Abstract
Background
Little is known of the mutation and tumor spectrum of Korean patients with Li-Fraumeni syndrome (LFS). Owing to the rarity of LFS, few cases have been reported in Korea thus far. This study aimed to retrospectively review the mutations and clinical characteristics of Korean patients with LFS.
Methods
TP53 mutation was screened in 89 unrelated individuals at the Samsung Medical Center in Korea, from 2004 to 2015. Six additional mutation carriers were obtained from the literature.
Results
We identified nine different mutations in 14 Korean patients (male to female ratio=0.3:1). Two such frameshift mutations (p.Pro98Leufs*25, p.Pro27Leufs*17) were novel. The recurrent mutations were located at codons 31 (n=2; p.Val31Ile), 175 (n=3; p.Arg175His), and 273 (n=4; p.Arg273His and p.Arg273Cys). The median age at the first tumor onset was 25 yr. Ten patients (71%) developed multiple primary tumors. A diverse spectrum of tumors was observed, including breast (n=6), osteosarcoma (n=4), brain (n=4), leukemia (n=2), stomach (n=2), thyroid (n=2), lung (n=2), skin (n=2), bladder (n=1), nasal cavity cancer (n=1), and adrenocortical carcinoma (n=1).
Conclusions
There was considerable heterogeneity in the TP53 mutations and tumor spectrum in Korean patients with LFS. Our results suggest shared and different LFS characteristics between Caucasian and Korean patients. This is the first report on the mutation spectrum and clinical characteristics from the largest series of Korean LFS patients.
Keywords: Li-Fraumeni syndrome, TP53, Germline mutation
INTRODUCTION
Li-Fraumeni syndrome (LFS, OMIM #151623) is an autosomal dominant cancer predisposition syndrome caused by a germline mutation in the TP53 tumor suppressor gene [1,2]. LFS is characterized by an early onset of tumors and a lifetime risk of developing multiple primary tumors, with individuals likely to develop osteosarcoma, soft tissue sarcoma, pre-menopausal breast cancer, brain tumors, adrenocortical carcinoma (ACC), and acute leukemia [1]. These core component tumors comprise ~70% of LFS-related tumors. In addition, there is growing evidence that a wide spectrum of cancers, including lung cancers or stomach cancers, is associated with LFS [3,4,5,6].
LFS diagnosis can be confirmed by a genetic mutation in TP53. According to the largest TP53 mutation database, the International Agency for Research on Cancer (IARC) database (version R18, April 2016, http://p53.iarc.fr/), over 700 germline mutations have been described to date [7]. Although most mu-tations are scattered throughout the gene, several hotspots such as codons 125, 158, 175, 196, 213, 220, 245, 248, 273, 282, and 337 have been reported [7,8]. Furthermore, the spectrum of LFS-related TP53 mutations can be categorized into two groups: (1) gain of function mutations (missense mutations) conferring a dominant-negative effect or promoting an oncogenic effect and (2) loss of function mutations such as nonsense and frameshift mutations [9,10]. A previous study reported that missense mutations rather than nonsense or frameshift mutations are associated with an earlier onset of cancer [8].
Although the IARC database provided essential information pertaining to the mutation distribution and cancer spectrum in LFS, the majority of data originated from Caucasian populations [7]. Currently, little is known about the mutation and tumor spectrum of Korean patients with LFS. Owing to the rarity of LFS, few case reports of Korean patients have been published [11,12,13,14,15,16]. However, the data presented in these case reports were too limited to assess the mutation spectrum in Korean patients with LFS. We aimed to evaluate the mutation features and clinical characteristics, including the tumor spectrum, of Korean patients with LFS.
METHODS
1. Study subjects
The Institutional Review Board of the Samsung Medical Center in Korea approved this study. This retrospective review was exempt from the requirement for informed consent. TP53 mutation was screened in 89 unrelated individuals at the Samsung Medical Center from 2003 to 2015. Among them, eight patients were selected as mutation carriers (9%, 8/89). Six additional mutation carriers were identified from case reports published in the literature [11,12,13,14,15,16]. We collected information on the age at the first tumor onset, sex, familial cancer history, and tumor spectrum from the 14 mutation-positive cases.
