Abstract
PURPOSE
Trends in sedentary lifestyle may have influenced adult body composition and metabolic health among individuals at presumably healthy weights. This study examines the nationally representative prevalence of prediabetes and abdominal obesity among healthy-weight adults in 1988 through 2012.
METHODS
We analyzed the National Health and Nutrition Examination Survey (NHANES) III (1988–1994) and NHANES for the years 1999 to 2012, focusing on adults aged 20 years and older who have a body mass index (BMI) of 18.5 to 24.99 and do not have diabetes, either diagnosed or undiagnosed. We defined prediabetes using glycated hemoglobin (HbA1c) level ranges from 5.7% to 6.4%, as specified by the American Diabetes Association. Abdominal obesity was measured by waist circumference and waist-to-height ratio.
RESULTS
The prevalence of prediabetes among healthy-weight adults, aged 20 years and older and without diagnosed or undiagnosed diabetes, increased from 10.2% in 1988–1994 to 18.5% in 2012. Among individuals aged 45 years and older, the prevalence of prediabetes increased from 22.0% to 33.1%. The percentage of adults aged 20 years and older with an unhealthy waist circumference increased from 5.6% in 1988–1994 to 7.6% in 2012. The percentage of individuals with an unhealthy waist-to-height ratio increased from 27.2% in 1988–1994 to 33.7% in 2012. Adjusted models found that measures of abdominal obesity were not independent predictors of prediabetes among adults with a healthy BMI.
CONCLUSIONS
Among individuals within a healthy BMI range, the prevalence of prediabetes and abdominal obesity has substantially increased. Abdominal obesity does not appear to be the primary cause of the increase.
Keywords: prediabetes, prevalence, prediabetic state, hyperglycemia, diabetes type II, prevention
INTRODUCTION
Prediabetes is a high-risk state for the development of diabetes and its associated complications.1–3 Recent data have shown that in developed countries, such as the Unites States and the United Kingdom, more than one-third of adults have prediabetes, but most of these individuals are unaware they have the condition.4–6 Once detected, prediabetes needs to be acknowledged with a treatment plan to prevent or slow the transition to diabetes.7,8 Treatment of prediabetes is associated with delay of the onset of diabetes.9 Detection and treatment of prediabetes is therefore a fundamental strategy in diabetes prevention.1 Moreover, prediabetes-aware adults are more likely than those with prediabetes who are unaware of their condition to engage in diabetes risk-reducing behaviors.10
Historical trends in the United States have indicated a substantial rise in sedentary lifestyle among adults in recent years.11 Comparing findings from the National Health and Nutrition Examination Survey (NHANES) 1988–1994 with NHANES 2009–2010, the proportion of adults who reported no leisure-time physical activity increased from 19.1% to 51.7%. Although these leisure-time activity findings have been criticized as a result of methodological changes in the NHANES, findings from other national surveys have shown decreasing trends in leisure-time physical activity from the 1990s into the 2000s.12,13 As trends in sedentary lifestyle increased, so did trends in the prevalence of obesity.11 In addition to the rise in obesity, this population-level increase in sedentary lifestyle may have influenced adult body composition and metabolic health among individuals at presumably healthy weights.
Current recommendations for prediabetes screening by the American Diabetes Association focus nearly exclusively on adults who are overweight or obese as defined by body mass index (BMI; kg/m2) until the patient meets the age-oriented screening at 45 years.1 Further, the recently released recommendation from the US Preventive Services Task Force regarding screening for abnormal glucose levels and type 2 diabetes limits screening to individuals who are overweight or obese.14 This focus on obese or overweight individuals, however, may lead to missed opportunities for investigation of undetected disease in healthy-weight individuals.
Although obesity and prediabetes have shown trends of increasing prevalence, it is unclear whether prediabetes has also increased among healthy-weight adults. Moreover, it is unclear whether abdominal obesity has increased among healthy-weight adults. Thus, the purpose of this study was to examine the nationally representative prevalence of prediabetes and abdominal obesity among healthy-weight adults reported in the 1988–1994 and 1999–2012 NHANES findings.
METHODS
We analyzed the NHANES III and NHANES data for the years 1999–2012. NHANES III was conducted in 1988–1994. The NHANES is a large, nationally representative survey that samples the noninstitutionalized population of the United States using a stratified multistage probability sample design. The application of weights and variables accounting for the complex survey design allows the study to provide nationally representative population estimates for the United States. The current study focused on adults aged 20 years and older who have a BMI of 18.5 to 24.99, the range considered healthy, and who have not had diabetes diagnosed or a glycated hemoglobin (HbA1c) level of 6.5% or greater. This study was approved by the Institutional Review Board at the University of Florida as exempt.
