Abstract
Background
The incidence and etiology of hepatocellular carcinoma (HCC) vary widely according to race and geographic regions. The insertional mutagenesis of adeno-associated virus 2 (AAV2) has recently been considered a new viral etiology of HCC. The aim of this study was to investigate the frequency and clinical characteristics of AAV2 in Korean patients with HCC.
Methods
A total of 289 unrelated Korean patients with HCC, including 159 Hepatitis-B-related cases, 16 Hepatitis-C-related cases, and 114 viral serology-negative cases, who underwent surgery at the Samsung Medical Center in Korea from 2009 to 2014 were enrolled in this study. The presence of AAV2 in fresh-frozen tumor tissues was investigated by DNA PCR and Sanger sequencing. The clinical and pathological characteristics of AAV2-associated HCC in these patients were compared with previous findings in French patients.
Results
The AAV2 detection rate in Korean patients (2/289) was very low compared with that in French patients (11/193). Similar to the French patients, the Korean patients with AAV2-related HCC showed no signs of liver cirrhosis. The Korean patients were younger than the French patients with the same AAV2-associated HCC; the ages at diagnosis of the two Korean patients were 47 and 39 yr, while the median age of the 11 French patients was 55 yr (range 43-90 yr).
Conclusions
AAV2-associated HCC was very rare in Korean patients with HCC. Despite a limited number of cases, this study is the first to report the clinical characteristics of Korean patients with AAV2-associated HCC. These findings suggest epidemiologic differences in viral hepatocarcinogenesis between Korean and European patients.
Keywords: Adeno-associated virus 2, Epidemiology, Hepatocellular carcinoma, Korean
INTRODUCTION
Epidemiologic studies have revealed that the incidence and etiology of hepatocellular carcinoma (HCC) vary widely according to race and geographic regions [1,2]. Globally, the incidence of HCC is high in East Asia, moderate in Southern Europe, and low in America and Northern Europe [1,2]. Hepatitis B virus (HBV) infection is the main contributing factor to the development of HCC in East Asian countries, including Korea [1,2]. In contrast, hepatitis C virus (HCV) and alcohol intake are the major risk factors in Japanese and European patients, respectively [1,2]. A recent time-trend analysis revealed that the incidence of HCC has decreased in Japan and Italy and increased in the United States, Germany, Brazil, and Taiwan [1,2]. This change can be explained partly by the finding that HCV-related HCC has increased and decreased in the United States and Japan, respectively [1,2]. The changes in the epidemiology of HCC seem related to the relative contribution of the viral etiology. An understanding of the burdens of different viral etiologies might be important for HCC control.
Recently, the insertional mutagenesis of adeno-associated virus 2 (AAV2), which is similar to HBV integration, has been reported as a new viral etiology of HCC [3]. To date, AAV2 has been considered a nonpathogenic virus because it requires a helper virus, such as adenovirus, for productive infection. Furthermore, several previous studies have demonstrated the oncosuppressive role of AAV2 infection in some cancer cell lines, such as human papillomavirus-positive cervical cancers, MKr melanoma, and breast cancer [4,5,6]. In contrast, few studies have examined the possibility of hepatocarcinogenesis after AAV vector-mediated gene therapy in mice [7,8]. No studies investigated the role of AAV2 in human HCC until a report of AAV2-associated HCC in French patients [3]. That study revealed that AAV2 integration occurred in approximately 5% (11/193) of French patients with HCC, and most (9/11) did not have liver cirrhosis (LC). Known risk factors, such as HCV (n=1), HBV (n=1), and alcohol intake (n=4), were identified in about 45% (5/11) of the patients with AAV2 integration.
According to a recent seroprevalence survey [9], the presence of the neutralizing antibody against AAV2 varies among populations worldwide. The prevalence of the antibody ranges from 30% in the United States to 60% in Africa [9]. It is unclear whether the major histocompatibility complex background and/or immune-related genetic factors are involved in these differences among populations. Determining the seroprevalence status might be important for understanding the mechanisms of AAV2-associated HCC because differences in the pre-existing immunity to AAV2 might contribute to the carcinogenesis of AAV2-associated HCC.
In this study, we aimed to investigate the epidemiology of viral HCC by examining the frequency of AAV2-associated HCC in Korean patients with HCC and comparing the clinical and pathological characteristics of Korean patients with those of French patients.