2. TP53 mutation analysis
All 11 exons and their flanking intron regions of TP53 were amplified by PCR using primer pairs designed with the Primer3 software in samples from 85 individuals. In the remaining four individuals, exons 4-9 and their flanking regions were targeted. Sanger sequencing was performed by using the BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) and an ABI Prism 3100 Genetic Analyzer (Applied Biosystems). The mutation status was assessed by using the Human Genome Mutation Database (HGMD, professional version 2014.01) and IARC TP53 database (version R18, April 2016, http://p53.iarc.fr/) [7]. The "A" of the ATG translation initiation codon is described as position number 1 in TP53 (NM_000546.4). Mutation group and domain were based on previously reported structural data and the Pfam database, respectively [8,17].
RESULTS
1. Spectrum of germline TP53 mutations
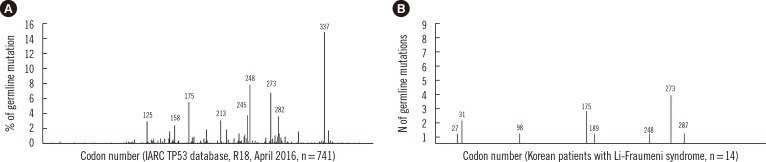
The mutations consisted of seven known mutations and two novel mutations in 14 unrelated Korean patients with 30 tumors. Six missense mutations (11/14, 79%), two frameshift deletions (2/14, 14%), and one nonsense mutation (1/14, 7%) were detected (Table 1). All mutations were distributed among exons 3 to 8; the DNA-binding domain (n=10) and transactivation domain (n=3) (Table 1). The most common mutation locations were exons 8 (n=5), 5 (n=3), and 3 (n=3) in TP53. The recurrent mutations were located at codons 31 (n=2; p.Val31Ile), 175 (n=3; p.Arg175His), and 273 [n=4; p.Arg273His (n=3), and p.Arg273Cys (n=1)] in TP53 (Fig. 1). Two novel frameshift mutations (p.Pro98Leufs*25 and p.Pro27Leufs*17) were identified (Table 1, Fig. 2).
Table 1. Clinical and TP53 mutation data of the 14 Korean patients with Li-Fraumeni syndrome.
| Patient | Age* | Sex | Tumor type (age at diagnosis)† | Criteria | Exon | Domain | Group** | NT alteration | Mutation type | AA alteration | HGMD | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | F | Br (7), Breast (23), Der (35), Leu (35)‡ | Chompret | 5 | DNA binding | L2 | c.524G > A | missense | p.Arg175His | CM951224 | This study |
| 2 | NA | F | Os (NA) | NA | 5 | DNA binding | L2 | c.524G > A | missense | p.Arg175His | CM951224 | This study |
| 3 | NA | F | Os (NA), Leu‡,§ | NA | 8 | DNA binding | S2-S2-H2 motif | c.818G > A | missense | p.Arg273His | CM920677 | This study |
| 4 | 45 | F | Thy (45)∥, Lm (47) | NA | 3 | Transactivation | NA | c.91G > A | missense | p.Val31Ile | CM074603 | This study |
| 5 | NA | F | Leu (NA), Breast (NA), Thy (NA) | Chompret | 8 | DNA biding | S2-S2-H2 motif | c.817C > T | missense | p.Arg273Cys | CM951233 | This study |
| 6 | 1 | F | ACC (1), Os, (4), Br (4), Leu (10)‡ | Chompret | 4 | NA | NA | c.293delC | frameshift | p.Pro98Leufs*25 | novel | This study |
| 7 | 28 | F | Breast (28,38)¶, Nf (33), Nasal cavity (38), Lung (43) | Chompret | 3 | Transactivation | NA | c.78delT | frameshift | p.Pro27Leufs*17 | novel | This study |
