Abstract
Bone morphogenetic proteins (BMPs) constitute the largest subdivision of the TGF-β family of ligands and are unequivocally involved in regulating stem cell behavior. Appropriate regulation of canonical BMP signaling is critical for the development and homeostasis of numerous human organ systems, as aberrations in the BMP pathway or its regulation are increasingly associated with diverse human pathologies. In this review, we provide a wide-perspective on strategies that increase or decrease BMP signaling. We briefly outline the current FDA-approved approaches, highlight emerging next-generation technologies, and postulate prospective avenues for future investigation. We also detail how activating other pathways may indirectly modulate BMP signaling, with a particular emphasis on the relationship between the BMP and Activin/TGF-β pathways.
1. Introduction
Bone morphogenetic proteins (BMPs) constitute the largest subdivision of the TGF-β family of ligands. To date, approximately thirty distinct human proteins are named BMPs and some have additionally been assigned as Growth/Differentiation Factors (GDFs). However, important differences exist among these molecules with regard to pathway mechanics and effects on cellular behavior. This imprecise nomenclature can cause confusion when discussing BMP ligands and their role in human physiology or disease. Clarification may come, however, by focusing on the downstream pathway activated by each ligand rather than name alone. The intracellular effectors SMAD1/5/8 actuate the “bone morphogenetic protein” activity (i.e., autoinduction of bone at extraskeletal sites) originally described by Urist [1, 2]. Proteins that participate in the activation of SMAD1/5/8, then, are bona fide components of the canonical BMP signaling cascade. On this basis, it is possible to identify approximately thirteen bone fide BMP ligands in humans. Bona fide human bone morphogenetic proteins (BMPs) (less common alternative names are in parentheses) are as follows:
-
BMP2 (BMP2A, BDA2A).
-
BMP4 (BMP2B, BMP2B1, MCOPS6, OFC11, and ZYME).
-
BMP5.
-
BMP6 (VGR, VGR1).
-
BMP7 (OP-1).
-
BMP8A.
-
BMP8B (OP-2).
-
BMP9 (GDF2, HHT5).
-
BMP10.
-
BMP15 (GDF9B, ODG2, and POF4).
-
GDF5 (BMP14, OS5, LAP4, BDA1C, CDMP1, SYM1B, and SYNS2).
-
GDF6 (BMP13, KFM, KFS, KFS1, KFSL, SGM1, CDMP2, LCA17, MCOP4, SCDO4, and MCOPCB6).
-
GDF7 (BMP12).
It is this narrow definition of BMP signaling that we utilize in this review article.
Bone morphogenetic proteins (BMPs) are unequivocally involved in the modulation of several stem cell populations including embryonic stem cells (ESCs), induced pluripotent stem cells, intestinal stem cells, and mesenchymal stem cells (reviewed in [3–6]). For instance, in embryonic primordial germ cell differentiation, BMP signaling activates a transcriptional network and reexpression of the pluripotency markers Nanog and Sox2 [7]. Mouse ESCs also require dose dependent BMP pathway activation to maintain pluripotency [7]. Genetic inactivation studies demonstrate that Bmp7 is essential for the maintenance of nephron progenitor cells and its absence promotes premature arrest of nephrogenesis [8]. Additionally, complete removal of BMP signaling sends inactive hair follicle (HF) stem cells into premature proliferation while ectopic expression of BMP4 reduces HF induction and leads to baldness [9]. These findings support the idea that BMP signaling acts as a gatekeeper in stem cells preventing execution of differentiation programs; however other studies demonstrate that BMPs may also elicit the opposite effect. This is often accomplished in collaboration with other signaling pathways. For example, in human ESCs BMPs work in concert with FGF2 to drive mesendoderm differentiation into cardiac, hematopoietic, pancreatic, and liver lineages [10]. The same study suggests that cells derived from mouse ESCs further differentiate into hematopoietic mesoderm cells driven by cooperation between BMP, TGF-β, and Wnt signals [10]. And, BMP pathway activation is a potent activator of osteochondral differentiation in mesenchymal stem cells [11]. Thus, depending on the stem cell population in question, BMP signaling may act in a context-specific manner to either stimulate differentiation or promote maintenance of pluripotency.