METHODS
1. Patients and samples
A total of 289 unrelated Korean patients with HCC, including 159 HBV-related HCC cases, 16 HCV-related HCC cases, and 114 viral serology-negative cases, were enrolled from the consecutive patients who underwent surgery at the Samsung Medical Center in Korea from 2009 to 2014. The following clinical and pathological data were reviewed: age at the time of operation, sex, HCC etiology, operation location, survival time, medical history, viral serology, HBV DNA quantitation, preoperative levels of alpha fetoprotein and prothrombin induced by vitamin K absence-II, Child-Pugh score, and pathologic findings in the tumor and nontumor tissues. The clinical and pathological characteristics of the Korean patients with AAV2-associated HCC were compared with those of the French patients [3]. The Institutional Review Board of the Samsung Medical Center in Korea approved this study. The study was considered exempt from the requirement for informed consent because of the use of stored biospecimens.
All fresh-frozen tissues were stored at -70℃ until the extraction of genomic DNA and RNA. To screen for the presence of AAV2 in the tumors, genomic DNA was extracted from 289 fresh-frozen tumor tissues. All the tumor specimens were examined by pathologists, and each tumor had a minimum tumor burden of 90%. In brief, the tumor tissues (15 mg) were incubated overnight at 55℃ in nuclei lysis solution (500 µL) and proteinase K (17.5 µL) with gentle shaking. After protein precipitation and vortexing, the tube was centrifuged at 13,000g for 4 min. The supernatant was transferred to a new tube, and isopropanol (500 µL) was added. The solution was mixed by inversion until the white thread-like strands of DNA were visible. After centrifugation at 13,000g for 1 min, the supernatant was discarded, and 70% ethanol (500 µL) was added. After additional centrifugation at 13,000g for 1 min, the pellet was air-dried for 10-15 min. DNA rehydration solution (100 µL) was added and incubated at 56℃ for 1 hr. The concentration of the extracted DNA was measured with a PicoGreen dsDNA assay (Quanti-iT PicoGreen dsDNA kit; Thermo Fisher Scientific Inc., Waltham, MA, USA). The RNA was extracted with TRIzol methods, and the RNA integrity number was measured as 7 with an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Palo Alto, CA, USA).
2. Virus detection
Viral DNA PCR was performed with nine primer pairs targeting the AAV2 genome (AF043303.1), as described previously [3]. The viral fragments were further investigated with subsequent Sanger sequencing.
In one tumor tissue sample with AAV2 DNA fragments, we performed mRNA sequencing (mRNA-seq) to investigate their functional impact on the tissue, such as fusion transcript generation events. cDNA library preparation (TruSeq Library preparation Kits, Illumina, Inc., San Diego, CA, USA) and mRNA-seq (100-bp paired-end reads, 40 million reads, on HiSeq2000, Illumina, Inc.) was done according to the manufacturer's instructions. VirusSeq (The University of Texas, Houston, TX, USA) was coupled with MOSAIK (Boston College, MA, USA) to identify the virus and host integration sites (http://odin.mdacc.tmc.edu/~xsu1/VirusSeq.html) [10,11]. The following criteria were applied for a fusion transcript: at least two discordant read pairs and at least one junction-spanning read.
RESULTS
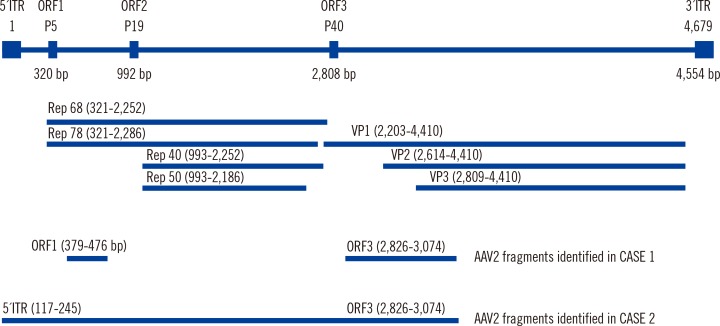
We found AAV2 fragments in the samples of only two of the 289 Korean patients (0.69%) by DNA PCR and Sanger sequencing. The viral fragments involved the 5' inverse tandem repeat (ITR), replication proteins (Rep), and capsid protein (VP1) (Fig. 1). The mRNA-seq results, which were available only in Case 1, showed no remarkable findings, such as fusion transcripts or RNA-processing events.
Fig. 1. Genomic sequences of the adeno-associated virus 2 (AAV2) and the viral fragments that were identified in the Korean patients with hepatocellular carcinoma. The reference genome of AAV2 is AF043303.1.
Abbreviations: ORF, open reading frame; ITR, inverse tandem repeat; Rep, replication protein; VP, viral capsid protein.
The clinicopathological features are described in Table 1. Although risk factors, such as chronic HBV infection and/or alcohol intake, were identified in both patients, there were no signs of LC (Table 1). Two patients with AAV2-associated HCC were relatively younger than the other patients with HCC, despite the limited number of cases; the ages at diagnosis of the two Korean patients were 47 and 39 yr, while the median age of the 11 French patients was 55 yr (range 43-90 yr). High levels of HBV replication were detected in two patients with AAV2-associated HCC (Table 1).