Previously Diagnosed Diabetes
Individuals were considered to have diabetes if they reported ever being told by a health care provider that they had diabetes, excluding gestational diabetes. We also removed individuals with an HbA1c of 6.5% or greater to account for undiagnosed diabetes.
Normoglycemia and Prediabetes
Individuals participating in the NHANES undergo a physical examination that includes laboratory analysis of blood. We defined normoglycemia as an HbA1c level between 4.0% and 5.6% (20–38 mmol/mol). To control for any potential effect of low HbA1c levels, we also removed individuals with an HbA1c level of less than 4.0% (20 mmol/mol), a level associated with increased all-cause mortality in adults without diabetes.15
We defined prediabetes among individuals without previously diagnosed or undiagnosed diabetes using HbA1c level ranges as specified by the American Diabetes Association, 5.7% to 6.4% (39–46 mmol/mol).1 This range has been shown in a meta-analysis to be predictive of progression to diabetes.16 We excluded individuals with previously diagnosed diabetes because the current glycemic status of those patients may simply represent diabetes control.
Abdominal Obesity
Abdominal obesity was measured by waist circumference and waist-to-height ratio. Waist circumference measured a horizontal line just above the uppermost lateral border of the right ilium for participants at standing position by using measuring tape. An unhealthy waist circumference was defined as a waist circumference of greater than 102 cm for men and greater than 88 cm for women. These measures are consistent with the levels for characterizing metabolic syndrome. An unhealthy waist-to-height ratio was defined as .53 or greater in men and .49 and greater in women, levels that are indicative of increased cardiometabolic health risk.17–19
First-Degree Relative With Diabetes
In NHANES III, respondents were asked whether any of their living or deceased blood relatives (including grandparents, parents, brothers, and sisters) were ever told by a physician that they had diabetes. In NHANES 1999–2012, respondents were asked whether any close biological or blood relatives (including father, mother, sisters, or brothers) were ever told by a health professional that they had diabetes.
Demographic Characteristics
Age was self-reported and categorized as 20 to 44 years, 45 to 64 years, and 65 years and older. Sex was self-reported. Race was self-reported and is categorized as non-Hispanic white, non-Hispanic black, Mexican American, and other. Education was categorized as less than high school (<12 years of education), high school (12 years of education), and some college/degree (>12 years of education). Poverty-to-income ratio is based on self-report and categorized as less than 1.0 (poverty) and 1.0 or greater (not in poverty).20 Health insurance status was self-reported and categorized as private, public, and none.
Analysis
The NHANES uses a stratified multistage probability design. To account for the complex sample design of the NHANES, SAS 9.4 (SAS Institute, Cary, NC) and SUDAAN 11.0.1 (RTI International, Research Triangle Park, NC) were used with the appropriate design and weighting variables provided by the National Center for Health Statistics.
We calculated prediabetes prevalence for NHANES III and for each 2-year NHANES cycle from 1999 to 2012, as well as for several demographic characteristics. We conducted trend analysis of the 1999–2012 data, using logistic regression to assess the impact of time on prediabetes prevalence, changes in waist circumference, and changes in waist-to-height ratio for all adults, as well as for adults aged 45 years and older. Time is modeled as a continuous variable. t Tests were used to calculate the mean difference in BMI, waist circumference, and waist-to-height ratio between individuals with and without prediabetes for 2011–2012 data. Forced inclusion logistic regressions were conducted using 2011–2012 data to assess the impact of waist circumference and waist-to-height ratio on prediabetes. Both unadjusted models and models adjusting for demographic factors and first-degree relative with diabetes were computed.
RESULTS
Table 1 shows the prevalence of prediabetes for each NHANES cycle, as well as the prevalence of prediabetes for various demographic characteristics. The prevalence of prediabetes varied by year, but tended to show an increase overall, with the percentage of those with prediabetes rising from 10.2% in 1988–1994 to a high of 18.5% in 2011–2012. Using HbA1c to measure prediabetes, the prevalence of prediabetes appears to have decreased from 1988–1994 to 1999–2000, but then increases with time.
Table 1.
