To detect changes in coastal ecosystems, we evaluated the variation over time in some vegetation features, such as species composition and structure (species richness, cover, growth forms). We found that ecological groups of species such as native focal species (species that provide essential ecological functions) and aliens (species that spread outside their natural distribution), and growth forms proved their efficacy in discriminating between habitat types and in describing their changes over time. The approach used in the current study may provide an instrument for the assessment of plant community quality that can be applied to other coastal ecosystems.
Keywords: Coastal dune ecosystems, community structure, community level variables, habitat monitoring, species ecological groups
Abstract
The monitoring of biodiversity has mainly focused on the species level. However, researchers and land managers are making increasing use of complementary assessment tools that address higher levels of biological organization, i.e. communities, habitats and ecosystems. Recently, a variety of frameworks have been proposed for assessing the conservation status of communities or ecosystems. Among the various criteria proposed, all the protocols suggest considering (i) spatial aspects (range and area), and (ii) qualitative aspects of specific structures and functions. However, changes to ecological function are difficult to quantify and many protocols end up by using qualitative criteria. The aim of this work was to test the efficacy of some plant community attributes for the detection of vegetation quality in sand dune plant communities. We chose plant community attributes that either help to distinguish a habitat from others (diagnostic components) or play a significant role in habitat function and persistence over time. We used a diachronic approach by contrasting up-to-date vegetation data with data from previous studies carried out within the same areas. Changes in species composition were detected through detrended correspondence analyses (detrended correspondence analyses), Multi-Response Permutation Procedures and Indicator Species Analysis, while structural changes were analyzed by comparing species richness, total species cover, ecological groups of species and growth forms through null models. Ecological groups such as native focal species and aliens, and growth forms proved their efficacy in discriminating between habitat types and in describing their changes over time. The approach used in this study may provide an instrument for the assessment of plant community quality that can be applied to other coastal ecosystems.
Introduction
Globally, countries are experiencing the degradation and loss of coastal habitats (Curr et al. 2000; Defeo et al. 2009; EEA 2009; Feagin et al. 2005). Economic progress, burgeoning human populations as well as the growing demand for coast-bound tourism opportunities have increased pressures on sandy beaches (Dugan and Hubbard 2010) and coastal sandy ecosystems are currently identified as one of the most threatened ecosystems prone to biodiversity loss (EEA 2009). Given the growing empirical and theoretical evidence that ecosystem functions and services are linked to biodiversity (Cardinale et al. 2012; Hooper et al. 2012), it can be expected that the loss of species and habitats will affect pivotal ecosystem functions which form the basis of the distinctive ecological services provided by coastal ecosystems, such as erosion and salt spray control, storm buffering, water filtration, nutrient recycling.
Hitherto, the monitoring of biodiversity has mainly focused on the species level, with species-level assessments of extinction risk having been used to set priorities for conservation (Mace et al. 2008; Margules and Pressey 2000). However, researchers and land managers are making increasing use of complementary assessment tools that address higher levels of biological organization, i.e. ecological communities, habitats and ecosystems (Keith 2009; Keith et al. 2013; Nicholson et al. 2009). Indeed, plant communities or vegetation types represent a key approach for biodiversity conservation above the species level and have been increasingly used as crucial units for inventory, planning and monitoring as they are good indicators of overall biodiversity. Moreover, they are able to provide information about underlying abiotic components and to document individual species’ ecological requirements (Benavent-González et al. 2014; Peet and Roberts 2012).
In Europe, vegetation types have achieved a legal status as they are used to define endangered habitats according to the Habitats Directive 92/43 (EEC 1992), which aims to ensure biodiversity by conserving natural habitats and wild fauna and flora in the territory of the Member States. The Directive requires governments to designate and protect a national network of sites (the Natura 2000 network) and to provide monitoring, management and all appropriate measures to maintain, or restore, habitats at a ‘Favourable Conservation Status’ (FCS). The concept of FCS is central to the EC Habitats Directive and means that a habitat’s natural range and area are stable or increasing and the species structure and functions which are necessary for its long term maintenance exist and are likely to continue to exist for the foreseeable future. Finally, the populations of its typical species are stable and self-maintaining (Jones 2002). The FCS issue is particularly challenging for sandy coastal ecosystems, where plant communities have long proved to be critical elements in relation to the morphology and dynamics of the entire dune system (Stallins 2005).
Recently, a variety of frameworks has been proposed for assessing the conservation status of habitats or ecosystems (e.g. Evans and Arvela 2011; Keith et al. 2013; New South Wales Scientific Committee 2012; Nicholson et al. 2009; Rodríguez et al. 2011, 2012; Walker et al. 2006). Although based on different approaches and different scales, all the protocols suggest considering both spatial (range and area, and rate of decline in distribution) and qualitative aspects. Stemming from the protocol for species risk assessment (Mace et al. 2008), spatial criteria make direct reference to declining population and small population paradigms. Qualitative aspects refer to specific structures (physical components) and functions (ecological processes) necessary for the long-term maintenance of the community, and relate to properties that involve manifold species and interactions between species and between species and their environment (Keith 2009).
Although reduction in distribution is relatively easily detected, the recognition of discrete thresholds and endpoints of the structural or functional decline of a vegetation type is difficult (Nicholson et al. 2009), since a vegetation type can undergo a slow decline that leads to transformation into a new one, with a different species composition and with weakened or altered functions (Hobbs et al. 2006). However, before extinction occurs, proxies can be used to evaluate changes to ecological functions. Such proxies can be related to community structure and species composition, e.g. focal, keystone or dominant species (e.g. Benson 2006; Keith et al. 2013; Lindgaard and Henriksen 2011), functions of component species, disruption of ecological processes, such as disturbance regimes, or alien invasion (e.g. Keith et al. 2013; Lindgaard and Henriksen 2011; New South Wales Scientific Committee 2012).
In this context, the concept of ‘diagnostic species composition’, a kind of ‘reference state’ (Reynoldson and Wright 2000), becomes central. Hence, such plant community attributes as the presence, abundance or dominance of key species, i.e. structural or functional unique elements, or groups of species that share ecological requirements and features of importance for determining habitat structure and composition (i.e. ecological groups or functional groups) may be used for a practical analysis of plant community quality (Cardinale et al. 2012; Keith et al. 2013). Many authors (Keith 2009; Keith et al. 2013; Nicholson et al. 2009) agree that when applied effectively, ecological communities or habitats can be powerful tools for achieving cost-effective outputs in land-use planning and biodiversity conservation. However, their recent application in different contexts has evidenced some critical aspects and it remains difficult to quantify the degradation of communities and incorporate it in assessment protocols. One solution could be to develop consistent and transparent sub-criteria for specific types of degradation (Nicholson et al. 2009), or for specific habitat types (Gigante et al. 2015). Such an approach to assessing habitat quality might help to implement strategic management plans underpinned by a sound theoretical background.
On this basis, the aim of this study was to test the efficacy of some plant community attributes for the detection of habitat quality in coastal environments. We analyzed both plant species composition and structure, considering variables that either help to distinguish a habitat from others (diagnostic components) or play a significant role in habitat function and persistence over time. To test the efficacy of chosen attributes in disclosing changes over time we used a diachronic approach by contrasting up-to-date vegetation data with data from previous studies carried out using the same field protocol and survey method and at the same sites.
Methods
Study area
The study area corresponds to the Venetian portion of the north Adriatic coast, delimited by the estuaries of the Adige and Tagliamento rivers, north-eastern Italy (Fig. 1). Sites consisted of narrow, recent dunes (Holocene), bordered by river mouths and tidal inlets, mostly fixed by docks (ARPAV 2008). Recent dunes at the sites are in contact with ancient dunes (Pleistocene), alluvial or lacustrine deposits, or run bordering the Venice Lagoon.
