We present a case of donor-derived coccidioidomycosis in a cardiac transplant patient and review previous cases of donor-derived coccidioidomycosis in solid organ transplant. Donor-derived coccidioidomycosis is rare, can be difficult to diagnose and has significant mortality.
Keywords: coccidioidomycosis, donor-derive, endocarditis, solid organ transplant
Abstract
Background. Endocarditis is a rare manifestation of infection with Coccidioides. This is the first reported case of donor-derived Coccidioides endocarditis obtained from a heart transplant.
Methods. We present a unique case of donor-derived Coccidioides immitis endocarditis and disseminated infection in a heart transplant patient. We also conducted a review of the literature to identify other cases of donor-derived coccidioidomycosis in solid organ transplant recipients and reviewed their clinical characteristics.
Results. Fifteen prior cases of donor-derived coccidioidomycosis were identified. A majority of these cases were diagnosed by positive culture (83%). Mortality was high at 58%.
Conclusions. Clinicians should maintain a high index of suspicion for disseminated coccidioidomycosis in patients who received transplants with organs from donors with a history of residing in endemic regions.
The patient was a 52-year-old white female with idiopathic nonischemic cardiomyopathy for which she underwent orthotopic heart transplant (recipient cytomegalovirus [CMV] immunoglobulin [Ig]G negative, donor CMV IgG positive). Pretransplant workup was notable for computerized tomography (CT) of the chest a few months before transplant that showed scattered ground-glass opacities, up to 4 mm in diameter. She had no perioperative complications, and she received antithymocyte globulin as induction immunosuppression. She recovered as expected in the immediate postoperative period. Six weeks after her transplant, she had a routine transthoracic echocardiogram that was notable for a new mobile mass in the left atrium. She complained only of fatigue at the time, and she denied fever or chills; blood cultures were negative for any microbial growth. She was started on anticoagulation for a presumed left atrial thrombus.
The patient's immunosuppressive regimen consisted of tacrolimus 3 mg twice daily (level ranged from 7.5 to 15.7 ng/mL), mycophenolic acid 720 mg twice daily, and prednisone at a total daily dose of 20 mg. She was taking trimethoprim/sulfamethoxazole 80 mg/400 mg daily, itraconazole 200 mg in the morning and 100 mg at night (serum level <0.1 mcg/mL), and valganciclovir 900 mg daily for antimicrobial prophylaxis. She was living in the coastal city of Morro Bay, California, but had previously resided in New Mexico for several years.
Ten weeks posttransplant, the patient represented with 2 weeks of progressive left ankle pain and swelling and was admitted to the hospital for further evaluation. She denied any associated fevers, chills, or rash, but complained of few days of severe headache located in occipital area radiating down her neck. The headache was worse with movement without associated photophobia, changes in vision, or focal neurologic complaints.
On admission, the patient was afebrile, hemodynamically stable, and in no acute distress. Her physical examination was notable for stiff neck with mild paraspinal tenderness to palpation. Cardiac exam revealed regular rhythm and no murmurs. The left ankle had an effusion but no erythema or warmth; tenderness to palpation was present, greatest over the lateral malleolus. The ankle joint had good range of motion. Neurological exam was nonfocal, and the remainder of physical exam was within normal limits.
Admission laboratory values were notable for white blood cell count 9.7 kg/µL with a normal differential, hemoglobin 10.0 g/dL, and platelets 297 kg/µL. The patient was hyponatremic with Na 127 mmol/L. Creatinine (Cr) was 1.4 mg/dL, and liver function tests were within normal limits. International normalized ratio (INR) was 8.3. Inflammatory markers were elevated with erythrocyte sedimentation rate 82 mm/hour and C-reactive protein 13.6 mg/dL. The patient underwent aspiration of left ankle, which revealed 256 nucleated cells and 46 000 red blood cells per µL, and no crystals were seen. Gram stain of synovial fluid was negative; bacterial and fungal cultures showed no growth. Lumbar puncture was not initially performed due to supratherapeutic INR.
