Abstract
The suprachiasmatic nucleus (SCN) regulates daily rhythms in physiology and behavior. Previous studies suggest a critical role for neurons expressing vasoactive intestinal peptide (VIP) in coordinating rhythmicity and synchronization in the SCN. Here we examined the firing properties of VIP-expressing SCN neurons in acute brain slices. Active and passive membrane properties were measured in VIP and in non-VIP neurons during the day and at night. Current-clamp recordings revealed that both VIP and non-VIP neurons were spontaneously active, with higher firing rates during the day than at night. Average firing frequencies, however, were higher in VIP neurons (3.1 ± 0.2 Hz, day and 2.4 ± 0.2 Hz, night) than in non-VIP neurons (1.8 ± 0.2 Hz, day and 0.9 ± 0.2 Hz, night), both day and night. The waveforms of individual action potentials in VIP and non-VIP neurons were also distinct. Action potential durations (APD50) were shorter in VIP neurons (3.6 ± 0.1 ms, day and 2.9 ± 0.1 ms, night) than in non-VIP neurons (4.4 ± 0.3 ms, day and 3.5 ± 0.2 ms, night) throughout the light-dark cycle. In addition, after hyper polarization (AHP) amplitudes were larger in VIP neurons (21 ± 0.8 mV, day and 24.9 ± 0.9 mV, night) than in non-VIP neurons (17.2 ± 1.1 mV, day and 20.5 ± 1.2 mV, night) during the day and at night. Furthermore, significant day/night differences were observed in APD50 and AHP amplitudes in both VIP and non-VIP SCN neurons, consistent with rhythmic changes in ionic conductances that contribute to shaping the firing properties of both cell types. The higher day and night firing rates of VIP neurons likely contribute to synchronizing electrical activity in the SCN.
Keywords: VIP neuropeptide, circadian rhythms, SCN, membrane excitability, rhythmic firing, action potential waveforms
The suprachiasmatic nucleus (SCN), a small, bilateral structure in the hypothalamus, contains a network of approximately 20,000 neurons that generate and synchronize circadian rhythms in physiology and behavior (Kalsbeek and Buijs, 2002; Colwell, 2011). In mammals, daily rhythms in the membrane properties of SCN neurons are observed. Input resistances and firing rates, for example, are reportedly higher during the day than at night (Inouye and Kawamura, 1979; Green and Gillette, 1982; Schaap et al., 1999; Pennartz et al., 2002). Although previous studies have shown that most SCN neurons can function as autonomous pacemakers (Welsh et al., 1995), the rhythmicity and synchrony of firing of isolated SCN neurons are reduced markedly compared to cells in an intact SCN network (Welsh et al., 1995; Herzog et al., 2004; Welsh et al., 2010), indicating that synaptic communication mediates the coherent, reliable, daily rhythms among SCN neurons. Previous studies have highlighted that between 60% and 90% of SCN neurons show circadian rhythms in electrical activity (Welsh et al., 1995; Herzog et al., 1997; Nakamura et al., 2002; Webb et al., 2009), including the vasopressinergic neurons (Schaap et al., 1999) but not the calbindin-positive neurons (Jobst and Allen, 2002). Because the intrinsic membrane properties of SCN neurons vary (Jiang et al., 1997; Pennartz et al., 1998; Kononenko and Dudek, 2004), we sought to characterize firing properties of identified SCN cell types.
We chose to focus on neurons that synthesize the neuropeptide, vasoactive intestinal peptide (VIP). Located primarily in the ventral-lateral region of the SCN, VIP neurons receive direct retinal input and project to other neurons throughout the SCN (Romijn et al., 1997; An et al., 2012). These neurons release VIP in response to light in vivo and in a circadian pattern in vitro (Shinohara et al., 1995; Shinohara et al., 1998; Francl et al., 2010). VIP mRNA and protein levels in the SCN have also been shown to vary with time of day depending on age and ambient lighting conditions (Okamoto et al., 1991; Takeuchi et al., 1992; Shinohara et al., 1993; Fukuhara et al., 1994; Okamura et al., 1995; Ban et al., 1997; Kawakami et al., 1997; Kunst et al., 2015). Studies conducted on animals in which either VIP or the VIP receptor (VPAC2) was genetically eliminated demonstrated that up to 70% of neurons in the SCN become arrhythmic and desynchronized (Aton et al., 2005; Brown et al., 2007). Co-culture and stimulation studies have placed VIP as the major agent for circadian synchrony in the SCN (Brown et al., 2005; Maywood et al., 2011; Pauls et al., 2014). In addition, exogenous application of VIP has been shown to shift and entrain daily rhythms in the SCN (Watanabe et al., 2000; Reed et al., 2001; Reed et al., 2002; An et al., 2011; Kudo et al., 2013). Taken together, these observations suggest that daily release of VIP from a subset of SCN neurons coordinates circadian rhythms in the SCN.
We tested the hypothesis that the firing properties of VIP neurons are distinct from those of other neurons in the SCN. To identify VIP-expressing neurons in acute SCN slices, we took advantage of a knock-in mouse that expresses a fluorescent reporter only in VIP neurons. Targeted recordings of VIP neurons revealed that VIP neurons, on average, have significantly higher firing rates during the day and at night than non-VIP neurons.
MATERIALS AND METHODS
All experiments were performed in accordance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Animal Studies Committee at Washington University approved all procedures involving animals.
VIP-tdTomato mice were generated by crossing VIP-ires-Cre knock-in mice (stock 010908; Jackson Laboratory, Bar Harbor, ME) (Taniguchi et al., 2011) with Rosa-CAG-LSL-tdTomato-WPRE reporter mice (stock 007908; Jackson Laboratory) (Madisen et al., 2010) and maintained on the C57BL/6N background for at least 3 generations. These animals were group-housed on either a standard 12:12-h light:dark (LD) schedule (lights on at 0700 h and off at 1900 h) or a reversed LD schedule (lights on at 1900 h and off at 0700 h) and were given access to food and water ad libitum. In both cases, the ambient temperature was 22 °C and the humidity was 50%. In addition, “nighttime” animals were maintained in the reversed LD schedule for at least 14 days prior to the preparation of acute SCN slices. The light intensity for the facility with the standard LD schedule was 333 lux, and the light intensity for the facility with the reversed LD schedule was 667 lux.
