Abstract
Purpose. Granulocyte transfusions have been used to treat immune cell dysfunction in sepsis. A granulocyte bioreactor for the extracorporeal treatment of sepsis was tested in a prospective clinical study focusing on the dosage of norepinephrine in patients and influence on dynamic and cell based liver tests during extracorporeal therapies. Methods and Patients. Ten patients with severe sepsis were treated twice within 72 h with the system containing granulocytes from healthy donors. Survival, physiologic parameters, extended hemodynamic measurement, and the indocyanine green plasma disappearance rate (PDR) were monitored. Plasma of patients before and after extracorporeal treatments were tested with a cell based biosensor for analysis of hepatotoxicity. Results. The observed mortality rate was 50% during stay in hospital. During the treatments, the norepinephrine-dosage could be significantly reduced while mean arterial pressure was stable. In the cell based analysis of hepatotoxicity, the viability and function of sensor-cells increased significantly during extracorporeal treatment in all patients and the PDR-values increased significantly between day 1 and day 7 only in survivors. Conclusion. The extracorporeal treatment with donor granulocytes showed promising effects on dosage of norepinephrine in patients, liver cell function, and viability in a cell based biosensor. Further studies with this approach are encouraged.
1. Introduction
Severe sepsis and septic shock remain a major cause of morbidity and mortality in critically ill patients and the treatment of these patients is very expensive [1–4]. The impairment of hemodynamics and liver function are major problems in patients with severe sepsis [5, 6]. In patients with septic shock a liver dysfunction or liver failure occurred in nearly 19% and lead to a poor prognosis of these patients [6, 7].
Extracorporeal therapies have been suggested to influence successfully immune imbalances and subsequently the clinical course of multiorgan failure and sepsis [8]. Some studies showed hemodynamic stabilization of patients during extracorporeal treatment of sepsis; however, no clear impact on survival was seen [8, 9]. The influence of extracorporeal therapies of sepsis on liver function has not been investigated yet, but it may be an important tool for the improvement of outcome in this high-risk cohort population with liver dysfunction or liver failure [6].
Extracorporeal bioreactors were studied in the treatment of liver failure and acute renal failure associated with sepsis using hepatocytes or renal tubular cells; the proper choice of the cell-source was of central importance [10–14]. The use of immune cells (leukocytes) to treat sepsis in an extracorporeal setting was reported from our group [15]. With regard to cellular immunocompetence, functional impairment of neutrophils and monocytes is associated with increased mortality in advanced stages of sepsis [16–23]. Therefore, we developed an extracorporeal granulocyte bioreactor system [24, 25]. The rationale for such an approach is that on one hand the plasma-modifying capacity of human phagocytes can be used (e.g., to remove antigenic material from the circulation) while on the other hand control over these cells can be maintained (e.g., retention of the cells and their release and break-down products, preventing local tissue effects; 15). In in vitro studies and two large animal models for septic shock, we were able to show the proof of principle and promising survival data [15, 24, 26]. Additionally, the granulocyte bioreactor was studied in a pilot phase I trial with ten septic shock patients and showed safety and compatibility of this complex therapy [25]. During extracorporeal granulocyte treatments, the dosage of norepinephrine could be significantly reduced, as blood pressure was stable in the treated septic patients.
The focus of the current work was to document the exact impact of the extracorporeal granulocyte treatment on the dosage of norepinephrine in patients and the liver function using extended hemodynamic monitoring with the PiCCO-System and dynamic measurement of the liver function with the LiMON-System [27, 28]; moreover, in this second clinical study, cell based analysis of hepatotoxicity of plasma of patients [29, 30] with severe sepsis or septic shock patients was done. Based on the results of the second study, a first controlled study with this new therapy should be designed.
2. Materials and Patients
The study was conducted in accordance with the Helsinki Declaration, received ethics approval from the local research ethics committee (reg.-no: II HV 28/2003), and was notified to the state authorities according to German pharmaceutical and medical device law. The trial was registered https://clinicaltrials.gov/ (reg.-no: NCT00818597). Written informed consent was obtained from all participants or from the patients' representatives if direct consent could not be received.
2.1. Patients
Between January 2010 and November 2011, ten patients of one medical and one surgical intensive care units of a tertiary care university hospital were enrolled in the study. During a 22-month period, all patients were screened for the parameters of severe sepsis or septic shock as defined by international consensus criteria [31]. Definitions of organ dysfunctions were adopted from the PROWESS study [32] with the difference that liver failure was not an exclusion criterion in this current study. The exclusion criteria were age under 18 years, hepatitis C, HIV infection, and active bleeding or contraindications against systemic heparinization.
2.2. Procedures
After inclusion of a patient, a healthy blood donor for obtaining an ABO-compatible granulocyte concentrate was identified and stimulated with corticosteroids (each 8 mg p.o. methylprednisolone, Sanofi-Aventis Deutschland GmbH, Frankfurt, Germany) and lenograstim (Granocyte, each 1.5 μg/kg s.c., Chugai Pharma Deutschland, Frankfurt, Germany) 16 h before donation. Granulocytes were collected by extracorporeal density gradient centrifugation using hydroxyethyl starch (HES 200/0.5 6%, Fresenius Kabi AG, Bad Homburg, Germany) and citrate in a cell separator (COBE Spectra, Gambro BCT, Planegg-Martinsried, Germany) according to standard procedures. Prior to the treatment, the inclusion criteria were reconfirmed and the patients were treated for up to six hours with an extracorporeal bioreactor (Figure 1) consisting of plasma separation and plasma perfusion through the cell compartment containing the donor cells. Blood access was venovenous via a Shaldon catheter. Plasma separation was carried out by a dialysis monitor (BM25, Edwards Lifesciences GmbH, Unterschleissheim, Germany) using a 0.5 μm pore-size plasma filter (PF 1000N, Gambro Hospal GmbH, Planegg-Martinsried, Germany). The plasma was infused into a continuously recirculating donor cell compartment that was prefilled with hemofiltration solution HF-BIC 35-410 (Fresenius Medical Care, Bad Homburg, Germany). Plasma reflux to the patient was done through a second PF 1000N plasma filter to withhold the donor cells from being infused into the patient. Total extracorporeal volume was 400 mL. The blood flow rate was 110–150 mL/minute with a plasma separation rate of 16.7–33.3 mL plasma/minute using the BM 25 monitor. The MARS-Monitor 1 TC (Gambro Rostock GmbH, Rostock, Germany) was used for the recirculating bioreactor circuit at a rate of 200 mL/minute and to maintain the temperature in the cell compartment at 37°C. Unfractionated heparin (40 IU/kg, Roche, Grenzach-Wyhlen, Germany) was given at the beginning of the extracorporeal treatment followed by a continuous infusion into the circuit. Heparin administration was adjusted to maintain activated clotting time (ACT) within 180–200 seconds. Following safety assessment of the first treatment, all patients were treated a second time 48 hours after the first treatment, again for up to six hours with granulocytes from the same donor.
