Abstract
Aims
The aim of this study was to investigate the association between hypothyroidism and major adverse cardiovascular and cerebral events (MACCE) in patients undergoing percutaneous coronary intervention (PCI).
Methods and results
Two thousand four hundred and thirty patients who underwent PCI were included. Subjects were divided into two groups: hypothyroidism ( n = 686) defined either as a history of hypothyroidism or thyroid-stimulating hormone (TSH) ≥5.0 mU/mL, and euthyroidism ( n = 1744) defined as no history of hypothyroidism and/or 0.3 mU/mL ≤ TSH < 5.0 mU/mL. Patients with hypothyroidism were further categorized as untreated ( n = 193), or those taking thyroid replacement therapy (TRT) with adequate replacement (0.3 mU/mL ≤ TSH < 5.0 mU/mL, n = 175) or inadequate replacement (TSH ≥ 5.0 mU/mL, n = 318). Adjusted hazard ratios (HRs) were calculated using Cox proportional hazards models. Median follow-up was 3.0 years (interquartile range, 0.5–7.0). After adjustment for covariates, the risk of MACCE and its constituent parts was higher in patients with hypothyroidism compared with those with euthyroidism (MACCE: HR: 1.28, P = 0.0001; myocardial infarction (MI): HR: 1.25, P = 0.037; heart failure: HR: 1.46, P = 0.004; revascularization: HR: 1.26, P = 0.0008; stroke: HR: 1.62, P = 0.04). Compared with untreated patients or those with inadequate replacement, adequately treated hypothyroid patients had a lower risk of MACCE (HR: 0.69, P = 0.005; HR: 0.78, P = 0.045), cardiac death (HR: 0.43, P = 0.008), MI (HR: 0.50, P = 0.0004; HR: 0.60, P = 0.02), and heart failure (HR: 0.50, P = 0.02; HR: 0.52, P = 0.017).
Conclusion
Hypothyroidism is associated with a higher incidence of MACCE compared with euthyroidism in patients undergoing PCI. Maintaining adequate control on TRT is beneficial in preventing MACCE.
Keywords: Hypothyroidism, Major adverse cardiovascular and cerebral events, Percutaneous coronary intervention
See page 2066 for the editorial comment on this article (doi:10.1093/eurheartj/ehv694)
Introduction
Thyroid hormone affects every cell, tissue, and organ in the body. 1 Minor changes in thyroid hormone levels may adversely affect the cardiovascular system. 2 The prevalence of hypothyroidism continues to be debated, 3 and the lack of consistency in results across studies may be due to varying definitions of hypothyroidism. 4 Most studies report a higher prevalence of hypothyroidism in elderly women, 5 , 6 with estimates as high as 24% in women >60 years of age. Up to 60% of those with hypothyroidism are unaware of their condition, yet the frequency of medical professionals prescribing thyroid medication has increased dramatically over time in the USA, from an estimated 49.8 million prescriptions in 2006 to 70.5 million in 2010. 7
Abnormal thyroid function has important public health consequences. 8 Patients with hypothyroidism have an increased risk of functional cardiovascular abnormalities, as well as accelerated atherosclerosis and an increased risk of coronary artery disease (CAD). 9 However, whether subclinical hypothyroidism (SCH) or overt hypothyroidism is independent risk factor for cardiovascular events or mortality remains uncertain. 10
Evaluating risk and medically optimizing patients prior to percutaneous coronary intervention (PCI) is essential to limiting complications and improving prognosis. 11 To date, no clinical trial has been undertaken to examine the relationship between hypothyroidism and cardiovascular events in patients undergoing PCI. The purpose of the present study was to examine the association between hypothyroidism and major adverse cardiovascular and cerebral events (MACCE) in patients undergoing PCI.
Methods
Study population and design
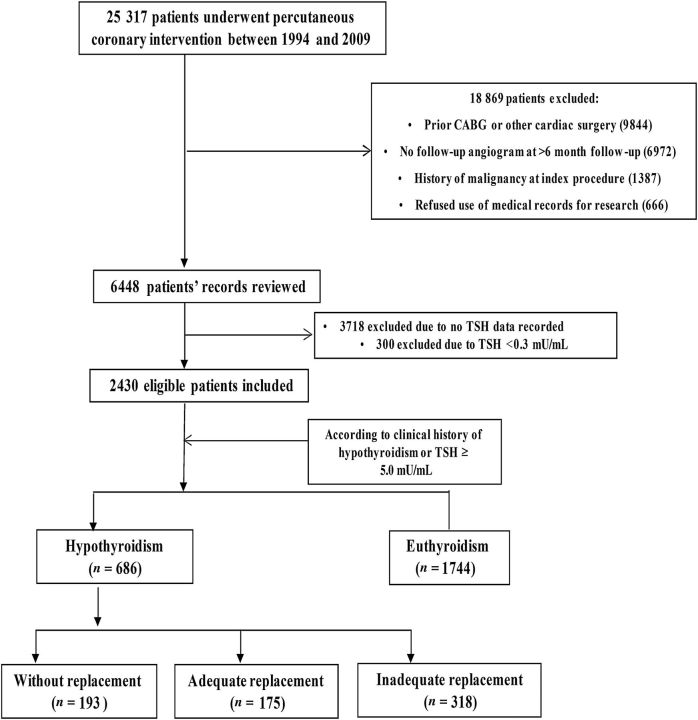
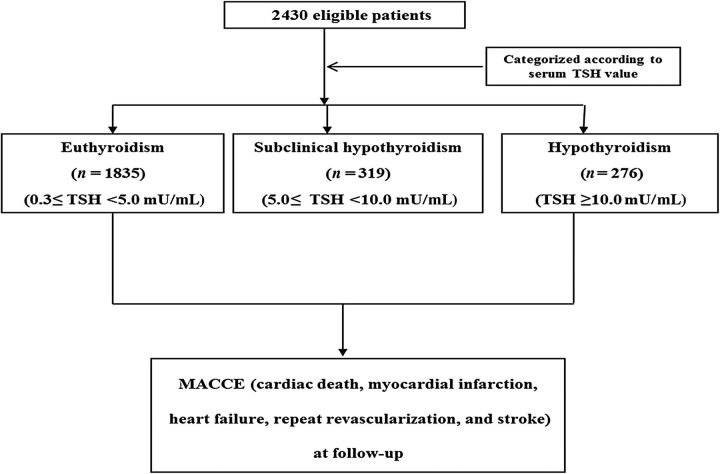
All patients who undergo PCI at Mayo Clinic have information relating to demographic, clinical, procedural, and angiographic data entered into a prospective database. A clinical research nurse contacts all patients after 6 and 12 months and annually thereafter to ascertain relevant follow-up information. Records from outside hospitals relating to adverse events are also collected. The supervisor for data integrity randomly audits 10% of all records to ensure data are collected accurately. Approval for this study was obtained from the Institutional Review Board of the Mayo Foundation. Figures 1 and 2 show the patient selection process.
