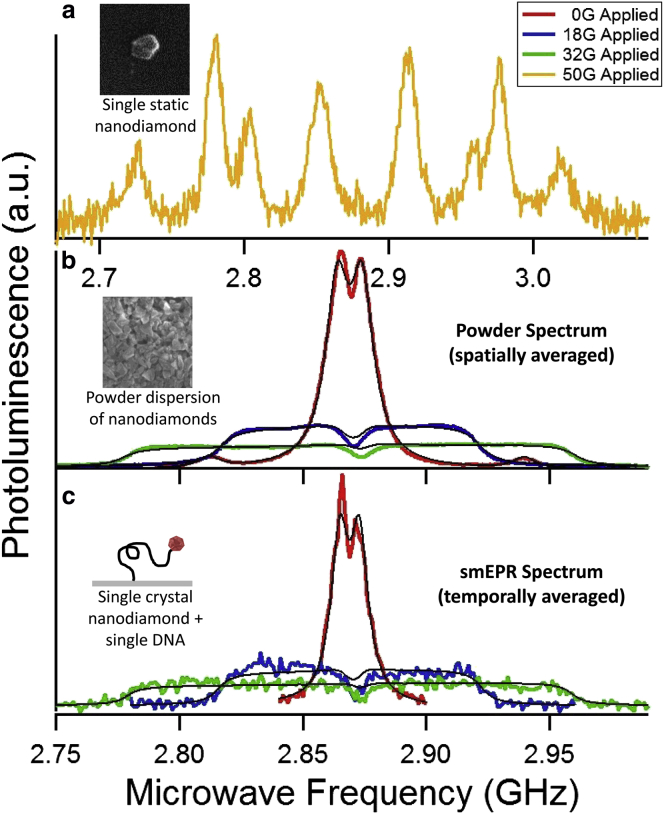
ODMR spectra. (a) ODMR spectrum of a single static nanodiamond. The eight distinct peaks arise from NV centers oriented in four directions allowed by the nanodiamond crystal lattice. (b) NV diamond powder dispersion, collected from a large population of randomly oriented nanodiamond crystals (top inset) under three different applied external fields: 0 G (red), 18.7 G (blue), and 32.6 G (green). The uniform intensity of this spectrum reflects the uniform probability of finding an NV center with any particular orientation relative to the applied field. The field values are extracted from the fit for each spectrum (black) based on our model and are in agreement with our field calibration. (c) smEPR spectrum of a nanodiamond crystal attached to a single DNA molecule (bottom inset) under three similar applied external fields (0 G (red), 19.0 G (blue), and 32.1 G (green)), again extracted from the fit (black) for each spectrum, also based on our model. The close similarity to the powder spectrum confirms our expectation that the nanodiamond probe will rotate isotropically, and hence freely, through all possible orientations on a timescale that is slow compared with the characteristic transverse spin relaxation time for NV centers. Each spectrum is the average of five frequency sweeps and each sweep takes 300 s. To see this figure in color, go online.
Diagonalization of the Hamiltonian yields the allowed eigenstates, which are sensitive to the direction of the applied magnetic field. Each NV center is oriented along one of diamond’s four crystallographic axes, and the zero-field splitting term quantizes the NV center’s z-component of spin along this axis. The eigenstates depend upon the angle between the NV axis and the applied magnetic field, which is important for fitting the powder of nanodiamonds, which contains NV centers randomly oriented in any direction.
We generate the powder pattern spectra shown in Fig. 5 by using the conventional formula described by Abragam (13). We find the energy splitting of the two allowed spin transitions, i.e., between the 0 and spin states, at a particular angle. We use a Lorentzian lineshape to describe the resonance, which yields a simulated resonance spectrum for a single nanodiamond. We then average over spectra obtained at all angles to simulate the full powder spectrum. We apply this method to simulate both the static spectrum of many nanodiamonds dispersed on a surface and the dynamic spectrum of a single nanodiamond attached to a DNA strand. Our experiment records the spectrum of the attached nanodiamond as it varies its orientation in time, exploring all orientations, such that a powder spectrum is a good approximation.