Figure 1.
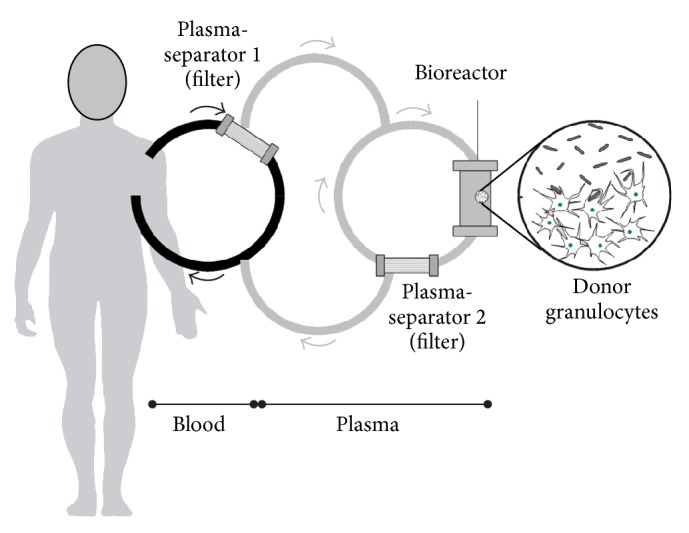
Schematic drawing of the extracorporeal plasma separation and cell perfusion model.
2.3. Measurements
We recorded basic demographic information, illness severity (APACHE II, SOFA, MODS, and SAPS II scores), microbiological results, premorbidity, and clinical outcome for study cohort (Tables 1 and 2). Patients were followed up for 28 days and hospital survival. At the days “inclusion”, 1–8, 10, 12, 14, 21, 28, and before/after an extracorporeal bioreactor-treatment, the patients were screened for clinical and immunological data: hemodynamic, inflammation, coagulation, hemolysis, temperature, organ function blood parameters, cytokines, complement (C3, C4), and number of HLA-DR molecules per monocyte surface. “Day 1” was defined as the day of the first bioreactor-treatment. At inclusion, at days 1 to 7 and before and after extracorporeal treatment, hemodynamic monitoring was done with the PiCCO-System (Table 4, PULSION Medical Systems, Feldkirchen, Germany; 27). The LiMON-System (based on the indocyanine green plasma disappearance rate, PULSION; 28) was used for the dynamic measurement of the liver function before and after extracorporeal treatment and on day 7.
Table 1.
Patients characteristics, illness severity, premorbidity, and clinical outcome for the study cohort (n = 10).
| Patient | Major diagnoses at inclusion | Source of infection | Organ failure | Premorbidity | Age (years) |
Sex (m) |
Hospital survival |
|---|---|---|---|---|---|---|---|
| 1 | SS, ALI | Pneumonia, bacteremia, infection of foot, and thoracic empyema | ARF, ALI | Diabetes mellitus, chronic heart failure |
66 | m | Died (day 62) |
| 2 | SS, ALI, and endocarditis | Bacteremia, infection of sternum | ARF, ALI, and liver failure |
Diabetes mellitus | 72 | m | Died (day 2) |
| 3 | Severe sepsis, thoracic empyema | Pneumonia, bacteremia | ALI | Alcohol abuse | 33 | m | Survived |
| 4 | SS, colitis | Peritonitis, colitis | ARF, ALI, DIC, and liver failure | Chronic heart failure | 78 | m | Died (day 4) |
| 5 | SS, ALI | Pneumonia, urinary tract infection | ARF, ALI | IHD, immunosuppression | 59 | m | Survived |
| 6 | SS, spondylodiscitis | Pneumonia, bacteremia, spondylodiscitis, and infection of arm | Liver failure, ALI, and ARF | COPD | 67 | m | Died (day 1) |
| 7 | SS, urosepsis | Pneumonia, bacteremia, and urinary tract infection | ALI, ARF, and DIC | Diabetes mellitus, IHD | 76 | m | Survived |
| 8 | SS, peritonitis | Bacteremia, pneumonia, and peritonitis | ALI | COPD, alcohol abuse | 62 | m | Died (day 22) |
| 9 | SS, perforated aortic aneurysm | Bacteremia, pneumonia, and peritonitis | ARF, ALI | IHD, cancer | 83 | m | Survived |
| 10 | SS, after urgent ACB surgery | Pneumonia | ALI | Diabetes mellitus, COPD, and IHD | 57 | m | Survived |
ACB: aortocoronary bypass.
ALI: acute lung injury.
ARF: acute renal failure.
COPD: chronic obstructive pulmonary disease.
DIC: disseminated intravascular coagulation.
IHD: ischemic heart disease.
m: male.
SS: septic shock.
Table 2.
Age, scores, and results of laboratory parameters, of hemodynamic monitoring (PiCCO-System), and of dynamic measurement of the liver function (LiMON-System) at inclusion or on day 1 (before granulocyte therapy) of survivors and nonsurvivors (median/0.25–0.75 quartile).
| Survivors (n = 5) |
Nonsurvivors (n = 5) |
Statistical significance (p) | |
|---|---|---|---|
| Age (years) | 59 (57–76) | 67 (66–72) | n.s. |
| Surgery/no surgery | 4/1 | 5/0 | |
| APACHE II at ICU arrival/at inclusion | 20.5 (17.8–25)/22 (22–34) | 27 (27–33)/30 (28–32) | n.s. |
| SOFA at inclusion | 8 (6–10) | 14 (13–14) | n.s. |
| SAPS II at inclusion | 47 (44–62) | 74 (73–78) | 0.032 |
| MODS at inclusion | 8 (7–12) | 12 (11–13) | n.s. |
| Bilirubin (µmol/L) | 12 (11–17) | 44 (30–145) | 0.016 |
| ALAT (U/L) | 32 (20–45) | 37 (30–219) | n.s. |
| ASAT (U/L) | 62 (44–74) | 105 (52–669) | n.s. |
| Ammonia (mmol/L) | 36 (32–62) | 39 (38–61) | n.s. |
| PCT (ng/mL) | 7.3 (0.5–43) | 10.4 (7.4–14.6) | n.s. |
| Leukocytes (GpT/L) | 18.1 (14.8–18.1) | 16.2 (13.9–22.2) | n.s. |
| Thrombocytes (GpT/L) | 159 (89–278) | 125 (47–269) | n.s. |
| Prothrombin time as INR | 1.09 (1.01–1.1) | 1.32 (1.11–1.33) | n.s. |
| Activated partial thromboplastin time (s) | 36 (33–49) | 48 (45–54) | n.s. |
| Creatinine (µmol/L) | 143 (79–238) | 178 (165–213) | n.s. |
| Urea (mmol/L) | 15.7 (5.7–16) | 14.7 (13.8–24.9) | n.s. |
| Lactate (mmol/L) | 1.0 (0.9–1.8) | 2.0 (1.6–2.1) | n.s. |
| Complement C3 (g/L) | 1.1 (1.0–1.8) | 0.7 (0.5–1.0) | n.s. |
| Complement C4 (g/L) | 0.5 (0.4–0.9) | 0.4 (0.2–0.5) | n.s. |
| HLA-DR/CD-14 positive cells (expression/cell) | 6413 (5890–6970) | 8980 (7640–9590) | n.s. |
| Cardiac index (L/m2/min) | 3.5 (3.3–4.0) | 2.5 (2.5–3.4) | n.s. |
| Stroke volume index (mL/m2) | 48 (43–51) | 29 (28–39) | n.s. |
| MAP (mmHg) | 92 (84–93) | 71 (69–85) | n.s. |
| Norepinephrine (µg/kg/min) | 0.13 (0.06–0.21) | 0.36 (0.29–0.51) | 0.032 |
| ICG-PDR on day 1 (%)/ICG-PDR/CI | 14.4 (13.3–17.2)/5.6 (4.9–5.7) | 5.3 (2.5–11.9)/2.9 (1.1–2.9) | 0.048/0.05 |
ALAT: alanine aminotransferase.