Figure 1.
Patient selection process and study protocol.
Figure 2.
Patient selection process by thyroid-stimulating hormone levels.
Definitions of hypothyroidism and subgroup classification
Patients were divided into subgroups as part of three separate analyses, as follows.
Clinical classification of hypothyroidism vs. euthyroidism
Hypothyroidism was defined as a documented history of hypothyroidism or SCH in the patients' clinical record or a thyroid-stimulating hormone (TSH) level ≥5.0 mU/mL at the time of the index PCI, n = 686. Euthyroidism was defined as no documented history of hypothyroidism in the patients' clinical record and/or 0.3 mU/mL ≤ TSH < 5.0 mU/mL at the time of the index PCI ( Figure 1 ).
Use of thyroid replacement therapy
Patients with a clinical diagnosis of hypothyroidism were further stratified according to those receiving no thyroid replacement therapy (TRT), n = 193; patients who had inadequate replacement on TRT (TSH < 0.3 or TSH ≥ 5.0 mU/mL at follow-up), n = 318, and patients with adequate replacement on TRT (0.3 ≤ TSH < 5.0 mU/mL at follow-up), n = 175 ( Figure 1 ).
Biochemical classification of hypothyroidism vs. subclinical hypothyroidism vs. euthyroidism
Patients were separately categorized based on TSH levels only, as follows: euthyroidism, 0.3 mU/mL ≤ TSH < 5.0 mU/mL, n = 1835; SCH, 5.0 mU/mL ≤ TSH < 10.0 mU/mL, n = 319; and overt hypothyroidism, TSH ≥ 10.0 mU/mL, n = 276 ( Figure 2 ). 12
Data collection and clinical follow-up
Information on patient demographics, conventional cardiovascular risk factors, laboratory data, and angiographic characteristics, as well as TSH values, history of hypothyroidism, and whether or not patients were prescribed TRT was abstracted from patients' electronic medical records by a single investigator (M.Z.) blinded to PCI data. Information on adverse cardiovascular events was collected prospectively as part of the Mayo Clinic PCI registry described above. Major adverse cardiovascular and cerebral event was defined as cardiac death, myocardial infarction (MI), target-vessel revascularization (TVR), stroke, and heart failure.
Follow-up angiogram
To compare coronary disease progression between patients with hypothyroidism and euthyroidism, we randomly selected 102 patients from the hypothyroid group and 306 patients from the euthyroid group (to maintain a 1:3 ratio between the two groups). Both baseline and follow-up coronary angiograms were analysed with quantitative coronary angiography (QCA) by a single trained investigator (M.Z.) blinded to each patient's hypothyroid status using QAngio XA software (Version 7.3, Medis Medical Imaging System BV, Leiden, The Netherlands). We examined disease progression in both the target lesion and in the segment downstream to the target lesion. Disease progression was defined as any of the following: (i) 30% luminal diameter reduction of a pre-existing stenosis of <50%, (ii) progression of any lesion to total occlusion, and (iii) ‘new’ lesions with a 30% luminal diameter reduction in a segment that was normal at the first angiogram. 13
Statistical analysis
Continuous variables are expressed as mean ± SD or median (25th, 75th percentile) and were compared across groups using Student's t -test or the Wilcoxon rank-sum test as appropriate. Categorical variables are presented as counts and percentages and were compared across groups using the χ2 test or Fisher's exact test as appropriate. Kaplan–Meier survival curves were generated and the log-rank test was used to assess for differences between the curves. Multivariate Cox proportional hazards models were developed to determine the hazards ratios for MACCE and its constituent parts in patients with hypothyroidism compared with those with euthyroidism, after adjusting for the following variables: age, gender, diabetes, hypertension, dyslipidaemia, family of CAD, renal failure, current smoking, heart failure, history of MI, number of diseased vessels, stent type used, and discharge medications, including aspirin, β-blockers, angiotensin-converting enzyme (ACE) inhibitors, or statins. These variables were selected from Table 1 due to the following: the variable is known to be related to both thyroid disease status and the risk of adverse cardiovascular events and thus may act as a potential confounder, including age and gender; the variable is strongly associated with the risk of adverse cardiovascular events and is therefore clinically important, including discharge medication or the variable differed significantly between the hypothyroid and euthyroid groups at a Type 1 error rate of 0.05. In addition, to ensure stability of our models, variables associated with a high degree of collinearity were excluded from our final model. All variables selected from Table 1 were entered into an adjusted model with backward selection, and those covariates associated with a variance inflation factor of ≥10 were excluded from final model.
Table 1.