Table 1. Clinical and pathological characteristics of the Korean patients with AAV2-associated HCC.
| Case 1 | Case 2 | |
|---|---|---|
| Age at diagnosis | 47 | 39 |
| Sex | M | F |
| Etiology | HBV, Heavy alcohol intake | HBV |
| Operation location | S4/S8, Central hepatectomy | Right liver, S7/S8 |
| Extended hemihepatectomy | ||
| Disease-free survival | 168 days | 71 days |
| Overall survival | 1,788 days | 449 days |
| Status | Alive | Death |
| Medical histories | Previously healthy | Tuberculosis (on medication) |
| Chronic hepatitis B (on Entecavir) | Chronic hepatitis B (on Entecavir) | |
| Radiofrequency ablation | Transarterial chemoembolization | |
| Liver cirrhosis | No | No |
| Viral serology | HBsAg (+), HBeAg (+), Anti-HCV Ab (-), Anti-HAV, IgG (+) | HBsAg (+), HBeAg (+), Anti-HCV Ab (-), Anti-HAV, IgG (-) |
| HBV DNA quantitation | 632,139 IU/mL | 1,675 IU/mL |
| AFP (RI, 0-8.1 ng/mL) | 19163 | 200000 |
| PIVKA-II (RI, 0-40 mAU/mL) | 122 | 1200 |
| Child-Pugh score | B | B |
| Total bilirubin (RI, 0.2-1.5 mg/dL) | 0.6 | 0.5 |
| Serum albumin (RI, 3.2-5.2 g/dL) | 4.5 | 4.1 |
| Prothrombin time (sec) | 16.2 | 12.7 |
| Ascites | None | Mild |
| Hepatic encephalopathy | None | None |
| Tumor tissues | ||
| Differentiation | Edmondson grade II | Edmondson grade II |
| Histologic type | Macrotrabecular | Macrotrabecular and microtrabecular |
| Cell type | Hepatic | Hepatic |
| Tumor size | 6 × 5.5 × 4 cm | 19 × 14 × 10.5 cm |
| Necrosis | 5% | 0% |
| Hemorrhage | 2% | 2% |
| Fibrous capsule | Partial | Partial |
| Tumor invasion in tumor capsule | Yes | Partial |
| Microvessel invasion | Capsule and peritumoral | Peritumoral |
| Portal vein invasion | No | Segmental |
| Bile duct invasion | No | No |
| Serosa invasion | No | No |
| Intrahepatic metastasis | Yes | Yes |
| Multicentric occurrence | No | No |
| Surgical margin invasion | No | No |
| Pathological stage | T3 | T4 |
| Nontumor tissues | ||
| Chronic hepatitis | Yes | Yes |
| Etiology | HBV | HBV |
| Lobular activity | Minimal | Mild |
| Portal/periportal activity | Minimal | Mild |
| Fibrosis | Septal | Septal |
| Cirrhosis | No | No |
| Dysplastic nodule | No | No |
Abbreviations: AAV2, adeno-associated virus 2; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B envelope antigen; HCV, hepatitis C virus; Ab, antibody; HAV, hepatitis A virus; AFP, Alpha-fetoprotein; RI, reference interval; PIVKA-II, prothrombin induced by vitamin K absence-II.
DISCUSSION
Nault et al [3] demonstrated the carcinogenic properties of AAV2 in human HCC with functional experiments. We found that AAV2-associated HCC was very rare in Korean patients with HCC through DNA PCR and subsequent Sanger sequencing. The mRNA-seq showed no remarkable findings, such as fusion transcripts. One possibility that may explain this discrepancy between the mRNA-seq and DNA sequencing is the extrachromosomal existence of viral DNA rather than its viral integration into the host genome. Currently, it is not clear if all viral DNA fragments in tumor tissues are necessarily associated with viral-human fusion transcripts. Furthermore, the oncogenic role of the extrachromosomal existence of viral DNA should be assessed in a context-dependent manner. It remains to be determined whether an extrachromosomal viral existence may lead to oncogenic effects through trans-effects or simply passenger effects in the background of uninvestigated driver mutations. In the current study, we did not investigate the carcinogenic roles and mechanisms of AAV2 fragments with functional experiments.
The viral fragments that were identified in the Korean patients were 5' ITR, Rep, and VP1 (Fig. 1). However, most of the fragments in the study of French patients were the 3' ITR or capsid proteins (with the exception of the identification of a Rep fragment in one patient) [3]. It remains to be determined how different viral fragments contribute to hepatocarcinogenesis.