APACHE: Acute Physiology and Chronic Health Evaluation.
ASAT: aspartate aminotransferase.
CI: cardiac index.
HLA: human leukocyte antigen.
ICG-PDR: indocyanine green plasma disappearance rate.
MAP: mean arterial pressure.
MODS: multiorgan dysfunction syndrome (score).
n.s.: not (statistically) significant.
PCT: procalcitonin.
SAPS: simplified acute physiology score.
SOFA: sequential organ failure assessment (score).
Table 4.
Hemodynamic parameters, central venous oxygen saturation, dynamic measurement of the liver function (LiMON-System), and lactate before and after the extracorporeal granulocyte therapy (median/0.25–0.75 quartile) measured with the PiCCO-System (under significant reduction of norepinephrine; see Figure 2).
| Before extracorporeal therapy (n = 9) | After extracorporeal therapy (n = 8) | Statistical significance | |
|---|---|---|---|
| CI (L/m2/min) | 3.1 (2.3–3.8) | 3.5 (2.9–3.7) | n.s. |
| SVI (mL/m2) | 42 (33–56) | 45 (40–52) | n.s. |
| MAP (mmHg) | 73 (65–83) | 74 (66–79) | n.s. |
| Heart rate (beat/min) | 73 (67–95) | 76 (70–85) | n.s. |
| SVRI (dyne × sec × cm−5/m2) | 1510 (1150–1730) | 1290 (1130–1760) | n.s. |
| ITBVI (mL/m2) | 1060 (930–1160) | 1000 (960–1120) | n.s. |
| EVLWI (mL/kg) | 7.6 (6.8–9.4) | 7.5 (6.5–8.3) | n.s. |
| cvSpO2 (%) | 73.5 (68.4–76.5) | 71.5 (68.6–79.1) | n.s. |
| Lactate (mmol/L) | 1.5 (1.2–3.0) | 1.3 (1.0–2.7) | n.s. |
| ICG-PDR (%)/ICG-PDR/CI | 13.8 (11.9–15.6.)/4.4 (3.1–5.1) | 14.2 (12.4–17.1)/4.6 (3.1–4.8) | n.s./n.s. |
CI: cardiac index.
cvSpO2: central venous oxygen saturation.
EVLWI: extravascular lung water index.
ICG-PDR: indocyanine green plasma disappearance rate.
ITBVI: intrathoracic blood volume index.
MAP: mean arterial pressure.
n.s.: not (statistically) significant.
SVI: stroke volume index.
SVRI: systemic vascular resistance index.
2.4. Cell Based Analysis of Hepatotoxicity: Cell Cultures and Test Methods
Before and after extracorporeal treatment, 10 mL plasma was drawn from each patient for testing with our hepatotoxicity test (biosensor; 29, 30). The method to determine the toxicity of patient plasma used the human hepatocytes cell line HepG2/C3A obtained from the American Type Culture Collection (ATCC CRL-10741). The cells were cultivated in Dulbecco's modified Eagle's Medium (GIBCO Life Technologies, Eggenstein, Germany). HepG2/C3A cells were seeded in 24-well cell culture plates in a density of 250.000 cells/well; then, the cells were cultured for three days with 1 mL heparinized plasma from subjects. Subsequently, cells were rinsed once with medium and incubated with fresh medium (1 mL) for three days. Cells, respectively, cell culture supernatants, were tested for viability (XTT-test: dehydrogenases activity in the mitochondria, cell-count and vitality with Trypan blue-staining), synthesis of microalbumin, and cytochrome 1A2 activity. Each test batch with plasma from test persons was duplicated (+medium control) and each measurement was taken twice. Microalbumin was determined nephelometrically from cell culture medium supernatant (Immage 800, Beckmann Coulter GmbH, Krefeld, Germany). The XTT-test was carried according to the protocol of Scudiero et al. [33]. The activity of cytochrome P450 1A2 was determined by means of O-demethylation of 7-ethoxyresorufine to resorufin. The measurement was carried according to the protocol of Kelly and Sussman [34].
2.5. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) was used to conduct nonparametric analyses using Friedman test and Wilcoxon test for the comparison of parameters in the course of disease; statistical significance between the survivors and nonsurvivors was analyzed with the Kruskal-Wallis one-way and the two-tailed Mann-Whitney U test. The results are expressed as the median with 0.25–0.75 quartile. Differences were considered significant at p < 0.05. Box Plots were used for graphics. The horizontal line within the boxes represents the median, whereas the upper part represents the 75th and the lower part the 25th percentiles. The whiskers represent the range of the values, whereas the circles and the asterisks show the outliers (extreme values that derivate from the rest of the sample).
3. Results
3.1. Clinical Characteristics of Patients and Survival
Nine patients with septic shock and one patient with severe sepsis were included in the study (all male, 9 out of 10 surgical patients). Details concerning diagnoses, source of infection, organ failure, age, sex, premorbidity, and survival are shown in Table 1. Nine out of ten patients had positive microbial tests; 18 Gram-positive bacteria, 12 Gram-negative bacteria, and 4 positive cultures with fungi were found.
The observed mortality rate was 40% within 28 days and 50% during stay in hospital. Five patients could be discharged from the hospital in stable condition. Patients 2 and 4 died after reduction of therapy on palliative care (on the very same day). During the first extracorporeal treatment, patient 6 died; an autopsy revealed an advanced and longer existing ischemia of the bowel. The time between beginning of shock and beginning of extracorporeal treatment was 5.5 (4.3–7.5) days. In Table 2, age, scores, and results of laboratory parameters, of hemodynamic monitoring (PiCCO-System), and of dynamic measurement of liver function (LiMON-System) at inclusion or at day 1 of survivors and nonsurvivors are shown. The dosage of norepinephrine, the values of bilirubin, and the median SAPS II were significantly higher and the indocyanine green plasma disappearance rate (ICG/PDR)/ICG-PDR cardiac index ratio was significantly lower in nonsurvivors than in survivors. Three patients developed a liver failure in the course of disease (Table 1, all nonsurvivors).