We then fit the simulated spectrum to the data to extract the relevant physical parameters. We first fit the zero-applied field powder spectrum data to extract the linewidth and strain, which causes a prominent dip in the center of the spectrum. The spectrum is the superposition of two spectra: NV centers without 13C interactions, and NV centers with 13C interactions that cause bumps on the edges of the spectra. The relative amplitudes for these two populations are also a fit parameter. We then fix the linewidth, strain, and relative amplitude of the 13C-interacting population, and only allow the applied magnetic field to be a fitting parameter for the spectra with applied fields. For the powder spectra in Fig. 5, the fits give estimated parameter errors that are <1 G for the three spectra with applied magnetic field.
Results
EPR measurements of the NV center in diamond
To perform a CW ODMR measurement, the NV center is irradiated with a microwave magnetic field whose frequency is varied until it matches the energy difference between its and states, thus driving one or both of these transitions. This increases the population of the states, which are able to decay through a nonradiative transition, thus reducing the fluorescence from the NV center (24, 25, 26, 27). These magnetic resonance frequencies are shifted in a magnetic field due to the Zeeman interaction with the electronic moment. The magnitude of this shift depends on the orientation of the magnetic field relative to the symmetry axis of the NV center, being largest when the field is parallel to this axis and vanishing when they are perpendicular. In Fig. 1 c, we show an ODMR spectrum from a single-crystal diamond that exhibits eight lines, two arising from each of the four orientations of the NV center symmetry axis allowed by the diamond crystalline structure (24, 25, 26, 27). We show the fluorescence quenching as peaks instead of valleys. In this work, we report ODMR measurements of an individual nanodiamond crystal attached to a single DNA molecule in which we spectroscopically measure the consequences of rotational motion of the nanodiamond crystal, and hence the molecule, relative to the applied magnetic field.
Experimental design for ODMR of a single biomolecule
An ODMR measurement of a single nanodiamond attached to a DNA molecule requires optical and microwave excitation combined with optical detection in a controlled aqueous environment. Our experimental setup (Fig. 4) integrates three key modules: 1) a confocal microscope that optically excites the nanodiamond and efficiently collects the NV fluorescence, 2) a coplanar waveguide microwave circuit that excites NV electron spins from the to the state, and 3) fluid circuitry that maintains and controls the DNA molecule-nanodiamond system during the ODMR measurement. Fig. 4 a shows a simplified schematic of the custom-built ODMR microscope used for the smEPR experiment. The optical setup (in gray) and beam path (green pump beam and red NV fluorescence) optically excite and collect the NV luminescence from the labeled sample in the flow-cell circuit (in blue). An expanded image of the sample mount (Fig. 4 b) shows the integrated microwave and fluid circuits. Eight fluid-flow channels to house the single-molecule experiments are aligned perpendicular to the Au microwave coplanar waveguide, which is photolithographically fabricated directly on the glass surface of the flow cell. The buffer and single DNA molecules labeled with a nanodiamond flow into the channel via a peristaltic pump, which pulls fluid into the channel through drilled holes in the back glass plane of the flow cell. The external magnetic field is applied by a set of rare-earth magnets that deliver a uniform, in-plane magnetic field at the focal spot of the objective. The magnetic field can be rotated 360° in the plane of the sample. Nanodiamond-labeled DNA molecules in the coplanar waveguide gap are selected for measurement to ensure sufficient microwave intensity.
Site-specific attachment of a single nanodiamond to a DNA molecule
The application of an NV nanodiamond as a spin probe requires that it be site-specifically attached to a DNA or protein molecule. We took advantage of the biotin-streptavidin-biotin linkage, which has been widely used in previous single-molecule measurements (1, 2, 4, 6, 10). We biotinylated the surfaces of the nanodiamonds (Fig. 2) prepared by HPHT methods. HPHT diamonds that have largely sp3 hybridized carbon are much easier to surface functionalize than detonation nanodiamonds, which can contain sp2 (graphitic) carbon surfaces (33). Nanodiamonds were subjected to repeated acid reflux cleaning and then incubated in glycidol to introduce alcohol groups onto the surface. The surface was then sequentially treated with DSC, TTDD, and finally NHS-dPEG-biotin. As a result, biotin was covalently attached to the diamond surface and sufficiently extended outward for efficient streptavidin binding. The nanodiamonds were then incubated with excess streptavidin, followed by two rounds of centrifugation to remove the unreacted streptavidin.