Magnetic resonance imaging (MRI) of the brain showed the presence of few nonspecific white matter lesions, which were thought to be secondary to chronic ischemic small vessel disease. Cardiac MRI was done to further evaluate her mobile left atrial mass and showed 2 highly mobile pedunculated masses in the left atrium, measuring 1 cm and 8 mm in diameter. There was also a lobulated pseudoaneurysm in the ascending aorta at the site of anastomosis. She had repeat blood cultures as well as Bartonella spp, Coxiella burnetii, and Brucella spp serologies to evaluate for culture-negative endocarditis, which were all negative. On hospital day 3, a positron emission tomography-CT was also ordered to better assess for underlying infection; however, before the test was performed, the patient had an acute change in mental status and was found unresponsive. A CT angiography of the head and neck revealed a large hemorrhage in the posterior fossa. Despite aggressive treatment, including reversal of anticoagulation and external ventricular drain placement, her condition did not improve and the patient died on hospital day 7.
Post mortem examination revealed a mycotic aortic aneurysm at the anastomotic site with infection extending to the pericardium, right ventricular myocardium, pulmonary artery trunk, and left atrial anastomosis with a 2 cm pedunculated left atrial mycotic thrombus (Figure 1). Histopathology revealed spherules and hyphal elements (arthroconidia) consistent with Coccidioides (Figure 2). Autopsy also showed evidence of disseminated fungal infection involving lungs and brain.
Figure 1.
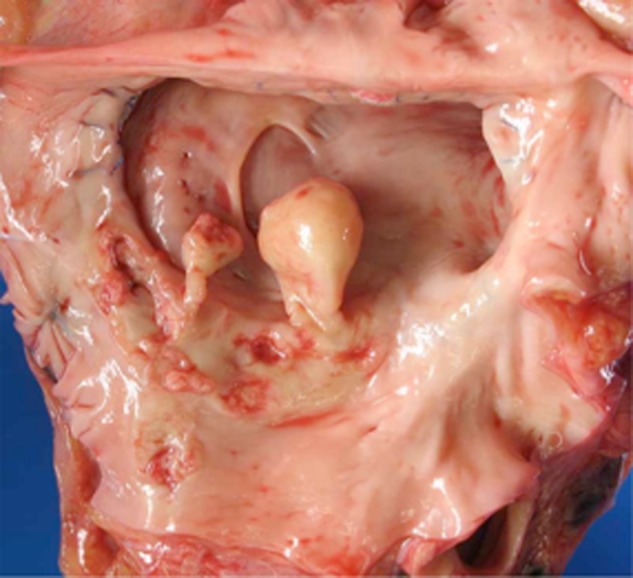
Gross pathologic specimen showing pedunculated mycotic thrombus at the left atrial anastamosis.
Figure 2.
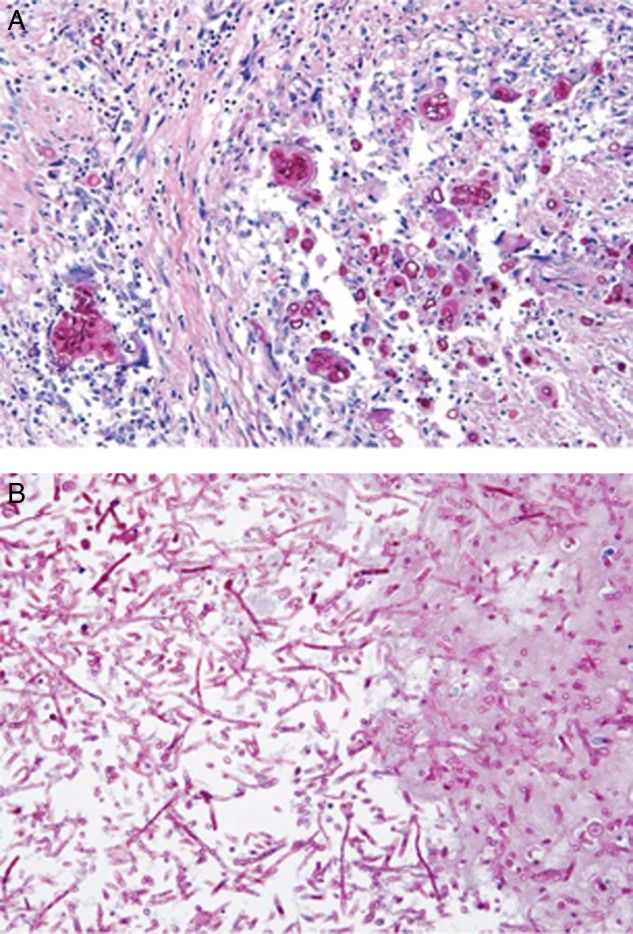
(A) Periodic acid-Schiff stain of epicardial tissue showing spherules consistent with Coccidioides infection. (B) Periodic acid-Schiff stain of left atrial mycotic thrombus showing fungal hyphal elements.
