Abstract
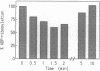
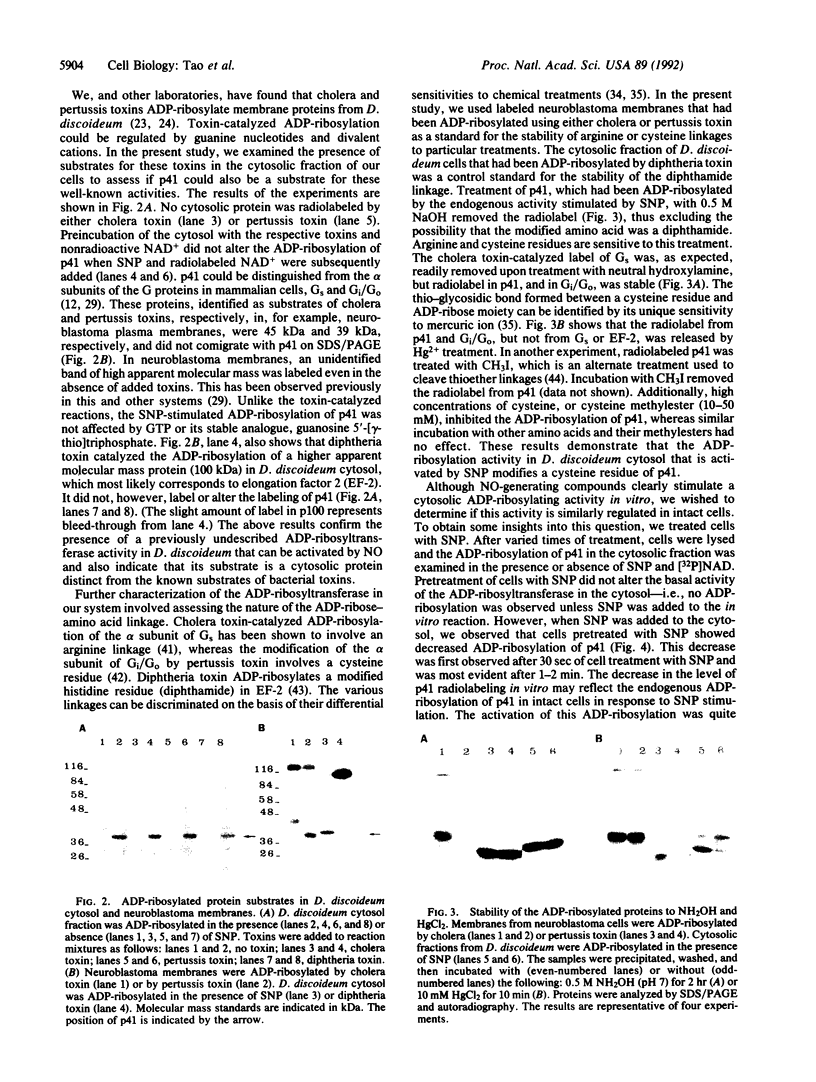
Nitric oxide-releasing compounds were shown to activate an ADP-ribosyltransferase activity in the cytosol of Dictyostelium discoideum. The enzyme ADP-ribosylated a cytosolic protein of approximately 41 kDa, p41. Neither cGMP nor GTP and its analogues affected this ADP-ribosylation. p41 differs from other substrates ADP-ribosylated by cholera, pertussis, or diphtheria toxins. Treatment of ADP-ribosylated p41 with snake venom phosphodiesterase released adenosine 5'-monophosphate, indicating a mono-ADP-ribose-protein linkage. This linkage was stable to neutral hydroxylamine but was sensitive to mercury ions and iodomethane, suggesting an attachment to a cysteine residue. Treatment of intact cells with nitric oxide-releasing compounds appeared to stimulate the ADP-ribosylation of p41 and this modification was reversible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L. G proteins in signal transduction. Annu Rev Pharmacol Toxicol. 1990;30:675–705. doi: 10.1146/annurev.pa.30.040190.003331. [DOI] [PubMed] [Google Scholar]
- Bodley J. W., Dunlop P. C., VanNess B. G. Diphthamide in elongation factor 2: ADP-ribosylation, purification, and properties. Methods Enzymol. 1984;106:378–387. doi: 10.1016/0076-6879(84)06040-7. [DOI] [PubMed] [Google Scholar]
- Braughler J. M., Mittal C. K., Murad F. Effects of thiols, sugars, and proteins on nitric oxide activation of guanylate cyclase. J Biol Chem. 1979 Dec 25;254(24):12450–12454. [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Duncan M. R., Rankin P. R., King R. L., Jacobson M. K., Dell'Orco R. T. Stimulation of mono (ADP-ribosyl)ation by reduced extracellular calcium levels in human fibroblasts. J Cell Physiol. 1988 Jan;134(1):161–165. doi: 10.1002/jcp.1041340121. [DOI] [PubMed] [Google Scholar]
- Fendrick J. L., Iglewski W. J. Endogenous ADP-ribosylation of elongation factor 2 in polyoma virus-transformed baby hamster kidney cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):554–557. doi: 10.1073/pnas.86.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide decreases cytosolic free calcium in Balb/c 3T3 fibroblasts by a cyclic GMP-independent mechanism. J Biol Chem. 1991 Jan 5;266(1):9–12. [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Howlett A. C., Qualy J. M., Khachatrian L. L. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986 Mar;29(3):307–313. [PubMed] [Google Scholar]
- Hsia J. A., Tsai S. C., Adamik R., Yost D. A., Hewlett E. L., Moss J. Amino acid-specific ADP-ribosylation. Sensitivity to hydroxylamine of [cysteine(ADP-ribose)]protein and [arginine(ADP-ribose)]protein linkages. J Biol Chem. 1985 Dec 25;260(30):16187–16191. [PubMed] [Google Scholar]