The DNA molecules that were the subject of our study were 16.5-μm-long λ DNA molecules labeled at one end with biotin and at the other end with digoxigenin for smEPR measurements. The biotin-labeled end of the λ DNA molecule was attached to a single biotinylated nanodiamond coated with streptavidin, and the digoxigenin-labeled end of the λ DNA molecules was tethered to the digoxigenin-coated glass surface in the smEPR flow cell (Fig. 3 a) (42). Epifluorescence microscopy was used to verify correct linkage between the antidigoxigenin-coated surface and a biotinylated NV nanodiamond (Fig. 3 b). Fluorescence images of SYBR Gold (Invitrogen, S11494)-labeled single λ DNA molecules tethered between the glass surface and a nanodiamond were acquired at two separate emission wavelengths. The images obtained at 570 nm (Fig. 3 b, top) show the labeled λ DNA molecule, and the images acquired at 670 nm (Fig. 3 b, bottom) show the nanodiamond fluorescence. At a low flow rate of ∼0.10 μL/s, the position of the nanodiamond shifts by 12.4 μm and the fluorophore-labeled λ DNA tether can be visualized (Fig. 3 b). High flow conditions were avoided to preserve the sample integrity and prevent the tethers from breaking off the flow-cell surface.
smEPR
Fig. 5 shows the results of this single-molecule measurement. Fig. 5 a shows the ODMR spectrum of a single static nanodiamond crystal. As discussed above, this results in well-defined peaks, and the largest shifts arise from NV centers having the largest projection of applied magnetic field parallel to their symmetry axis. In contrast, a collection of stationary but randomly oriented nanodiamonds exhibit a distribution of shifts because all possible orientations are represented (Fig. 5 b), resulting in a powder spectrum. The uniform intensity of the spectrum reflects the uniform probability of finding an NV center with any particular orientation relative to the applied field. The spectrum cuts off at a maximum frequency associated with the subset of NV centers whose axes are parallel to the applied field; thus, the magnitude of the cutoff is equal to the full Zeeman shift in the particular applied magnetic field.
A similar spectrum results from a single nanodiamond crystal if it dynamically rotates through all possible orientations during the acquisition of the spectrum. Fig. 5 c shows the ODMR spectrum obtained from the single nanodiamond tethered to the end of the DNA molecule. The close similarity to the powder spectrum confirms our expectation that the nanodiamond probe will rotate isotropically, and hence freely, through all possible orientations. It is also consistent with the fact that, due to its ∼100 nm diameter, its characteristic rotational fluctuation timescale, τR, is slow compared with the characteristic transverse spin relaxation time T2, which is typically in the range of 0.25–1.4 μs or longer for NV centers in nanodiamond samples (35). For smaller nanodiamonds with a τR smaller than T2, the short correlation time would lead to motional averaging, that is, a reduction of the net effect of motion on the evolution of the NV spin under the influence of the applied magnetic field, leading to a reduction of the width of the resonance line and hence a spectrum with sharper lines.
The ODMR spectra of both the powder and the single nanodiamond tethered to the DNA molecule were measured at three different applied fields: 0 G, 19 G, and 32 G. The solid lines in Fig. 5 c show the results of our simulation of the spatially averaged powder dispersion spectrum for nanodiamonds that was fit to both data sets. The details of this data fitting can be found in the Materials and Methods section. The linewidth and strain extracted from the 0 G spectrum are 8.57 MHz and 4 MHz, respectively. The field values extracted from these fits are 0 G (red, fixed), 18.7 ± 0.05 G (blue), and 32.6 ± 0.09 G (green) for the powder spectra, and 0 G (red, fixed), 19.0 ± 0.26 G (blue), and 32.1 ± 0.28 G (green), for the smEPR spectra. The fitted values agree well, within the error, with our determinations of the fields applied by means of a set of moveable rare-earth magnets.