Baseline patient characteristics
| Variable | All ( n = 2430) |
Euthyroidism ( n = 1744) |
Hypothyroidism ( n = 686) |
P -value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age | 64.6 ± 11.6 | 63.9 ± 11 | 66.4 ± 12.4 | <0.0001 |
| Female, n (%) | 870 (36) | 499 (29) | 371 (54) | <0.0001 |
| Body mass index (kg/m 2 ) | 30.2 ± 6.0 | 30.1 ± 5.7 | 30.4 ± 6.4 | 0.27 |
| Diabetes mellitus, n (%) | 640 (27) | 418 (24.1) | 222 (33) | <0.0001 |
| Hypertension, n (%) | 1658 (769) | 1161 (68) | 497 (74) | 0.003 |
| Current smoking, n (%) | 324 (15) | 241 (16) | 83 (14) | 0.24 |
| Hyperlipidaemia, n (%) | 1656 (81) | 1207 (82) | 450 (79) | 0.08 |
| Myocardial infarction, n (%) | 933 (39) | 643 (37) | 290 (43) | 0.02 |
| F/H CAD, n (%) | 678 (36) | 490 (36) | 188 (36.2) | 0.99 |
| Peripheral arterial disease, n (%) | 201 (8.4) | 136 (8) | 65 (9.6) | 0.2 |
| Chronic lung disease, n (%) | 239 (10) | 174 (10) | 65 (10) | 0.77 |
| EF_le40, n (%) | 155 (6) | 116 (6.7) | 39 (5.7) | 0.37 |
| Chronic heart failure, n (%) | 249 (10.6) | 160 (9.5) | 89 (13.5) | 0.005 |
| Renal failure, n (%) | 168 (7) | 128 (7) | 40 (6) | 0.2 |
| TSH, median (Q1, Q3) | 2.5 (1.5, 4.7) | 2 (1.3, 2.8) | 7.8 (5.5, 13.5) | <0.0001 |
| FT4, median (Q1, Q3) | 1.1 (0.9, 1.3) | 1.15 (1, 1.3) | 1.1 (0.9, 1.3) | 0.0009 |
| Primary hypothyroidism | 465 (19) | NA | 465 (93) | NA |
| Laboratory characteristics | ||||
| White blood cells, median (Q1, Q3) | 7.5 (6.2, 9.4) | 7.5 (6.1, 9.3) | 7.5 (6.3, 10) | 0.3 |
| Haemoglobin (g/dL), median (Q1, Q3) | 13 (11.8, 14.1) | 13.2 (12, 14.2) | 12.6 (11.3, 13.6) | <0.0001 |
| Platelet (g/L), median (Q1, Q3) | 211 (176, 252) | 210 (176, 249) | 215 (177, 256) | 0.08 |
| Random glucose (mg/dL), median (Q1, Q3) | 107 (96, 130) | 106 (96, 127) | 109 (97, 135) | 0.008 |
| Creatinine (mg/dL), median (Q1, Q3) | 1.1 (1, 1.3) | 1.1 (1, 1.3) | 1.1 (0.9, 1.3) | 0.67 |
| Total cholesterol (mg/dL), median (Q1, Q3) | 185 (158, 219) | 184 (158, 218) | 190 (160, 223) | 0.03 |
| Triglyceride (mg/dL), median (Q1, Q3) | 145 (101, 206) | 144 (100, 205) | 149 (103, 209) | 0.32 |
| LDL (mg/dL), median (Q1, Q3) | 109 (85, 137) | 108 (84, 137) | 111 (87, 138) | 0.09 |
| HDL (mg/dL), median (Q1, Q3) | 43 (36, 52) | 42 (35, 51) | 44 (37, 55) | <0.0001 |
| Medications at discharge | ||||
| Aspirin, n (%) | 2216 (92) | 1578 (91) | 638 (94) | 0.05 |
| Clopidogrel, n (%) | 1187 (49) | 849 (49) | 338 (50) | 0.84 |
| β-Blockers, n (%) | 1811 (75) | 1310 (76) | 501 (73) | 0.24 |
| ACEI/ARBs, n (%) | 1169 (49) | 812 (50) | 357 (52) | 0.02 |
| Calcium channel antagonist, n (%) | 684 (28) | 477 (28) | 207 (30) | 0.18 |
| Nitrates, n (%) | 1247 (52) | 873 (51) | 374 (55) | 0.05 |
| Statins, n (%) | 1822 (76) | 1289 (75) | 533 (78) | 0.06 |
| Amiodarone, n (%) | 39 (1.6) | 19 (1) | 20 (3) | 0.002 |
| PCI characteristics | ||||
| Diseased vessels, n (%) | <0.0001 | |||
| 1-vessel | 557 (23) | 353 (20) | 204 (30) | |
| 2-vessel | 1076 (58) | 813 (47) | 263 (38) | |
| 3-vessel | 797 (42) | 577 (33) | 220 (32) | |
| Left main disease, n (%) | 46 (2.0) | 33 (1.9) | 13 (1.9) | 0.99 |
| Status of procedure | 0.05 | |||
| Selective | 1030 (42) | 753 (43) | 277 (40) | |
| Urgent | 1102 (45) | 794 (46) | 308 (45) | |
| Emergent | 297 (12) | 195 (12) | 102 (15) | |
| Drug-eluting stent | 620 (26) | 431 (25) | 189 (28) | 0.16 |
| Stent number, median (Q1, Q3) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.10 |
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PCI, percutaneous coronary intervention; TSH, thyroid-stimulating hormone; ACEI/ARBs, ACE inhibitors/angiotensin receptor blockers.
Prior to reporting hazard ratios, the proportional hazards assumption was verified in each model graphically by plotting the log hazard vs. time by levels of the covariates separately for hypothyroidism and euthyroidism. Each set of plotted lines was then visually assessed to ensure that the same vertical distance was maintained over time.
To ensure we did not violate the assumption of independent observations of Cox models, disease progression, when related to target-lesion or distal-to-target-lesion disease progression, was categorized as a binary event for each individual patient regardless of the number of coronary vessels affected. All tests were two tailed with a Type 1 error rate of 0.05. All analyses were undertaken using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
Two thousand four hundred and thirty patients met the inclusion criteria for this study, of which 686 individuals (28%) with hypothyroidism. The median (Q1, Q3) follow-up for all patients was 3.0 years (0.5, 7.0). Baseline clinical, laboratory, and angiographic characteristics of the study groups are presented in Table 1 . Compared with the euthyroid group, patients with hypothyroidism were significantly older and more likely to be female. The prevalence of hypertension (74.0 vs. 68.0%, P = 0.003), diabetes mellitus (33.0 vs. 24.0%, P < 0.001), previous MI (43.0 vs. 37.0%, P = 0.02), and heart failure (13.5 vs. 9.5%, P = 0.001) were significantly higher in patients with hypothyroidism. Levels of random glucose and high-density lipoprotein cholesterol were significantly higher, and levels of haemoglobin were significantly lower, in patients with hypothyroidism. Patients with hypothyroidism were also more likely to be taking nitrates, ACE inhibitors/angiotensin receptor blockers, and amiodarone.
Cardiovascular events and hypothyroidism
The frequency of in-hospital MACCE and its constituent components are shown in Table 2 . The incidence of heart failure was significantly higher in patients with hypothyroidism; however, the frequency of MI, cardiac death, and TVR did not differ significantly between groups.
Table 2.