The patient's donor was a 58-year-old female, from Las Vegas, Nevada, with a past medical history of substance use and renal artery aneurysm, and she died of an unexplained subarachnoid hemorrhage. On CT of the chest, she was found to have a 2.3 × 3.8 × 2.8 cm cavitary lesion in her right upper lung that was biopsied during organ procurement, and pathological examination showed inflammation and fibrosis but no evidence of malignancy and no granulomas. Although the tissue was not cultured and no stains for microorganisms were performed, a bronchoalveolar lavage specimen was negative for bacterial, fungal, or mycobacterial growth. The only other organ procured from this donor was the liver, and the recipient had immediate graft failure and required retransplant.
Post mortem, the patient's pretransplant serum was obtained and testing for Coccidioides by immunodiffusion and compliment fixation was negative. Further investigation on the donor after our patient's death revealed that the donor serum was positive for Coccidioides by immunodiffusion but had a negative complement fixation titer, most consistent with localized disease. The donor's lung pathology was reviewed and showed arthroconidia in deep tissue block samples consistent with fungal disease, likely coccidioidomycosis in this clinical context (Figure 3). The original pathology review was not performed at our institution so it is difficult to speculate why the diagnosis was not made initially. In retrospect, it is possible that knowing the diagnosis allowed pathologists to locate arthroconidia that were seen deep in the tissue block.
Figure 3.
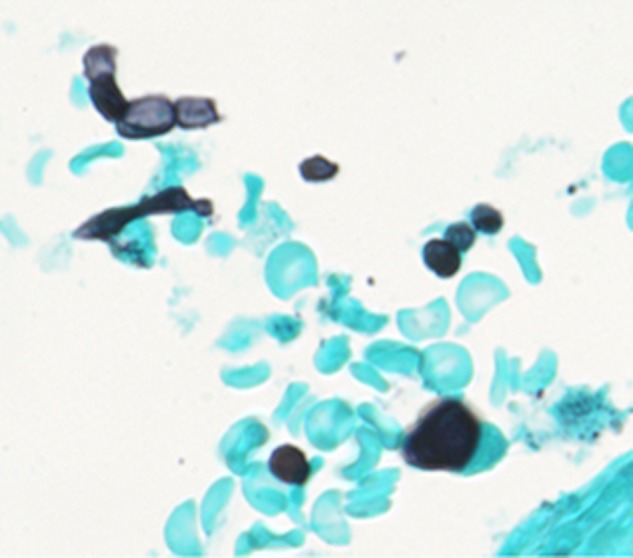
Grocott's methenamine silver stain of donor lung tissue showing arthroconidia.
Our patient had expressed desire to be an organ donor herself before her death, and she was taken for organ procurement after she passed away. At the time of surgery, she was noted to have a small amount of purulence deep to the sternum. This was sent for culture and ultimately grew Coccidioides immitis. Her kidneys were removed, and initial review of histopathology on frozen section did not reveal evidence of infection. Her 2 kidneys were subsequently transplanted into a single recipient before availability of the results of the culture of the retrosternal specimen. A subsequent review of sections from the donated kidney revealed evidence of spherules consistent with Coccidioides infection. With these findings, the recipient transplant center was contacted immediately; the recipient was started on fluconazole 400 mg daily and continues to do well with Cr near baseline 6 months posttransplant.
DISCUSSION
Coccidioidomycosis is an endemic fungal disease found in the desert regions of southwestern United States, northern Mexico, and parts of Central and South America. In immunocompetent adults, it can be asymptomatic in up to 60% of cases; if symptomatic, it usually manifests with influenza-like symptoms or pneumonia. Less often, it can disseminate to extrapulmonary sites in approximately 1%–5% of infections, most commonly to the meninges, bone, joints, and skin. Dissemination is more common in those of African or Filipino ancestry, pregnant women, or those who are immunosuppressed [1].