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Immunohistochemistry
Adult (10–12 weeks) VIP-tdTomato knock-in male mice were anesthetized with 1.25% Avertin (2,2,2-tri-bromoethanol and tert-amyl alcohol in 0.9% NaCl; 0.025 mL/g body weight) and were transcardially perfused with ice-cold phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were rapidly removed and placed in 30% sucrose for 2 days. Frozen coronal sections (30 μm) containing the SCN were cut on a cryostat and placed in a 12-well tissue culture dish. Following washing for 1 h in PBS at room temperature (RT), sections were incubated for 30 min at 37 °C in 3% Triton in PBS and subsequently blocked in PBS containing 10% bovine serum albumin (BSA) and 0.3% Triton for 1 h at RT. Sections were then incubated overnight at 4 °C in rabbit anti-VIP primary antibody (1/1000; Immunostar, Hudson, WI) in PBS with 2% BSA and 0.25% Triton. Following washing with PBS containing 0.25% Triton for 30 min at RT, sections were incubated for 2 h at RT in donkey anti-rabbit Alexa 488 secondary antibody (1/500; Jackson Immunoresearch, West Grove, PA) in PBS with 2% BSA and 0.25% Triton. Sections were washed again in PBS for 30 min, mounted, allowed to air dry overnight in the dark, and coverslipped with DABCO (1,4-Diazobicyclo-[2,2,2]-octane) mounting medium.
Fluorescence images were acquired on a Nikon A1 confocal microscope using the NIS Elements software (Nikon Instruments, Melville, NY). Z-stacks (8–10 μm), composed of 1024 × 1024 (1 μm) optical sections, were collected and projected onto a single optical slice. Cells expressing tdTomato and/or immunolabeled for VIP in every fourth section containing the SCN were counted (ImageJ; National Institutes of Health, Bethesda, MD). A total of 8 sections were used from 4 mice (2 sections/mouse). The numbers of tdTomato-expressing cells that were also VIP positive and, conversely, the number of VIP-positive neurons that express tdTomato were determined.
Preparation of Acute SCN Slices
Acute SCN slices were prepared from adult (4–12 weeks) male mice maintained in either a standard or a reversed 12:12-h LD cycle (Granados-Fuentes et al., 2012). Time referred to here is zeitgeber time (ZT); ZT0 corresponds to the time of lights on and ZT12 corresponds to lights off in the animal facility. Daytime slices were routinely prepared at ZT5 from mice maintained in the standard LD cycle, and nighttime slices were prepared at ZT15 from mice maintained in the reversed LD cycle.
For the preparation of (daytime) slices, brains were rapidly removed in the light from animals anesthetized with 1.25% Avertin (2,2,2-tribromoethanol and tert-amyl alcohol in 0.9% NaCl; 0.025 mL/g body weight) and placed in ice-cold cutting solution containing (in mM) the following: sucrose, 240; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; CaCl2, 0.5; and MgCl2 7, saturated with 95% O2/5% CO2. For the preparation of nighttime slices, mice in the reversed LD cycle were removed from their cages at ZT15 under infrared illumination to avoid exposure to visible light during the preparation of acute SCN slices. Animals were anesthetized with isoflorane and enucleated using a previously described procedure (Aton et al., 2004; Hattar et al., 2006). Briefly, following the induction of anesthesia and wiping the head with Betadine, gentle pressure was applied to the sides of the head. Using sterile scissors, a single cut was made through the ocular muscles and optic nerve and the eyes were rapidly removed. Cotton gauze was placed in the orbits to stop bleeding. Animals were allowed to recover from the anesthesia (for approximately 1 h) during the transport to the laboratory for the preparation of slices. At ZT16, these animals were anesthetized with 1.25% Avertin; brains were rapidly removed and placed in ice-cold cutting solution. An additional series of experiments was also completed in which animals in the normal LD cycle were anesthetized and enucleated (at ZT5) prior to transport to the laboratory for the preparation of SCN slices (at ZT6) and electrophysiological recordings.
For all experiments, coronal slices (300 μm) were cut on a Leica VT1000 S vibrating blade microtome (Leica Microsystems, Buffalo Groves, IL) and incubated in a holding chamber with oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM) the following: NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; CaCl2, 2; MgCl2, 1; and dextrose, 25 (~310 mosmol l−1), saturated with 95% O2/5% CO2, at room temperature (~25 °C) for at least 1 h before transfer to the recording chamber.