Frequency of in-hospital clinical outcomes in patients undergoing percutaneous coronary intervention
| Variable | Euthyroidism ( n = 1744) |
Hypothyroidism ( n = 686) |
P -value |
|---|---|---|---|
| Myocardial infarction | 85 (4.9) | 36 (5.2) | 0.71 |
| Heart failure | 160 (9.5) | 89 (13.5) | 0.005 |
| Revascularization with PCI | 69 (4) | 25 (3.6) | 0.71 |
| Revascularization target vessel with PCI | 21 (1.2) | 13 (1.9) | 0.2 |
| Cardiac death | 0 (0) | 0 (0) | NA |
| Revascularization with CABG | 13 (0.8) | 8 (0.9) | 0.33 |
NA, no statistical testing undertaken as no events recorded; CABG, coronary artery bypass grafting.
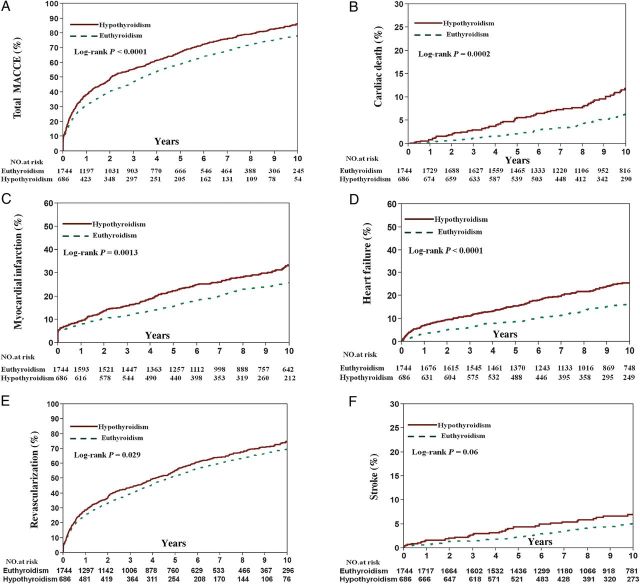
The relationship between hypothyroidism and MACCE at follow-up was evaluated using the Kaplan–Meier method. In patients with hypothyroidism, unadjusted Kaplan–Meier estimates for MACCE (86.5 vs. 78.3%, log-rank P < 0.0001), cardiac death (11.9 vs. 6.4%, log-rank P = 0.0002), MI (33.5 vs. 25.8%, log-rank P = 0.001), TVR (75.0 vs. 69.6%, Log-rank P = 0.021), and heart failure (26.6 vs. 16.3%, log-rank P < 0.0001) at 10-year follow-up were significantly higher than for patients with euthyroidism ( Figure 3 ). After adjusting for covariates, the risk of MACCE (HR: 1.28, P = 0.0001), MI (HR: 1.25, P = 0.037), heart failure (HR: 1.46, P = 0.004), TVR (HR: 1.26, P = 0.0008), and stroke (HR: 1.62, P = 0.04) was higher in the hypothyroid group compared with the euthyroid group ( Table 3 ).
Figure 3.
Frequency of adverse cardiovascular events at follow-up in patients with hypothyroidism and euthyroidism. Unadjusted Kaplan–Meier curves during 10-year follow-up for ( A ) major adverse cardiovascular and cerebral events, ( B ) cardiac death, ( C ) myocardial infarction, ( D ) heart failure, ( E ) target-vessel revascularization,and ( F ) stroke.
Table 3.
Hazard ratios for major adverse cardiovascular and cerebral events in patients with hypothyroidism vs. euthyroidism
| Variable | Adjusted HR a | 95% CI | P -value |
|---|---|---|---|
| MACCE | 1.28 | 1.13–1.45 | 0.0001 |
| Cardiac death | 1.14 | 0.75–1.69 | 0.54 |
| Myocardial infarction | 1.25 | 1.01–1.53 | 0.037 |
| Heart failure | 1.46 | 1.13–1.88 | 0.004 |
| Revascularization | 1.26 | 1.10–1.43 | 0.0008 |
| Stroke | 1.62 | 1.04–2.49 | 0.04 |
a Adjusted for age, gender, diabetes, hypertension, dyslipidaemia, family of CAD, renal failure, current smoking, heart failure, history of MI, number of diseased vessels, stent type, aspirin, β-blockers, ACE inhibitors, and statins.
Thyroid status by thyroid-stimulating hormone levels and cardiovascular outcomes
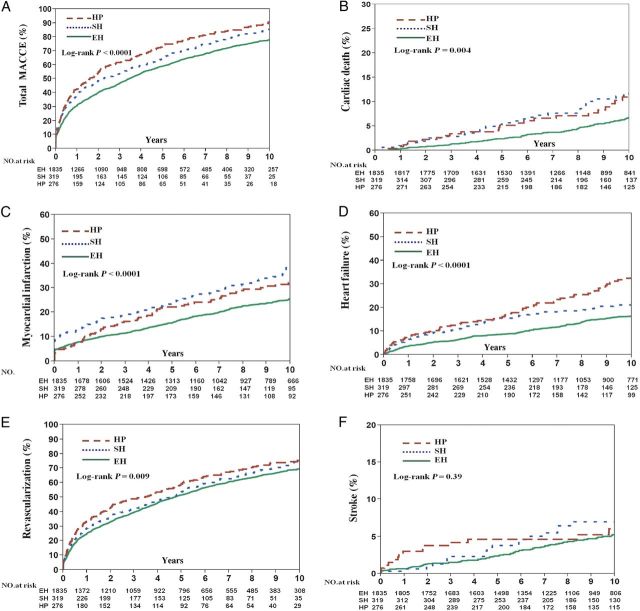
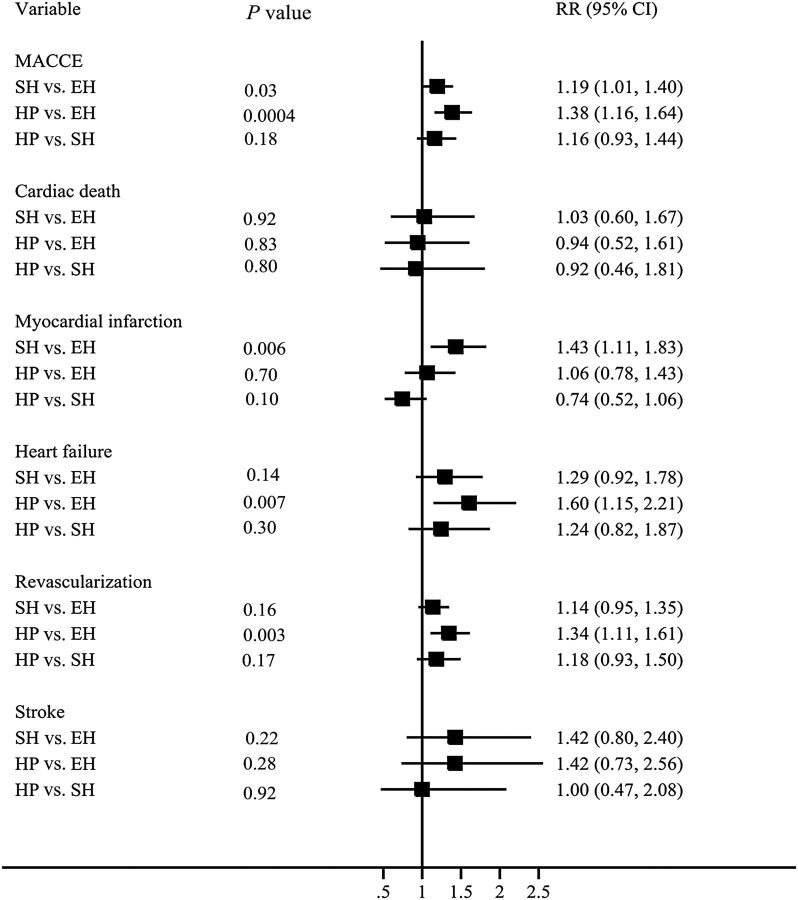