Endocarditis is a rare manifestation of coccidioidomycosis, and to date there have been only 7 other reported cases [2, 3]. To our knowledge, this is the first reported case of Coccidioides endocarditis acquired through the cardiac allograft. The involvement of the anastomotic site in particular suggests either fungemia, which allowed the fungus to seed injured endothelium at the anastomosis, or infection derived from the donor organ, which then disseminated widely. On subsequent pathology review of the donor's lung tissue, infectious arthroconidia were seen. Therefore, it is also conceivable that at the time of organ procurement, resection of the lungs might have led to a small amount of organisms contaminating the donated heart and then subsequently infecting the recipient. In either of these scenarios, the donor was the source of the C immitis in this recipient.
The incidence of coccidioidomycosis after solid organ transplantation in endemic areas has been reported to be from 3.8% to 8.7% [4, 5]. The highest risk of coccidioidomycosis after solid organ transplant occurs in the first year posttransplant, accounting for as many as 70% of cases in one series [4, 6]. In areas of high endemicity, the risk of symptomatic posttransplant coccidioidomycosis is increased with history of prior infection, positive serology just before transplant, or antirejection therapy [4]. An intact and effective cellular immune response is paramount in controlling Coccidioides infection, and thus immunosuppressive agents used to prevent rejection impair the immune response to coccidioidomycosis [5]. As such, dissemination is much more common in transplant patients compared to immunocompetent patients, occurring in up to 75% of cases [7]. Mortality is reported to be from 30% to 63% [5, 7, 8].
Coccidioidomycosis infection after transplant is a result of either de novo acquisition of the infection posttransplant, reactivation of previously acquired infection in the recipient, or infection transmitted through the donor organ. The most common mechanism is reactivation of previously acquired infection [5]. Our patient had lived in New Mexico and had pulmonary nodules on pretransplant chest imaging, making reactivation of previous infection plausible. However, after her death, saved pretransplant serum was tested for Coccidioides serology and results were negative. Based on (1) the donor's positive Coccidioides serology and residence in an endemic area and (2) arthroconidia found in her cavitary lung lesion, we concluded that this was most likely a donor-derived infection.
Visualization of arthroconidia in the atrial thrombus in this patient as well as in the cavitary lesion of the donor is unusual because C immitis has the characteristic thermal dimorphism of endemic fungi, growing as yeast form at body temperature (37°C) and as mold at lower temperatures (25–30°C). Exception to this behavior has been observed in patients with chronic cavitary pulmonary lesions [9, 10], ventricular peritoneal shunt specimens [11, 12], and a previously reported case of coccidioidomycosis also presenting as left atrial thrombus endocarditis [13]. It is possible that endothelialization that takes place during formation of vegetations creates the environment of a biofilm that facilitates transition from yeast to mold morphology.
Donor-derived Coccidioides infections are rare with only 15 previous reported cases (Table 1) [14–21]. Only 12 cases have clinical data available for review: 4 lung transplants, 1 heart transplant, 1 liver transplant, 1 liver/kidney transplant, 1 kidney/pancreas transplant, and 4 kidney transplants. Three of 9 cases for which race was reported were of the higher risk African or Filipino ethnicity, possibly contributing to their susceptibility to disseminated infection. Also of note, 5 of 7 donors came from or visited an endemic area. Symptom onset in most cases (9 of 11 of cases with symptoms) was within 1 month after transplant. The 2 cases that presented later than 1 month had recent increase in immunosuppression due to concern for graft rejection. Of these 12 cases, 7 patients (58%) who received organs from infected donors died of Coccidioides infection [14–20]. Of the 5 patients who survived, 4 had active disease and 1 had asymptomatic seroconversion. In these reports, another 3 recipients of organs from infected donors never developed signs or symptoms of infection, were started on appropriate antifungal prophylaxis, and survived [16, 19]. Ten of 12 (83%) cases were diagnosed by positive culture, and 7 of 12 (58%) patients had positive blood cultures. Coccidioides fungemia is a rare entity but has been found more commonly in the setting of fulminant, disseminated coccidioidomycosis [22]. The high proportion of transplant recipients with positive culture results suggests that cultures can significantly aid in diagnosing Coccidioides infection in this patient population.