- Janssens P. M., Van Haastert P. J. Molecular basis of transmembrane signal transduction in Dictyostelium discoideum. Microbiol Rev. 1987 Dec;51(4):396–418. doi: 10.1128/mr.51.4.396-418.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliani M. H., Klein C. Photoaffinity labeling of the cell surface adenosine 3':5'-monophosphate receptor of Dictyostelium discoideum and its modification in down-regulated cells. J Biol Chem. 1981 Jan 25;256(2):613–619. [PubMed] [Google Scholar]
- Kawata M., Farnsworth C. C., Yoshida Y., Gelb M. H., Glomset J. A., Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachatrian L., Klein C., Howlett A. Pertussis and cholera toxin ADP-ribosylation in Dictyostelium discoideum membranes. Biochem Biophys Res Commun. 1987 Dec 31;149(3):975–981. doi: 10.1016/0006-291x(87)90504-3. [DOI] [PubMed] [Google Scholar]
- Khachatrian L., Klein C., Howlett A. Regulation of Dictyostelium discoideum adenylate cyclase by manganese and adenosine analogs. Biochim Biophys Acta. 1987 Feb 18;927(2):235–246. doi: 10.1016/0167-4889(87)90140-6. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Shall S., Whish W. J. The relationship between cell growth, macromolecular synthesis and poly ADP-ribose polymerase in lymphoid cells. Exp Cell Res. 1974 Jan;83(1):63–72. doi: 10.1016/0014-4827(74)90688-0. [DOI] [PubMed] [Google Scholar]
- Leichtling B. H., Coffman D. S., Yaeger E. S., Rickenberg H. V., al-Jumaliy W., Haley B. E. Occurrence of the adenylate cyclase "G protein" in membranes of Dictyostelium discoideum. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1187–1195. doi: 10.1016/s0006-291x(81)80137-4. [DOI] [PubMed] [Google Scholar]
- Leno G. H., Ledford B. E. Reversible ADP-ribosylation of the 78 kDa glucose-regulated protein. FEBS Lett. 1990 Dec 10;276(1-2):29–33. doi: 10.1016/0014-5793(90)80499-9. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Fowler V. M., Swanson J., Branton D., Taylor D. L. A membrane cytoskeleton from Dictyostelium discoideum. I. Identification and partial characterization of an actin-binding activity. J Cell Biol. 1981 Feb;88(2):396–409. doi: 10.1083/jcb.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989 Dec;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Meyer T., Koch R., Fanick W., Hilz H. ADP-ribosyl proteins formed by pertussis toxin are specifically cleaved by mercury ions. Biol Chem Hoppe Seyler. 1988 Jul;369(7):579–583. doi: 10.1515/bchm3.1988.369.2.579. [DOI] [PubMed] [Google Scholar]