To further evaluate the effect of thyroid status on MACCE, all patients were separately divided into three groups according only to their TSH level at the time of the index procedure as follows: euthyroidism, SCH, and hypothyroidism. At 10-year follow-up, the unadjusted Kaplan–Meier estimates for MACCE (86.1 vs. 78.1%, P = 0.0008; 90.7 vs. 78.1%, P < 0.0001), cardiac death (11.8 vs. 6.7%, P = 0.014; 11.7 vs. 6.7%, P = 0.014), MI (38.3 vs. 25.6%, P < 0.0001; 32.1 vs. 25.6%, P = 0.14), heart failure (21.1 vs. 16.4%, P = 0.003; 33.0 vs. 16.4%, P < 0.0001), and TVR (74.6 vs. 69.6%, P < 0.0001;77.0 vs. 69.6%, P < 0.0001) were significantly higher in the SCH and hypothyroidism groups, respectively, compared with the euthyroid group ( Figure 4 ). After adjusting for covariates, the risk of MACCE (HR SCH : 1.19, P = 0.033 and HR hypothyroidism : 1.38, P = 0.0004), MI (HR SCH : 1.43, P = 0.006), heart failure (HR hypothyroidism :1.60, P = 0.0007), and TVR (HR SCH : 1.34, P = 0.003) were higher in the SCH and hypothyroid groups compared with the euthyroid group. However, there were no significant differences between SCH and overt hypothyroidism with respect to any outcome ( Figure 5 ).
Figure 4.
Frequency of adverse cardiovascular events at follow-up in patients with hypothyroidism, subclinical hypothyroidism, and euthyroidism according to serum thyroid-stimulating hormone levels. Unadjusted Kaplan–Meier curves during 10-year follow-up for ( A ) major adverse cardiovascular and cerebral events, ( B ) cardiac death, ( C ) myocardial infarction, ( D ) heart failure, ( E ) revascularization, and ( F ) stroke. EH, euthyroidism; SH, subclinical hypothyroidism; HP, hypothyroidism.
Figure 5.
Hazard ratios for major adverse cardiovascular and cerebral events in patients with hypothyroidism, subclinical hypothyroidism, and euthyroidism.
Thyroid replacement therapy and cardiovascular outcomes
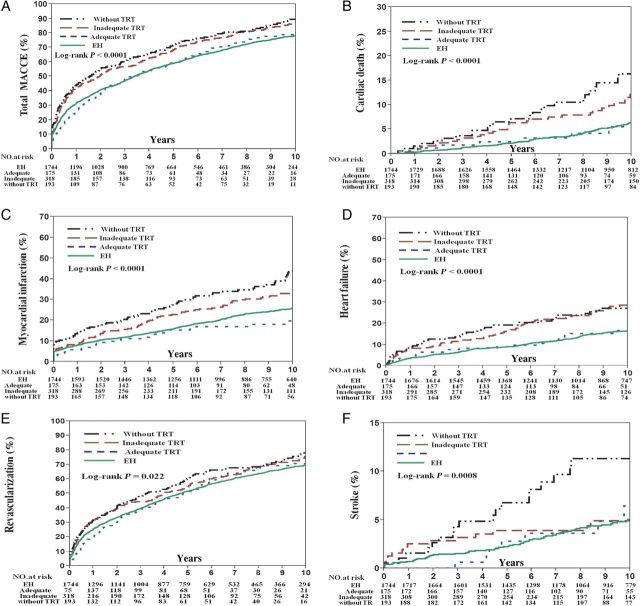
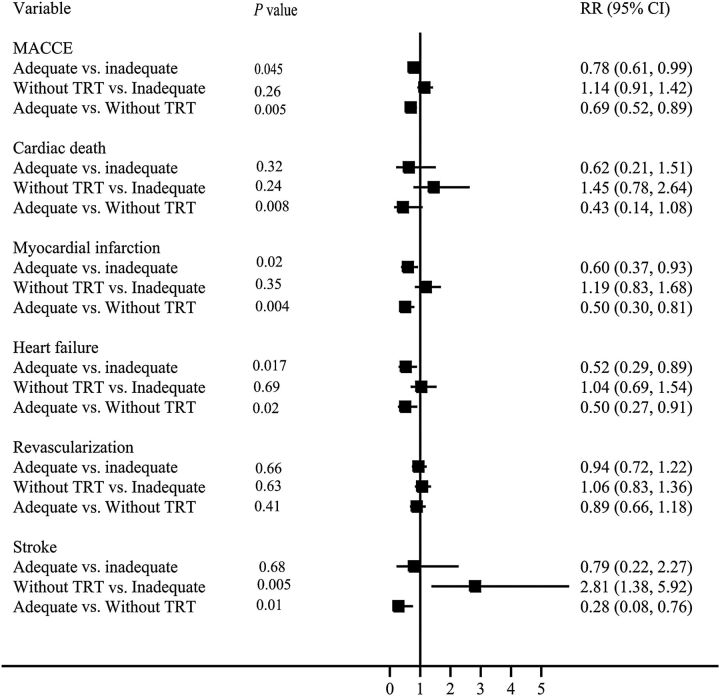
For patients on adequate replacement with TRT, the unadjusted Kaplan–Meier estimates at 10-year follow-up for MACCE (78.8 vs. 90.4%, P = 0.001; 78.8 vs. 87.5%, P = 0.002), cardiac death (5.5 vs. 16.3%, P = 0.002; 5.5 vs. 12.1%, P = 0.003), MI (19.7 vs. 43.8%, P < 0.0001; 19.7 vs. 33.5%, P = 0.004), heart failure (16.6 vs. 27.1%, P = 0.009; 16.6 vs. 28.6%, P = 0.001), TVR (70.6 vs. 78.0%, P = 0.04; 70.6 vs. 75.6%, P = 0.12), and stroke (6.5 vs. 2.6%, P = 0.009; 6.5 vs. 4.9%, P = 0.68) were lower compared with patients taking no TRT and patients with inadequate replacement on TRT, respectively ( Figure 6 ). After adjusting for covariates, patients with adequate replacement on TRT had a lower risk of MACCE (HR: 0.69, P = 0.005; HR: 0.78, P = 0.045), cardiac death (HR: 0.43, P = 0.008), MI (HR: 0.50, P = 0.0004; HR: 0.60, P = 0.02), and heart failure (HR: 0.50, P = 0.02; HR: 0.52, P = 0.017) compared with patients not taking TRT and patients with inadequate replacement on TRT, respectively. When comparing patients prescribed no TRT to patients with inadequate replacement on TRT, there were no differences with respect to any outcome except stroke (HR: 2.81, P = 0.005) ( Figure 7 ).