| 8 | 26 | M | St (26), Br (26) | LFL | 3 | Transactivation | NA | c.91G > A | missense | p.Val31Ile | CM074603 | This study |
| 9 | 25 | F | Breast (25), Lm (25) | LFL | 7 | DNA binding | L3 | c.742C > T | missense | p.Arg248Trp | CM900211 | [11] |
| 10 | 38 | M | St (38) | LFS | 8 | DNA binding | NA | c.859G > T | nonsense | p.Glu287* | CM044948 | [12] |
| 11 | 24 | F | Breast (25), Br (25) | LFL | 5 | DNA binding | L2 | c.524G > A | missense | p.Arg175His | CM951224 | [13] |
| 12 | 2 | M | Rhabdomyosarcoma (2), Br (4) | LFS | 8 | DNA binding | S2-S2-H2 motif | c.818G > A | missense | p.Arg273His | CM920677 | [14] |
| 13 | 47 | F | Breast (47) | LFL | 6 | DNA binding | L2 | c.566C > T | missense | p.Ala189Val | CM031755 | [15] |
| 14 | 17 | F | Os (17), Lung (17) | LFL | 8 | DNA binding | S2-S2-H2 motif | c.818G > A | missense | p.Arg273His | CM920677 | [16] |
The reference sequence of TP53 was NM_000546.4. Recurrent mutations are in bold. All mutations were heterozygous.
*Age at first tumor diagnosis; †In the order of tumor diagnosis; ‡Therapy-related myeloid neoplasm; §positive for chromosomal breakage test; ∥BRAF V600E mutation positive case; ¶BRCA 1 and BRCA 2 mutation negative and positive for hormone receptors (estrogen and progesterone) and ERBB2 (formerly HER2/neu); **Based on structural data from [8,17].
Abbreviations: NT, nucleotide; AA, Amino acid; LFS, Li-Fraumeni syndrome; LFL, Li-Fraumeni syndrome like; NA, not applicable, Br; brain; Der, dermatofibrosarcoma; Leu, leukemia; Os, Osteosarcoma; Thy, thyroid carcinoma; Lm, leiomyoma; ACC, adrenocortical carcinoma; Nf, neurofibroma; St, stomach; HGMD, Human Genome Mutation Database.
Fig. 1. Frequency distribution of TP53 germline mutations by codon. (A) Data reproduced from the International Agency for Research on Cancer (IARC) TP53 database (version R18, April 2016, http://p53.iarc.fr/) [7]. (B) Data from Korean patients with Li-Fraumeni syndrome. Figures above the vertical line represent the codon locations of the mutations.
Fig. 2. Sequence chromatogram of two novel frameshift mutations in TP53. (A) Heterozygous mutation of c.293delC (p.Pro98Leufs*25) identified in patient 6, who was treated for adrenocortical carcinoma, osteosarcoma, brain cancer, and therapy-related neoplasm. (B) Heterozygous mutation of c.78delT (p.Pro27Leufs*17) identified in patient 7 with multiple primary tumors comprising left breast cancer (28 yr), neurofibroma (33 yr), right breast cancer (38 yr), right nasal cavity squamous cell carcinoma (38 yr), and lung adenocarcinoma (43 yr). Forward and reverse sequences are shown at the top and bottom, respectively.
2. Clinical characteristics of Korean TP53 mutation carriers
The TP53 mutation carriers included three males and 11 females (male to female ratio of 0.3:1). Clinical criteria were fulfilled in 11 patients, with the exception of three patients for whom familial cancer history was not available (Table 1). The median age at the first tumor manifestation was 25 yr, regardless of the mutation type.