3.2. Extracorporeal Treatment
All extracorporeal treatments were carried out for 6 h without technical problems. Eight patients were treated twice within 72 h with an extracorporeal bioreactor containing 12.3 (10.4–14.4) x10E10 granulocytes from healthy donors. On average, 11.7 (10.3–12.0) liters of separated plasma were treated by the therapeutic donor cells. To test whether the donor cells were still functional, every two hours cells from the cell circuit were evaluated for viability and functionality. For the whole treatment, the cells showed a viability of more than 90% and unimpaired cellular functions like phagocytosis and oxidative burst (data not shown).
There was no significant change in coagulations markers (platelet counts, antithrombin, prothrombin time, and fibrinogen) within 12 h after the extracorporeal circulation. D-dimers did not increase significantly during the extracorporeal treatment (data not shown). No hemorrhages and no signs of hemolysis were observed. Haptoglobin remained within the normal range and no significant change in values of lactatedehydrogenase and potassium was seen during the treatments (data not shown). Moreover, no allergic reactions were recognized.
3.3. Inflammation
During the six-hour treatment, a dramatic increase in the number of leukocytes was observed (before: 14.9 (13.8–19.4); after 6 hours: 18.2 (14.6–24.8); x10E9/l; p = 0.002); after 12 hours, however, a decrease to baseline was seen. This increase during extracorporeal treatment was not due to changes in a particular subset of WBC, the ratio of segmented to banded neutrophils remained unchanged. During the extracorporeal treatments, the complement factors C3 and C4 decreased slightly but were even in normal ranges during the whole observation time (data not shown). The values of procalcitonin decreased significantly during the six-hour treatment (before: 6.9 (0.3–11.8); after 6 hours: 6.2 (0.3–9.4); ng/mL; p = 0.003) and between days 3 and 28 (all time points) compared with day 1 (before extracorporeal treatment, p < 0.05, data not shown). The expression per cell of HLA-DR on monocytes increased significantly from inclusion [6974 (5888–9119)] to day 4 [9278 (6394–12526)] and between inclusion and days 5 to 28 (all time points, p < 0.05, data not shown). Between day 1 (before extracorporeal treatment) and day 3 after extracorporeal treatment, the values of IL-6 and IL-8 increased; these changes, however, were not significant and the values normalized on day 8 (Table 3). The values of IL-10 and TNF-alpha were lower and decreased between day 1 and day 8 (Table 3).
Table 3.
Cytokines values before the first extracorporeal granulocyte therapy, after the second extracorporeal granulocyte therapy, and after 7 days (median/0.25–0.75 quartile).
| Cytokine | Before first extracorporeal therapy (day 1) (n = 10, pg/mL) | After second extracorporeal therapy (day 3) (n = 8, pg/mL) | After 7 days (day 8) (n = 7, pg/mL) |
|---|---|---|---|
| IL-6 | 57 (41–159) | 78 (45–154) | 28 (26–62)# |
| IL-8 | 40 (28–53) | 57 (38–118) | 30 (29–36) |
| TNF-alpha | 30 (22–40) | 22 (19–26) | 15 (13–17)∗# |
| IL-10 | 15 (8–20) | 11 (8–13) | 3 (3–8) |
∗Statistically significant (p = 0.028) compared to day 1 (before first extracorporeal treatment).
#Statistically significant (p < 0.05) compared to the end of second extracorporeal treatment.
IL: interleukin.
TNF: tumor necrosis factor.
3.4. Hemodynamic Parameters during Extracorporeal Treatment
The dosage of norepinephrine could be reduced significantly during the extracorporeal treatments (Figure 2). Additionally, during the extracorporeal treatments, the mean arterial pressure, the heart rate, the parameters of hemodynamic monitoring using the PiCCO-System, the central venous oxygen saturation, and values of lactate showed no significant changes; but the cardiac index and stroke volume index increased (Table 4). After the second extracorporeal treatments of patients, the use of norepinephrine could reduce continue in the course of the observation time (data not shown).
Figure 2.
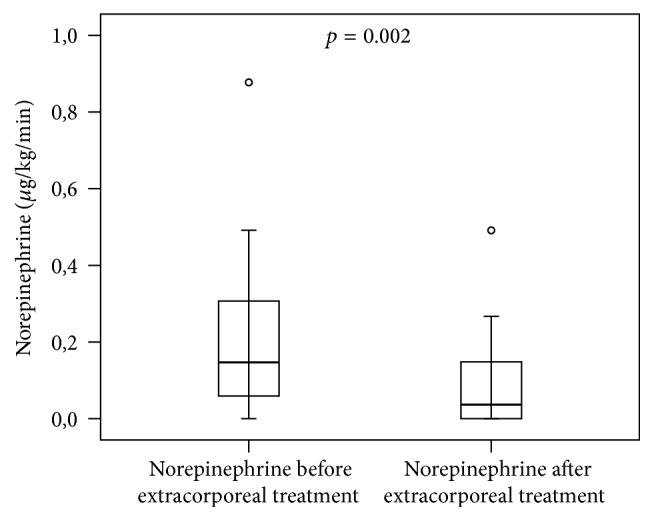
The dosage of norepinephrine (μg/kg/min) could be reduced significantly during the extracorporeal granulocyte treatments (Mann-Whitney U test; n = 10; median/0.25–0.75 quartile).
3.5. Liver Function Markers and Tests
The dynamic measurement of liver function used the LiMON-System (based on the indocyanine green plasma disappearance rate, ICG-PDR) and the ICG-PDR cardiac index ratio which both on day 1 showed significantly lower values in nonsurvivors than in survivors (Table 2). During the extracorporeal treatments, no significant increases of PDR-values and of ICG-PDR cardiac index ratio could be observed (Table 4). The PDR-values increased significantly (p = 0.027) between day 1 [11.9 (5.3–13.8)] and day 7 [23.3 (20.2–23.8)] only in surviving patients.
At inclusion, the values of bilirubin were significantly lower in survivors than in nonsurvivors (Table 2). In the course of disease, no significant differences of bilirubin-values were seen between the survivors and nonsurvivors and during extracorporeal treatments.