Discussion
The combination of optical detection of EPR within a buffered biocompatible solution with the site-specific attachment of an NV nanodiamond to a DNA molecule demonstrates the feasibility of performing EPR measurements on a single spin-labeled biomolecule. Given the wide use of ensemble EPR measurements on spin-labeled biomolecules (15, 16, 17, 18), the realization of smEPR should enable the development of a broadly applicable methodology for investigating single biomolecular structures and their dynamics. Furthermore, our strategy of using biotin-streptavidin-biotin to attach a nanodiamond to a DNA molecule for site-specific labeling is compatible with RNA, proteins, and lipid vesicles (1, 2, 3, 4, 6, 8, 9, 10). This suggests that our approach to NV diamond site-specific labeling can be used with a wide variety of biomolecular systems.
Given the potential of this single-molecule methodology, we should consider the ultimate scope of this CW EPR approach. To accurately follow the molecular motion, one must use rigidly attached smaller nanodiamond labels with intrinsic rotational timescales faster than that of the molecule. Rigid attachment of a single DNA molecule to a nanoparticle is routinely achieved by preparing DNA molecules with multiple biotin molecules (43, 44). The rotational correlation time of the nanodiamond can be estimated using the Stokes-Einstein equation, , where is the radius, is the viscosity of water (10−3 Pa s), and is the thermal energy unit at room temperature. The data in Fig. 5 c represent a measurement on a ∼100 nm nanodiamond, which implies ≈ 100 μs. However, commercially available nanodiamonds with nm will have = 100 ns.
The influence of rotational motion on magnetic resonance spectra is determined by three parameters: the rotational time , which ultimately is determined by molecular motions; the ensemble spin lifetime, (35), which can, in general, be orientation dependent; and the anisotropic broadening that results from hyperfine and Zeeman interactions. Thus, the data in Fig. 5 c are in the regime of slow rotation, i.e., is much larger than both T2 and the timescale set by the Zeeman anisotropy. Hence, we observe a powder pattern for the nanodiamond. (This is in contrast to measurements on ensembles of nitroxide spin labels (45), where a powder pattern is seen in the rigid limit. For a single nanodiamond in the rigid limit, we see a spectrum of eight peaks, as shown in Fig. 5 a.) To measure faster timescales, we can use smaller nanodiamonds with much faster correlation times and longer spin lifetimes (coherence times of 0.7 ms have been reported in aqueous environments (46)). The fastest motion that can be measured is set by the anisotropy, determined here by the Zeeman interaction, which can be made much larger than = 100 ns. Measuring motions slower than this would require narrower lines than shown in Fig. 5; narrower lines have been reported previously (46). These limits indicate a useful range of timescales in which motional measurements could be made. Although a detailed discussion of the complex dependence of the spectra on these parameters, as described by the slow tumbling theory (45), is beyond the scope of this work, the computation of the dependence of spectra on these parameters has been well studied (15, 16, 17, 18) for other spin labels.
Beyond these CW applications to individual biomolecules, many of the advances described above have important implications for related techniques and experimental geometries. For example, the attachment chemistry developed for the nanodiamonds could be used in conjunction with methods described in a recent study (47) that employed in vivo measurements to achieve targeted labeling of cellular structures. The same study also demonstrated in vivo measurements on NV nanodiamonds. A separate study demonstrated CW EPR of a single nanodiamond within an optical trap (48). The ability to site-specifically attach a biomolecule to a nanodiamond, as demonstrated here, provides a key step toward the integration of force spectroscopy via optical trapping (2, 3, 6, 8) and EPR spectroscopy.
Finally, we note that the CW EPR techniques employed in this study provide a foundation for developing more sophisticated pulsed EPR techniques in much the same way that ensemble CW EPR studies paved the way for pulsed studies of large ensembles. A promising next step would be to apply pulsed ODMR techniques to this measurement, which would enable substantially improved spectral resolution (49). In combination with smaller nanodiamond labels, this could enable quantitative measurements of both the motional timescales and the rotational freedom of the single molecules to which the probe is attached.