Weighted Total Prediabetes Prevalence, % (95% CI) of Adults Aged 20 Years or Older in the US Population Using Hemoglobin A1c Levels to Define Diabetes; NHANES 1988–1994 and 1999–2012
| Characteristic | 1988–1994 (n = 5,667) | 1999–2000 (n = 1,265) | 2001–2002 (n = 1,382) | 2003–2004 (n = 1,319) | 2005–2006 (n = 1,272) | 2007–2008 (n = 1,335) | 2009–2010 (n = 1,415) | 2011–2012 (n = 1,347) |
|---|---|---|---|---|---|---|---|---|
| Prediabetes cases, No. (weighted total No.) | 917 (7,086,925) | 107 (3,318,187) | 119 (3,375,785) | 126 (3,963,758) | 138 (4,847,946) | 265 (9,029,950) | 292 (10,239,137) | 292 (10,869,265) |
| Prevalence of prediabetes | 10.2 (8.8–11.7) | 5.6 (3.7–8.4) | 5.9 (4.9–6.9) | 6.8 (5.4–8.5) | 8.3 (6.2–10.9) | 15.5 (13.0–18.4) | 17.7 (15.6–20.1) | 18.5 (15.1–22.4) |
| Age, y | ||||||||
| 20–44 | 4.2 (3.2–5.4) | 1.3 (0.5–3.3) | 2.7 (1.5–4.7) | 0.8 (0.4–1.8) | 1.9 (1.1–3.4) | 4.9 (3.5–6.8) | 5.1 (4.1–6.4) | 6.8 (4.7–9.7) |
| 45–64 | 16.5 (14.2–19.0) | 9.9 (5.2–18.0) | 7.7 (5.5–10.8) | 11.0 (7.6–15.5) | 11.9 (9.0–15.6) | 22.8 (18.0–28.3) | 30.4 (24.0–37.7) | 26.8 (20.3–34.6) |
| ≥65 | 30.6 (26.9–34.6) | 17.6 (12.7–23.9) | 18.3 (13.4–24.5) | 23.7 (18.5–29.8) | 25.5 (20.3–31.5) | 40.6 (32.7–48.9) | 42.6 (37.2–48.2) | 44.9 (36.1–54.1) |
| Sex | ||||||||
| Male | 12.7 (11.0–14.6) | 6.6 (3.9–10.8) | 6.90 (5.3–8.9) | 7.7 (6.0–9.9) | 9.0 (6.3–12.7) | 16.0 (12.8–19.8) | 17.1 (14.0–20.8) | 17.7 (13.0–23.6) |
| Female | 8.18 (6.7–10.0) | 4.7 (2.7–8.1) | 5.1 (3.6–7.2) | 6.1 (4.2–8.7) | 7.8 (5.7–10.6) | 15.2 (11.8–19.4) | 18.1 (15.5–21.2) | 19.1 (15.3–23.6) |
| Race | ||||||||
| Non-Hispanic white | 8.4 (6.9–10.2) | 4.7 (2.6–8.4) | 5.1 (3.8–6.8) | 5.4 (4.0–7.3) | 6.7 (4.2–10.8) | 14.8 (11.7–18.5) | 16.4 (13.8–19.3) | 18.3 (14.2–23.1) |
| Non-Hispanic Black | 20.4 (18.4–22.6) | 9.1 (6.0–13.4) | 10.2 (6.6–15.4) | 10.8 (7.1–16.1) | 19.8 (13.4–28.4) | 25.6 (17.8–35.2) | 22.7 (16.0–31.1) | 24.6 (17.6–33.2) |
| Mexican American | 9.9 (8.1–12.1) | 4.3 (2.2–8.1) | 3.6 (1.5–8.5) | 3.8 (2.0–7.0) | 6.1 (3.0–12.1) | 12.2 (8.4–17.3) | 13.5 (8.8–20.0) | 16.8 (10.2–26.5) |