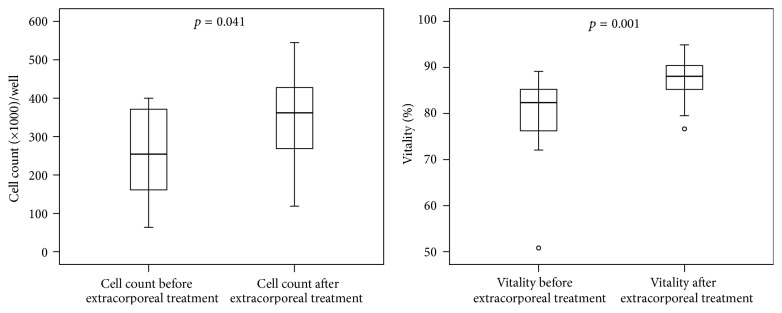
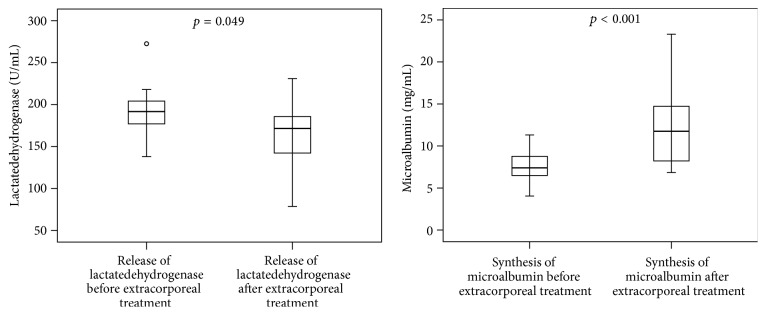
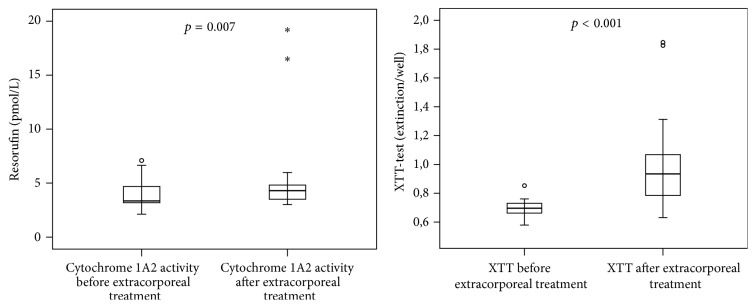
In the cell based analysis of hepatotoxicity (biosensor), the HepG2/C3A cells were incubated with plasma of the patients before and after each extracorporeal granulocyte treatment. The cell-count and vitality (Figure 3), the synthesis of microalbumin (Figure 4), and the activities of cytochrome 1A2 and mitochondrial dehydrogenases (XTT-test, Figure 5) increased significantly during extracorporeal treatment. In addition, the values of lactatedehydrogenase (LDH) were significantly lower after extracorporeal treatment than the values of LDH before extracorporeal treatment (Figure 4). Only in survivors were significant increases of all biosensor parameters observed between day 1 (before extracorporeal treatment) and day 3 after extracorporeal granulocyte treatment (p < 0.05, data not shown), with the exception of LDH (significant decrease in survivors).
Figure 3.
Cell-count and vitality (Trypan blue-staining) of HepG2/C3A cells (biosensor-test) incubated with plasma from septic patients before extracorporeal treatments compared to plasma from patients after extracorporeal granulocyte treatments (Mann-Whitney U test; n = 10; median/0.25–0.75 quartile).
Figure 4.
Release of lactatedehydrogenase and synthesis of microalbumin of HepG2/C3A cells (biosensor-test) incubated with plasma from septic patients before extracorporeal treatments compared to plasma from patients after extracorporeal granulocyte treatments (Mann-Whitney U test; n = 10; median/0.25–0.75 quartile).
Figure 5.
Activity of cytochrome 1A2 (metabolism of ethoxyresorufin to resorufin) and the XTT-test (dehydrogenases activity in the mitochondria) of HepG2/C3A cells (biosensor-test) incubated with plasma from septic patients before extracorporeal treatments compared to plasma from patients after extracorporeal granulocyte treatments (Mann-Whitney U test; n = 10; median/0.25–0.75 quartile). ∗Whisker.
4. Discussion
The therapy with the extracorporeal granulocyte bioreactor system led to a reduction of norepinephrine dosage; moreover, the liver cell function and the viability in the biosensor-test were improved. We showed as before in the first clinical study [25] a good compatibility of the system. No significant changes in coagulation markers or any hemorrhages and signs of hemolysis were observed.
The extracorporeal granulocyte therapy influenced the immune system of the treated patients. During the six-hour treatment, an increase in the number of leukocytes and a decrease of procalcitonin were observed in the sera of the patients. The expression per cell of HLA-DR on monocytes increased significantly from inclusion to day four; additionally, between day 1 (before extracorporeal treatment) and day 3 after extracorporeal treatment, the values of IL-6 and IL-8 increased slowly. Immunomodulation has been introduced as a supportive therapy to overcome immune system dysfunction and could show positive impact on survival of patients with severe sepsis in some studies [35] but failed in a number of other studies [36, 37]. Extracorporeal blood detoxification methods have also been suggested to successfully influence immune imbalances and subsequently clinical course and outcome of multiorgan failure and sepsis [8, 9, 38, 39], because immunosuppression seems to be the main course of mortality of patients with sepsis and multiple organ failure [40].
The transfusion of granulocyte preparations (GTx) failed to improve survival in sepsis and neutropenic patients [41, 42]. However, there is some indication that steroid- or G-CSF-stimulated high-yield granulocyte donations might result in better survival in severe infections associated with neutropenia and cancer [42, 43]. In order to deploy the beneficial features of neutrophils such as phagocytosis of cellular debris, antigenic material, or pathogens and at the same time to circumvent the possible damaging local effects of systemically transfused neutrophils, a bed-side bioreactor was built, which uses granulocytes in a strictly extracorporeal mode. The bioreactor-cells are retained in the extracorporeal system and discarded after the treatment.
The 28-day mortality was 40% in the studied patients, consisting of nine patients with septic shock and one patient with severe sepsis, and is comparable with the results of other studies [1, 2]. Patients 2 and 4 died after reduction of therapy on palliative care (on the very same day and during the observation time). During the first extracorporeal treatment, patient 6 died; an autopsy revealed an advanced and longer existing ischemia of the bowel. No conclusions about survival can be drawn based on this (uncontrolled) study. The aim of the study was to document the impact of the extracorporeal granulocyte treatment on the dosage of norepinephrine in patients and the liver function using extended hemodynamic monitoring, dynamic measurement of the liver function with the LiMON-System, and, additionally, cell based analysis of hepatotoxicity of patient's plasma. Both need of vasopressors and the occurrence of liver dysfunction or failure are well known to impair the prognosis of patients with severe sepsis [5–7, 44]. The general idea of this work was to postulate that a new therapy of severe sepsis, for example, extracorporeal treatments, should be proven for a positive influence of hemodynamics and the liver in the septic organism.
Under reduction of vasopressor administration, the mean arterial pressure was stable and the cardiac index and the stroke volume index increased slightly during treatments measured with a dynamic monitoring system (PiCCO, 27). These results are the second report of a decreasing dosage of norepinephrine in patients using this extracorporeal granulocyte treatment system [25].