This widespread yet context-dependent role of BMP signaling in modulating stem cell behavior requires appropriate regulation of BMP signaling for the development and homeostasis of numerous human organ systems [12]. Aberrations in the BMP pathway or its regulation are increasingly associated with diverse human pathologies (reviewed in [13–16]). Concomitant with this increased clinical significance, there is a growing need to develop effective strategies that modulate BMP signaling as a means of regulating stem cell populations. Tremendous gains have been made in recent years, but these exciting advances have often occurred within areas that may have been overlooked by nonspecialists. Thus, in this review we wish to provide a wide-perspective on the modulation of BMP signaling by paying particular attention to strategy rather than specific application per se, though numerous reported applications are noted in the main text and supplemental tables. We briefly outline the current FDA-approved approaches, highlight emerging technologies, and postulate prospective avenues for future investigation. We also detail how activating other pathways may indirectly modulate BMP signaling, with a particular emphasis on the relationship between the BMP and Activin/TGF-β pathways.
2. Strategies to Activate the BMP Pathway
In this section, we highlight several strategies to activate the BMP pathway. These different approaches are schematized in Figure 1.
Figure 1.
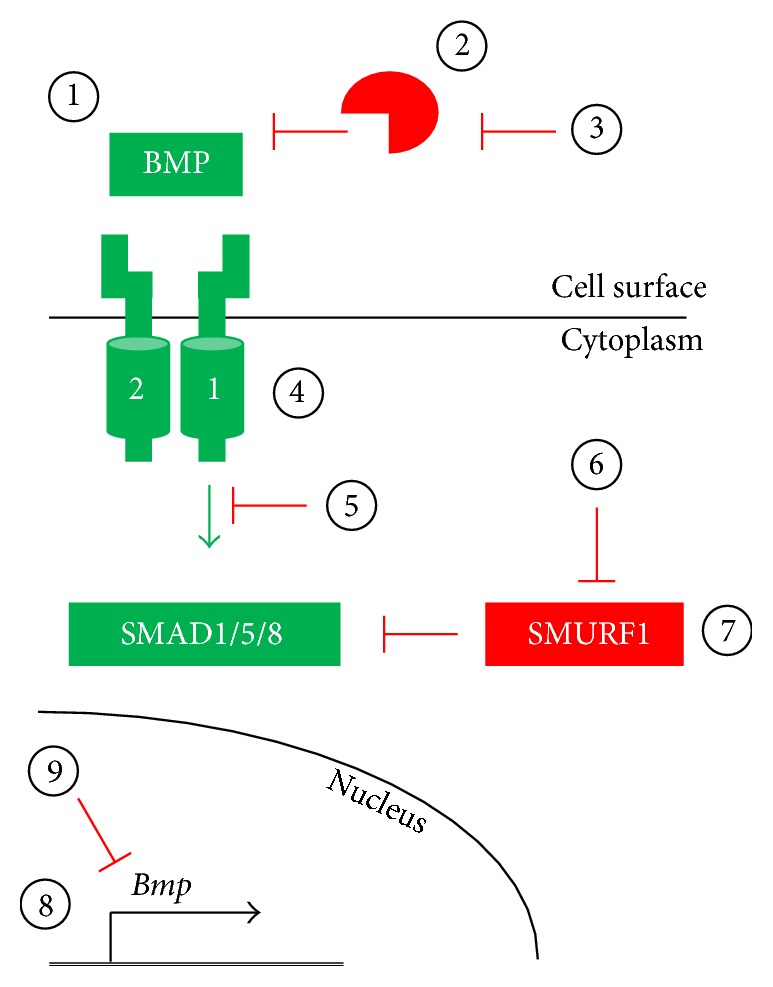
Potential strategies for modulating the BMP pathway. (1–3) The BMP pathway may be activated by exogenous natural or engineered BMP ligands or by expression of such ligands via gene transfer techniques (1). Ligand-induced BMP pathway activation may be inhibited by extracellular ligand traps, such as naturally-occurring antagonists or neutralizing antibodies, via delivery of recombinant protein or expression via gene transfer techniques (2). Endogenous extracellular BMP antagonists, such as Noggin or Chordin, may be inhibited via neutralizing antibodies or small molecules, resulting in increased BMP signaling (3). (4-5) The endogenous BMP pathway inhibitors FKBP12 and Casein Kinase 2 may be inactivated by delivery of FK506 and CK2.3, respectively, thereby increasing signal transduction (4). Alternatively, BMP receptor-mediated activation of the SMAD effectors may be blocked by kinase inhibitors (5). (6-7) Persistence of BMP signaling may be modulated by regulating the SMURF1-mediated ubiquitination of SMAD effector proteins by disrupting SMURF1 interaction with SMADs by small molecule inhibitors (6) or by increasing SMURF1 protein levels (7). (8-9) BMP pathway component expression may be elevated by increasing transcription or alleviating microRNA-mediated translational silencing (8). Alternatively, BMP pathway component levels may be reduced by reducing transcription and/or translation rates (9).