| Other | 16.4 (11.0–23.6) | 8.7 (4.2–17.2) | 9.8 (4.5–19.8) | 14.8 (9.1–23.3) | 12.0 (7.1–19.6) | 14.8 (8.7–24.1) | 23.2 (17.8–30.0) | 17.2 (13.2–22.1) |
| Poverty to income ratio (PIR) | ||||||||
| Poverty (PIR <1.0) | 14.5 (10.9–18.9) | 7.3 (4.5–11.6) | 5.1 (3.2–8.0) | 7.8 (5.3–11.3) | 9.8 (6.3–15.0) | 15.2 (10.5–21.5) | 18.4 (13.8–24.2) | 16.3 (6.8–34.3) |
| Not in poverty (PIR ≥1.0) | 9.3 (8.1–10.7) | 5.5 (3.5–8.4) | 5.9 (4.7–7.4) | 6.7 (5.2–8.6) | 7.6 (5.4–10.4) | 14.9 (12.4–17.8) | 17.0 (14.6–19.7) | 18.1 (15.2–21.4) |
| Health insurance | ||||||||
| Private | 9.1 (7.7–10.7) | 4.9 (2.8–8.3) | 4.6 (3.6–5.8) | 6.7 (5.1–8.7) | 6.9 (5.0–9.6) | 14.6 (11.8–17.9) | 16.2 (13.6–19.3) | 17.5 (13.7–22.1) |
| Public | 21.5 (17.3–26.5) | 13.3 (7.7–22.1) | 13.5 (9.6–18.7) | 11.5 (7.3–17.7) | 17.5 (13.6–22.2) | 25.5 (19.4–32.6) | 24.3 (19.5–29.8) | 27.9 (20.2–37.0) |
| None | 8.3 (5.0–13.6) | 4.5 (3.2–6.2) | 6.7 (4.7–9.4) | 3.7 (1.9–7.0) | 6.5 (3.5–11.6) | 12.4 (8.3–18.3) | 18.6 (13.8–24.5) | 13.0 (9.6–17.4) |
| Education | ||||||||
| <High school | 17.9 (15.4–20.7) | 10.6 (7.5–14.9) | 8.6 (5.8–12.6) | 9.8 (7.1–13.5) | 14.6 (10.1–20.7) | 21.9 (16.6–28.3) | 25.6 (21.2–30.4) | 31.6 (21.4–43.9) |
| High school | 10.0 (7.8–12.8) | 6.4 (3.5–11.6) | 8.1 (5.7–11.2) | 8.0 (5.7–11.0) | 10.7 (6.5–17.3) | 23.4 (17.6–30.4) | 16.3 (11.2–23.2) | 23.1 (16.3–31.7) |
| Some college/college degree | 6.7 (5.6–8.0) | 3.3 (1.7–6.4) | 4.2 (3.0–5.9) | 5.2 (3.6–7.6) | 5.7 (4.1–7.9) | 10.8 (8.1–14.3) | 16.3 (14.5–18.3) | 14.9 (11.3–19.3) |
| First-degree relative with diabetes | ||||||||
| Family history | 10.0 (8.5–11.8) | 7.1 (4.4–11.3) | 6.5 (5.1–8.3) | 7.0 (4.8–10.3) | 10.1 (6.7–14.8) | 21.0 (17.1–25.5) | 18.1 (14.8–21.9) | 21.2 (14.4–30.0) |
| No family history | 10.4 (8.7–12.4) | 4.6 (2.9–7.1) | 5.2 (4.0–6.8) | 6.9 (5.0–9.4) | 7.5 (5.4–10.4) | 13.2 (10.5–16.4) | 17.6 (15.3–20.1) | 17.6 (14.5–21.2) |
NHANES = National Health and Nutritional Examination Survey.