- Moss J., Jacobson M. K., Stanley S. J. Reversibility of arginine-specific mono(ADP-ribosyl)ation: identification in erythrocytes of an ADP-ribose-L-arginine cleavage enzyme. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5603–5607. doi: 10.1073/pnas.82.17.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. ADP-ribosylation of guanyl nucleotide-binding regulatory proteins by bacterial toxins. Adv Enzymol Relat Areas Mol Biol. 1988;61:303–379. doi: 10.1002/9780470123072.ch6. [DOI] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Peterson J. E., Larew J. S., Graves D. J. Purification and partial characterization of arginine-specific ADP-ribosyltransferase from skeletal muscle microsomal membranes. J Biol Chem. 1990 Oct 5;265(28):17062–17069. [PubMed] [Google Scholar]
- Rankin P. W., Jacobson E. L., Benjamin R. C., Moss J., Jacobson M. K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J Biol Chem. 1989 Mar 15;264(8):4312–4317. [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Mattera R., Hildebrandt J. D., Codina J., Field J. B., Birnbaumer L., Sekura R. D. ADP-ribosylation of membrane components by pertussis and cholera toxin. Methods Enzymol. 1985;109:566–572. doi: 10.1016/0076-6879(85)09115-7. [DOI] [PubMed] [Google Scholar]
- Ross F. M., Newell P. C. Streamers: chemotactic mutants of Dictyostelium discoideum with altered cyclic GMP metabolism. J Gen Microbiol. 1981 Dec;127(2):339–350. doi: 10.1099/00221287-127-2-339. [DOI] [PubMed] [Google Scholar]
- Sayhan O., Ozdemirli M., Nurten R., Bermek E. On the nature of cellular ADP-ribosyltransferase from rat liver specific for elongation factor 2. Biochem Biophys Res Commun. 1986 Sep 30;139(3):1210–1214. doi: 10.1016/s0006-291x(86)80306-0. [DOI] [PubMed] [Google Scholar]
- Tanuma S., Kawashima K., Endo H. Eukaryotic mono(ADP-ribosyl)transferase that ADP-ribosylates GTP-binding regulatory Gi protein. J Biol Chem. 1988 Apr 15;263(11):5485–5489. [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Kanaho Y., Halpern J. L., Moss J. Immunological and biochemical differentiation of guanyl nucleotide binding proteins: interaction of Go alpha with rhodopsin, anti-Go alpha polyclonal antibodies, and a monoclonal antibody against transducin alpha subunit and Gi alpha. Biochemistry. 1987 Jul 28;26(15):4728–4733. doi: 10.1021/bi00389a020. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Van Ness B. G., Howard J. B., Bodley J. W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem. 1980 Nov 25;255(22):10710–10716. [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Wood K. S., Ignarro L. J. Hepatic cyclic GMP formation is regulated by similar factors that modulate activation of purified hepatic soluble guanylate cyclase. J Biol Chem. 1987 Apr 15;262(11):5020–5027. [PubMed] [Google Scholar]