Author Contributions
R.M.T.-S., Y.W.J., E.J.-H., M.G.P., and P.C.H. designed the study. R.M.T.-S., J.C., Y.W.J., E.J.-H., M.G.P., and P.C.H. wrote the manuscript. R.M.T.-S., Y.W.J., and V.P.B. built the smEPR experimental system. R.M.T.-S., I.R., and A.R. prepared the biotin-functionalized nanodiamond. R.M.T.-S., J.A.N., and M.S. prepared the DNA and functionalized flow cells. R.M.T.-S., Y.W.J., and V.P.B. acquired the EPR data. N.S. and J.C. modeled the ensemble and single nanodiamond EPR spectra. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
This study was supported by the Center for Emergent Materials, a Materials Research Science and Engineering Center funded by the National Science Foundation, under award number DMR-1420451; the Army Research Office through award W911NF-12-1-0587; and the National Institutes of Health through award GM083055.
Editor: David Cafiso.
References
- 1.Cornish P.V., Ha T. A survey of single-molecule techniques in chemical biology. ACS Chem. Biol. 2007;2:53–61. doi: 10.1021/cb600342a. [DOI] [PubMed] [Google Scholar]
- 2.Allemand J.-F., Bensimon D., Croquette V. Stretching DNA and RNA to probe their interactions with proteins. Curr. Opin. Struct. Biol. 2003;13:266–274. doi: 10.1016/s0959-440x(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 3.Haustein E., Schwille P. Single-molecule spectroscopic methods. Curr. Opin. Struct. Biol. 2004;14:531–540. doi: 10.1016/j.sbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Michalet X., Weiss S., Jäger M. Single-molecule fluorescence studies of protein folding and conformational dynamics. Chem. Rev. 2006;106:1785–1813. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos N.C., Castanho M.A.R.B. An overview of the biophysical applications of atomic force microscopy. Biophys. Chem. 2004;107:133–149. doi: 10.1016/j.bpc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Bockelmann U. Single-molecule manipulation of nucleic acids. Curr. Opin. Struct. Biol. 2004;14:368–373. doi: 10.1016/j.sbi.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Bustamante C., Chemla Y.R., Izhaky D. Mechanical processes in biochemistry. Annu. Rev. Biochem. 2004;73:705–748. doi: 10.1146/annurev.biochem.72.121801.161542. [DOI] [PubMed] [Google Scholar]
- 8.Ha T. Structural dynamics and processing of nucleic acids revealed by single-molecule spectroscopy. Biochemistry. 2004;43:4055–4063. doi: 10.1021/bi049973s. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang X. Single-molecule RNA science. Annu. Rev. Biophys. Biomol. Struct. 2005;34:399–414. doi: 10.1146/annurev.biophys.34.040204.144641. [DOI] [PubMed] [Google Scholar]
- 10.Deniz A.A., Mukhopadhyay S., Lemke E.A. Single-molecule biophysics: at the interface of biology, physics and chemistry. J. R. Soc. Interface. 2008;5:15–45. doi: 10.1098/rsif.2007.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal A., Deo R., Nie S. Nanometer-scale mapping and single-molecule detection with color-coded nanoparticle probes. Proc. Natl. Acad. Sci. USA. 2008;105:3298–3303. doi: 10.1073/pnas.0712351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slichter C.P. Springer; Berlin/Heidelberg: 1996. Principles of Magnetic Resonance. [Google Scholar]
- 13.Abragam A. Clarendon Press; Oxford, UK: 1961. The Principles of Nuclear Magnetism. [Google Scholar]
- 14.John E., Wertz J.R.B. McGraw-Hill Book Company; New York: 1986. Electron Spin Resonance: Elementary Theory and Practical Applications. [Google Scholar]