Figure 1.
Location of the six investigated protected sites (dark grey) within the study area. The arrows indicate the extension of the coast occupied by sand dune environments, where the plot were collected. For each site, the number of plots used in the analysis is also reported; filled circle = 2000s; filled square = 2012.
Sediments on the backshore and dunes are similar at all sites and are in the range of fine sand (Bezzi et al. 2009). Carbonate dominates the mineralogical composition of sands (especially in the northernmost area) due to the lithology of the catchment areas of corresponding rivers. Southwards a slight magmatic component arises (Zunica 1971). The predominant winds are from the northeast and east (Bezzi et al. 2009). Annual average wave heights are <0.50 m (Dal and Simeoni 1994) with semi-diurnal tides ranging from 1.0 m (spring) to a neap range of ∼0.20 m (Polli 1970). The combination of spring tides, winds and low atmospheric pressure can exceptionally raise sea level up to 1.60 m.
The vegetation zonation follows a typical sea-inland ecological gradient, spanning annual dominated plant communities on the strandline zone of the beach to shrubby or forest communities on the stabilized dunes (Buffa et al. 2012).
Until the 1950s, the Venetian coast was almost entirely fronted by dunes up to 10-m high (Bezzi and Fontolan 2003; Pignatti 2009), but few of these still survive. The coastline suffers from increasing erosion, reduction in sand supply, alteration of geomorphic processes and heavy human use, in the form of housing, resort development and road construction (Nordstrom et al. 2009). Summer beach tourism has become one of the region’s main sources of income and in 2011, from May to September, numbered more than 25 million visitors (Romano and Zullo 2014). In particular, trampling by beach visitors is considered one of the principal causes of degradation, affecting dune vegetation both at the species and community level (Santoro et al. 2012).
Despite this situation, the Venetian coastline represents one of the regional and national biodiversity hotspots, hosting many rare plant and animal species of biogeographical importance, one Protected Area (National Law 394/91), two Important Plant Areas (Blasi et al. 2011), and several Faunal Oases. The Natura 2000 Network includes six coastal sand dune sites (Buffa and Lasen 2010), distributed along a coastal strip of about 100 km (Fig. 1), covering a total area of about 8300 ha. These dune systems host a number of plant communities endemic to the north Adriatic coastline (Buffa et al. 2012; Sburlino et al. 2008, 2013), and are included as ‘Natural habitats of European Community interest’ in Annex I of the Habitat Directive (EEC 2013). Three of these, ‘Fixed coastal dunes with herbaceous vegetation (grey dunes)’, ‘Coastal dunes with Juniperus spp.’ and ‘Wooded dunes with Pinus pinea and/or Pinus pinaster’, are considered within the category of ‘priority habitats’.
Data collection
Vegetation sampling was carried out between April and July 2012, using a stratified random sampling design on dunes that had historical vegetation survey records (Fig. 1).
The study focused on plant communities of two coastal zones: foredunes and semi-fixed or transition dunes. Foredunes (hereafter FD), which comprise embryonic and mobile dunes (habitat 2110 ‘Embryonic shifting dunes’, and 2120 ‘Shifting dunes along the shoreline with Ammophila arenaria—white dunes’ of the Habitat Directive 92/43/ECC, respectively). FD are the most dynamic part of the dune system and occupy the area directly behind the beach. FD are very sensitive to topographical and coastline dynamics (Bitton and Hesp 2013). They develop on poor sandy substrata, with high salinity content. Local scale sand movements cause burial and sand blasting of vegetation, and communities are characterized by low species richness and the percentage cover of vegetation is normally around 50–70% (García-Mora et al. 2000; Prisco et al. 2012). Tufted plants, such as the grasses Elymus farctus and A. arenaria dominate. Species with vegetative below-ground organs, as bulbs or rhizomes, proved particularly successful in withstanding sand burial (Brown and McLachlan 2002; Maun 2009). The semi-fixed or transition dunes (hereafter TD) (habitat 2130 ‘Fixed coastal dunes with herbaceous vegetation—grey dunes) occupy a zone between the mobile dunes with A. arenaria, and the more inland dune scrub and woodland habitats (Doing 1985). TD are harsh environments which favour drought tolerant plants. However, compared to the FD zone, TD are less exposed to salt winds, coastal erosion and sand burial, and so are more stable, supporting the highest number of plant species of the dry dune series (Isermann et al. 2010). Total percentage cover values are higher than in FD, often reaching 100%. The community is dominated by dwarf shrubs, perennial herbaceous erect leafy species, mosses and lichens (Sburlino et al. 2013).
The three considered habitats (2110, 2120 and 2130) are the most widespread and evenly distributed habitats in the study area (Buffa et al. 2012). Moreover, they have already been used as indicators of coastal dune conservation status at a landscape scale (Carboni et al. 2009). In addition, the presence of the habitat 2130 (grey dunes) represents one of the study area’s most interesting features. Although relatively common along the Atlantic, Baltic and North Sea coasts (Houston 2008), in Italy this habitat has been detected only along the North Adriatic coast (Prisco et al. 2012), where it is represented by an endemic perennial plant community (Sburlino et al. 2013).
All plant species were recorded and their projected cover was visually estimated by using the Braun-Blanquet seven-degree scale of abundance and dominance (Westhoff and van der Maarel 1978) in 2 × 2 m sample plots, a size commonly judged adequate in Mediterranean coastal dune ecosystems (Carboni et al. 2009). Altogether 60 plots were surveyed in 2012. Nomenclature and taxonomy followed Conti et al. (2005). Data collected in 2012 have been compared with data from previous studies carried out using the same surveying method and at the same areas in the 2000s (mostly the authors’ unpublished surveys and a small number from Gamper (2002) and Poldini et al. (1999). In order to obtain a homogeneous data set in terms of plot size (Haveman and Janssen 2008), only 2 × 2 m plots were selected. Overall, we obtained a matrix of 115 species × 103 plots (43 from 2000s + 60 from 2012). Plots were distributed as reported in Table 1. Plots location and number are indicated in Figure 1.
Table 1.
List and number of plots used in the analysis. FD, embryonic and mobile dune communities; TD, transition dune communities.
| Dune zone | 2000s | 2012 |
|---|---|---|
| FD | 7 | 8 |
| 8 | 22 | |
| TD | 28 | 30 |
Selection of community level variables
According to the Habitat Directive, a habitat type can be considered to be at a ‘FCS’ when its ‘typical species’ are at FCS, although no clear definition of ‘typical species’ is provided (Evans and Arvela 2011). For the purposes of this study, we considered ‘typical species’ to be those that exhibited high abundances or frequencies within a vegetation type, relative to other types (Chytrý et al. 2002), or were structural dominants or functionally distinct elements, governing vegetation dynamics and reflecting vegetation properties such as space occupancy pattern or resistance to disturbance (De Bello et al. 2010; Moretti et al. 2009; Noss 1990).
Regarding composition, we first identified two groups of species based on species’ origin: native vs. alien or allochthonous species, the latter identified according to Celesti-Grapow et al. (2010). Following an approach adopted in other research (Bartha et al. 2008; Buffa and Villani 2012), native species were then grouped according to species’ affinity to a given habitat (ecological groups). The three resulting groups were as follows: (i) focal species, i.e. the key species pivotal to habitat structure and function, identified with reference to the list of diagnostic and characteristic species listed in Biondi et al. (2009), EEC (2013) and Prisco et al. (2012); (ii) generalist species, i.e. all native opportunistic species not specific to dune environments; and (iii) species of other habitats, i.e. all the native species that were descriptors of dune habitats other than FD and TD.
Structural features were analyzed by grouping species according to their leaf-stem architecture (growth forms), as an indicator of adaptation to both biotic and abiotic environmental conditions. Following Perez-Harguindeguy et al. (2013), six groups were identified: (i) erect leafy; (ii) creeping; (iii) rosette; (iv) tussocks; (v) dwarf shrubs; and (vi) shrubs and trees.