Electrophysiological Recordings
Whole-cell current-clamp recordings were obtained at ZT7-12 (daytime) or ZT19-24 (nighttime) from visually identified SCN neurons using a Nikon FN-S2N microscope equipped with differential interference contrast (DIC) optics with infrared illumination. Slices were perfused continuously with ACSF saturated with 95% O2/5% CO2 at room temperature (~25 °C). Recordings were obtained using glass pipettes (4–5 MΩ) filled with intracellular solution containing (in mM) the following: KMeSO4, 120; KCl, 20; HEPES, 10; EGTA, 0.2; NaCl, 8; Mg-ATP, 4; Tris-GTP, 0.3; and phosphocreatine, 14 (pH 7.3–7.5; ~300 mosmol l−1). Experiments were controlled and data were collected using a Multiclamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA) interfaced to a Dell personal computer with a Digidata 1332 and pCLAMP 10 (Molecular Devices). Tip potentials were zeroed before recordings were obtained. For each cell, a “loose patch” cell-attached recording was first obtained, and spontaneous activity was recorded for ~1 min. A gigaOhm seal (≥2 GΩ) was then formed, and the whole-cell configuration was established. Following compensation of whole-cell membrane capacitances and series resistances, spontaneous firing was again recorded for ~1 min. Access resistances of these cells were 15 to 23 MΩ. Data were not collected if the value significantly (20%) changed during the course of the experiment. Voltage signals were acquired at 100 kHz, filtered at 10 kHz, and stored for offline analysis. Input resistances (Rin) were determined by measuring the steady-state voltage changes produced by ±5 pA current injections from a hyperpolarized membrane potential. Action potential thresholds (APTs) were determined in each cell as the point during the upstroke (depolarizing phase) of the action potential at which the second derivative of the voltage was zero. After hyper polarization (AHP) amplitudes were measured in each cell as the difference between the APT for action potential generation and the most negative membrane potential. Action potential durations were measured at 50% repolarization (APD50). The interspike membrane potential was also measured in each cell to provide an estimate of the resting membrane potential. In some recordings (n = 52), designed to determine the resting membrane potentials of VIP and non-VIP SCN neurons directly, a second glass pipette containing tetrodotoxin (TTX, 0.1 μM in ACSF) was brought near (20–30 μm) the recorded cell, and the TTX-containing solution was applied with positive pressure for 10 sec; resting potentials were measured for 15 sec directly when the cell stopped firing. In separate voltage-clamp experiments, whole-cell membrane capacitances were determined from analyses of capacitive currents elicited by brief (25-msec) voltage steps (±20 mV) from the holding potential of −70 mV.
The mean whole-cell capacitances of VIP cells during the day (n = 17) and at night (n = 24) were 12.4 ± 0.9 pF and 12.6 ± 0.8 pF, respectively. In non-VIP cells, the mean whole-cell capacitance during the day (n = 25) was 12.5 ± 0.9 pF, and at night, the mean whole-cell capacitance was 10.4 ± 0.6 pF.
Data Analysis
Electrophysiological data (Table 1) were compiled and analyzed using ClampFit (version 10.2; Molecular Devices), Microsoft Excel (Microsoft Corp., Redmond, WA), Mini Analysis (version 6.0.7; Synaptosoft, Decatur, GA), Prism (version 6; GraphPad Software, La Jolla, CA), and OriginLab (version 9.1; Northampton, MA).
Table 1.
Resting and Active Membrane Properties of VIP and Non-VIP SCN Neurons.
| Firing Rate (Hz) | APD50 (ms) | AHP (mV) | AHP Duration (ms) | Rin (MΩ) | APT (mV) | Vr (mV) | |
|---|---|---|---|---|---|---|---|
| Day ZT7-12 | |||||||
| VIP | 3.1 ± 0.21,4 n = 41 |
3.6 ± 0.12,4 n = 41 |
21.9 ± 0.81,4 n = 41 |
135.3 ± 7.12 n = 41 |
1308 ± 1764 n = 20 |
−30.7 ± 0.9 n = 41 |
−40.4 ± 0.9 n = 16 |
| Non-VIP | 1.8 ± 0.21,5 n = 36 |
4.4 ± 0.32,5 n = 36 |
17.2 ± 1.11,5 n = 36 |
177.6 ± 11.22 n = 22 |
1350 ± 2085 n = 20 |
−29.9 ± 1.2 n = 36 |
−43.3 ± 1.5 n = 15 |
| Night ZT19-24 | |||||||
| VIP | 2.4 ± 0.23,4 n = 46 |
2.9 ± 0.23,4 n = 46 |
24.9 ± 1.13,4 n = 46 |
157.6 ± 9.9 n = 44 |
856 ± 564 n = 23 |
−30.2 ± 0.7 n = 46 |
−42.7 ± 0.9 n = 11 |
| Non-VIP | 0.9 ± 0.23,5 n = 34 |
3.5 ± 0.23,5 n = 26 |
20.5 ± 1.23,5 n = 26 |
179.8 ± 14.8 n = 20 |
703 ± 765 n = 22 |
−28.3 ± 0.9 n = 26 |
−45.5 ± 2.1 n = 10 |
n = number of cells; VIP = vasoactive intestinal peptide; APT = action potential threshold; APD50 = action potential duration at 50% repolarization; AHP = after hyper polarization amplitude; AHP duration = after hyper polarization duration; Rin = input resistance; Vr = resting membrane potential measured after TTX application.
Values in VIP and non-VIP neurons are significantly different during the day (1p < 0.001; 2p < 0.01) and at night (3p < 0.05).
Values in VIP neurons during the day and at night (4p < 0.05) are significantly different.
Values in non-VIP neurons during the day and at night (5p < 0.05) are significantly different. Values are means ± SEM.
Statistics
All data are presented as means ± standard error of the mean (SEM). All data were collected from 17 slices/animals for daytime recordings and from 13 slices/animals for nighttime recordings. Statistical analyses were performed using 1-way analysis of variance (ANOVA) with Newman-Kuels post hoc pairwise comparisons or 2-sample Kolmogorov-Smirnov test. All data were analyzed using GraphPad Prism software with the exception of the cumulative distribution plots, which were analyzed using OriginLab with the 2-sample Kolmogorov-Smirnov test. Statistical significance was set at p < 0.05; p values are reported in the text, Table 1, and figure legends.