2.1. Natural and Engineered Ligands
The potential for clinical application of the BMP pathway was discovered decades prior to the identification of the BMP ligands [1, 2]. In these original reports, BMP activity liberated from the bone matrix was shown to promote ectopic bone formation. Several osteogenic proteins were then cloned, expressed as recombinant human proteins, and demonstrated to induce bone formation [17], heralding the potential for clinical applicability in orthopedics, which came to actualization in 2001 when recombinant human (rh) BMP7 (OP-1, Stryker) received a humanitarian device exemption (HDE) from the US FDA “for use as an alternative to autograft in recalcitrant long bone nonunions where use of autograft is unfeasible and alternative treatments have failed” (FDA). This was followed in 2002 when rhBMP2 (InFuse Bone Graft, Medtronic) received FDA medical device approval for use in anterior lumbar interbody fusion. The FDA subsequently approved rhBMP2 for use in several additional spine fusion approaches. rhBMP7 received a second HDE in 2004 for use in posterolateral lumbar fusion, and rhBMP2 received additional FDA approval for use in open tibial fractures in 2004 and oral-maxillofacial applications including sinus augmentation and localized alveolar ridge augmentation in 2007 (FDA). Several ongoing or upcoming clinical trials evaluate the usefulness of rhBMP2 and rhBMP7 in additional orthopedic/dental applications (https://clinicaltrials.gov/).
Recombinant BMPs have a high production cost for clinical use, which raises concern about their cost-effectiveness [18, 19]. As detailed in Table 1, this has prompted several groups to produce relatively short biomimetic peptides and/or to optimize BMP sequences for synthesis in E. coli [20–40]. Additionally, numerous studies have demonstrated the feasibility of a gene transfer approach for production of natural or engineered BMP ligands in vivo (Tables S1–S7 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/7290686). Several of these studies accomplished cell type specific and/or regulated BMP synthesis. One very interesting idea put forth involves ingesting bacteria that express BMPs for localized production in the gastrointestinal tract [41], which might be advantageous for treating conditions like inflammatory bowel disease (Table S7).
Table 1.