For each habitat, we calculated the following variables: (i) mean number of species per plot; (ii) mean total species cover per plot (calculated by summing the percentage cover of each species, thus obtaining a figure that can exceed 100%) (Chytrý et al. 2005; Del Vecchio et al. 2015b); (iii) mean evenness index J per plot, as H’/ln S, where H’ is the Shannon diversity index and S the number of species; (iv) mean percent cover per plot of alien species; (v) mean percent cover per plot of each ecological group (focal species, generalist species and species of other habitats); and (vi) mean percent cover per plot of the individual growth forms.
Data analysis
To analyze and compare species composition of FD and TD, we performed an ordination on species cover (DCA on a matrix of 103 plots × 115 species; software Juice; Tichý 2002).
To test the changes in species composition over time, we performed a Multi-Response Permutation Procedure (MRPP; PC-ORD 5.10; McCune and Mefford 2006) using the year as a grouping variable. Furthermore, we applied the Indicator Species Analysis (grouping variable = year; PC-ORD 5.10; Dufrêne and Legendre 1997) to detect the species that underwent major changes over time. This method combines information on species abundance and occurrence in a particular group, produces indicator values (IVs) for each species in each group, and tests it for statistical significance using a randomization technique. The IVs range from zero (no indication) to 100 (perfect indication).
To test the variation in the structure of vegetation over time for each dune zone (FD and TD), we ran separate null models on each of the 13 variables described above in Selection of community level variables section (species richness, species cover, evenness index, alien species, focal species, generalist species, species of other habitats and the six growth forms). We applied the Monte Carlo F-test for two groups (Ecosim; Gotelli and Entsminger 2004), using the year as the grouping variable (2000s and 2012; factors with two levels). The test calculates the observed F-value (Fobs) and a simulated average F-value (Fexp), computed on 30 000 randomly permutated matrices. This number of permutation avoids algorithm biases (Lehsten and Harmand 2006). Fobs and Fexp were then compared, calculating the probability (P) of the null hypothesis that Fobs was drawn at random from the distribution of the simulated F indexes (Fexp). Non-random differences were assumed when PFobs ≥ Fexp ≤ 0.05. In interpreting the P values we applied the Bonferroni correction for multiple comparisons to reduce type I errors, and statistical significance was consequently set at P < 0.003.
Results
Species composition: diagnostic components
The ordination scatter diagram (Fig. 2) indicated groups of surveys according to their floristic composition. In particular, axis one shows a distinctive separation between FD and TD, reflecting the strong coastal dune zonation along the sea-inland environmental gradient.
Figure 3.
Distribution of growth forms within the selected species groups in the two zones. FD, fore dune zone; TD, transition dune zone.
Figure 2.
Ordination scatter diagram of sampled plots, using species as explanatory variables. Only the first two axes are represented. Number ‘1’: 2000s plots; number ‘2’: 2012 plots.
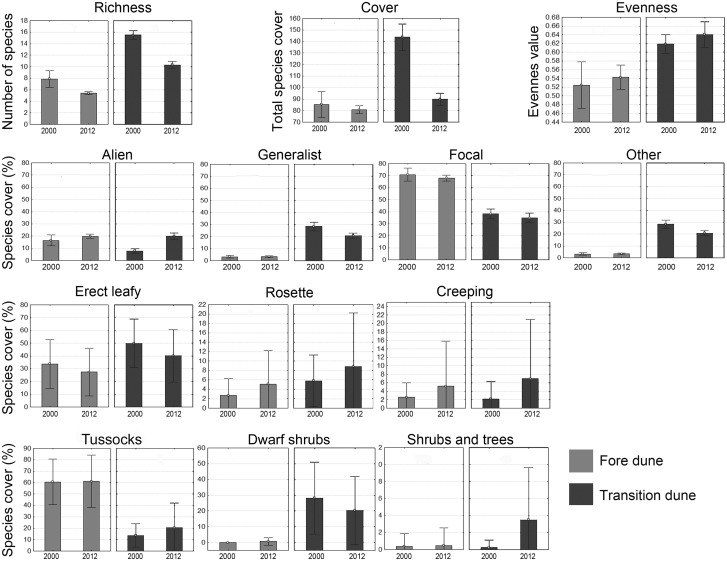
The two zones demonstrated strong differences both in composition and structure, with TD showing higher mean number of species per plot and higher mean percentage cover per plot compared with FD [see Supporting Information].
Focal species were rarely shared between the two zones and only focal species of FD were recorded inland. Among the diagnostic component (focal species), those that contributed the most to distinguish the two habitats were tussocks of A. arenaria (Pearson correlation with ordination axis 1: r = 0.7), E. farctus (r = 0.3) and erect leafy species such as Echinophora spinosa (r = 0.3) in FD, while TD showed a more complex structure dominated by dwarf shrubs of Fumana procumbens (r = 0.6), Helianthemum nummularium ssp. obscurum (r = −0.3), Teucrium capitatum (r = −0.3), Teucrium chamaedrys (r = −0.3), and perennial herbs (erect leafy, rosette and tussocky plants) as Petrorhagia saxifraga (r = −0.5), Koeleria macrantha (r = −0.4), Stachys recta (r = −0.3), Silene otites (r = −0.3), Lomelosia argentea (r = −0.2).
Native species of other habitats showed a similar distribution. Nitrophilous annuals typical of the upper zone of the beach (Cakile maritima or Salsola kali) colonized only the FD zone. Conversely, species of other habitats found in TD mostly came from the shrub-covered fixed dunes and for the most part were represented by woody perennials (e.g. Helichrysum italicum, Asparagus acutifolius, Juniperus communis) [see Supporting Information].
The group of alien species contributed to homogenize the two zones. Albeit with some exceptions, alien species colonized both zones with comparable percentage cover, irrespective of their identity, growth form and life span. Among the alien species, the most frequent and abundant growth form was that of erect leafy, e.g. Ambrosia psilostachya, Cenchrus longispinus or Erigeron canadensis, followed by that of rosettes, such as Oenothera stucchii.
Temporal trends
FD and TD zones showed analogous trends of variation with respect to the variables analyzed. However, only the TD zone revealed significant differences in the Monte Carlo test (Table 2 and Fig. 4). Overall, the TD zone showed a significant reduction in the mean species richness per plot and mean species cover per plot. These overall changes in community attributes reflected more fine-grained modification and turnover in species composition, ecological groups and growth forms.
Table 2.
Results of Monte Carlo test for two groups. The critical P-value of 0.05 yielded a value of 0.003 according to the Bonferroni correction.