Figure 6.
Frequency of adverse cardiovascular events at follow-up between patients not taking thyroid replacement therapy and those taking thyroid replacement therapy with and without adequate replacement. Unadjusted Kaplan–Meier curves during 10-year follow-up for ( A ) major adverse cardiovascular and cerebral events, ( B ) cardiac death, ( C ) myocardial infarction, ( D ) heart failure, ( E ) target-vessel revascularization, and ( F ) stroke.
Figure 7.
Hazard ratios for major adverse cardiovascular and cerebral events in patients taking thyroid replacement therapy with adequate replacement vs. inadequate replacement and no replacement therapy.
Coronary disease progression
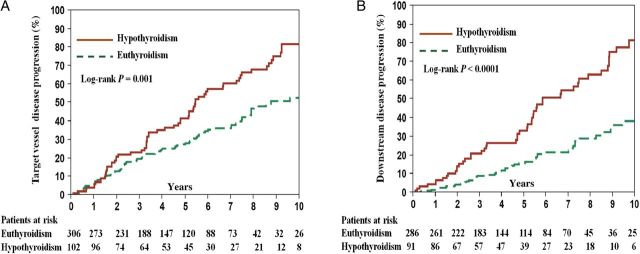
Four hundred and eight patients were analysed for CAD progression. The Kaplan–Meier-estimated frequency of cumulative target-lesion disease progression at 10-year follow-up was higher for patients with hypothyroidism compared with those with euthyroidism (79.9 vs. 52.6%, log-rank P = 0.001) ( Figure 8 A ). After adjusting for covariates, hypothyroidism was associated with a higher risk of target-lesion disease progression compared with euthyroidism (HR: 1.43, P = 0.044) ( Table 4 ).
Figure 8.
Kaplan–Meier-estimated cumulative target and downstream disease progression frequencies during 10-year follow-up. ( A ) Target-lesion disease progression and ( B ) downstream disease progression.
Table 4.
Hazard ratios for coronary disease progression in patients with hypothyroidism vs. euthyroidism
| Variable | Adjusted HR a | 95% CI | P -value |
|---|---|---|---|
| Target lesion | 1.43 | 1.01–1.99 | 0.04 |
| Downstream lesion | 1.90 | 1.21–2.95 | 0.006 |
| Target-lesion total occlusion | 1.25 | 0.57–2.59 | 0.57 |
a Adjusted for age, gender, diabetes, hypertension, dyslipidaemia, family of CAD, renal failure, current smoking, heart failure, history of MI, number of diseased vessels, stent type, aspirin, β-blockers, ACE inhibitors, and statins.
Thirty-one (7.6%) had total conduit occlusion at follow-up, and were therefore excluded from the downstream disease progression analysis. The Kaplan–Meier-estimated frequency of cumulative downstream disease progression at 10-year follow-up was significantly higher in patients with hypothyroidism (79.5 vs. 43.2%, log-rank P < 0.001) ( Figure 8 B ). After adjusting for covariates, hypothyroidism was associated with a higher risk for downstream disease progression compared with euthyroidism (HR: 1.90, P = 0.006) ( Table 4 ).
Discussion
The present study demonstrates that patients with a clinical history of hypothyroidism have a higher risk of MACCE, cardiac death, MI, heart failure, and TVR following PCI compared with euthyroid patients. Hypothyroidism is also associated with a higher incidence of coronary target-lesion and downstream disease progression. In addition, our findings suggest that adequate replacement with TRT among patients with a clinical history of hypothyroidism significantly reduces the risk of MACCE compared with patients with inadequate replacement or no TRT. The latter finding highlights the importance of monitoring TSH levels and maintaining a biochemically euthyroid state and supports a role for the routine testing of TSH levels in hypothyroid patients presenting for PCI as part of standard laboratory testing.
Hypothyroidism and major adverse cardiovascular and cerebral events
Previous studies have linked hypothyroidism with accelerated atherosclerosis and an increased risk of CAD, 9 congestive heart failure, 14 and diabetes mellitus. 15 The present study extends these findings by also demonstrating a higher prevalence of hypertension, diabetes mellitus, previous MI, and heart failure in patients with hypothyroidism.