Table 1.
Previous Reported Cases of Donor-Derived Coccidioidomycosis
| Author | Donor | Transplanted Organ | Recipient | Manifestation | Timing of Symptom Onseta | Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|
| Wright et al [16] | Incarcerated in AZ, history of extrapulmonary disease | Liver | 46-yr-old white male with cryptogenic cirrhosis | Bone marrow, lungs, kidneys, heart, thyroid, pancreas, brain, liver, spleen | 13 | Blood culture, bronchial wash culture | Deceased |
| Kidney | 26-yr-old African American male with ESRD | Bone marrow, lungs, kidneys, heart, thyroid, pancreas, brain, testes, liver, spleen | 17 | Blood culture, bronchial wash culture | Deceased | ||
| Miller et al [14] | 30-yr-old female who had visited Mexico | Bilateral Lung | 61-yr-old male with COPD | Lung | 14 | Bronchial wash culture | Deceased |
| Tripathy et al [15] | Female from Arizona | Lung | 21-yr-old French male with pulmonary hypertension | Lung | 6 | Bronchial wash culture | Survived |
| Blodget et al [18] | 52-yr-old African American woman from Southern California | Heart | 66-yr-old Hispanic with ischemic cardiomyopathy | Pericardium, liver, lungs, spleen, pancreas, adrenals | 16 | Blood cultures, cytology of pericardial fluid | Deceased |
| Kidney | 40-yr-old African American male with postobstructive glomerulonephritis | Liver, spleen, bone marrow, thyroid, pancreas | 13 | Blood cultures, sputum cytology | Deceased | ||
| Kidney/Liver | 23-yr-old Hispanic male with cryptogenic cirrhosis | Lung, liver, bone marrow, blood | 14 | Blood cultures, bronchial wash culture, transbronchial biopsy culture, pleural fluid culture | Survived | ||
| Dierberg et al [19] | 22-yr-old male from Jamaica, living in Maryland, no known travel to endemic area | Kidney | 19-yr-old African American Male with FSGS | Lung, blood | 29 | Blood cultures, bronchial wash culture, transbronchial biopsy culture | Survived |
| Kidney/Pancreas | 45-yr-old white female with DM and HTN | Lung, blood | 26 | Blood cultures, bronchial wash culture | Deceased | ||
| Bilateral Lung | 62-yr-old white female with COPD | asymptomatic seroconversion | n/a | serology | Survived | ||
| Brugiere et al [17] | Male who had visited Arizona months before organ donation | Lung | 58-yr-old French male with IPF | Lung | 1050 | Bronchial wash culture | Survived |
| Carvahlo et al [20] | 38-yr-old female with unknown travel history | Kidneyb | 62-yr-old white male with ESRD | CNS | 210 | Brain biopsy with spherules on path and PCR | Deceased |
Abbreviations: AZ, Arizona; CNS, central nervous system; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESRD, end-stage renal disease; FSGS, focal segmental glomerulosclerosis; HTN, hypertension; IPF, idiopathic pulmonary fibrosis; PCR, polymerase chain reaction.
a Days posttransplant.
b Presumed donor derived, not confirmed.
Recently published guidelines suggest that live donors from endemic areas be tested for coccidioidomycosis before donating organs and that deceased donors have serology performed if pathology from suspicious lung lesions shows Coccidioides on fungal stain [23]. Furthermore, any recipients of organs from deceased donors who are found to have evidence of coccidioidomycosis should (1) have baseline Coccidioides serological testing before transplantation if possible and with development of any suspicious clinical symptoms and (2) receive prolonged prophylaxis [23]. However, it is important to realize that serologies can also be falsely negative in this population given their immunosuppression. One study showed that in 27 cases of newly acquired coccidioidomycosis posttransplant in an endemic area, single serologic test was positive in only 21%–56% of patients, depending on the test. Sensitivity improved if multiple assays were performed (77%) and if repeated 1 month later (92%) [24]. The Coccidioides skin hypersensitivity test is now available again as an adjunctive diagnostic test; however, given limitations similar to tuberculin skin testing, it is unclear how reliable this test will be in the immunocompromised patient population [25].