RESULTS
Identification of VIP Neurons in the SCN
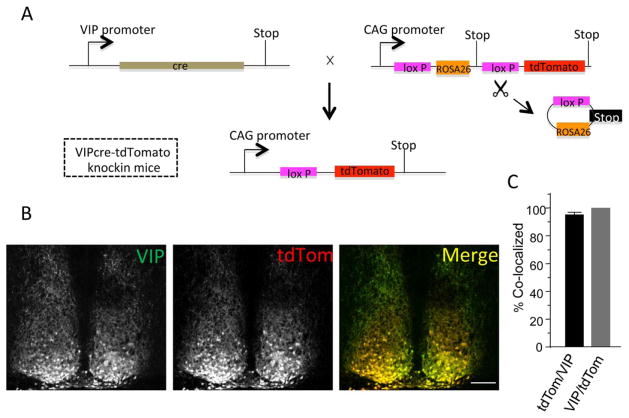
The strategy for identification of SCN neurons in vitro is illustrated in Figure 1A. A mouse line expressing the red fluorescent protein, tdTomato (tdTom), in VIP-expressing neurons of the SCN was generated by crossing mice (VIP-Cre) expressing Cre-recombinase driven by a knock-in fragment of the VIP promoter (Taniguchi et al., 2011) with mice containing the Rosa-CAG-LSL-tdTomato-WPRE conditional floxed allele (Madisen et al., 2010). Coronal sections containing the SCN were cut from the brains of the resulting VIP-tdTom knock-in mice and stained with an anti-VIP antibody. The specificity of the anti-VIP antibody was previously validated in SCN sections from mice (VIP−/−) harboring a targeted disruption of the VIP locus: no anti-VIP immunolabeling was evident in VIP−/− SCN sections with this antibody (An et al., 2012). VIP immunolabeling and tdTom fluorescence, however, were readily detected in a subpopulation of SCN neurons from VIP-tdTom knock-in mice (Figure 1B). We found that an average of 95% of the tdTom-expressing SCN neurons also immunolabeled for VIP. Conversely, we found that all VIP-immunolabeled SCN cells expressed tdTom (Figure 1C). The VIP-tdTom knock-in mouse, therefore, reliably identifies VIP neurons in the SCN.
Figure 1.
Generation and validation of tdTomato expression in vasoactive intestinal peptide (VIP) SCN neurons. (A) Schematic representation of the breeding strategy used to generate VIPcre-tdTomato knock-in mice: VIP-cre mice (Taniguchi et al., 2011) were bred with mice hemizygous for Rosa-CAG-LSL-tdTomato-WPRE (Madisen et al., 2010). In tissues expressing Cre-recombinase driven by the VIP promoter, the STOP cassette is deleted and the fluorescent tdTomato (tdTom) reporter protein is expressed. (B) Immunolabeling with an anti-VIP antibody in a representative coronal SCN section from a VIPcre-tdTomato knock-in mouse is shown. Robust VIP expression (left panel) is evident in cells that also express tdTom (middle panel). The merged image illustrates the overlap of VIP and tdTom expression (right panel); scale bar = 100 μm. (C) Cell counts revealed that nearly all of the tdTom-expressing SCN neurons were also labeled with the anti-VIP antibody (tdTom/VIP) and, in addition, that all VIP-positive neurons also express tdTom (VIP/tdTom). Values presented are means ± SEM of 1539 neurons counted in 8 sections from 4 animals.
Spontaneous Firing Properties of VIP and Non-VIP SCN Neurons Are Distinct
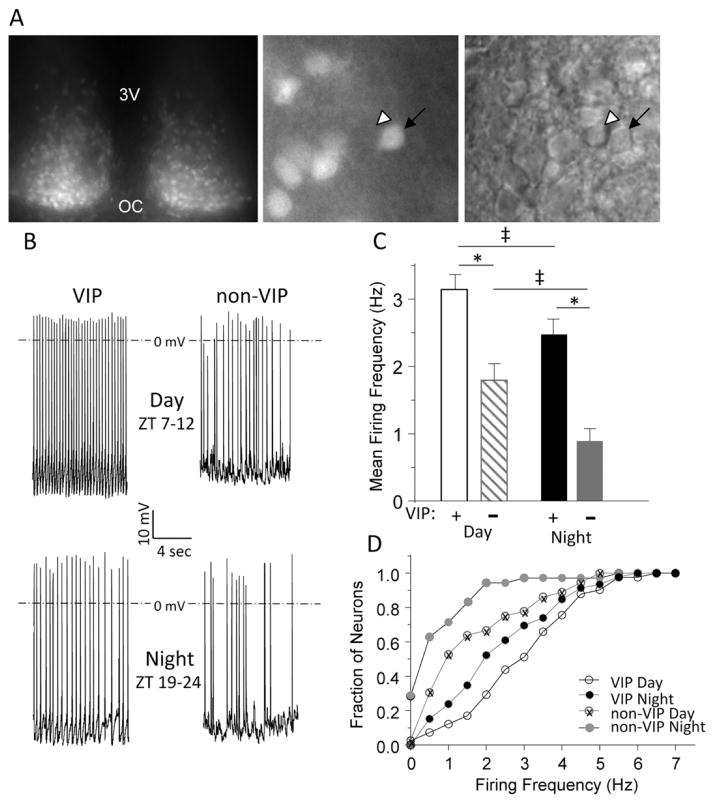
We visually identified VIP and non-VIP SCN neurons by the presence and absence of tdTom fluorescence, respectively (Figure 2A). In all experiments, cell-attached recording (Perkins, 2006) was obtained prior to establishing the whole-cell configuration, and spontaneous firing was recorded for 1 min. After a gigaOhm seal was formed and the whole-cell configuration was obtained, spontaneous firing was again recorded for ~1 min. Analysis of the records obtained in the cell-attached and the whole-cell configuration from the same cells revealed no significant (p > 0.05) differences in firing rates during the day (n = 77) or night (n = 80), validating the use of whole-cell recordings to quantify the resting and active membrane properties of VIP and non-VIP SCN neurons. Most (149 of 157; 95%) of the (VIP and non-VIP) SCN neurons examined were spontaneously active, and higher firing rates were observed during the day than at night (Figure 2B). In addition, average firing rates of both VIP and non-VIP neurons were significantly (p < 0.05) higher during the day than at night (Figure 2C and Table 1). We do note here that, while this manuscript was under review, Fan and colleagues reported no day-night differences in intrinsic membrane properties or repetitive firing rates in VIP neurons (Fan et al., 2015). It is unclear if these unexpected findings reflect differences in the strain, age, numbers of animals used, and/or in the variability of the whole-cell current clamp data.