Examples of engineered BMP pathway activators.
| Category | Engineered version | Modification(s) | Reference(s) |
|---|---|---|---|
| BMP2-based | B2A (B2A2-K-NS) | BMP2-based peptide with heparin-binding domain that augments activity of BMP2 but has no signaling ability alone | [54–59, 151] |
| BMP2-L51P | BMP2 mutant that augments activity of BMP2 but has no signaling ability alone | [51–53] | |
| BMP2_108 | BMP2-based peptide; mimics activity of BMP2 | [20] | |
| mBMP | BMP2-based peptide with mineral-binding domain; mimics activity of BMP2 | [21] | |
| OPD | BMP2-based peptide; mimics or presumed to mimic activity of BMP2 | [22] | |
| P1 | BMP2-based peptide; mimics or presumed to mimic activity of BMP2 | [23] | |
| P2 | BMP2-based peptide; mimics or presumed to mimic activity of BMP2 | ||
| P24 | BMP2-based peptide; mimics or presumed to mimic activity of BMP2 | [24, 25] | |
| PEP7 | BMP2-based peptide; mimics or presumed to mimic activity of BMP2 | [26] | |
| Unnamed | BMP2-based peptide; mimics or presumed to mimic activity of BMP2 | [27–34] | |
|
| |||
| BMP2/Activin A chimerae | AB204 | Segmental-chimera of BMP2 and Activin A with enhanced activity over BMP2; Noggin resistant | [35, 42–46] |
| AB204-I103Y | Variant of AB204; enhanced activity over BMP2 and AB204 | [42] | |
| AB211 | Segmental-chimera of BMP2 and Activin A with enhanced activity over BMP2; Noggin resistant | [35] | |
| AB215 | Segmental-chimera of BMP2 and Activin A with enhanced activity over BMP2; Noggin resistant | [35, 47] | |
|
| |||
| BMP2/BMP9 chimera | BB29 | Segmental-chimera of BMP2 and BMP9 with enhanced folding when produced in E. coli | [35] |
|
| |||
| BMP6/BMP7 chimera | 80-1 | Segmental-chimera of BMP6 and BMP7 with reduced Noggin binding when compared to BMP7 | [48] |
|
| |||
| BMP7-based | BMP7-E60K | BMP6-informed mutant with reduced Noggin binding | [48] |
| THR-123 | BMP7-based peptide | [36] | |
| Unnamed | BMP7-based peptide; mimics activity of BMP7 | [27] | |
|
| |||
| BMP9-based | MB109 | BMP9-based peptide optimized for production in E. coli | [37] |
| pBMP9 | BMP9-based peptide with enhanced activity over BMP9 | [38–40] | |
| SpBMP9 | BMP9-based peptide with enhanced activity over BMP9 | [40] | |
| Unnamed | BMP9-based peptide; mimics activity of BMP9 | [27] | |
|
| |||
| GDF5-based | GDF5-S94N | Naturally-occurring mutant with enhanced activity due to decreased inhibition by Noggin | [48] |
| GDF5-N445K | Naturally-occurring mutant with enhanced activity due to decreased inhibition by Noggin | [49] | |
| GDF5-N445T | Naturally-occurring mutant with enhanced activity due to decreased inhibition by Noggin | [49, 50] | |
| GDF5-V453/V456 | BMP2-informed variant of GDF5; enhanced activity over GDF5 and BMP2 | [152, 153] | |
|
| |||
| Heterodimers | BMP2/6 | Heterodimer with enhanced activity over BMP2 and BMP6 | [60, 61] |
| BMP2/7 | Heterodimer with enhanced activity over BMP2 and BMP7 | [62–67] | |
| BMP4/7 | Heterodimer with enhanced activity over BMP4 and BMP7 | [68–70] | |
Part of the high cost of rhBMPs is related to the fact that large amounts of protein have been required for clinical use, leading multiple groups to engineer versions that have higher activity than the naturally-occurring ligand (Table 1). For instance, BMP2 chimerae containing segments from Activin A have been shown to be resistant to sequestration by the antagonist Noggin [35, 42–47], leading to greater signaling activity. Noggin-resistant versions of BMP7 and GDF5 bearing enhanced activity have also been described [48–50]. Other studies have utilized nonsignaling ligand decoys to neutralize Noggin [51–53] or potentiate receptor complex assembly [54–59]. In addition, heterodimeric ligands, such as BMP2/6, BMP2/7, and BMP4/7, have been designed to optimize receptor:ligand interactions and each of these display greater activity than the respective homodimer [60–70]. To the best of our knowledge, there are no ongoing clinical trials in humans with these second-generation ligands. One can envision combining the best features of these intelligently engineered molecules and/or production methods into an optimized BMP pathway activator best-suited for specific clinical uses.