A diverse spectrum of tumors was detected, including breast cancer (n=6), osteosarcoma/rhabdomyosarcoma (n=4/n=1), brain tumor (n=4), leukemia (n=2), stomach cancer (n=2), thyroid cancer (n=2), lung cancer (n=2), skin cancer (n=2), nasal cavity cancer (n=1), and ACC (n=1) (Table 1). Twelve patients (86%) had at least one of the core spectrum cancers (breast cancer, brain tumors, osteosarcoma, rhabdomyosarcoma, and ACC) (Table 1). Ten patients (71%) developed multiple primary tumors (range of 1-5). Therapy-related neoplasm was observed in three patients (Patients 1, 3, and 6) (Table 1). Among them, one patient (Patient 3) treated with radiation therapy was positive for chromosomal breakage analysis.
One novel mutation, p.Pro98Leufs*25 was observed in patient 6, who was treated for ACC (at the age of 1 yr) and later developed osteosarcoma (4 yr), brain cancer (4 yr), and therapy-related neoplasm (10 yr) (Table 1). The other novel mutation, p.Pro27Leufs*17 was identified in patient 7 (Table 1). After the age of 28 yr, five primary tumors developed, including left breast cancer (28 yr), neurofibroma (33 yr), right breast cancer (38 yr), right nasal cavity squamous cell carcinoma (38 yr), and EGFR-mutated (p.Leu858Arg) lung adenocarcinoma (43 yr) in patient 7 (Table 1). The bilateral breast cancers of patient 7 were positive for hormone receptors (estrogen and progesterone) and ERBB2 (formerly HER2/neu) while negative for BRCA1 and BRCA2 mutations.
DISCUSSION
In this study, we investigated the mutation and tumor spectrums of Korean patients with LFS. Nine different mutations, including seven known mutations and two novel mutations, were identified in 14 unrelated patients. The codon and exon distribution of some mutations (especially codons 175 and 273) in Korean patients was similar to that in Caucasians. A notable exception was codon 337 (p.Arg337His), which was the previously reported founder mutation in Brazilian patients [18]. The mutation of p.Arg337His has been reported to be the most common mutation in the IARC database, but it was not identified in the Korean patients analyzed in this study.
Furthermore, missense mutations located at the loop, which were demonstrated to bind to the minor groove of DNA (L2: codons 164 to 194, L3: codons 237 to 250) and major groove of DNA (L1: codons 115 to 135, S2-S2-H2 motif: codons 273 to 286), were associated with brain cancer and ACC, respectively [8]. Here, we noted that the mutations of two brain cancer carriers and four breast cancer carriers were located at loop L2 and L3. However, no association was evident between tumor spectrum and mutation location, owing to the limited number of cases.
This study reported two novel frameshift mutations located at exons 3 and 4. The mutation locations and types were not present in the IARC database. The majority of previously reported mutations are missense mutations. In addition, previously reported mutations have been located mainly within exons 5-8 that encode the DNA-binding region. Despite the limited number of cases, identification of these frameshift mutations provides further insights into the TP53 mutation spectrum from Korean patients.
The predominance of women among Korean patients was not necessarily associated with breast cancer. A previous study reported a significant gender difference, possibly due to the high incidence of breast cancer among women with LFS [19]. However, in this study, the predominance of women was observed for a variety of tumors, including osteosarcoma and brain tumors as well as breast cancer.
There was no significant difference in age at the first tumor diagnosis between mutation types. A previous study reported that missense variants appear to be associated with an earlier onset of cancer [8]. However, this study demonstrated that missense mutations (e.g., p.Val31Ile) were identified in patients with a late-onset of cancer, while frameshift mutations (e.g. p.Pro98Leufs*25) were identified in patients with an early-onset of cancer. The age at tumor onset in TP53 mutation carriers could be influenced by genetic modifiers such as SNP309 (T>G), rs2279744 in MDM2 as well as PIN3 in TP53, together with environmental factors [20,21]. Moreover, this might be possibly explained by variable expressivity.