Figure 2.
Spontaneous firing rates are higher in vasoactive intestinal peptide (VIP) than in non-VIP SCN neurons. (A) An acute SCN slice from a VIPcre-tdTomato knock-in mouse is shown. In the low-magnification fluorescence image on the left, tdTom expression is evident in both hemispheres; the third ventricle (3V) and optic chiasm (OC) are marked. In the higher magnification fluorescence image in the center, tdTom-positive neurons (arrow) are easily distinguished from nonlabeled neurons (arrowhead); the corresponding differential interference contrast (DIC) image is shown on the right. (B) Representative daytime (top) and nighttime (bottom) whole-cell current clamp recordings obtained from VIP and non-VIP neurons are shown; mean ± SEM firing frequencies are presented in (C). The mean ± SEM daytime firing rate is significantly (*p < 0.001, 1-way analysis of variance [ANOVA]) higher in VIP (n = 41) than in non-VIP (n = 36) SCN neurons. The mean ± SEM nighttime firing frequency is also significantly (*p < 0.001, one-way ANOVA) higher in VIP neurons (n = 46) than in non-VIP SCN neurons (n = 34). In addition, the mean ± SEM firing frequencies in both VIP and non-VIP SCN neurons are significantly (‡p < 0.05, 1-way ANOVA) higher during the day than at night. (D) Cumulative distribution plots illustrate that the firing frequencies of VIP SCN neurons are shifted significantly (*p < 0.001, 2-sample Kolmogorov-Smirnov test) to the right (toward higher frequencies) compared to non-VIP SCN neurons during the day and at night.
Day or night, VIP neurons had significantly (p < 0.001) higher mean firing rates than non-VIP neurons (Figure 2C and Table 1). Additional recordings from acute SCN slices prepared from daytime enucleated mice also revealed that the mean firing frequency was significantly (p < 0.001) higher in VIP (4.7 ± 0.3 Hz; n = 28) than in non-VIP (2.9 ± 0.3 Hz; n = 23) neurons.
Furthermore, whereas 90% of non-VIP neurons fired at 2 Hz or less at night, approximately 50% of VIP cells fired at similar frequencies (Figure 2D). We also found that, while 25% (8 of 34) of the non-VIP SCN neurons were electrically silent at night, none (0 of 46) of the VIP SCN neurons studied were quiescent at night (Figure 2D). This cell type–specific difference remained during the day when 65% of the non-VIP cells fired at 2 Hz or less, whereas only 30% of the VIP cells fired at 2 Hz or less (Figure 2D). Therefore, although daily rhythms in firing rates are clearly observed in both VIP and non-VIP neurons in acute SCN slices, on average, the firing rates of VIP neurons are higher than non-VIP neurons throughout the LD cycle.
Action Potential Waveforms in VIP and Non-VIP SCN Neurons Are Distinct
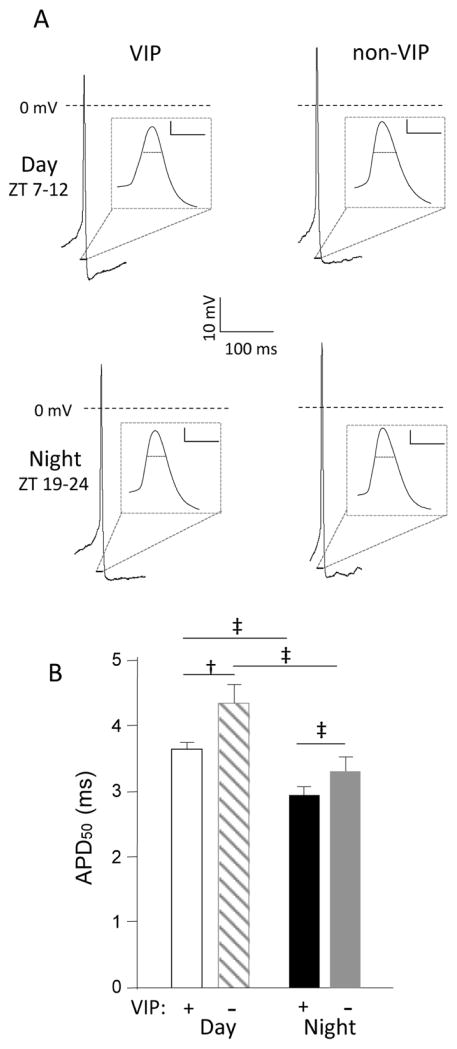
The waveforms of individual action potentials recorded in VIP and non-VIP SCN neurons, however, were distinct (Figures 3 and 4). During the day and at night, for example, action potentials were significantly (p < 0.05) briefer in VIP than in non-VIP SCN neurons (Figure 3A,B). In addition, the average APD50 was significantly (p < 0.05) shorter at night than during the day in both VIP and non-VIP neurons (Figure 3B and Table 1).
Figure 3.
Action potentials are briefer in vasoactive intestinal peptide (VIP) than in non-VIP SCN neurons. (A) Representative action potential waveforms recorded in VIP and non-VIP SCN neurons during the day (top) or at night (bottom) are illustrated; the records are shown on an expanded time scale in the insets (scale bars = 10 mV and 5 ms). (B) For daytime recordings, the mean ± SEM action potential duration (APD50) is significantly (‡p < 0.05, 1-way analysis of variance [ANOVA]) shorter in VIP SCN neurons (n = 41) than in non-VIP SCN neurons (n = 36). The mean ± SEM nighttime APD50 values in VIP (n = 46) and non-VIP (n = 26) neurons are significantly (‡p < 0.05, 1-way ANOVA) different. In addition, the mean ± SEM APD50 values determined in VIP and non-VIP SCN neurons during the day and at night are also significantly (‡p < 0.05, 1-way ANOVA) different (†p < 0.01, 1-way ANOVA).