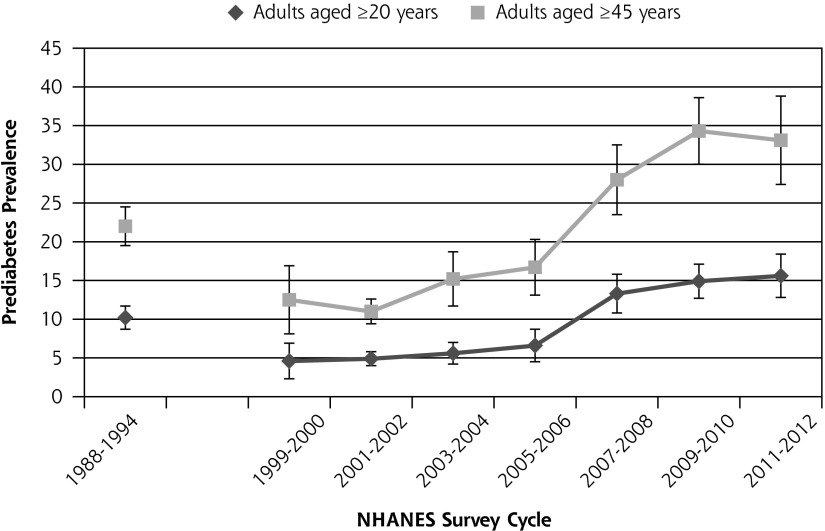
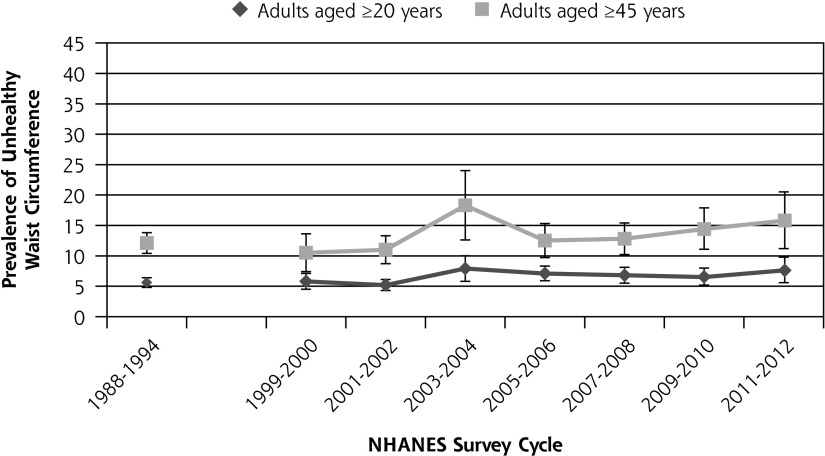
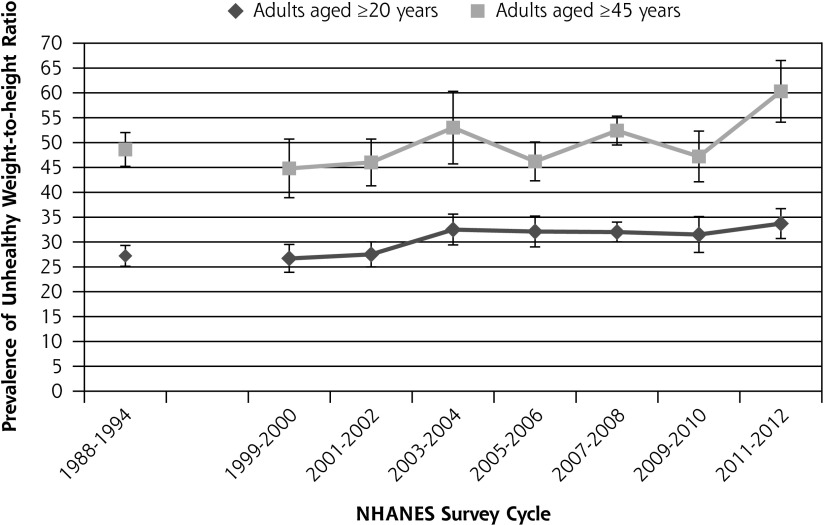
Figure 1 shows the prevalence of prediabetes from 1988 to 2012. The prevalence of prediabetes among healthy-weight adults aged 20 years and older increased from 10.2% in 1988–1994 to 18.5% in 2012. Among individuals aged 45 years and older, the prevalence of prediabetes increased from 22.0% to 33.1%. Trend analysis for 1999–2012 data shows that the increase in prediabetes during that time was statistically significant both for adults aged 20 years and older (P <.0001) and for adults aged 45 years and older (P <.0001). Figure 2 shows the change in the percentage of adults with an unhealthy waist circumference from 1988 to 2012 for adults aged 20 years and older and for adults aged 45 years and older. The percentage of adults aged 20 years and older with an unhealthy waist circumference increased from 5.6% in 1988–1994 to 7.6% in 2012. The change in prevalence of unhealthy waist circumference from 1999 to 2012 was not statistically significant either for adults aged 20 years and older (P = .38) or for adults aged 45 years and older (P = .39). Figure 3 shows the change in the percentage of adults with an unhealthy waist-to-height ratio from 1988 to 2012. The percentage of adults aged 20 years and older with an unhealthy waist-to-height ratio increased from 27.2% in 1988–1994 to 33.7% in 2012. The change in prevalence of unhealthy waist-to-height ratio was significant for adults aged 20 years and older (P = .01), as well as for adults aged 45 years and older (P = .007), in the 1999 to 2012 data.
Figure 1.