| Fore dune |
Transition dune |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed mean |
F-obs | F-exp | P (O > E) | Observed mean |
F-obs | F-exp | P (O > E) | ||||
| 2000 | 2012 | 2000 | 2012 | ||||||||
| Richness | 7.9 | 5.2 | 5.46 | 1.04 | 0.0206 | 15.5 | 10.5 | 27.09 | 1.02 | 0.0000 | |
| Cover | 85.3 | 76.1 | 0.66 | 1.06 | 0.4264 | 143.8 | 89.1 | 19.60 | 1.03 | 0.0001 | |
| Evenness | 0.5 | 0.5 | 0.03 | 1.06 | 0.8563 | 0.6 | 0.7 | 0.68 | 1.03 | 0.4075 | |
| Alien | 16.5 | 20.1 | 0.55 | 1.04 | 0.4625 | 7.8 | 20.3 | 12.67 | 1.03 | 0.0007 | |
| Generalist | 3.2 | 4.1 | 0.18 | 1.02 | 0.6993 | 28.3 | 20.3 | 3.28 | 1.03 | 0.0774 | |
| Focal | 70.8 | 64.9 | 0.62 | 1.04 | 0.4401 | 38.2 | 33.6 | 0.72 | 1.03 | 0.3967 | |
| Other | 9.5 | 10.9 | 0.09 | 1.04 | 0.7712 | 25.7 | 25.2 | 0.01 | 1.04 | 0.9245 | |
| Erect leafy | 33.7 | 26.9 | 1.43 | 1.06 | 0.2382 | 49.9 | 40.8 | 3.09 | 1.04 | 0.0850 | |
| Rosette | 2.7 | 7.7 | 4.46 | 1.06 | 0.0452 | 5.8 | 9.1 | 1.89 | 1.04 | 0.1775 | |
| Creeping | 2.6 | 1.5 | 0.82 | 1.04 | 0.3949 | 2.2 | 7.2 | 3.21 | 1.02 | 0.0762 | |
| Tussocks | 60.6 | 62.7 | 0.04 | 1.06 | 0.7424 | 13.7 | 18.4 | 0.18 | 1.02 | 0.2573 | |
| Dwarf shrubs | 0.0 | 0.3 | 1.00 | 1.03 | 0.5471 | 28.2 | 20.9 | 1.54 | 1.04 | 0.2168 | |
| Shrubs and trees | 0.4 | 1.0 | 0.49 | 1.03 | 0.5930 | 0.2 | 3.6 | 7.96 | 1.03 | 0.0008 | |
Figure 4.
Means ± SD of the analysed fine-scale biotic variables in the FD and in the TD.
When comparing the two time steps, MRPP test revealed significant differences in species composition (A = 0.010; P < 0.001) between past and present surveys. The Indicator Species Analysis confirmed the trend, indicating that all the species that were best represented in the 2000s underwent major decline over time, as indicated by the change in their IV: F. procumbens (IV2000s = 36.1 P = 0.001; IV2012 = 3), E. spinosa (IV2000s = 28.7 P = 0.007; IV2012 = 4), Scabiosa triandra (IV2000s = 24 P = 0.001; IV2012 = 1), K. macrantha (IV2000s = 19.9; P = 0.004; IV2012 = 0), Medicago marina (IV2000s = 17.8 P = 0.006; IV2012 = 1), E. farctus (IV2000s = 19.9 P = 0.049; IV2012 = 4), Medicago minima (IV2000s = 16.4 P = 0.008; IV2012 = 1), S. otites (IV2000s = 14.0 P = 0.004; IV2012 = 3), T. capitatum (IV2000s = 14.0 P = 0.004; IV2012 = 0). Although the mean cover of focal species remained more or less constant in both zones, some focal species were no longer recorded in 2012 compared with the 2000s, or showed a drop in their percentage cover. The decreasing trend was particularly evident for erect leafy species and dwarf shrubs [see Supporting Information].
In both zones, alien species showed a rising trend with respect to the past plots (Fig. 4), but only TD faced a significant increase in their mean percentage cover per plot, due to both an increased cover of some species (A. psilostachya and O. stucchii) and the arrival of new species (e.g. Senecio inaequidens; IV2000s = 0; IV2012 = 10 P = 0.048) in 2012.
Growth forms showed a similar trend with respect to the past plots in both zones, but only the cover of shrubs and trees in TD showed a significant increase (Fig. 4).
Discussion
Our study showed distinct patterns of habitat modification over time. The most evident result was an overall tendency towards a shift in the plant communities’ attributes, with lower mean species richness per plot, reduced mean species cover, increased cover of alien species and, in general, shifts in the cover of different groupings of plants, so mirroring an overall shift in both the composition and structure of the analyzed plant communities. Overall, our results are in line with other previous studies, reporting habitat degradation as a result of the increasing intensity of coast-bound tourism, excessive visitor pressure and trampling (Acosta et al. 2013; Davenport and Davenport 2006; Faggi and Dadon 2011; Grunewald and Schubert 2007; Provoost et al. 2011; Rodgers 2003; Santoro et al. 2012).
Given the current consensus on the positive effect of biodiversity on ecosystem functioning (Balvanera et al. 2006; Cardinale et al. 2012), species richness and loss are among the simplest and most used measures of decline in vegetation quality. Indeed, species loss under increasing land-use intensity has already been reported for different species groups (Hoffmann and Zeller 2005; Kleijn et al. 2009; Msuha et al. 2012; Verhulst et al. 2004). However, species richness and loss indices assign an equal weight to all species, and as such they may fail in detecting changes if coupled with the increase in other species such as alien species. Arguably, by leaving vacant niches, the disappearance of native species may contribute to making plant communities more susceptible to the invasion of alien or generalist weeds (Elton 2000; Gerhold et al. 2011). Hence, by overlooking the identity of species, species richness and loss indices fail in evidencing species turnover and changes in species composition resulting from some species becoming locally extinct and others entering the community. Thus, even when richness or total cover remains constant over the decades, some replacement of species can take place. Besides, not only the disappearance but also small changes or a decline in species’ abundance can lead to the disruption of a community’s structure and function, even before any characteristic component is actually lost (Keith et al. 2013). Ultimately, not only the total species richness but also the identity of species present, the abundance of each species, and the species’ pattern of space occupancy are crucial in determining the relationships between species diversity and ecological functions.
Species are different and do not contribute equally to ecosystem functioning (Lefcheck et al. 2015). Thus, the effect of species loss is likely to depend on the range of function of species in any particular community (Rosenfeld 2002). Recent research has pointed out that functional diversity, rather than the simple number of species, plays a crucial role in regulating ecosystem processes (Diaz and Cabido 2001; Hooper et al. 2005; Lavorel and Garnier 2002). Undoubtedly, increasing land-use intensity affects the functional diversity, and thus the stability of ecosystems and their adaptability to future changes (Cadotte et al. 2009; Forest et al. 2007; Hooper et al. 2005). We argue that the analysis of suitable species groups (e.g. focal species, aliens, and growth forms) is more effective in describing the relationship between disturbance patterns and biodiversity than the use of species richness per se (Carranza et al. 2012; Vandewalle et al. 2010). As for the studied coastal plant communities, focal species, which define the habitat identity, proved to be very helpful in discriminating between habitat types and zones, as they actually exhibited higher abundances or frequencies within a habitat type, relative to other habitats. Moreover, the detailed analysis of focal species abundance and turnover can be used as a short-term alert of plant community disruption, before the effects of disturbance become fully evident.
However, in coastal ecosystems, growth forms play a key role in ecosystem organization and functioning, representing essential components of vegetation identity (Maun 2004, 2009). Furthermore, being easily observable, they may provide readily discernible evidence of ongoing processes of habitat modification (Espejel et al. 2004; Noss 1997). In particular, when integrated with compositional indicators, they can help evaluate changes and trends caused by disturbance and anthropogenic stresses (Espejel et al. 2004).
Although spatially close, investigated systems showed some distinctive features in terms of composition, structure and pattern of spatial occupancy, and a high level of distinctiveness. These peculiar features determined the different behaviour which the two zones exhibited in response to pressure exerted along the temporal sequence. Indeed, our results showed similar trends in the selected variables comparing former vs. current situation in both zones, but with different statistical significance. This suggests that the ability of the selected variables to identify changes is related to the structural complexity of the vegetation type under evaluation.
Several authors agree in assuming that the alteration of plant communities and, eventually, the disappearance of the most sensitive are generally linked to the alteration of the morphology of dune systems (Acosta et al. 2007; Nordstrom et al. 2007). In the Mediterranean coasts, especially tourism, and associated trampling and beach cleaning operations, appears to have a detrimental impact on sand dunes habitats (Ciccarelli 2014; Farris et al. 2013; Pinna et al. 2015; Santoro et al. 2012).