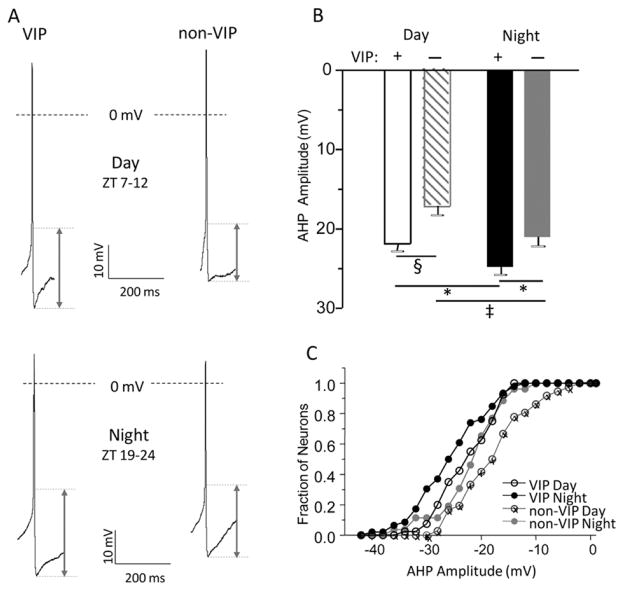
Figure 4.
After hyper polarization (AHP) amplitudes are larger in vasoactive intestinal peptide (VIP) than in non-VIP SCN neurons. (A) Representative action potential waveforms recorded in VIP and non-VIP SCN neurons during the day (top) and at night (bottom) are shown. AHP amplitudes were measured as the difference between the voltage threshold for action potential generation and the most negative membrane potential reached during the AHP. (B) The mean ± SEM AHP amplitudes measured in VIP SCN neurons (n = 36–41) during the day (*p < 0.001, 1-way analysis of variance [ANOVA]) or at night (‡p < 0.05, 1-way ANOVA) are significantly larger than in non-VIP (n = 26–46) SCN neurons. In addition, both VIP and non-VIP SCN neurons’ mean ± SEM AHP amplitudes measured at night were significantly (‡p < 0.05, 1-way ANOVA) larger than during the day. (C) The cumulative distribution plots of AHP amplitudes illustrate that the distribution of AHP amplitudes in VIP SCN neurons during the day is shifted significantly (p < 0.03, 2-sample Kolmogorov-Smirnov test) to the left compared to non-VIP SCN neurons during the day. Similarly, the distribution of AHP amplitudes in VIP SCN neurons at night is shifted (p < 0.07, 2-sample Kolmogorov-Smirnov test) to the left compared to non-VIP SCN neurons at night.
The average AHP amplitude measured in VIP neurons was significantly (p < 0.05) larger than in non-VIP neurons during the day and at night (Figure 4B and Table 1). Furthermore, the average AHP amplitudes measured in both VIP and non-VIP neurons were significantly (p < 0.05) larger at night than during the day (Figure 4B). Consequently, approximately 30% of the non-VIP neurons but ~60% of VIP neurons had AHP amplitudes greater than 20 mV during the day (p < 0.03; Figure 4C). At night, AHP amplitudes in ~60% of non-VIP neurons were greater than 20 mV, whereas AHP amplitudes greater than 20 mV were observed in ~75% of the VIP neurons (p < 0.05; Figure 4C). In addition, analysis of AHP durations in VIP and non-VIP revealed significantly (p < 0.002) shorter mean AHP duration in VIP neurons (135.3 ± 7.1 msec) compared to non-VIP neurons (177.6 ± 11.2 msec) during the day and revealed a trend at night (VIP neurons: 157.6 ± 9.9 msec and non-VIP neurons 179.8 ± 14.8 msec; Table 1).
Other parameters such as action potential thresholds (APTs) and resting membrane potentials (Vm) were not different in VIP and non-VIP neurons of the SCN either at night or during the day (Table 1). The mean input resistances (Rin) of VIP neurons and non-VIP neurons were significantly (p < 0.05) lower at night than during the day (Table 1) but did not differ in the 2 cell types.
DISCUSSION
The results presented here demonstrated that the repetitive firing properties of VIP-expressing neurons in the SCN differ from those of other SCN neurons throughout the LD cycle. Whereas both VIP and non-VIP neurons fire more during the day than at night, consistent with prior recordings from unidentified SCN neurons (Inouye and Kawamura, 1979; Green and Gillette, 1982; Welsh et al., 1995; Pennartz et al., 2002), the firing rates of VIP neurons were 40% higher during the day and at night than non-VIP neurons. In addition, all VIP neurons continued to fire repetitively at night, whereas only 75% of non-VIP neurons fired at night; 25% of the non-VIP neurons were electrically silent. These observations may reflect cellular heterogeneity in the population of SCN neurons classified here as “non-VIP” neurons, a hypothesis worth testing experimentally.
Intrinsic Membrane Properties of VIP SCN Neurons Are Distinct
Similar to results obtained in studies on unidentified SCN neurons (de Jeu et al., 1998; Kuhlman and McMahon, 2004, 2006; Belle et al., 2009), day-night differences in the input resistances (Rin) of VIP and non-VIP neurons were observed in the experiments presented here. In both groups of cells, Rin values were significantly higher during the day than at night, consistent with daily rhythmic changes in K+ conductance(s) (Kuhlman and McMahon, 2004, 2006). Comparisons of Rin values measured in VIP and non-VIP neurons during the day (as well as at night) revealed no significant differences, suggesting that both cell types undergo similar changes in the ionic conductance(s) mediating the daily change in firing frequencies observed.