The prevalence of prediabetes in the United States among adults without diagnosed or undiagnosed diabetes from 1988 to 2012.
Note: Trend from 1999–2012 age ≥20 years, P <.0001; trend from 1999–2012 age ≥45 years, P <.0001. Vertical bars = 95% CI.
Figure 2.
The prevalence of unhealthy waist circumference in the United States among healthy weight adults without diagnosed or undiagnosed diabetes from 1988 to 2012.
Note: Trend from 1999–2012 age ≥20 years (P = .34); trend from 1999–1912 age ≥45 years, P = .40. Vertical bars = 95% CI.
Figure 3.
The prevalence of unhealthy waist-to-height ratio in the United States among healthy weight adults without diagnosed or undiagnosed diabetes from 1988 to 2012.
Note: Trend from 1999–2012 ages ≥20 years, P =.007; trend from 1999–2012 ages ≥45, years P = .007. Vertical bars = 95% CI.
The mean BMI for individuals without prediabetes was 22.2, and mean BMI for individuals with prediabetes was 22.6. The difference in means was statistically significant (P = .03). The mean waist circumference for individuals without prediabetes was 81.3 cm. For individuals with prediabetes, the mean waist circumference was 84.9 cm (P <.0001). Mean waist-to-height ratio for individuals without prediabetes was 0.48. The mean waist-to-height ratio for individuals with prediabetes was 0.51 (P <.0001).
Table 2 shows the unadjusted and adjusted logistic regression results for the impact of unhealthy waist circumference and unhealthy waist-to-height ratio on risk of prediabetes for adults aged 20 years and older and for adults aged 45 years and older. In unadjusted analyses, only unhealthy waist-to-height ratio was predictive of prediabetes. After adjustment for demographics and having a first-degree relative with diabetes, however, unhealthy waist-to-height ratio did not predict prediabetes.
Table 2.
Logistic Regressions Examining the Relation of Abdominal Obesity to Risk of Prediabetes in 2011–2012, NHANES
| Characteristic | Unadjusted OR (95% CI) | Adjusted OR (95% CI)a |
|---|---|---|
| Aged ≥20 years | ||
| Unhealthy waist circumference | 2.00 (0.76–5.28) | 1.10 (0.45–2.66) |
| Unhealthy waist-to-height ratio | 2.34 (1.39–3.95) | 1.06 (0.65–1.73) |
| Age ≥45 years | ||
| Unhealthy waist circumference | 1.27 (0.46–3.54) | 1.00 (0.42–2.37) |
| Unhealthy waist-to-height ratio | 1.40 (0.90–2.18) | 1.04 (0.65–1.66) |
NHANES = National Health and Nutritional Examination Survey; OR = odds ratio.
Controls for age, sex, race/ethnicity, education, poverty-to-income ratio, health insurance, and presence of a first-degree relative with diabetes.
DISCUSSION
To our knowledge, this study is the first that has examined the trends in prediabetes prevalence among individuals with a BMI in the healthy range. The key findings of this study are that among individuals with a BMI in the healthy range, the prevalence of prediabetes and abdominal obesity has increased from 1988–1994 to 2012.
In addition to the population change in the metabolic marker of HbA1c, one measure of abdominal obesity has also increased among individuals of healthy BMI. Previous studies found that people with abdominal obesity are more likely to have diabetes21,22 and to be at increased risk for cardiovascular disease.23,24 Also, as abdominal obesity in midlife may develop an elevated risk of diabetes in older age, it is an important factor in predicting diabetes.25 It is important to note that abdominal obesity is just one of several factors that are associated with prediabetes. In fact, although our findings reported trends of increased waist-to-height ratio along with increasing prevalence of prediabetes, abdominal obesity was not independently associated with prediabetes after accounting for other variables in a multivariate model.
The factors driving the increase in prediabetes and waist-to-height ratio among adults with a healthy BMI is unclear. Recent studies utilizing NHANES data showed that whereas overall dietary quality remains poor, there have been improvements to the quality of diet in America.26–28 The increase in sedentary lifestyle could in part be responsible, as there has been a documented increase in sedentary lifestyle.11,12 Sedentary lifestyle has been shown to negatively affect insulin sensitivity29 and increase the relative risk of diabetes.30 Sedentary lifestyle is also associated with increases in abdominal obesity.31–33
The increase of prediabetes in individuals with a healthy BMI is a concern. The recommendations of the US Preventive Services Task Force for screening for abnormal blood glucose levels and type 2 diabetes suggest screening for abnormal blood glucose levels in adults aged 40 to 70 years who are overweight or obese.14 These guidelines, along with those from the American Diabetes Association, make it less likely that individuals with a healthy BMI will be screened, despite the increasing prevalence of prediabetes among this group.