There was a considerable heterogeneity in the relationship between TP53 mutations and tumor spectrum. Of note, breast cancer (n=6) was the most common tumor in Korean patients, followed by osteosarcoma/rhabdomyosarcoma (n=5), brain (n=4), and others. Our findings revealed that non-core tumors such as stomach cancer (n=2), lung cancer (n=2), and thyroid cancer (n=2) were recurrently present in Korean patients. A recent study reported that stomach cancer is more frequently detected in Asian patients compared with Caucasian patients [22]. This suggested differences in the tumor spectrum of LFS between Caucasian and Asian patients [22].
Previous studies reported that, when BRCA mutations are not identified in patients with breast cancer diagnosed before 30 yr, the likelihood of having a TP53 mutation is estimated to be 4-8% [19,23,24]. The likelihood could increase with a family history of LFS-related cancers, personal history of a breast cancer positive for hormone receptors, and/or ERBB2, or additional LFS-related cancers [24,25]. This is in agreement with the findings from patient 7 with bilateral breast cancers.
Lastly, we reported patients with EGFR-mutated (p.Leu858Arg) lung adenocarcinoma (in patient 7) as well as therapy-related neoplasm. To date, little data on EGFR-mutated lung cancer in the context of LFS have been published [5,6]. It is assumed that patient 7 had a germline TP53 mutation (p.Pro27Leufs*17) as the first genetic hit and a somatic EGFR mutation (p.Leu858Arg) as the second hit.
This study had several limitations. This study was performed by retrospective review in a single institution. The small number of patients as well as incomplete information might have had an impact on the results. In addition, we did not compare clinical data between TP53 mutation-positive and -negative cases in the current study. Lastly, we did not consider genetic modifiers and environmental factors that influence the clinical phenotype. Despite these limitations, it is important that these are the first data from the largest case series of Korean patients with LFS.
In summary, this study provides the mutation features and tumor spectrum of Korean patients with LFS. There was a considerable heterogeneity in TP53 mutations and tumor spectrum among Korean patients. This study suggests shared and different features of LFS between Caucasian and Korean patients. Further large studies are warranted to stratify the clinical management and to establish surveillance strategies specific for Korean patients with LFS.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120030).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Li FP, Fraumeni JF, Jr, Mulvihill JJ, Blattner WA, Dreyfus MG, Tucker MA, et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988;48:5358–5362. [PubMed] [Google Scholar]
- 2.Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 3.Ruijs MW, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet. 2010;47:421–428. doi: 10.1136/jmg.2009.073429. [DOI] [PubMed] [Google Scholar]
- 4.Masciari S, Dewanwala A, Stoffel EM, Lauwers GY, Zheng H, Achatz MI, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651–657. doi: 10.1097/GIM.0b013e31821628b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalarea V, Calcasola M, Cane P, Tobal K, Izatt L, Spicer J. EGFR-mutated lung cancer in Li-Fraumeni syndrome. Lung Cancer. 2014;85:485–487. doi: 10.1016/j.lungcan.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Ricordel C, Labalette-Tiercin M, Lespagnol A, Kerjouan M, Dugast C, Mosser J, et al. EFGR-mutant lung adenocarcinoma and Li-Fraumeni syndrome: report of two cases and review of the literature. Lung Cancer. 2015;87:80–84. doi: 10.1016/j.lungcan.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 8.Bougeard G, Renaux-Petel M, Flaman JM, Charbonnier C, Fermey P, Belotti M, et al. Revisiting Li-Fraumeni syndrome from TP53 mutation carriers. J Clin Oncol. 2015;33:2345–2352. doi: 10.1200/JCO.2014.59.5728. [DOI] [PubMed] [Google Scholar]