The most important finding of our study was that hypothyroidism was associated with an increased risk of MACCE at follow-up in patients who underwent PCI. This may in part be attributable to the higher prevalence of conventional cardiovascular risk factors in the hypothyroid cohort compared with the euthyroid cohort, particularly with respect to an atherogenic lipid profile. 16 However, the association between hypothyroidism and MACCE remained even after adjusting for cardiovascular risk factors, suggesting that other factors may be involved. In this study, we showed that patients with hypothyroidism have a higher risk of target-lesion and downstream disease progression compared with patients with euthyroidism, which could partly explain hypothyroidism's association with a higher incidence of MACCE. The mechanism of this accelerated disease progression may relate to other factors such as endothelial dysfunction 17 and low-grade systemic inflammation, 18 both of which are associated with atherosclerosis. Endothelial dysfunction is an early marker of atherosclerosis 19 and arises in part due to the impaired bioavailability of nitric oxide and increased platelet adherence and smooth muscle cell migration and proliferation, both of which are implicated in the pathogenesis of atherosclerosis. 20 In addition, endothelial dysfunction is associated with coronary plaque progression and necrotic plaque, which are vulnerable to rupture and is also linked to an increased risk of adverse cardiovascular events. Recent studies have suggested that hypothyroidism may have adverse effects on endothelial function independent of other well-known atherosclerotic risk factors. 17 , 21 We recently showed that hypothyroidism is associated with microvascular endothelial dysfunction in women with non-obstructive CAD. 22 It has also been shown that the TSH receptor is expressed on vascular endothelial cells and elevated TSH can promote endothelial dysfunction by altering gene expression. 23 Thus, hypothyroidism may lead to an increased risk of adverse cardiovascular events through endothelial dysfunction; however, the precise mechanisms require further clarification.
Thyroid status by thyroid-stimulating hormone and major adverse cardiovascular and cerebral events
We carried out a separate analysis evaluating the relationship between hypothyroidism, SCH and euthyroidism, and MACCE. Our results showed that patients with overt hypothyroidism and SCH were at increased risk of MACCE at follow-up compared with patients with euthyroidism. Although some studies do not support the routine screening of individuals for thyroid disease, 6 these results suggest that routinely screening patients using TSH as a simple biomarker may be beneficial in identifying subjects with SCH or undiagnosed overt hypothyroidism who are at increased risk of cardiovascular events. This is particularly important as these patients may benefit from TRT to reduce the risk of adverse health outcomes 24 as well as cardiovascular disease. 25 Though there is controversy as to whether or not SCH merits treatment, our findings support previous lines of evidence that suggest that SCH is indeed a clinically important entity that requires identification and management, as its risk is comparable with that of overt hypothyroidism. However, given that other studies have found no differences in risk between patients with SCH and euthyroidism, 10 the clinical implications of SCH require further investigation.
Thyroid replacement therapy
Among 686 participants with hypothyroidism, 493 were prescribed TRT at the time of index PCI. It remains unclear as to whether treatment with TRT can offset the increased risk of cardiovascular disease in patients with hypothyroidism. Some studies suggest that TRT reverses this risk 25 and improves the symptoms of patients with hypothyroidism while also improving the health status of patients with SCH. 24 Other studies, however, do not support the treatment of individuals with SCH to prevent cardiovascular events. 10 , 26 The aim of treating hypothyroidism is to achieve euthyroidism, and TRT is considered safe providing there is close monitoring of serum TSH levels. 27 However, despite frequent monitoring and dose adjustment, inadequate thyroid hormone replacement remained a problem in over a third of levothyroxine users in this population. 28 In our study, among the 72% of patients with hypothyroidism taking TRT, 65% had TSH levels outside the target range at follow-up. Other studies have reported that up to 40% of patients taking TRT have abnormal TSH levels. 29 We found that the risk of MACCE was significantly lower in patients with adequate replacement on TRT. However, the risk of MACCE was not significantly different between patients with inadequate replacement on TRT and those not taking TRT, further underscoring the importance of maintaining the euthyroid state. Our findings highlight the need to evaluate the adequacy of treatment with TRT among patients with hypothyroidism by the routine monitoring of serum TSH levels.
Study limitations
First, the present study was a prospective cohort study and our results may be influenced by confounding variables not accounted for in our analyses. There may also be a selection bias as patients must have had TSH levels drawn at the time of the index PCI to be eligible for this study. Second, most subjects who had documented TSH levels had no FT4 levels available, limiting the assessment of thyroid status to a single TSH value, which may result in a misclassification bias, particularly when diagnosing SCH. Thyroid-stimulating hormone levels are also known to fluctuate with time adding to this problem. Third, this study is limited by its observational design and its relatively modest sample size, thus further studies evaluating a larger sample size in the context of a randomized clinical trial are required. Nevertheless, the present study is, to our knowledge, the only prospective cohort study to have evaluated the relationship between hypothyroidism and adverse cardiovascular events in patients undergoing PCI and, despite the modest sample size, was still able to demonstrate a significant difference in the incidence of MACCE in patients with hypothyroidism compared with those with euthyroidism. Finally, when collecting our data, we were not able to identify other non-cardiac causes of death such as malignancy, which may be competing risks against cardiovascular death, and should also be considered.
Conclusions
Hypothyroidism is associated with a higher incidence of MACCE compared with euthyroidism in patients undergoing PCI. Maintaining adequate control on TRT is beneficial in preventing cardiovascular events.
Authors’ contributions
M.Z., J.D.S.S., Y.M., H.G., M.R.B., R.G., L.O.L., and A.L. performed statistical analysis. A.L. handled funding and supervision. M.Z. and A.L. acquired the data. M.Z., J.D.S.S., Y.M., H.G., M.R.B., R.G., L.O.L., and A.L. conceived and designed the research. M.Z. and A.L. drafted the manuscript. M.Z. and A.L. made critical revision of the manuscript for key intellectual content.
Funding
The work was supported by the National Institute of Health (NIH Grants HL-92954 and AG-31750 to A.L.) and Beijing Municipal Training Foundation for Highly-Qualified and Technological Talents of Health System (2014-3-038), China.
Conflict of interest : none declared.
References
- 1. Kahaly GJ , Dillmann WH . Thyroid hormone action in the heart . Endocr Rev 2005. ; 26 : 704 – 728 . [DOI] [PubMed] [Google Scholar]
- 2. Polikar R , Burger AG , Scherrer U , Nicod P . The thyroid and the heart . Circulation 1993. ; 87 : 1435 – 1441 . [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner C , Blum MR , Rodondi N . Subclinical hypothyroidism: summary of evidence in 2014 . Swiss Med Wkly 2014. ; 144 : w14058 . [DOI] [PubMed] [Google Scholar]
- 4. Rugge JB , Bougatsos C , Chou R . Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force . Ann Intern Med 2015. ; 162 : 35 – 45 . [DOI] [PubMed] [Google Scholar]
- 5. Boelaert K . Thyroid dysfunction in the elderly . Nat Rev Endocrinol 2013. ; 9 : 194 – 204 . [DOI] [PubMed] [Google Scholar]
- 6. Tabatabaie V , Surks MI . The aging thyroid . Curr Opin Endocrinol Diabetes Obes 2013. ; 20 : 455 – 459 . [DOI] [PubMed] [Google Scholar]
- 7. ATA . Prevalence and impact of thyroid disease . http://www.thyroid.org/ (29 September 2014) . 2014. .