According to Cole (1995), the variability of the response to human disturbance, e.g. trampling, was mainly due to plant morphological characteristics, namely growth forms, than to site characteristics such as topography. Overall, tolerance, i.e. the ability of plants and plant communities to withstand a cycle of disturbance and recover, seemed primarily a function of stature, erectness and growth form. The most tolerant plants were in order tufted graminoids, rosette and creeping plants, and woody plants (shrubs and trees), while the least tolerant were the dwarf shrubs. As resistance was mainly a function of vegetation stature and erectness, erect leafy plants turned out to be the least resistant growth form.
Thus, given the intrinsic low species richness and low percentage cover (Del Vecchio et al. 2015b; Prisco et al. 2012) coupled with the leaf-stem architecture of their resident focal species, mainly tufted species, FD appear to be more capable of withstanding and recovering after disturbance events (Lucas and Carter 2013) and changes may be expected to occur more slowly compared with TD. Conversely, TD which are dominated by dwarf shrubs (Sburlino et al. 2013), are amongst the sand dunes communities most sensitive to trampling.
The group of alien species partially represents an exception to the rule, demonstrating an increasing trend irrespective of species’ identity and growth form. In fact, as well as a trampling tolerant rosette species (O. stucchii), erect leafy species (e.g. A. psilostachya or C. longispinus), dwarf shrubs (e.g. S. inaequidens) and shrubs and trees (e.g. Amorpha fruticosa, Elaeagnus angustifolia) also showed stable or increasing mean percentage cover. Indeed, increasing alien population growth and dispersal have been proved to be favoured by widespread anthropogenic environmental alterations which create new, suitable habitats and ensure human-assisted dispersal, reducing the distinctiveness of plant communities (Del Vecchio et al. 2015a; Pyšek and Hulme 2005). Thus, also the proportion and cover of alien species can provide diagnostic information, which would be disregarded when considering diversity pattern alone.
Conclusions
Our study may help to highlight some challenging points. First, although species richness is traditionally the most widely considered component of biodiversity in conservation planning, our study emphasized that species richness on its own cannot explain all patterns of biodiversity (Bartha et al. 2008; Van Meerbeek et al. 2014). The analysis of community level variables, in particular ecological groups such as native focal species and aliens, and growth forms, seems to be a practical tool for assessing and monitoring vegetation quality in coastal environments. In this context, the most interesting take home message stemming from the current study is that the analysis of suitable species groups may provide an instrument for the assessment of decline that can be applied to other coastal systems. Moreover, the plant community level proved to be effective in detecting the fine-scale habitat heterogeneity and the diversity of species assemblages in sandy coastal systems, which, in many cases, are addressed at a too coarse scale and disappear in large scale management plans.
Second, at the local scale, our study underlines the need for monitoring and active management of these environments particularly where TD are concerned. Across Europe transition and fixed dunes are the most threatened and exploited part of the dune system (Genovesi et al. 2014; Houston 2008). Although plant communities are expected to possess a certain capability of resisting external fluctuations, that is a ‘biological inertia’ (Gorham 1957), TD have shown changes in species’ composition and abundance over a short period of time, a process that should not be underestimated. In fact, for vegetation types, disappearance could be incremental, due to gradual changes in their characteristic features; as such they may not become extinct, but rather turn into a new type with a new species combination and potentially new functions (Hobbs et al. 2006).
Finally and on a broader scale our results contribute to underlining a noteworthy aspect concerning the Natura 2000 network, which is considered one of the most important and largest conservation networks worldwide (Hochkirch et al. 2013; Maiorano et al. 2007) and one of the most important tools that could allow to improve the existing networks of conservation areas and to meet the target of halting biodiversity loss. One of the principal debates in the field of conservation ecology is the monitoring of results obtained by conservation targets and the evaluation of their efficacy, i.e. the ability to achieve conservation targets, of existing protected areas (Jackson et al. 2009; Vellak et al. 2009). As required by the EC Habitats Directive, Natura 2000 sites in the North-Adriatic dune system were selected in order to ensure the long-term persistence of its native biodiversity, i.e. species and habitats of European interest. Despite this, since their formal establishment as Natura 2000 sites no official management plans have been approved, competition over land-use allocation still remains a problem and most dune habitats, as many other habitat types (Bagella et al. 2013), are still under heavy pressure. According to data reported by the Italian Ministry of the Environment and Protection of Land and Sea (Genovesi et al. 2014), 67.6% of habitats are currently characterized by inadequate or very bad conservation status, percentage that rises to 86.7% for coastal habitats, which are threaten mostly by urban sprawl, linked to the explosion of mass tourism.
Although recreational tourism is economically stimulating in terms of income, employment and development, it is also inevitably associated with a heavy impact. Intense tourism pressure coupled with a general lack of ecological consciousness of the value of these ecosystems may ultimately compromise not only the natural value and the ecological functionality of these systems, but also the quality of the recreational experience itself. Thus, in contexts where high-value natural ecosystems and socio-economic interests coexist, halting biodiversity loss requires substantial societal and political consensus as well as the determination to implement conservation strategies at all administrative levels, from national authorities to regional administrations, and local stakeholders.
Sources of Funding
This study was supported by the internal research grant for Department of Environmental Science, Informatics and Statistics, Ca’ Foscari University of Venice, Italy.
Conflicts of Interest
None declared
Supplementary Material
Acknowledgements
We would like to thank Martin Bennett for language editing. We are grateful to Hall Cushman, Katharine Dickinson and the anonymous reviewers for their useful comments that have considerably improved the article.
Supporting Information
The following [Supporting Information] is available in the online version of this article –
File 1. Table. List of species surveyed in the FD and TD zones in 2000 and 2012. Species with frequency values <1% are not displayed in the table. Ecological group symbols mean as follows: F-focal; A-aliens; G-generalist; OH-other habitats. Growth forms are indicated in number as follows: 1-erect leafy; 2-creeping; 3-rosette; 4-tussock; 5-dwarf shrubs; 6-shrubs and trees. Cumulative species number refers to the overall pool of species surveyed in both time steps. Braun-Blanquet rank cover scale was converted in percentage as follows: 5 = 87.5%; 4 = 62.5%; 3 = 37.5%; 2 = 15%; 1 = 2.5%; + =1%; r = 0.1% (van der Maarel 1979).
Literature Cited
- Acosta A, Ercole S, Stanisci A, De Patta Pillar V, Blasi C. 2007. Coastal vegetation zonation and dune morphology in some Mediterranean ecosystems. Journal of Coastal Research 23:1518–1524. [Google Scholar]
- Acosta A, Jucker T, Prisco I, Santoro R. 2013. Passive recovery of Mediterranean coastal dunes following limitations to human trampling In: Martínez MI, Hesp P, Gallego-Fernandez JB, eds. Restoration of Coastal Dunes. Heidelberg, Berlin: Springer, 187–198. [Google Scholar]
- ARPAV. 2008. I suoli della provincia di Venezia. Grafiche Erredici, Padova.
- Bagella S, Caria MC, Filigheddu R. 2013. Gap analysis revealed a low efficiency of Natura 2000 network for the conservation of endemic species in Mediterranean temporary wet habitats. Plant Biosystems 147:1092–1094. [Google Scholar]
- Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecology Letters 9:1146–1156. [DOI] [PubMed] [Google Scholar]
- Bartha S, Merolli A, Campetella G, Canullo R. 2008. Changes of vascular plant diversity along a chronosequence of beech coppice stands, central Apennines, Italy. Plant Biosystems 142:572–583. [Google Scholar]
- Benavent-González A, Lumbreras A, Molina JA. 2014. Plant communities as a tool for setting priorities in biodiversity conservation: a novel approach to Iberian aquatic vegetation. Biodiversity and Conservation 23:2135–2154. [Google Scholar]
- Benson JS. 2006. New South Wales vegetation classification and assessment: introduction – the classification, database, assessment of protected areas and threat status of plant communities. Cunninghamia 9:331–382. [Google Scholar]
- Bezzi A, Fontolan G. 2003. Foredunes classification and morphodynamic processes along the Veneto coasts (northern Adriatic, Italy). In: Ozhan E, ed. Proceedings of the 6th International Conference on the Mediterranean Coastal Environment MEDCOAST’03, Ravenna, 1425–1434.