In interpreting the results of this study, we need to consider several limitations. First, although we were able to assess some common abdominal obesity measures, specifically, waist circumference and waist-to-height ratio, hip measurement was not included in both NHANES. Thus, we were unable to assess the changes in a third, common measure of abdominal obesity, waist-to-hip ratio.
Second, this study utilizes 2 iterations of the NHANES with different sampling methodologies. Data from 1988–1994 cannot be included in the trend analysis along with data from 1999–2012, which limits our ability to estimate the significance of the changes in prediabetes and abdominal obesity prevalence to only the 1999–2012 data. Even so, we were still able to document an increase in prediabetes and waist-to-height ratio using only that data.
Third, the NHANES is a cross-sectional survey that assesses the health status of different individuals in every cycle. The data are weighted, however, to allow for analysis of the entire population of the United States, rather than relying on idiosyncratic patient populations.
Fourth, the data showed a decrease in prediabetes prevalence between 1988–1994 and 1999–2000. The reason for this decrease is unclear. It is possible that clumping data into a 6-year group for the NHANES III led to an artifact when compared with clumping data into a 2-year group for the NHANES 1999–2000, but the reason for the decrease from the NHANES III to NHANES 1999–2000 requires additional exploration.
Fifth, as was noted earlier, some authors have criticized comparing leisure time physical activity assessments between the NHANES III and NHANES from 2007 forward, because leisure time physical activity questions were not the same in the 2 data sets.12 We were concerned about artifactual differences between the 2 periods, so we were unable to assess the impact of leisure time physical activity on prediabetes.
Sixth, although abdominal obesity can differ significantly among different ethnic groups, in particular South and East Asians that tend to show a lower BMI and higher waist circumference than North Americans, NHANES is limited in how it distinguishes certain ethnic groups.
Seventh, this report uses only HbA1c to measure prediabetes prevalence. Although there is evidence that the use of HbA1c to identify patients with prediabetes may lead to underdiagnosis of patients with prediabetes,34–36 HbA1c is the only measure of glucose metabolism available for all participants in the NHANES. Fasting plasma glucose and oral glucose tolerance testing are available only for subsets of respondents. Finally, we are unable to account for the apparent decrease in prediabetes that occurred from 1999 to 2006.
This nationally representative study provides evidence of a substantial proportion of individuals with a healthy weight BMI having prediabetes. Moreover, it indicates a secular increase in prediabetes in the population designated as a healthy weight. Diabetes prevention efforts will benefit from future research focused on determining the primary cause of this rise and efficient ways to detect prediabetes in primary care among healthy weight adults.
Footnotes
Conflicts of interest: authors report none.
Funding support: Stephen Anton, PhD, was supported by a grant from the National Institutes of Health and the National Institute on Aging (grant P30AG028740).
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mainous AG, III, Tanner RJ, Coates TD, Baker R. Prediabetes, elevated iron and all-cause mortality: a cohort study. BMJ Open. 2014;4(12):e006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Player MS, Diaz VA, Mainous AG, III, Gregorie SH, Knoll ME, Everett CJ. Ethnic differences in the relationship of prediabetes with the presence of target-organ disease. Diabetes Metab. 2011;37(5):403–409. [DOI] [PubMed] [Google Scholar]
- 4.Bullard KM, Saydah SH, Imperatore G, et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care. 2013;36(8):2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainous AG, III, Tanner RJ, Baker R, Zayas CE, Harle CA. Prevalence of prediabetes in England from 2003 to 2011: population-based, cross-sectional study. BMJ Open. 2014;4(6):e005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Awareness of prediabetes—United States, 2005–2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209–212. [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922–933. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R. Screening for type 2 diabetes mellitus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(11):765–776. [DOI] [PubMed] [Google Scholar]
- 10.Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of prediabetes and engagement in diabetes risk–reducing behaviors. Am J Prev Med. 2015. June 16 pii: S0749-3797(15)00124-5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med. 2014;127(8):717–727, e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassett DR, Lee IM. Trends in physical inactivity. Am J Med. 2015; 128(5):e21. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Trends in leisure-time physical inactivity by age, sex, and race/ethnicity—United States, 1994–2004. MMWR Morb Mortal Wkly Rep. 2005;54(39):991–994. [PubMed] [Google Scholar]
- 14.Final Update Summary: Abnormal Blood Glucose and Type 2 Diabetes Mellitus: Screening. U.S. Preventive Services Task Force. December 2015. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes?ds=1&s=diabetes Accessed Dec 16, 2015.