2.2. Neutralizing Antibody and Small Molecule Approaches
BMP pathway activation is regulated by a large number of soluble antagonists [71]. Because these proteins operate in the extracellular space, they are attractive targets for strategies aimed at blocking their interaction with BMPs. The feasibility of this approach has been demonstrated by studies using neutralizing antibodies against Noggin or Gremlin in the contexts of pulmonary arterial hypertension (PAH) and spinal cord injury [72–74]. Additionally, the peptide CK2.3 reportedly disrupts the inhibitory interaction between Casein Kinase 2 and the BMP type 1 receptor BMPR1A [75]. Similarly, an in silico screen has identified several compounds that could bind to Noggin to disrupt its interaction with BMP ligands [76] and lead candidates have emerged from a screen for small molecules that potentially inhibit the E3 ubiquitin ligase SMURF1 by preventing its interaction with the BMP effectors SMAD1/5 and targeting them for degradation [77–79]. We are not aware of clinical trials of these antibodies or small molecules for increasing BMP signaling in vivo at present. The FDA-approved immunosuppressant tacrolimus (Astellas Pharma), which is also known as FK506, activates BMP signaling by inhibiting FKBP12 and is being tested in a clinical trial for the treatment of PAH (NCT01647945).
2.3. Regulation of Expression and/or Potentiating Activity
Enhancing the expression of BMP pathway components could serve as a means to increase signaling. Numerous stimuli have been reported to increase expression levels of BMP ligands or receptors (Table S8). Notably, several kinds of clinically relevant physical stimuli, such as pulsed electromagnetic fields, ultrasound, and mechanical loading, can positively modulate the BMP pathway at multiple levels [80–89]. Additionally, several FDA-approved drugs have been shown to regulate expression of BMP pathway components and/or potentiate BMP signaling. For instance, the statin drugs lovastatin and simvastatin increase BMP2 expression and signaling in several cell types and in vivo [90–95]. BMP2 expression and signaling are also increased by the Rho-kinase inhibitor fasudil [96, 97]. Pan-phosphodiesterase inhibition with pentoxifylline or selective inhibition with rolipram or sildenafil has been reported to potentiate BMP signaling as well [98–104].
Recent years have brought considerable attention to the role that microRNAs (miRNAs) play in gene expression, and several miRNAs have been implicated in negatively regulating the expression of BMP pathway components (Table 2 and Section 3). This opens the door, then, to an RNA interference strategy called “anti-miR” or “antagomiR” that targets miRNA and thereby alleviates translation repression. To date, a handful of studies have demonstrated the feasibility of anti-miRs to augment BMP pathway activity in vitro and in animal models (Table 2). This technology could prove useful as a means to increase expression of BMP pathway members, especially in scenarios where abnormal miRNA expression is involved in disease pathogenesis [105].
Table 2.
Examples of microRNAs targeting BMP pathway components and their inhibition via anti-miR RNA interference.