- Bezzi A, Fontolan G, Nordstrom KF, Carrer D, Jackson NL. 2009. Beach nourishment and foredune restoration: practices and constraints along the Venetian shoreline, Italy. Journal of Coastal Research 56:287–291. [Google Scholar]
- Biondi E, Blasi C, Burrascano S, Casavecchia S, Copiz R, Del Vico E, Galdenzi E, Gigante D, Lasen C, Spampinato G, Venanzoni R, Zivkovic L. 2009. Italian interpretation manual of the 92/43/EEC Directive Habitats Società Botanica Italiana. Ministero dell’Ambiente e della Tutela del Territorio e del Mare, D.P.N. http://vnr.unipg.it/habitat/.
- Bitton MCA, Hesp PA. 2013. Vegetation dynamics on eroding to accreting beach-foredune systems, Florida panhandle. Earth Surface Processes and Landforms 38:1472–1480. [Google Scholar]
- Blasi C, Marignani M, Copiz R, Fipaldini M, Bonacquisti S, Del Vico E, Rosati L, Zavattero L. 2011. Important plant areas in Italy: from data to mapping. Biological Conservation 144:220–226. [Google Scholar]
- Brown AC, McLachlan A. 2002. Sandy shore ecosystems and the threats facing them: some predictions for the year 2025. Environmental Conservation 29:62–77. [Google Scholar]
- Buffa G, Fantinato E, Pizzo L. 2012. Effects of disturbance on sandy coastal ecosystems of N-Adriatic coasts (Italy). In: Lameed GA, ed. Biodiversity Enrichment in a Diverse World Rijeka: InTech, 339–372. [Google Scholar]
- Buffa G, Lasen C. 2010. Atlante dei siti Natura 2000 del Veneto, Regione del Veneto - Direzione Pianificazione Territoriale e Parchi, Venezia. [Google Scholar]
- Buffa G, Villani M. 2012. Are the ancient forests of the Eastern Po Plain large enough for a long term conservation of herbaceous nemoral species? Plant Biosystems 146:970–984. [Google Scholar]
- Cadotte MW, Cavender-Bares J, Tilman D, Oakley TH. 2009. Using Phylogenetic, Functional and Trait Diversity to Understand Patterns of Plant Community Productivity. PLoS One 4:e5695.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni M, Carranza ML, Acosta A. 2009. Assessing conservation status on coastal dunes: A multiscale approach. Landscape and Urban Planning 91:17–25. [Google Scholar]
- Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486:59–67. [DOI] [PubMed] [Google Scholar]
- Carranza ML, Frate L, Paura B. 2012. Structure, ecology and plant richness patterns in fragmented beech forests. Plant Ecology and Diversity 5:541–551. [Google Scholar]
- Celesti-Grapow L, Pretto F, Carli E, Blasi C. 2010. Flora vascolare alloctona e invasiva delle regioni d'Italia. Roma: Università La Sapienza.
- Chytrý M, Tichy L, Holt J, Botta-Dukat Z. 2002. Determination of diagnostic species with statistical fidelity measures. Journal of Vegetation Science 13:79–90. [Google Scholar]
- Chytrý M, Pyšek P, Tichý L, Knollová I, Danihelka AJ. 2005. Invasions by alien plants in the Czech Republic: a quantitative assessment across habitats. Preslia 77:339–354. [Google Scholar]
- Ciccarelli D. 2014. Mediterranean coastal sand dune vegetation: influence of natural and anthropogenic factors. Environmental Management 54:194–204. [DOI] [PubMed] [Google Scholar]
- Cole DN. 1995. Experimental trampling of vegetation. 2. Predictors of resistance and resilience. Journal of Applied Ecology 32:215–224. [Google Scholar]
- Conti F, Abbate G, Alessandrini A, Blasi C. 2005. An Annotated Checklist of the Italian Vascular Flora. Roma: Palombi.
- Curr RHF, Koh A, Edwards E, Williams AT, Davis P. 2000. Assessing anthropogenic impact on Mediterranean sand dunes from aerial digital photography. Journal of Coastal Conservation 6:15–22. [Google Scholar]
- Dal CR, Simeoni U. 1994. A model for determining the classification, vulnerability and risk in the Southern coastal zone of the Marche (Italy). Journal of Coastal Research 10:18–29. [Google Scholar]
- Davenport J, Davenport JL. 2006. The impact of tourism and personal leisure transport on coastal environments: A review. Estuarine Coastal and Shelf Science 67:280–292. [Google Scholar]
- De Bello F, Lavorel S, Gerhold P, Reier U, Partel M. 2010. A biodiversity monitoring framework for practical conservation of grasslands and shrublands. Biological Conservation 143:9–17. [Google Scholar]
- Defeo O, McLachlan A, Schoeman DS, Schlacher TA, Dugan J, Jones A, et al. 2009. Threats to sandy beach ecosystems: A review. Estuarine Coastal and Shelf Science 81:1–12. [Google Scholar]
- Del Vecchio S, Pizzo L, Buffa G. 2015a. The response of plant community diversity to alien invasion: evidence from a sand dune time series. Biodiversity and Conservation 24:371–392. [Google Scholar]
- Del Vecchio S, Prisco I, Acosta A, Stanisci A. 2015b. Changes in plant species composition of coastal dune habitats over a 20-years period. AoB Plants 7:plv018.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz S, Cabido M. 2001. Vive la difference: plant functional diversity matters to ecosystem processes. Trends in Ecology & Evolution 16:646–655. [DOI] [PubMed] [Google Scholar]
- Doing H. 1985. Coastal foredune zonation and succession in various parts of the world. Vegetatio 61:65–75. [Google Scholar]
- Dufrêne M, Legendre P. 1997. Species assemblages and Indicator Species: the need for a flexible asymmetrical approach. Ecological Monographs 67:345–366. [Google Scholar]
- Dugan JE, Hubbard DM. 2010. Loss of Coastal Strand Habitat in Southern California: The Role of Beach Grooming. Estuaries and Coasts 33:67–77. [Google Scholar]
- EEA (European Environment Agency). 2009. Progress towards the European 2010 biodiversity target – indicator factsheets. Technical Report No 04/2009. European Environment Agency, Copenhagen. http://www.eea.europa.eu/publications/
- EEC. 1992. Council Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora. Official Journal of the European Communities No L206 of 22 July 1992.
- EEC. 2013. Interpretation Manual of European Union Habitats – EUR28. European Commission DG Environment, Nature ENV B.3.
- Elton S. 2000. The Ecology of Invasions by Animals and Plants. Chicago: University of Chicago Press. [Google Scholar]
- Espejel I, Ahumada B, Cruz Y, Heredia A. 2004. Coastal Vegetation as Indicators for Conservation In Martínez ML, Psuty NP, eds. Coastal Dunes. Ecology and Conservation. Ecological Studies, vol. 171 Heidelberg, Germany: Springer-Verlag,, 297–318. [Google Scholar]
- Evans D, Arvela M. 2011. Assessment and Reporting Under the Habitats Directive. Paris, France: European Topic Centre on Biological Diversity. [Google Scholar]
- Faggi A, Dadon J. 2011. Temporal and spatial changes in plant dune diversity in urban resorts. Journal of Coastal Conservation 15:585–594. [Google Scholar]
- Farris E, Pisanu S, Ceccherelli G, Filigheddu R. 2013. Human trampling effects on Mediterranean coastal dune plants. Plant Biosystems 147:1043–1051. [Google Scholar]
- Feagin RA, Sherman DJ, Grant WE. 2005. Coastal erosion, global sea-level rise, and the loss of sand dune plant habitats. Frontiers in Ecology and the Environment 7:359–364. [Google Scholar]
- Forest F, Grenyer R, Rouget M, Davies TJ, Cowling RM, Faith DP, et al. 2007. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445:757–760. [DOI] [PubMed] [Google Scholar]
- Gamper U. 2002. Caratteristiche ecologiche della vegetazione a carattere mediterraneo presente sul litorale sedimentario nord-adriatico (Veneto–NE-Italia), con particolare riguardo alle problematiche di conservazione della biodiversità fitocenotica. PhD Thesis, University of Catania, Italy.