- 15.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes. 2010;3(6):661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris DH, Khunti K, Achana F, et al. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia. 2013;56(7):1489–1493. [DOI] [PubMed] [Google Scholar]
- 17.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13(3):275–286. [DOI] [PubMed] [Google Scholar]
- 18.Schneider HJ, Friedrich N, Klotsche J, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95(4):1777–1785. [DOI] [PubMed] [Google Scholar]
- 19.MacKay MF, Haffner SM, Wagenknecht LE, D’Agostino RB, Jr, Hanley AJ. Prediction of type 2 diabetes using alternate anthropometric measures in a multi-ethnic cohort: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32(5):956–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Census Bureau. Poverty Definitions. Retrieved from https://www.census.gov/hhes/www/poverty/methods/definitions.html#poverty%20thresholds.
- 21.Gómez-Ambrosi J, Silva C, Galofré JC, et al. Body adiposity and type 2 diabetes: increased risk with a high body fat percentage even having a normal BMI. Obesity (Silver Spring). 2011;19(7):1439–1444. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81(3):555–563. [DOI] [PubMed] [Google Scholar]
- 23.Lukich A, Gavish D, Shargorodsky M. Normal weight diabetic patients versus obese diabetics: relation of overall and abdominal adiposity to vascular health. Cardiovasc Diabetol. 2014;13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SA, Hergenroeder AC. Waist circumference predicts increased cardiometabolic risk in normal weight adolescent males. Int J Pediatr Obes. 2011;6(2–2):e307–11. [DOI] [PubMed] [Google Scholar]
- 25.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA. 2010;303(24):2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang DD, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements In US Diet Helped Reduce Disease Burden And Lower Premature Deaths, 1999–2012; Overall Diet Remains Poor. Health Aff (Millwood). 2015; 34(11):1916–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson MM, Reedy J, Krebs-Smith SM. American Diet Quality: Where It Is, Where It Is Heading, and What It Could Be. J Acad Nutr Diet. 2015. pii: S2212-2672(15)01511-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brocklebank LA, Falconer CL, Page AS, Perry R, Cooper AR. Accelerometer-measured sedentary time and cardiometabolic biomarkers: A systematic review. Prev Med. 2015;76:92–102. [DOI] [PubMed] [Google Scholar]
- 30.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–2905. [DOI] [PubMed] [Google Scholar]
- 31.Golubic R, Wijndaele K, Sharp SJ, et al. ; ProActive Study Group. Physical activity, sedentary time and gain in overall and central body fat: 7-year follow-up of the ProActive trial cohort. Int J Obes (Lond). 2015;39(1):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Júdice PB, Silva AM, Sardinha LB. Sedentary Bout Durations Are Associated with Abdominal Obesity in Older Adults. J Nutr Health Aging. 2015;19(8):798–804. [DOI] [PubMed] [Google Scholar]
- 33.Nicholas JA, Lo Siou G, Lynch BM, Robson PJ, Friedenreich CM, Csizmadi I. Leisure-time physical activity does not attenuate the association between occupational sedentary behaviour and obesity: results from the tomorrow project in Alberta, Canada. [Epub 2015 Apr 1] J Phys Act Health. 2015;12(12):1589–1600. [DOI] [PubMed] [Google Scholar]
- 34.Bersoux S, Cook CB, Wu Q, et al. Hemoglobin A1c testing alone does not sufficiently identify patients with prediabetes. Am J Clin Pathol. 2011;135(5):674–677. [DOI] [PubMed] [Google Scholar]
- 35.Cosson E, Hamo-Tchatchouang E, Banu I, et al. A large proportion of prediabetes and diabetes goes undiagnosed when only fasting plasma glucose and/or HbA1c are measured in overweight or obese patients. Diabetes Metab. 2010;36(4):312–318. [DOI] [PubMed] [Google Scholar]
- 36.Heianza Y, Hara S, Arase Y, et al. HbA1c 5·7–6·4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378(9786):147–155. [DOI] [PubMed] [Google Scholar]