- 15.Rieger P.H. RSC Publishing; Cambridge, UK: 2007. Electron Spin Resonance: Analysis and Interpretation. [Google Scholar]
- 16.Dalton L.R. CRC Press; Boca Raton, FL: 1985. EPR and Advanced EPR Studies of Biological Systems. [Google Scholar]
- 17.Fajer P.G. Electron Spin Resonance Spectroscopy Labeling in Peptide and Protein Analysis. In: Meyers R.A., editor. Encyclopedia of Analytical Chemistry. John Wiley & Sons Ltd; Chichester, UK: 2000. pp. 5725–5761. [Google Scholar]
- 18.Hubbell W.L., Cafiso D.S., Altenbach C. Identifying conformational changes with site-directed spin labeling. Nat. Struct. Biol. 2000;7:735–739. doi: 10.1038/78956. [DOI] [PubMed] [Google Scholar]
- 19.Blank A., Dunnam C.R., Freed J.H. High resolution electron spin resonance microscopy. J. Magn. Reson. 2003;165:116–127. doi: 10.1016/s1090-7807(03)00254-4. [DOI] [PubMed] [Google Scholar]
- 20.Poole C.P. Dover Publications; New York: 1996. Electron Spin Resonance: A Comprehensive Treatise on Experimental Techniques. [Google Scholar]
- 21.Hoult D.I., Richards R.E. The signal-to-noise ratio of the nuclear magnetic resonance experiment. 1976. J. Magn. Reson. 2011;213:329–343. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Shi F., Zhang Q., Du J. Protein imaging. Single-protein spin resonance spectroscopy under ambient conditions. Science. 2015;347:1135–1138. doi: 10.1126/science.aaa2253. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer-Nolte E., Schlipf L., Wrachtrup J. Tracking temperature-dependent relaxation times of ferritin nanomagnets with a wideband quantum spectrometer. Phys. Rev. Lett. 2014;113:217204. doi: 10.1103/PhysRevLett.113.217204. [DOI] [PubMed] [Google Scholar]
- 24.Gruber A., Drabenstedt A., vonBorczyskowski C. Scanning confocal optical microscopy and magnetic resonance on single defect centers. Science. 1997;276:2012–2014. [Google Scholar]
- 25.Jelezko F., Wrachtrup J. Single defect centres in diamond: a review. Phys. Status Solidi Rapid Res. Lett. 2006;203:3207–3225. [Google Scholar]
- 26.Balasubramanian G., Chan I.Y., Wrachtrup J. Nanoscale imaging magnetometry with diamond spins under ambient conditions. Nature. 2008;455:648–651. doi: 10.1038/nature07278. [DOI] [PubMed] [Google Scholar]
- 27.Doherty M.W., Manson N.B., Hollenberg L.C.L. The nitrogen-vacancy colour centre in diamond. Phys. Rep. 2013;528:1–45. [Google Scholar]
- 28.Newton M.E., Campbell B.A., Anthony T.R. Recombination-enhanced diffusion of self-interstitial atoms and vacancy-interstitial recombination in diamond. Diamond and Related Materials. 2002;11:618–622. [Google Scholar]
- 29.Hunt D.C., Twitchen D.J., Vagarali S.S. Identification of the neutral carbon 〈100〉-split interstitial in diamond. Phys. Rev. B. 2000;61:3863–3876. [Google Scholar]
- 30.Campbell B., Mainwood A. Radiation damage of diamond by electron and gamma irradiation. Physica Status Solidi A Appl. Res. 2000;181:99–107. [Google Scholar]
- 31.Acosta V.M., Bauch E., Budker D. Diamonds with a high density of nitrogen-vacancy centers for magnetometry applications. Phys. Rev. B. 2009;80:115202. [Google Scholar]
- 32.Bradac C., Gaebel T., Rabeau J.R. Observation and control of blinking nitrogen-vacancy centres in discrete nanodiamonds. Nat. Nanotechnol. 2010;5:345–349. doi: 10.1038/nnano.2010.56. [DOI] [PubMed] [Google Scholar]
- 33.Krueger A., Lang D. Functionality is key: recent progress in the surface modification of nanodiamond. Adv. Funct. Mater. 2012;22:890–906. [Google Scholar]