- 9.Malkin D. Li-fraumeni syndrome. Genes Cancer. 2011;2:475–484. doi: 10.1177/1947601911413466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols KE. Genotype versus phenotype: The yin and yang of germline TP53 mutations in Li-Fraumeni syndrome. J Clin Oncol. 2015;33:2331–2333. doi: 10.1200/JCO.2015.61.5757. [DOI] [PubMed] [Google Scholar]
- 11.Bang YJ, Kang SH, Kim TY, Jung CW, Oh SM, Choe KJ, et al. The first documentation of Li-Fraumeni syndrome in Korea. J Korean Med Sci. 1995;10:205–210. doi: 10.3346/jkms.1995.10.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim IJ, Kang HC, Shin Y, Park HW, Jang SG, Han SY, et al. A TP53-truncating germline mutation (E287X) in a family with characteristics of both hereditary diffuse gastric cancer and Li-Fraumeni syndrome. J Hum Genet. 2004;49:591–595. doi: 10.1007/s10038-004-0193-9. [DOI] [PubMed] [Google Scholar]
- 13.Hwang SM, Lee ES, Shin SH, Kong SY. Genetic counseling can influence the course of a suspected familial cancer syndrome patient: from a case of Li-Fraumeni like syndrome with a germline mutation in the TP53 gene. Korean J Lab Med. 2008;28:493–497. doi: 10.3343/kjlm.2008.28.6.493. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, Kwon YJ, Lim YJ, Park BK, Ghim TT, Shin SH, et al. Family of Li-Fraumeni syndrome with a germline mutation in the p53 gene. Clin Pediatr Hematol Oncol. 2009;16:38–42. [Google Scholar]
- 15.Cho Y, Kim J, Kim Y, Jeong J, Lee KA. A case of late-onset Li-Fraumenilike syndrome with unilateral breast cancer. Ann Lab Med. 2013;33:212–216. doi: 10.3343/alm.2013.33.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh CS, Lee JH, Jung ST, Na BR. Osteosarcoma with adenocarcinoma of lung in Li-Fraumeni syndrome: a case report. J Korean Bone Joint Tumor Soc. 2014;20:99–103. [Google Scholar]
- 17.Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 18.Achatz MI, Olivier M, Le Calvez F, Martel-Planche G, Lopes A, Rossi BM, et al. The TP53 mutation, R337H, is associated with Li-Fraumeni and Li-Fraumeni-like syndromes in Brazilian families. Cancer Lett. 2007;245:96–102. doi: 10.1016/j.canlet.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni Syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27:1250–1256. doi: 10.1200/JCO.2008.16.6959. [DOI] [PubMed] [Google Scholar]
- 20.Sagne C, Marcel V, Bota M, Martel-Planche G, Nobrega A, Palmero EI, et al. Age at cancer onset in germline TP53 mutation carriers: association with polymorphisms in predicted G-quadruplex structures. Carcinogenesis. 2014;35:807–815. doi: 10.1093/carcin/bgt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Ariffin H, Chan AS, Oh L, Abd-Ghafar S, Ong GB, Mohamed M, et al. Frequent occurrence of gastric cancer in Asian kindreds with Li-Fraumeni syndrome. Clin Genet. 2015;88:450–455. doi: 10.1111/cge.12525. [DOI] [PubMed] [Google Scholar]
- 23.Mouchawar J, Korch C, Byers T, Pitts TM, Li E, McCredie MR, et al. Population-based estimate of the contribution of TP53 mutations to subgroups of early-onset breast cancer: Australian Breast Cancer Family Study. Cancer Res. 2010;70:4795–4800. doi: 10.1158/0008-5472.CAN-09-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCuaig JM, Armel SR, Novokmet A, Ginsburg OM, Demsky R, Narod SA, et al. Routine TP53 testing for breast cancer under age 30: ready for prime time? Fam Cancer. 2012;11:607–613. doi: 10.1007/s10689-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 25.Melhem-Bertrandt A, Bojadzieva J, Ready KJ, Obeid E, Liu DD, Gutierrez-Barrera AM, et al. Early onset HER2-positive breast cancer is associated with germline TP53 mutations. Cancer. 2012;118:908–913. doi: 10.1002/cncr.26377. [DOI] [PMC free article] [PubMed] [Google Scholar]