In this study, different parameters of liver function and hepatotoxicity were measured. The static parameter bilirubin was significantly lower in survivors than in nonsurvivors at the beginning of the extracorporeal treatment. During the observation time of 28 days, however, no significant differences of bilirubin-values were seen between the survivors and nonsurvivors and during extracorporeal treatments. Bilirubin is known as a good prognostic parameter for liver dysfunction or failure. In contrast, for acute changes in liver function, bilirubin seems to be not a valuable parameter [45, 46].
For the measurement of acute impairment of the liver function, dynamic measurement with tracer-substances was introduced [28]. We used the LiMON-System based on the indocyanine green plasma disappearance rate (PDR) and calculated the ICG-PDR cardiac index ratio. In accordance with other studies [47, 48] we found significant lower values in nonsurvivors than in survivors on day 1 before extracorporeal treatment and an increase of PDR from day 1 to day 7 only in surviving patients. With regard to clinical characteristics, three patients of the nonsurvivor group developed a (sepsis-induced) liver failure in the course of the disease; therefore, the time between beginning of shock and beginning of extracorporeal treatment was 5.5 days. During the extracorporeal treatments, however, no significant increases of PDR-values or ICG-PDR cardiac index ratio were observed. The cause of this could be the low number of patients. Patients with markedly decreased PDR-values (<8%) did not respond well to the extracorporeal granulocyte treatment. The PDR of indocyanine green provides information for the assessment of mortality and morbidity in critically ill patients, in patients with severe sepsis, and in patients underlying cardiac surgery [47–50]. Limitations of the LiMON-System are the nonexistent discrimination between liver function and perfusion of the splanchnic tract and false data in cases of hyperdynamic sepsis and hyperbilirubinaemia [51, 52].
In the cell based analysis of hepatotoxicity (biosensor; 29, 30), the HepG2/C3A hepatocyte cell line was incubated with plasma of the patients before and after each extracorporeal granulocyte treatment. During the extracorporeal treatments, a significant increase of vitality and function of the test cells was seen. These results suggest a positive impact of the extracorporeal granulocyte treatment on the liver cell vitality and function measured in this indirect cytotoxicity test. The reasons for this are still not clear but may be a result of reduction of toxins and drugs and the influence of chemokines and cytokines of the patients by the use of the extracorporeal bioartificial therapy. These factors are known to impair the cell functions and viability in HepG2/C3A cells [53–56]. Proinflammatory cytokines, for instance, are known to cause a dysfunction of mitochondria [50], to downregulate albumin synthesis [54], and to diminish function of some P450 cytochromes like CYP 1A2, CYP 2E1, and CYP 7A1 [55, 56].
Using the cell based analysis of hepatotoxicity of patient's plasma, only in survivors were significant increases of all biosensor parameters observed between day 1 (before extracorporeal treatment) and day 3 after extracorporeal granulocyte treatment. These data suggest a prognostic property of the biosensor in patients with severe sepsis, similarly to the PDR of indocyanine green [47]. In a previous prospective clinical study, the hepatotoxicity of plasma from septic and nonseptic patients was tested with this biosensor [29]. We found that the plasma of patients with septic shock impaired cellular functions and viability of HepG2/C3A cells. These values of biosensor parameters were increased only in survivors compared to nonsurvivors in this study. Additionally, only in the septic shock group were negative correlations found between cytochrome 1A2 activity and synthesis of albumin with APACHE II and SOFA scores (correlation coefficient: <0.7, p < 0.05).
5. Conclusions
In summary, there are three significant findings based on the results of the present study: (a) extracorporeal granulocyte treatment led to immunomodulation and a reduction of norepinephrine-dosage in patients during extracorporeal therapy, (b) patients with markedly decreased indocyanine green PDR-values did not respond well to the extracorporeal granulocyte treatment and an increase of PDR was only seen in survivors, and (c) a positive impact on the viability and function of hepatocyte senor cells (hepatotoxicity test of patient's plasma) were seen during the extracorporeal treatments.
Further studies should focus on changes of the hemodynamic system and the liver function in septic patients because of the prognostic value of these organ systems. Additionally, studies investigating the role of extracorporeal cell-therapies for sepsis are encouraged and should also address the influence on the survival of patients with severe sepsis.
Acknowledgments
The authors want to thank Heike Potschka and Helga Weiss-Reining for valuable technical support and Volker Kiefel for his support (Department of Transfusion Medicine, Medical Faculty of the University of Rostock) in the delivery of the granulocyte donations. This study relied solely on financial resources of the University of Rostock and the Artcline GmbH, Rostock, Germany. Additionally, the authors thank the European Regional Development Fund (EFRE) and European Social Fund (ESF), Grant no. AU 09 046:ESF/IV-BM-B35-0005/12, for financial support.
Abbreviations
- ACT:
Activated clotting time
- ALT:
Alanine transaminase
- AST:
Aspartate transaminase
- APACHE:
Acute Physiology and Chronic Health Evaluation (Score)
- G-CSF:
Granulocyte-colony stimulating factor
- GTx:
Granulocyte transfusion
- HLA-DR:
Human leukocyte antigen DR
- IL:
Interleukin
- INR:
International Normalized Ratio
- MAP:
Mean arterial pressure
- MODS:
Multiple Organ Dysfunction Score
- PCT:
Procalcitonin
- PDR:
Plasma disappearance rate
- SAPS:
Simplified Acute Physiology Score
- SOFA:
Sequential Organ Failure Assessment (Score)
- TNF:
Tumor necrosis factor.
Competing Interests
Jens Altrichter and Steffen R. Mitzner have filed patents on the technology of extracorporeal cell perfusion technology and own shares of and work as part-time consultants for Artcline GmbH, a university spin off that now owns the patent rights. Martin Sauer, Thomas Wild, and Fanny Doß work as a part-time consultants for Artcline GmbH. Martin Sauer has filed patents on the technology of the hepatocyte based biosensor. Cristof Haubner, Annette Pertschy, Thomas Mencke, Maren Thomsen, Johannes Ehler, Jörg Henschel, Sandra Doß, Stephanie Koch, Georg Richter, and Gabriele Nöldge-Schomburg declare that they have no competing interests.
Authors' Contributions
Martin Sauer, Jens Altrichter, and Steffen R. Mitzner participated in the design of the study. Martin Sauer did the regulatory work and coordinated the preparation of the paper. Martin Sauer, Cristof Haubner and Georg Richter did the data analysis. Martin Sauer, Annette Pertschy, Jörg Henschel, Thomas Mencke, Maren Thomsen, Johannes Ehler, Georg Richter, Gabriele Nöldge-Schomburg, and Steffen R. Mitzner were clinical investigators of the study and performed the treatments. Fanny Doß, Sandra Doß, and Stephanie Koch analyzed the clinical probes from patients and samples from the extracorporeal circuit.