Currently, the establishment of the oncogenic roles of AAV2 faces formidable challenges because of the limited data. This is because oncoviruses are necessary but possibly not sufficient for carcinogenesis. Literature reviews have suggested that the pathogenicity of AAV2 in cancers should be determined based on context-dependent interpretations, such as the cell specificity, interaction with other risk factors, and immune status of the host [4,5,6,8]. Indeed, the immune system of the host can play dual roles in carcinogenesis. For instance, the incidence of HCC could increase in the context of immunosuppression as well as in the presence of hyperactivation of the immune system, such as with chronic inflammation.
It is difficult to separate the role of AAV2 from those of other risk factors, such as HBV or heavy alcohol intake, in the development of HCC, which is characterized by a multistep process. According to the French study, known risk factors, such as HCV (n=1), HBV (n=1), and/or alcohol intake (n=4), were identified in more than 50% of the patients with AAV2 integration [3]. In addition, it is noteworthy that alcohol intake (n=4) and HBV infection (n=2) were relatively frequently associated with the development of AAV2-associated HCC in French and Korean patients, respectively.
The present results suggested the possibility of an interaction between HBV and AAV2 infection. Currently, little is known about the interactions of risk factors and AAV2 in HCC and whether they have subadditive or supra-additive effects. The interaction between HBV and AAV2 could be important on the basis of the previous findings that 1) AAV2 inhibits adenovirus replication, although it requires adenovirus for productive infection, and 2) AAV2 infection in osteosarcoma cells, which fail to trigger an innate immune response, has been reported to initiate replication even without adenovirus [12]. It remains to be determined how the interaction between viruses contributes to hepatocarcinogenesis according to immune status.
Some limitations of this study need to be acknowledged. Although we compared the frequency of AAV2-associated HCC to those reported in the French study, we did not investigate the frequency with all of the same strategies that were used in the French study. Unfortunately, a number of processes, such as viral capture sequencing and exome sequencing, were not conducted in this study. Of note, screening methods, as well as ethnic backgrounds, could influence the results. In addition, the viral integration site and the functional oncogenic effects of viral fragments were not investigated.
In summary, the DNA PCR and subsequent Sanger sequencing revealed that AAV2-associated HCC was very rare in Korean patients with HCC [13,14]. Despite the limited number of cases, this study is the first to report the clinical and pathological characteristics of Korean patients with AAV2-associated HCC, and it provides further information regarding the epidemiologic differences in viral hepatocarcinogenesis between Korean and European patients. Further studies regarding the interactions between the risk factors for HCC, the specific roles of the viral fragments, and the seroprevalence and effects of the AAV2-neutralizing antibody are warranted.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A120030).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200. doi: 10.1002/hep.27969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nault JC, Datta S, Imbeaud S, Franconi A, Mallet M, Couchy G, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 4.Alam S, Meyers C. Adeno-associated virus type 2 induces apoptosis in human papillomavirus-infected cell lines but not in normal keratinocytes. J Virol. 2009;83:10286–10292. doi: 10.1128/JVI.00343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bantel-Schaal U. Integration of adeno-associated virus 2 DNA in human MKR melanoma cells induces a peptide with oncosuppressive properties. Int J Cancer. 2001;92:537–544. doi: 10.1002/ijc.1230. [DOI] [PubMed] [Google Scholar]
- 6.Alam S, Bowser BS, Israr M, Conway MJ, Meyers C. Adeno-associated virus type 2 infection of nude mouse human breast cancer xenograft induces necrotic death and inhibits tumor growth. Cancer Biol Ther. 2014;15:1013–1028. doi: 10.4161/cbt.29172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 8.Bell P, Moscioni AD, McCarter RJ, Wu D, Gao G, Hoang A, et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther. 2006;14:34–44. doi: 10.1016/j.ymthe.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Yao H, Thompson EJ, Tannir NM, Weinstein JN, Su X. VirusSeq: software to identify viruses and their integration sites using next-generation sequencing of human cancer tissue. Bioinformatics. 2013;29:266–267. doi: 10.1093/bioinformatics/bts665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WP, Stromberg MP, Ward A, Stewart C, Garrison EP, Marth GT. MOSAIK: a hash-based algorithm for accurate next-generation sequencing short-read mapping. PLoS One. 2014;9:e90581. doi: 10.1371/journal.pone.0090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laredj LN, Beard P. Adeno-associated virus activates an innate immune response in normal human cells but not in osteosarcoma cells. J Virol. 2011;85:13133–13143. doi: 10.1128/JVI.05407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nault JC, Datta S, Imbeaud S, Franconi A, Mallet M, Couchy G, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet. 2015;47:1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 14.Russell DW, Grompe M. Adeno-associated virus finds its disease. Nat Genet. 2015;47:1104–1105. doi: 10.1038/ng.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]