- 34.Smith B.R., Gruber D., Plakhotnik T. The effects of surface oxidation on luminescence of nano diamonds. Diam. Relat. Mater. 2010;19:314–318. [Google Scholar]
- 35.Tisler J., Balasubramanian G., Wrachtrup J. Fluorescence and spin properties of defects in single digit nanodiamonds. ACS Nano. 2009;3:1959–1965. doi: 10.1021/nn9003617. [DOI] [PubMed] [Google Scholar]
- 36.Toshihiro A. Vapour-phase oxidation of diamond surfaces in O2 studied by diffuse reflectance Fourier-transform infrared and temperature-programmed desorption spectroscopy. J. Chem. Soc. Faraday Trans. 1993;89:3635–3640. [Google Scholar]
- 37.Ushizawa K.S.Y., Mitsumori T., Ando T. Covalent immobilization of DNA on diamond and its verification by diffuse reflectance infrared spectroscopy. Chem. Phys. Lett. 2002;351:105–108. [Google Scholar]
- 38.Russo S., Barnard A., Snook I. Hydrogenation of nanodiamond surfaces: structure and effects on crystalline stability. Surf. Rev. Lett. 2003;10:233–239. [Google Scholar]
- 39.Osswald S., Yushin G., Gogotsi Y. Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J. Am. Chem. Soc. 2006;128:11635–11642. doi: 10.1021/ja063303n. [DOI] [PubMed] [Google Scholar]
- 40.Boudou J.-P., David M.-O., Curmi P.A. Hyperbranched polyglycerol modified fluorescent nanodiamond for biomedical research. Diam. Relat. Mater. 2013;38:131–138. [Google Scholar]
- 41.Zhao L., Takimoto T., Komatsu N. Chromatographic separation of highly soluble diamond nanoparticles prepared by polyglycerol grafting. Angew. Chem. Int. Ed. Engl. 2011;50:1388–1392. doi: 10.1002/anie.201006310. [DOI] [PubMed] [Google Scholar]
- 42.Simon M., North J.A., Poirier M.G. Histone fold modifications control nucleosome unwrapping and disassembly. Proc. Natl. Acad. Sci. USA. 2011;108:12711–12716. doi: 10.1073/pnas.1106264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosconi F., Allemand J.F., Croquette V. Measurement of the torque on a single stretched and twisted DNA using magnetic tweezers. Phys. Rev. Lett. 2009;102:078301. doi: 10.1103/PhysRevLett.102.078301. [DOI] [PubMed] [Google Scholar]
- 44.Strick T.R., Allemand J.F., Croquette V. The elasticity of a single supercoiled DNA molecule. Science. 1996;271:1835–1837. doi: 10.1126/science.271.5257.1835. [DOI] [PubMed] [Google Scholar]
- 45.Freed J.H. Theory of slow tumbling ESR spectra for nitroxides. In: Berliner L., editor. Spin Labelling: Theory and Applications. Academic Press; New York: 1976. [Google Scholar]
- 46.Andrich P., Alemán B.J., Awschalom D.D. Engineered micro- and nanoscale diamonds as mobile probes for high-resolution sensing in fluid. Nano Lett. 2014;14:4959–4964. doi: 10.1021/nl501208s. [DOI] [PubMed] [Google Scholar]
- 47.McGuinness L.P., Yan Y., Hollenberg L.C.L. Quantum measurement and orientation tracking of fluorescent nanodiamonds inside living cells. Nat. Nanotechnol. 2011;6:358–363. doi: 10.1038/nnano.2011.64. [DOI] [PubMed] [Google Scholar]
- 48.Horowitz V.R., Alemán B.J., Awschalom D.D. Electron spin resonance of nitrogen-vacancy centers in optically trapped nanodiamonds. Proc. Natl. Acad. Sci. USA. 2012;109:13493–13497. doi: 10.1073/pnas.1211311109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maze J.R., Stanwix P.L., Lukin M.D. Nanoscale magnetic sensing with an individual electronic spin in diamond. Nature. 2008;455:644–647. doi: 10.1038/nature07279. [DOI] [PubMed] [Google Scholar]