In contrast to the similarities in Rin values, action potential waveforms in VIP and non-VIP neurons were distinct throughout the LD cycle. Action potentials recorded during the day in VIP neurons, for example, were briefer than non-VIP neurons. In addition, AHP amplitudes in VIP neurons were significantly larger than in non-VIP neurons during the day and at night. Furthermore, analysis of the mean AHP durations in VIP and non-VIP neurons revealed significantly shorter AHP durations in VIP neurons than in non-VIP neurons during the day. Shorter APD50, larger AHP amplitudes, and faster AHP durations are differences that likely contribute to the higher repetitive firing rates observed in VIP neurons. Taken together, these observations suggest that there are differences in the properties and/or the densities of the K+ currents that control the rates of action potential repolarization and AHP amplitudes in VIP compared with non-VIP SCN neurons.
As in many other types of GABAergic neurons in the central nervous system (Wang et al., 1998; Baranauskas et al., 2003), fast delayed rectifier K+ currents, encoded by voltage-gated K+ (Kv) channel pore-forming (α) submits of the Kv3 family, have been suggested to be important determinants of rapid action potential repolarization and the repetitive firing properties of SCN neurons (Itri, 2005; Kudo et al., 2011; Kudo et al., 2013). Although cell type–specific differences in the expression of Kv3-encoded fast delayed rectifier K+ currents could certainly underlie the observed differences in action potential waveforms and repetitive firing rates observed in VIP and non-VIP SCN neurons, other Kv- and/or Ca2+-dependent K+ currents (Cloues and Sather, 2003; Meredith et al., 2006; Belle et al., 2009) could also play important roles. Additional experiments, particularly voltage-clamp experiments focused on characterizing the K+ currents contributing to the control of action potential durations and the amplitudes of after hyper polarizations in VIP neurons and other identified cell types in the SCN, will be needed to provide further molecular insights.
Functional Implications of the Distinct Firing Properties of VIP SCN Neurons
Many studies have implicated VIP signaling in the modulation and synchronization of the rhythmic electrical activity of the SCN and behavior (Reed et al., 2001; Harmar et al., 2002; Cutler et al., 2003; Aton et al., 2005; Maywood et al., 2006; Brown et al., 2007). The findings here that the repetitive firing rates of VIP neurons are significantly higher than non-VIP neurons throughout the LD cycle are consistent with a model in which VIP-expressing neurons have a large influence on synchronizing electrical activity in the SCN. In this model, VIP-expressing neurons in the SCN play a role reminiscent of that ascribed to pigment dispersing factor (PDF)–expressing neurons in Drosophila (Renn et al., 1999). The PDF-expressing neurons have been shown to regulate the overall circadian period of Drosophila clock neurons (Stoleru et al., 2005), particularly clock neurons that express the canonical PDF receptor (Yao and Shafer, 2014). Additional experiments, in which the firing rates of VIP neurons are selectively controlled, are needed to test directly the hypothesis that VIP neurons can regulate the synchronization of electrical activity in the SCN.
Daily oscillations in the expression of the transcript encoding VIP, as well as in VIP protein expression and VIP release in the SCN, have been reported (Okamoto et al., 1991; Shinohara et al., 1993; Francl et al., 2010). Indeed, it has been shown that the peak expression of both VIP mRNA and protein occurs during the subjective night (Okamoto et al., 1991; Shinohara et al., 1993; Ban et al., 1997). VIP release, however, increases during the day (Francl et al., 2010), likely reflecting the daytime increase in the repetitive firing in VIP neurons. Interestingly, bath application of VIP alters the membrane and firing properties of SCN neurons for hours (Kudo et al., 2013), which may result in continued VIP release. Taken together, these observations suggest a model in which the increased daytime firing activity of VIPergic neurons leads to more VIP release, resulting in synchronization of electrical activity in the SCN. Further experiments focused on exploring this hypothesis directly will also be of interest.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- An S, Irwin RP, Allen CN, Tsai C, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105:2289–2296. doi: 10.1152/jn.00966.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Tsai C, Ronecker J, Bayly A, Herzog ED. Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus. J Comp Neurol. 2012;520:2730–2741. doi: 10.1002/cne.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Block GD, Tei H, Yamazaki S, Herzog ED. Plasticity of circadian behavior and the suprachiasmatic nucleus following exposure to non-24-hour light cycles. J Biol Rhythms. 2004;19:198–207. doi: 10.1177/0748730404264156. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban Y, Shigeyoshi Y, Okamura H. Development of vasoactive intestinal peptide mRNA rhythm in the rat suprachiasmatic nucleus. J Neurosci. 1997;17:3920–3931. doi: 10.1523/JNEUROSCI.17-10-03920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ. Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci. 2003;6:258–266. doi: 10.1038/nn1019. [DOI] [PubMed] [Google Scholar]
- Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science. 2009;326:281–284. doi: 10.1126/science.1169657. [DOI] [PubMed] [Google Scholar]
- Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Hughes AT, Piggins HD. Gastrin-releasing peptide promotes suprachiasmatic nuclei cellular rhythmicity in the absence of vasoactive intestinal polypeptide-VPAC2 receptor signaling. J Neurosci. 2005;25:11155–11164. doi: 10.1523/JNEUROSCI.3821-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. After hyper polarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci. 2011;12:553–569. doi: 10.1038/nrn3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. Eur J Neurosci. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport. 1998;9:3725–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- Fan J, Zeng H, Olson DP, Huber KM, Gibson JR, Takahashi JS. Vasoactive intestinal polypeptide (VIP)-expressing neurons in the suprachiasmatic nucleus provide sparse GABAergic outputs to local neuorns with circadian regulation occurring distal to the opening of postsynaptic GABAA iontropic receptors. J Neurosci. 2015;35:1905–1920. doi: 10.1523/JNEUROSCI.2661-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francl JM, Kaur G, Glass JD. Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. Neuroreport. 2010;21:1055–1059. doi: 10.1097/WNR.0b013e32833fcba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara C, Nishiwaki T, Cagampang FR, Inouye ST. Emergence of VIP rhythmicity following somatostatin depletion in the rat suprachiasmatic nucleus. Brain Res. 1994;645:343–346. doi: 10.1016/0006-8993(94)91671-3. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Norris AJ, Carrasquillo Y, Nerbonne JM, Herzog ED. I(A) channels encoded by Kv1.4 and Kv4.2 regulate neuronal firing in the suprachiasmatic nucleus and circadian rhythms in locomotor activity. J Neurosci. 2012;32:10045–10052. doi: 10.1523/JNEUROSCI.0174-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Geusz ME, Khalsa SB, Straume M, Block GD. Circadian rhythms in mouse suprachiasmatic nucleus explants on multi microelectrode plates. Brain Res. 1997;757:285–290. doi: 10.1016/s0006-8993(97)00337-5. [DOI] [PubMed] [Google Scholar]
- Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JNMS, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:7. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Yang Y, Liu ZP, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. J Physiol. 1997;499(Pt 1):141–159. doi: 10.1113/jphysiol.1997.sp021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst EE, Allen CN. Calbindin neurons in the hamster suprachiasmatic nucleus do not exhibit a circadian variation in spontaneous firing rate. Eur J Neurosci. 2002;16:2469–2474. doi: 10.1046/j.1460-9568.2002.02309.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Buijs RM. Output pathways of the mammalian suprachiasmatic nucleus: coding circadian time by transmitter selection and specific targeting. Cell Tissue Res. 2002;309:109–118. doi: 10.1007/s00441-002-0577-0. [DOI] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222:99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- Kononenko NI, Dudek FE. Mechanism of irregular firing of suprachiasmatic nucleus neurons in rat hypothalamic slices. J Neurophysiol. 2004;91:267–273. doi: 10.1152/jn.00314.2003. [DOI] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci. 2011;31:2746–2755. doi: 10.1523/JNEUROSCI.5792-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Tahara Y, Gamble KL, McMahon DG, Block GD, Colwell CS. Vasoactive intestinal peptide produces long-lasting changes in neural activity in the suprachiasmatic nucleus. J Neurophysiol. 2013;110:1097–1106. doi: 10.1152/jn.00114.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21:470–481. doi: 10.1177/0748730406294316. [DOI] [PubMed] [Google Scholar]
- Kunst M, Tso MC, Ghosh DD, Herzog ED, Nitabach MN. Rhythmic control of activity and sleep by class B1 GPCRs. Crit Rev Biochem Mol Biol. 2015;50:18–30. doi: 10.3109/10409238.2014.985815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci. 2002;5:399–400. doi: 10.1038/nn843. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Okamura H, Miyake M, Takahashi Y, Takagi S, Akagi Y, Fukui K, Okamoto H, Ibata Y. A diurnal variation of vasoactive intestinal peptide (VIP) mRNA under a daily light-dark cycle in the rat suprachiasmatic nucleus. Histochemistry. 1991;95:525–528. doi: 10.1007/BF00315750. [DOI] [PubMed] [Google Scholar]
- Okamura H, Kawakami F, Tamada Y, Geffard M, Nishiwaki T, Ibata Y, Inouye ST. Circadian change of VIP mRNA in the rat suprachiasmatic nucleus following p-chlorophenylalanine (PCPA) treatment in constant darkness. Brain Res Mol Brain Res. 1995;29:358–364. doi: 10.1016/0169-328x(94)00278-m. [DOI] [PubMed] [Google Scholar]
- Pauls S, Foley NC, Foley DK, LeSauter J, Hastings MH, Maywood ES, Silver R. Differential contributions of intra-cellular and inter-cellular mechanisms to the spatial and temporal architecture of the suprachiasmatic nucleus circadian circuitry in wild-type, crypto-chrome-null and vasoactive intestinal peptide receptor 2-null mutant mice. Eur J Neurosci. 2014;40:2528–2540. doi: 10.1111/ejn.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, de Jeu MT, Geurtsen AM, Sluiter AA, Hermes ML. Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus. J Physiol. 1998;506(Pt 3):775–793. doi: 10.1111/j.1469-7793.1998.775bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KL. Cell-attached voltage-clamp and current-clamp recording and stimulation techniques in brain slices. J Neurosci Methods. 2006;154:1–18. doi: 10.1016/j.jneumeth.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed HE, Cutler DJ, Brown TM, Brown J, Coen CW, Piggins HD. Effects of vasoactive intestinal polypeptide on neurones of the rat suprachiasmatic nuclei in vitro. J Neuroendocrinol. 2002;14:639–646. doi: 10.1046/j.1365-2826.2002.00826.x. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13:839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM. Evidence from confocal fluorescence microscopy for a dense, reciprocal innervation between AVP-, somatostatin-, VIP/PHI-, GRP-, and VIP/PHI/GRP-immunoreactive neurons in the rat suprachiasmatic nucleus. Eur J Neurosci. 1997;9:2613–2623. doi: 10.1111/j.1460-9568.1997.tb01691.x. [DOI] [PubMed] [Google Scholar]
- Schaap J, Bos NP, de Jeu MT, Geurtsen AM, Meijer JH, Pennartz CM. Neurons of the rat suprachiasmatic nucleus show a circadian rhythm in membrane properties that is lost during prolonged whole-cell recording. Brain Res. 1999;815:154–166. doi: 10.1016/s0006-8993(98)01025-7. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci U S A. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Inouye ST. Luminance-dependent decrease in vasoactive intestinal polypeptide in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;251:21–24. doi: 10.1016/s0304-3940(98)00491-1. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Takeuchi J, Nagasaki H, Shinohara K, Inouye ST. A circadian rhythm of somatostatin messenger RNA levels, but not of vasoactive intestinal polypeptide/peptide histidine isoleucine messenger RNA levels in rat suprachiasmatic nucleus. Mol Cell Neurosci. 1992;3:29–35. doi: 10.1016/1044-7431(92)90005-m. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol. 1998;509(Pt 1):183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 2000;877:361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc Natl Acad Sci U S A. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science. 2014;343:1516–1520. doi: 10.1126/science.1251285. [DOI] [PMC free article] [PubMed] [Google Scholar]