References
- 1.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Engel C., Brunkhorst F. M., Bone H.-G., et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Medicine. 2007;33(4):606–618. doi: 10.1007/s00134-006-0517-7. [DOI] [PubMed] [Google Scholar]
- 3.Schmid A., Burchardi H., Clouth J., Schneider H. Burden of illness imposed by severe sepsis in Germany. European Journal of Health Economics. 2002;3(2):77–82. doi: 10.1007/s10198-002-0095-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee H., Doig C. J., Ghali W. A., Donaldson C., Johnson D., Manns B. Detailed cost analysis of care for survivors of severe sepsis. Critical Care Medicine. 2004;32(4):981–985. doi: 10.1097/01.ccm.0000120053.98734.2c. [DOI] [PubMed] [Google Scholar]
- 5.Takala J. Should we target blood pressure in sepsis? Critical Care Medicine. 2010;38(10):S613–S619. doi: 10.1097/CCM.0b013e3181f2430c. [DOI] [PubMed] [Google Scholar]
- 6.Kramer L., Jordan B., Druml W., Bauer P., Metnitz P. G. H. Incidence and prognosis of early hepatic dysfunction in critically ill patients—a prospective multicenter study. Critical Care Medicine. 2007;35(4):1099–1104. doi: 10.1097/01.ccm.0000259462.97164.a0. [DOI] [PubMed] [Google Scholar]
- 7.Bakker J., Grover R., McLuckie A., et al. Administration of the nitric oxide synthase inhibitor NG-methyl- L-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of a randomized, double-blind, placebo-controlled multicenter study. Critical Care Medicine. 2004;32(1):1–12. doi: 10.1097/01.ccm.0000105118.66983.19. [DOI] [PubMed] [Google Scholar]
- 8.Rimmelé T., Kellum J. A. Clinical review: blood purification for sepsis. Critical Care. 2011;15(1, article 205) doi: 10.1186/cc9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla A. M. Extracorporeal therapy in sepsis: are we there yet. Kidney International. 2012;81(4):336–338. doi: 10.1038/ki.2011.375. [DOI] [PubMed] [Google Scholar]
- 10.Ellis A. J., Hughes R. D., Wendon J. A., et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24(6):1446–1451. doi: 10.1053/jhep.1996.v24.pm0008938179. [DOI] [PubMed] [Google Scholar]
- 11.Sauer I. M., Zeilinger K., Obermayer N., et al. Primary human liver cells as source for modular extracoporeal liver support-a preliminary report. International Journal of Artificial Organs. 2002;25(10):1001–1005. doi: 10.1177/039139880202501015. [DOI] [PubMed] [Google Scholar]
- 12.Humes H. D., Buffington D. A., Lou L., et al. Cell therapy with a tissue-engineered kidney reduces the multiple-organ consequences of septic shock. Critical Care Medicine. 2003;31(10):2421–2428. doi: 10.1097/01.CCM.0000089644.70597.C1. [DOI] [PubMed] [Google Scholar]
- 13.Humes H. D., Weitzel W. F., Bartlett R. H., Swaniker F. C., Paganini E. P. Renal cell therapy is associated with dynamic and individualized responses in patients with acute renal failure. Blood Purification. 2003;21(1):64–71. doi: 10.1159/000067864. [DOI] [PubMed] [Google Scholar]
- 14.Tumlin J., Wali R., Williams W., et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. Journal of the American Society of Nephrology. 2008;19(5):1034–1040. doi: 10.1681/asn.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitzner S. R., Freytag J., Sauer M., et al. Use of human preconditioned phagocytes for extracorporeal immune support: introduction of a concept. Therapeutic Apheresis. 2001;5(5):423–432. doi: 10.1046/j.1526-0968.2001.00378.x. [DOI] [PubMed] [Google Scholar]
- 16.Caille V., Chiche J.-D., Nciri N., et al. Histocompatibility leukocyte antigen-D related expression is specifically altered and predicts mortality in septic shock but not in other causes of shock. Shock. 2004;22(6):521–526. doi: 10.1097/01.shk.0000143410.63698.57. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann I., Hoelzl A., Schliephake F., et al. Polymorphonuclear leukocyte dysfunction syndrome in patients with increasing sepsis severity. Shock. 2006;26(3):254–261. doi: 10.1097/01.shk.0000223131.64512.7a. [DOI] [PubMed] [Google Scholar]
- 18.Pachot A., Monneret G., Brion A., et al. Messenger RNA expression of major histocompatibility complex class II genes in whole blood from septic shock patients. Critical Care Medicine. 2005;33(1):31–38. doi: 10.1097/01.ccm.0000150958.20209.a3. [DOI] [PubMed] [Google Scholar]
- 19.Tavares-Murta B. M., Zaparoli M., Ferreira R. B., et al. Failure of neutrophil chemotactic function in septic patients. Critical Care Medicine. 2002;30(5):1056–1061. doi: 10.1097/00003246-200205000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Keel M., Ungethüm U., Steckholzer U., et al. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90(9):3356–3363. [PubMed] [Google Scholar]
- 21.Egger G., Aigner R., Glasner A., Hofer H. P., Mitterhammer H., Zelzer S. Blood polymorphonuclear leukocyte migration as a predictive marker for infections in severe trauma: comparison with various inflammation parameters. Intensive Care Medicine. 2004;30(2):331–334. doi: 10.1007/s00134-003-2111-6. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann L., Novender U., Schroeder S., et al. Plasma levels of macrophage migration inhibitory factor are elevated in patients with severe sepsis. Intensive Care Medicine. 2001;27(8):1412–1415. doi: 10.1007/s001340101022. [DOI] [PubMed] [Google Scholar]
- 23.Wenisch C., Parschalk P., Hasenhündl M., Griesmacher A., Graninger W. Polymorphonuclear leucocyte dysregulation in patients with gram-negative septicaemia assessed by flow cytometry. European Journal of Clinical Investigation. 1995;25(6):418–424. doi: 10.1111/j.1365-2362.1995.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 24.Sauer M., Altrichter J., Kreutzer H. J., et al. Extracorporeal cell therapy with granulocytes in a pig model of Gram-positive sepsis. Critical Care Medicine. 2009;37(2):606–613. doi: 10.1097/CCM.0b013e318194aa77. [DOI] [PubMed] [Google Scholar]
- 25.Altrichter J., Sauer M., Kaftan K., et al. Extracorporeal cell therapy of septic shock patients with donor granulocytes: a pilot study. Critical Care. 2011;15(2, article R82) doi: 10.1186/cc10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauer M., Altrichter J., Kreutzer H.-J., et al. Safety evaluation for a cell-based immune support system in an ex vivo rat model of gram-negative sepsis. Therapeutic Apheresis and Dialysis. 2009;13(5):444–450. doi: 10.1111/j.1744-9987.2009.00764.x. [DOI] [PubMed] [Google Scholar]
- 27.Liton E., Morgan M. The PiCCO monitor: a review. Anaesthesia and Intensive Care. 2012;40(3):393–409. doi: 10.1177/0310057X1204000304. [DOI] [PubMed] [Google Scholar]
- 28.Sakka S. G. Assessing liver function. Current Opinion in Critical Care. 2007;13(2):207–214. doi: 10.1097/mcc.0b013e328012b268. [DOI] [PubMed] [Google Scholar]
- 29.Sauer M. The use of human hepatocytes to determine liver function and liver regeneration. PCT/EP 2007/001047; DE/2006 102006005526.