- García-Mora MR, Gallego-Fernandez JB, García-Novo F. 2000. Plant diversity as a suitable tool for coastal dune vulnerability assessment. Journal of Coastal Research 16:990–995. [Google Scholar]
- Genovesi P, Angelini P, Bianchi E, Duprè E, Ercole S, Giacanelli V, et al. 2014. Specie e habitat di interesse comunitario in italia: distribuzione, stato di conservazione e trend. ISPRA, Serie Rapporti, 194/2014.
- Gerhold P, Paertel M, Tackenberg O, Hennekens SM, Bartish I, Schaminee JHJ, et al. 2011. Phylogenetically Poor Plant Communities Receive More Alien Species, Which More Easily Coexist with Natives. American Naturalist 177:668–680. [DOI] [PubMed] [Google Scholar]
- Gigante D, Foggi B, Venanzoni R, Viciani D, Fantinato E, Buffa G. 2015. Habitat red-listing: the pattern of spatial occupancy does matter. In: Chytrý M, Zelený D, Hettenbergerová E. eds. 58th Annual Symposium of the International Association for Vegetation Science: Understanding broad-scale vegetation patterns. 19–24 July 2015. Brno, Czech Republic, 134.
- Gorham E. 1957. Development of peatlands. Quarterly Review of Biology 32:145–166. [Google Scholar]
- Gotelli NJ, Entsminger GL. 2004. EcoSim: Null models software for ecology. Version 7. Acquired Intelligence Inc. & Kesey-Bear. Jericho, VT 05465. http://garyentsminger.com/ecosim/index.htm.
- Grunewald R, Schubert H. 2007. The definition of a new plant diversity index “H'(dune)” for dune assessing human damage on coastal dunes - Derived from the Shannon index of entropy H'. Ecological Indicators 7:1–21. [Google Scholar]
- Haveman R, Janssen JAM. 2008. The analysis of long-term changes in plant communities using large databases: The effect of stratified resampling. Journal of Vegetation Science 19:355–397. [Google Scholar]
- Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, Ewel JJ, Klink CA, Lugo AE, Norton D, Ojima D, Richardson DM, Sanderson EW, Valladares F, Vila M, Zamora R, Zobel M. 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography 15:1–7. [Google Scholar]
- Hochkirch A, Schmitt T, Beninde J, Hiery M, Kinitz T, Kirschey J, Matenaar D, Rohde K, Stoefen A, Wagner N, Zink A, Lotters S, Veith M, Proelss A. 2013. Europe needs a new vision for a Natura 2020 network. Conservation Letters 6:462–467. [Google Scholar]
- Hoffmann A, Zeller U. 2005. Influence of variations in land use intensity on species diversity and abundance of small mammals in the Nama Karoo, Namibia. Belgian Journal of Zoology 135:91–96. [Google Scholar]
- Hooper DU, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA, Matulich KL, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–129. [DOI] [PubMed] [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, et al. 2005. Effects of biodiversity on ecosystem functioning. A consensus of current knowledge. Ecological Monographs 75:3–35. [Google Scholar]
- Houston J. 2008. Management of Natura 2000 habitats. 2130 *Fixed coastal dunes with herbaceous vegetation (‘grey dunes’). European Commission. Technical Report 2008 04/24.
- Isermann M, Koehler H, Mühl M. 2010. Interactive effects of rabbit grazing and environmental factors on plant species-richness on dunes of Norderney. Journal of Coastal Conservation 14:103–114. [Google Scholar]
- Jackson SF, Walker K, Gaston KJ. 2009. Relationship between distributions of threatened plants and protected areas in Britain. Biological Conservation 142:1515–1522. [Google Scholar]
- Jones W. 2002. EC Habitats Directive: Favourable Conservation Status. JNCC 02 D07.
- Keith DA. 2009. The interpretation, assessment and conservation of ecological communities. Ecological Management and Restoration 10:3–15. [Google Scholar]
- Keith DA, Rodriguez JP, Rodriguez-Clark KM, Nicholson E, Aapala K, Alonso A, Asmussen M, Bachman S, Basset A, Barrow EG, Benson JS, Bishop MJ, Bonifacio R, Brooks TM, Burgman MA, Comer P, Comin FA, Essl F, Faber-Langendoen D, Fairweather PG, Holdaway RJ, Jennings M, Kingsford RT, Lester RE, Mac Nally R, McCarthy MA, Moat J, Oliveira-Miranda MA, Pisanu P, Poulin B, Regan TJ, Riecken U, Spalding MD, Zambrano-Martinez S. 2013. Scientific Foundations for an IUCN Red List of Ecosystems. PLoS One 8:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn D, Kohler F, Baldi A, Batary P, Concepcion ED, Clough Y, Díaz M, Gabriel D, Holzschuh A, Knop E, Kovács A, Marshall EJ, Tscharntke T, Verhulst J. 2009. On the relationship between farmland biodiversity and land-use intensity in Europe. Proceedings of the Royal Society B-Biological Sciences 276:903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology 16:545–556. [Google Scholar]
- Lefcheck JS, Bastazini VAG, Griffin JN. 2015. Choosing and using multiple traits in functional diversity research. Environmental Conservation 42:104–107. [Google Scholar]
- Lehsten V, Harmand P. 2006. Null models for species co-occurrence patterns: assessing bias and minimum iteration number for the sequential swap. Ecography 29:786–792. [Google Scholar]
- Lindgaard A, Henriksen S. (eds.) 2011. The 2011 Norvegian red list for ecosystems and habitat types Norwegian Biodiversity Information Centre, Trondheim: Artsdatabanken, 120. [Google Scholar]
- Lucas KL, Carter GA. 2013. Change in distribution and composition of vegetated habitats on Horn Island, Mississippi, northern Gulf of Mexico, in the initial five years following Hurricane Katrina. Geomorphology 199:129–137. [Google Scholar]
- Mace GM, Collar NJ, Gaston KJ, Hilton-Taylor C, Akcakaya HR, Leader-Williams N, Milner-Gulland EJ, Stuart SN. 2008. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conservation Biology 22:1424–1442. [DOI] [PubMed] [Google Scholar]
- Maiorano L, Falcucci A, Garton EO, Boitani L. 2007. Contribution of the Natura 2000 Network to Biodiversity Conservation in Italy. Conservation Biology 21:1433–1444. [DOI] [PubMed] [Google Scholar]
- Margules CR, Pressey RL. 2000. Systematic conservation planning. Nature 405:243–253. [DOI] [PubMed] [Google Scholar]
- Maun MA. 2004. Burial of plants as a selective force in sand dunes In: Martínez ML, Psuty NP, eds. Coastal Dunes. Ecology and Conservation. Ecological Studies, vol. 171. Berlin: Springer-verrlag, 119–136. [Google Scholar]
- Maun MA. 2009. The Biology of Coastal Sandy Dunes. Oxford: Oxford University Press. [Google Scholar]
- McCune B, Mefford MJ. 2006. PC-ORD Multivariate Analysis of Ecological Data. Version 5. MjM Software. Oregon: Gleneden Beach.