Despite the recommendations listed above, a review at a center where Coccidioides is endemic showed a relatively low rate of seropositive live organ donor candidates (2.1%). Of these, 4 donors did go onto donate organs (kidney or liver). Recipients received prophylactic fluconazole for range of 1 to 8 months, and none developed coccidioidomycosis [26]. Blair and Mulligan [26] concluded that it is not clear, based on the relatively low rate of seropositivity, whether it can be recommended to perform serology on all donors from endemic areas. However, as is illustrated in our patient, coccidioidomycosis posttransplant can be difficult to diagnose and is associated with high rates of dissemination and mortality. Furthermore, in prior cases of suspected donor transmitted infection as well as in the case of the recipient of our deceased patient's kidneys, when infection is caught early and when antifungal prophylaxis is initiated, outcomes improved.
To be maximally effective, antifungal prophylaxis for recipients from suspected donor cases should be started before clinical symptoms are present. Our patient was on prophylactic itraconazole per our institutional protocol, but her itraconazole serum level when checked was <0.1 mcg/mL and unlikely to be therapeutic. Earlier awareness of a positive donor serology could have appropriately intensified prophylactic antifungal therapy and monitoring. In general, fluconazole is the recommended agent for prophylaxis, but other azoles also have activity against Coccidioides [23]. Based on this case, if concern for donor-derived Coccidioides is raised, it is now our policy to monitor itraconazole levels; if therapeutic level is unobtainable, we switch to an alternate agent such as fluconazole. Voriconazole or posaconazole would be substituted if prophylaxis against molds is also needed.
CONCLUSIONS
This case is especially unique in that it highlights a chain of donor-derived coccidioidomycosis from one donor to organ recipient and then to her subsequent recipient. As is illustrated by this case, organ transplant recipients who die of unexplained causes should not be considered as donors. In addition, it is important to maintain a high index of suspicion for coccidioidomycosis in patients with solid organ transplants who come from, or have donors from, endemic areas. Consideration should be given to perform routine serologic testing on donors from endemic areas, especially for donors with suspicious lung lesions found on imaging. This could direct appropriate prophylaxis in organ recipients, raise awareness of the possibility of disseminated infection, and hopefully prevent further morbidity and mortality from donor-derived coccidioidomycosis. The initiation of effective prophylaxis with good outcome in the recipient of our patient's kidneys later known to be infected with Coccidioides highlights the effectiveness of appropriate antifungal prophylaxis in this setting. Further study is needed to assess the usefulness of testing for all donors from endemic areas as well as improve diagnostics in transplant recipients.
Acknowledgments
We acknowledge Dr. Pappagianas for assistance with further testing of serum samples for serology. We also acknowledge Dr. Donald Regula for assistance with pathology review.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Stevens DA. Coccidioidomycosis. N Engl J Med 1995; 332:1077–82. [DOI] [PubMed] [Google Scholar]
- 2.Reuss CS, Hall MC, Blair JE et al. Endocarditis caused by Coccidioides species. Mayo Clin Proc 2004; 79:1451–4. [DOI] [PubMed] [Google Scholar]
- 3.Horng LM, Johnson R, Castro L et al. Endocarditis due to Coccidioides spp. - the seventh case. Open Forum Infect Dis 2015; 2:ofv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation. Clin Infect Dis 2001; 33:1536–44. [DOI] [PubMed] [Google Scholar]
- 5.Vikram HR, Blair JE. Coccidioidomycosis in transplant recipients: a primer for clinicians in nonendemic areas. Curr Opin Organ Transplant 2009; 14:606–12. [DOI] [PubMed] [Google Scholar]