- 30.Sauer M., Haubner C., Mencke T., et al. Impaired cell functions of hepatocytes incubated with plasma of septic patients. Inflammation Research. 2012;61(6):609–616. doi: 10.1007/s00011-012-0451-9. [DOI] [PubMed] [Google Scholar]
- 31.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Critical Care Medicine. 1992;20:864–874. [PubMed] [Google Scholar]
- 32.Bernard G. R., Vincent J.-L., Laterre P.-F., et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. New England Journal of Medicine. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 33.Scudiero D. A., Shoemaker R. H., Paull K. D., et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Research. 1988;48(17):4827–4833. [PubMed] [Google Scholar]
- 34.Kelly J. H., Sussman N. L. A fluorescent cell-based assay for cytochrome P-450 isozyme 1A2 induction and inhibition. Journal of Biomolecular Screening. 2000;5(4):249–253. doi: 10.1177/108705710000500407. [DOI] [PubMed] [Google Scholar]
- 35.Kreymann K. G., de Heer G., Nierhaus A., Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Critical Care Medicine. 2007;35(12):2677–2685. doi: 10.1097/01.ccm.0000295263.12774.97. [DOI] [PubMed] [Google Scholar]
- 36.Bochud P.-Y., Calandra T. Science, medicine, and the future: pathogenesis of sepsis: new concepts and implications for future treatment. British Medical Journal. 2003;326(7383):262–266. doi: 10.1136/bmj.326.7383.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasraway S. A. The problems and challenges of immunotherapy in sepsis. Chest. 2003;123(5):451–459. doi: 10.1378/chest.123.5_suppl.451s. [DOI] [PubMed] [Google Scholar]
- 38.Ronco C., Inguaggiato P., D'Intini V., et al. The role of extracorporeal therapies in sepsis. Journal of Nephrology. 2003;16(supplement 7):S34–S41. [PubMed] [Google Scholar]
- 39.Bellomo R., Honoré P., Matson J., Ronco C., Winchester J. Extracorporeal blood treatment (EBT) methods in SIRS/Sepsis. International Journal of Artificial Organs. 2005;28(5):450–458. doi: 10.1177/039139880502800505. [DOI] [PubMed] [Google Scholar]
- 40.Boomer J. S., To K., Chang K. C., et al. Immunosuppression in patients who die of sepsis and multiple organ failure. The Journal of the American Medical Association. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohan P., Brocklehurst P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropaenia. Cochrane Database of Systematic Reviews. 2004;4 doi: 10.1002/14651858.CD003956.CD003956 [DOI] [PubMed] [Google Scholar]
- 42.Stanworth S. J., Massey E., Hyde C., et al. Granulocyte transfusions for treating infections in patients with neutropenia or neutrophil dysfunction. Cochrane Database of Systematic Reviews. 2005;(3) doi: 10.1002/14651858.CD005339.CD005339 [DOI] [PubMed] [Google Scholar]
- 43.Safdar A., Hanna H. A., Boktour M., et al. Impact of high-dose granulocyte transfusions in patients with cancer with candidemia: retrospective case-control analysis of 491 episodes of candida species bloodstream infections. Cancer. 2004;101(12):2859–2865. doi: 10.1002/cncr.20710. [DOI] [PubMed] [Google Scholar]
- 44.Dünser M. W., Ruokonen E., Pettilä V., et al. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Critical Care. 2009;13(6, article R181) doi: 10.1186/cc8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastor C. M., Suter P. M. Hepatic hemodynamics and cell functions in human and experimental sepsis. Anesthesia and Analgesia. 1999;89(2):344–352. doi: 10.1097/00000539-199908000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Moreno R., Vincent J.-L., Matos R., et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive Care Medicine. 1999;25(7):686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 47.Kimura S., Yoshioka T., Shibuya M., Sakano T., Tanaka R., Matsuyama S. Indocyanine green elimination rate detects hepatocellular dysfunction early in septic shock and correlates with survival. Critical Care Medicine. 2001;29(6):1159–1163. doi: 10.1097/00003246-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Sakka S. G., Reinhart K., Meier-Hellmann A. Prognostic value of the indocyanine green plasma disappearance rate in critically ill patients. Chest. 2002;122(5):1715–1720. doi: 10.1378/chest.122.5.1715. [DOI] [PubMed] [Google Scholar]
- 49.Weis F., Kilger E., Beiras-Fernandez A., et al. Indocyanine green clearance as an outcome prediction tool in cardiac surgery: a prospective study. Journal of Critical Care. 2014;29(2):224–229. doi: 10.1016/j.jcrc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Zoller B., Spanaus K., Gerster R., et al. ICG-liver test versus new biomarkers as prognostic markers for prolonged length of stay in critically ill patients—a prospective study of accuracy for prediction of length of stay in the ICU. Annals of Intensive Care. 2014;4, article 19 doi: 10.1186/s13613-014-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stehr A., Ploner F., Traeger K., et al. Plasma disappearance of indocyanine green: a marker for excretory liver function? Intensive Care Medicine. 2005;31(12):1719–1722. doi: 10.1007/s00134-005-2826-7. [DOI] [PubMed] [Google Scholar]
- 52.Halle B. M., Poulsen T. D., Pedersen H. P. Indocyanine green plasma disappearance rate as dynamic liver function test in critically ill patients. Acta Anaesthesiologica Scandinavica. 2014;58(10):1214–1219. doi: 10.1111/aas.12406. [DOI] [PubMed] [Google Scholar]
- 53.Regueira T., Lepper P. M., Brandt S., et al. Hypoxia inducible factor-1α induction by tumour necrosis factor-α, but not by toll-like receptor agonists, modulates cellular respiration in cultured human hepatocytes. Liver International. 2009;29(10):1582–1592. doi: 10.1111/j.1478-3231.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 54.El-Saadany M. A., Rawel H. M., Raila J., El-Dashloty M. S., Schweigert F. J. Antioxidants modulate the IL-6 induced inhibition of negative acute-phase protein secretion in HepG2 cells. Cell Biochemistry and Function. 2008;26(1):95–101. doi: 10.1002/cbf.1405. [DOI] [PubMed] [Google Scholar]
- 55.Nakai K., Tanaka H., Hanada K., et al. Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metabolism & Disposition. 2008;36(9):1786–1793. doi: 10.1124/dmd.107.020073. [DOI] [PubMed] [Google Scholar]
- 56.Li T., Jahan A., Chiang J. Y. L. Bile acids and cytokines inhibit the human cholesterol 7α-hydroxylase gene via the JNK/c-Jun pathway in human liver cells. Hepatology. 2006;43(6):1202–1210. doi: 10.1002/hep.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]