- 8. Taylor PN , Iqbal A , Minassian C , Sayers A , Draman MS , Greenwood R , Hamilton W , Okosieme O , Panicker V , Thomas SL , Dayan C . Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study . JAMA Intern Med 2014. ; 174 : 32 – 39 . [DOI] [PubMed] [Google Scholar]
- 9. Grais IM , Sowers JR . Thyroid and the heart . Am J Med 2014. ; 127 : 691 – 698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappola AR , Fried LP , Arnold AM , Danese MD , Kuller LH , Burke GL , Tracy RP , Ladenson PW . Thyroid status, cardiovascular risk, and mortality in older adults . JAMA 2006. ; 295 : 1033 – 1041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silber S , Albertsson P , Avilés FF , Camici PG , Colombo A , Hamm C , Jørgensen E , Marco J , Nordrehaug JE , Ruzyllo W , Urban P , Stone GW , Wijns W , Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology . Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology . Eur Heart J 2005. ; 26 : 804 – 847 . [DOI] [PubMed] [Google Scholar]
- 12. McQuade C , Skugor M , Brennan DM , Hoar B , Stevenson C , Hoogwerf BJ . Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study . Thyroid 2011. ; 21 : 837 – 843 . [DOI] [PubMed] [Google Scholar]
- 13. Zouridakis EG , Schwartzman R , Garcia-Moll X , Cox ID , Fredericks S , Holt DW , Kaski JC . Increased plasma endothelin levels in angina patients with rapid coronary artery disease progression . Eur Heart J 2001. ; 22 : 1578 – 1584 . [DOI] [PubMed] [Google Scholar]
- 14. Biondi B . Mechanisms in endocrinology: heart failure and thyroid dysfunction . Eur J Endocrinol 2012. ; 167 : 609 – 618 . [DOI] [PubMed] [Google Scholar]
- 15. Radaideh AR , Nusier MK , Amari FL , Bateiha AE , El-Khateeb MS , Naser AS , Ajlouni KM . Thyroid dysfunction in patients with type 2 diabetes mellitus in Jordan . Saudi Med J 2004. ; 25 : 1046 – 1050 . [PubMed] [Google Scholar]
- 16. Ichiki T . Thyroid hormone and atherosclerosis . Vascul Pharmacol 2010. ; 52 : 151 – 156 . [DOI] [PubMed] [Google Scholar]
- 17. Papaioannou GI , Lagasse M , Mather JF , Thompson PD . Treating hypothyroidism improves endothelial function . Metabolism 2004. ; 53 : 278 – 279 . [DOI] [PubMed] [Google Scholar]
- 18. Taddei S , Caraccio N , Virdis A , Dardano A , Versari D , Ghiadoni L , Ferrannini E , Salvetti A , Monzani F . Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto's thyroiditis . J Clin Endocrinol Metab 2006. ; 91 : 5076 – 5082 . [DOI] [PubMed] [Google Scholar]
- 19. Elesber AA , Solomon H , Lennon RJ , Mathew V , Prasad A , Pumper G , Nelson RE , McConnell JP , Lerman LO , Lerman A . Coronary endothelial dysfunction is associated with erectile dysfunction and elevated asymmetric dimethylarginine in patients with early atherosclerosis . Eur Heart J 2006. ; 27 : 824 – 831 . [DOI] [PubMed] [Google Scholar]
- 20. Lüscher TF , Barton M . Biology of the endothelium . Clin Cardiol 1997. ; 20 : II-3 –II- 10 . [PubMed] [Google Scholar]
- 21. Völzke H , Robinson DM , Spielhagen T , Nauck M , Obst A , Ewert R , Wolff B , Wallaschofski H , Felix SB , Dörr M . Are serum thyrotropin levels within the reference range associated with endothelial function ? Eur Heart J 2009. ; 30 : 217 – 224 . [DOI] [PubMed] [Google Scholar]
- 22. Sara JD , Zhang M , Gharib H , Lerman LO , Lerman A . Hypothyroidism is associated with coronary endothelial dysfunction in women . J Am Heart Assoc 2015. ; 4 : e002225 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian L , Zhang L , Liu J , Guo T , Gao C , Ni J . Effects of TSH on the function of human umbilical vein endothelial cells . J Mol Endocrinol 2014. ; 52 : 215 – 222 . [DOI] [PubMed] [Google Scholar]
- 24. McDermott MT , Ridgway EC . Subclinical hypothyroidism is mild thyroid failure and should be treated . J Clin Endocrinol Metab 2001. ; 86 : 4585 – 4590 . [DOI] [PubMed] [Google Scholar]
- 25. Razvi S , Ingoe L , Keeka G , Oates C , McMillan C , Weaver JU . The beneficial effect of l -thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial . J Clin Endocrinol Metab 2007. ; 92 : 1715 – 1723 . [DOI] [PubMed] [Google Scholar]
- 26. Chu JW , Crapo LM . The treatment of subclinical hypothyroidism is seldom necessary . J Clin Endocrinol Metab 2001. ; 86 : 4591 – 4599 . [DOI] [PubMed] [Google Scholar]
- 27. Thvilum M , Brandt F , Brix TH , Hegedüs L . A review of the evidence for and against increased mortality in hypothyroidism . Nat Rev Endocrinol 2012. ; 8 : 417 – 424 . [DOI] [PubMed] [Google Scholar]
- 28. Okosieme OE , Belludi G , Spittle K , Kadiyala R , Richards J . Adequacy of thyroid hormone replacement in a general population . QJM 2011. ; 104 : 395 – 401 . [DOI] [PubMed] [Google Scholar]
- 29. Canaris GJ , Manowitz NR , Mayor G , Ridgway EC . The Colorado thyroid disease prevalence study . Arch Intern Med 2000. ; 160 : 526 – 534 . [DOI] [PubMed] [Google Scholar]