- Moretti M, De Bello F, Roberts SPM, Potts SG. 2009. Taxonomical vs. functional responses of bee communities to fire in two contrasting climatic regions. Journal of Animal Ecology 78:98–108. [DOI] [PubMed] [Google Scholar]
- Msuha MJ, Carbone C, Pettorelli N, Durant SM. 2012. Conserving biodiversity in a changing world: land use change and species richness in northern Tanzania. Biodiversity and Conservation 21:2747–2759. [Google Scholar]
- New South Wales Scientific Committee. 2012. Guidelines for interpreting listing criteria for species, populations and ecological communities under the NSW Threatened Species Conservation Act. Listing guidelines version 1.3, January 2012.
- Nicholson E, Keith DA, Wilcove DS. 2009. Assessing the threat status of ecological communities. Conservation Biology 23:259–274. [DOI] [PubMed] [Google Scholar]
- Nordstrom KF, Hartman JM, Freestone AL, Wong M, Jackson NL. 2007. Changes in topography and vegetation near gaps in a protective foredune. Ocean and Coastal Management 50:945–959. [Google Scholar]
- Nordstrom KF, Gamper U, Fontolan G, Bezzi A, Jackson NL. 2009. Characteristics of coastal dune topography and vegetation in environments recently modified using beach fill and vegetation plantings, Veneto, Italy. Environmental Management 44:1121–1135. [DOI] [PubMed] [Google Scholar]
- Noss RF. 1990. Indicators for monitoring biodiversity - a hierarchical approach. Conservation Biology 4:355–364. [Google Scholar]
- Noss RF. 1997. Hierarchical indicators for monitoring changes in biodiversity In: Mcffe KG, Carroll CR, eds. Principles of conservation biology. New York: Sinauer, 88–92. [Google Scholar]
- Peet RK, Roberts D. 2012. Classification of natural and semi-natural vegetation In: Van der Maarel E, Franklin J, eds. Vegetation Ecology. New York: John Wiley & Sons, 28–70. [Google Scholar]
- Perez-Harguindeguy N, Diaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quetier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61:167–234. [Google Scholar]
- Pignatti S. 2009. Com'è triste Venezia, soltanto mezzo secolo dopo. Parchi 58:59–70. [Google Scholar]
- Pinna MS, Cañadas EM, Fenu G, Bacchetta G. 2015. The European Juniperus habitat in the Sardinian coastal dunes: Implication for conservation Estuar. Coast Shelf Sci 164:214–220. [Google Scholar]
- Poldini L, Vidali M, Fabiani ML. 1999. La vegetazione del litorale sedimentario del Friuli-Venezia Giulia (NE Italia) con riferimenti alla regione alto-Adriatica. Studia Geobotanica 17:3–68. [Google Scholar]
- Polli S. 1970. Tabelle di previsione delle maree per Trieste e l’Adriatico Settentrionale per l’anno 1971. Trieste: Istituto Talassografico Sperimentale. [Google Scholar]
- Prisco I, Acosta A, Ercole S. 2012. An overview of the Italian coastal dune EU habitats. Annali di Botanica 2:39–48. [Google Scholar]
- Provoost S, Jones MLM, Edmondson SE. 2011. Changes in landscape and vegetation of coastal dunes in northwest Europe: a review. Journal of Coastal Conservation 15:207–226. [Google Scholar]
- Pyšek P, Hulme PE. 2005. Spatio-temporal dynamics of plant invasions: linking pattern to process. Ecoscience 12:302–315. [Google Scholar]
- Reynoldson TB, Wright JF. 2000. The Reference Condition: Problems and Solutions In: Wright JF, Sutcliffe DW, Furse MT. eds. Assessing the Biological Quality of Fresh Waters. RIVPACS and Other Techniques. Ambleside, UK: Freshwater Biological Association, 293–303. [Google Scholar]
- Rodgers JC. 2003. Effects of human disturbance on the dune vegetation of the Georgia Sea Islands. Physical Geography 23:79–94. [Google Scholar]
- Rodríguez JP, Rodríguez-Clark K, Baillie JE, Ash N, Benson J, Boucher T, et al. 2011. Establishing IUCN Red List Criteria for Threatened Ecosystems. Conservation Biology 25:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JP, Rodríguez-Clark KM, Keith DA, Barrow EG, Benson J, Nicholson E, Wit P. 2012. IUCN Red List of Ecosystems. S.A.P.I.EN.S 5.2. http://sapiens.revues.org.1286.
- Romano B, Zullo F. 2014. The urban transformation of Italy’s Adriatic coastal strip. Land Use Policy 38:26–36. [Google Scholar]
- Rosenfeld JS. 2002. Functional redundancy in ecology and conseration. Oikos 98:156–162. [Google Scholar]
- Santoro R, Jucker T, Prisco I, Carboni M, Battisti C, Acosta ATR. 2012. Effects of trampling limitation on coastal dune plant communities. Environmental Management 49:534–542. [DOI] [PubMed] [Google Scholar]
- Sburlino G, Buffa G, Filesi L, Gamper U. 2008. Phytocoenotic originality of the N-Adriatic coastal sand dunes (Northern Italy) in the European context: The Stipa veneta-rich communities. Plant Biosystems 142:533–539. [Google Scholar]
- Sburlino G, Buffa G, Filesi L, Gamper U, Ghirelli L. 2013. Phytocoenotic diversity of the N-Adriatic coastal sand dunes - The herbaceous communities of the fixed dunes and the vegetation of the interdunal wetlands. Plant Sociology 50:57–77. [Google Scholar]
- Stallins JA. 2005. Stability domains in barrier island dune systems. Ecological Complexity 2:410–430. [Google Scholar]
- Tichý L. 2002. JUICE, software for vegetation classification. Journal of Vegetation Science 13:451–453. [Google Scholar]
- van der Maarel E. 1979. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 39:97–114. [Google Scholar]
- Van Meerbeek K, Helsen K, Hermy M. 2014. Impact of land-use intensity on the conservation of functional and phylogenetic diversity in temperate semi-natural plant communities. Biodiversity and Conservation 23:2259–2272. [Google Scholar]
- Vandewalle M, de Bello F, Berg MP, Bolger T, Doledec S, Dubs F, Feld CK, Harrington R, Harrison PA, Lavorel S, da Silva PM, Moretti M, Niemela J, Santos P, Sattler T, Sousa JP, Sykes MT, Vanbergen AJ, Woodcock BA. 2010. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodiversity and Conservation 19:2921–2947. [Google Scholar]
- Vellak A, Tuvi EL, Reier U, Kalamees R, Roosaluste E, Zobel M, Partel M. 2009. Past and Present Effectiveness of Protected Areas for Conservation of Naturally and Anthropogenically Rare Plant Species. Conservation Biology 23:750–757. [DOI] [PubMed] [Google Scholar]
- Verhulst J, Baldi A, Kleijn D. 2004. Relationship between land-use intensity and species richness and abundance of birds in Hungary. Agriculture Ecosystems Environment 104:465–473. [Google Scholar]
- Walker S, Price R, Rutledge D, Stephens RTT, Lee WG. 2006. Recent loss of indigenous cover in New Zealand. New Zealand Journal of Ecology 30:169–177. [Google Scholar]
- Westhoff V, van der Maarel E. 1978. The Braun-Blanquet approach In: Whittaker RH, ed. Classification of Plant Communities. The Hague: Junk, 287–399. [Google Scholar]
- Zunica M. 1971. Evoluzione dei litorali dal Tagliamento all’Adige con particolare riguardo ai lidi della Laguna di Venezia (Relazione definitiva). Min. Lav. Pubbl. Com. St. Provv. Venezia, Padova, Italy.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.