- 6.Blair JE. Approach to the solid organ transplant patient with latent infection and disease caused by Coccidioides species. Curr Opin Infect Dis 2008; 21:415–20. [DOI] [PubMed] [Google Scholar]
- 7.Cohen IM, Galgiani JN, Potter D et al. Coccidioidomycosis in renal replacement therapy. Arch Intern Med 1982; 142:489–94. [PubMed] [Google Scholar]
- 8.Holt CD, Winston DJ, Kubak B et al. Coccidioidomycosis in liver transplant patients. Clin Infect Dis 1997; 24:216–21. [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Hernandez B, Martinez-Rivera MA, Palma Cortés G et al. Mycelial forms of Coccidioides spp. in the parasitic phase associated to pulmonary coccidioidomycosis with type 2 diabetes mellitus. Eur J Clin Microbial Infect Dis 2008; 27:817–20. [DOI] [PubMed] [Google Scholar]
- 10.Nosanchuk JD, Snedeker J, Nosanchuk JS. Arthroconidia in coccidioidoma: case report and literature review. Int J Infect Dis 1998; 3:32–5. [DOI] [PubMed] [Google Scholar]
- 11.Hagman HM, Madnick EG, D'Agostino AN et al. Hyphal forms in the central nervous system of patients with coccidioidomycosis. Clin Infect Dis 2000; 30:349–53. [DOI] [PubMed] [Google Scholar]
- 12.Davis LE, Cook G, Costerton JW. Biofilm on ventriculo-peritoneal shunt tubing as a cause of treatment failure in coccidioidal meningitis. Emerg Infect Dis 2002; 8:376–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Via WV, Koulouri S, Ross LA et al. Right atrial mass in a child with disseminated coccidioidomycosis. Pediatr Infect Dis J 2005; 24:470–2. [DOI] [PubMed] [Google Scholar]
- 14.Miller MB, Hendren R, Gilligan PH. Posttransplantation disseminated coccidioidomycosis acquired from donor lungs. J Clin Microbiol 2004; 42:2347–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathy U, Yung GL, Kriett JM et al. Donor transfer of pulmonary coccidioidomycosis in lung transplantation. Ann Thorac Surg 2002; 73:306–30. [DOI] [PubMed] [Google Scholar]
- 16.Wright PW, Pappagianis D, Wilson M et al. Donor-related coccidioidomycosis in organ transplant recipients. Clin Infect Dis 2003; 37:1265–9. [DOI] [PubMed] [Google Scholar]
- 17.Brugiere O, Forget E, Biondi G et al. Coccidioidomycosis in a lung transplant recipient acquired from the donor graft in France. Transplantation 2009; 88:1319–20. [DOI] [PubMed] [Google Scholar]
- 18.Blodget E, Geiseler PJ, Larsen RA et al. Donor-derived Coccidioides immitis fungemia in solid organ transplant recipients. Transpl Infect Dis 2012; 14:305–10. [DOI] [PubMed] [Google Scholar]
- 19.Dierberg KL, Marr KA, Subramanian A et al. Donor-derived organ transplant transmission of coccidioidomycosis. Transpl Infect Dis 2012; 14:300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho C, Ferreira I, Gaiao S et al. Cerebral coccidioidomycosis after renal transplantation in a non-endemic area. Transpl Infect Dis 2010; 12:151–4. [DOI] [PubMed] [Google Scholar]
- 21.Engelthaler DM, Chiller T, Schupp JA et al. Next-generation sequencing of Coccidioides immitis isolated during cluster investigation. Emerg Infect Dis 2011; 17:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keckich DW, Blair JE, Vikram HR. Coccidioides fungemia in six patients, with a review of the literature. Mycopathologia 2010; 170:107–15. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Huprikar S, Burdette SD et al. Donor-derived fungal infections in organ transplant recipients: guidelines of the American Society of Transplantation, infectious diseases community of practice. Am J Transplant 2012; 12:2414–28. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza N, Blair JE. The utility of diagnostic testing for active coccidioidomycosis in solid organ transplant recipients. Am J Transplant 2013; 13:1034–9. [DOI] [PubMed] [Google Scholar]
- 25.Wack EE, Ampel NM, Sunenshine RH et al. The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clin Infect Dis 2015; 61:787–91. [DOI] [PubMed] [Google Scholar]
- 26.Blair JE, Mulligan DC. Coccidioidomycosis in healthy persons evaluated for liver or kidney donation. Transpl Infect Dis 2007; 9:78–82. [DOI] [PubMed] [Google Scholar]