| miRNA | Target(s)/notes | Reference(s) | Anti-miR |
|---|---|---|---|
| miR-17-5p | Bmpr2, Smad7 | [154, 155] | NR |
| miR-20a | Bmpr2, Bambi, Crim1 | [154, 156] | [157] |
| miR-23b | Smad4, Smad5; also Smad3 | [158] | NR |
| miR-26a | Smad1, Smad4, Tob1 | [159–161] | [159, 160] |
| miR-27 | Acvr2a; also Tgfβr1 and Smad2 | [162] | NR |
| miR-30a/b/c/d | Bmp7, Smad1 | [163, 164] | [164] |
| miR-100 | Bmpr2 | [165] | NR |
| miR-122 | Hemojuvelin | [166] | [166] |
| miR-125 | Bmpr2 | [167] | [167] |
| miR-130a | Alk2 | [168] | NR |
| miR-135b | Bmpr2, Smad5; also Alk4 and Tgfβr2 | [169, 170] | NR |
| miR-140 | Bmp2 | [171] | NR |
| miR-145 | Undetermined (possibly Bmp4 indirectly) | [172] | NR |
| miR-148a | ALK2 | [173] | NR |
| miR-153 | Bmpr2 | [174] | NR |
| miR-155 | Smad1, Smad5 | [175, 176] | NR |
| miR-199a∗ | Smad1 | [177] | [177] |
| miR-200 | Bmp4, indirectly | [178] | NR |
| miR-205 | Smad1, Smad4 | [179] | NR |
| miR-302 | Bmpr2 | [180] | NR |
| miR542-3p | Bmp7 | [181] | NR |
NR: not reported.
3. Strategies to Inhibit the BMP Pathway
In this section, we will highlight several strategies to inhibit the BMP pathway. These different approaches are schematized in Figure 1.
3.1. Natural and Engineered Antagonists and Small Molecule Inhibitors
The fact that BMP ligands are present in the extracellular environment makes them vulnerable to sequestration upstream of receptor binding on target cells, and the extracellular antagonists Noggin, Gremlin, and Chordin might be used to regulate BMP signaling in this manner [71]. Numerous studies have exploited this relationship by administering recombinant BMP antagonists or delivering them via gene transfer (Tables S2, S4, and S6–S8). Once delivered, these antagonists typically sequester multiple BMP isoforms, which, depending on the specific application, may be advantageous or not. An alternative approach to enhance BMP:BMP antagonist interactions would be to employ soluble decoy receptors that comprise only the ligand binding domain of individual BMP receptors and, therefore, interact with ligands according to particular affinities (Table 3). An example of this kind of specificity can be observed with the soluble ALK1 (ALK1-ECD, Dalantercept, Acceleron Pharma), which is currently in clinical trials as a cancer therapy (NCT01458392, NCT01642082, NCT01720173, NCT01727336, and NCT02024087); ALK1-ECD preferentially sequesters BMP9 and BMP10 [106–111]. Greater specificity in ligand sequestration may also be achieved by using neutralizing antibodies raised against individual BMP ligands (Table 3). Investigators should be aware, however, that a high degree of homology exists between certain BMP ligands, such as BMP2 and BMP4 which are 92% identical, and this could make it challenging to specifically neutralize only one isoform when others are present. It is possible, also, that a specific BMP ligand could be inactivated via interaction with its prodomain [112] or via bespoke DNA aptamers [113].
Table 3.
Examples of BMP pathway modulation by receptor ECDs or neutralizing antibodies.
| Molecule | Reference(s) |
|---|---|
| ACVR2A-ECD | [182] |
| ACVR2B-ECD | [182, 183] |
| Anti-ALK1 Ab | [184] |
| ALK1-ECD | [106–110] |
| ALK3-ECD | [185–188] |
| Anti-BMP2 Ab | [189, 190] |
| Anti-BMP4 Ab | [190–192] |
| Anti-BMP6 Ab | [193–195] |
| Anti-BMP7 Ab | [196, 197] |
| Anti-BMP10 Ab | [111] |
| BMPR2-ECD | [198] |
| Dragon-ECD | [194] |
| Anti-gremlin Ab | [72] |
| Hemojuvelin-ECD | [193, 199, 200] |
| Anti-noggin Ab | [73, 74] |
Ab: antibody; ECD: extracellular domain.
BMP receptors are serine/threonine kinases, which makes them attractive targets for small molecules that block the kinase pocket and inhibit their activity. Considerable attention has been focused upon type 1 BMP receptors (ALK1/2/3/6) and the first kinase inhibitor reported was Dorsomorphin [114]. Though significant off-target effects are now noted for Dorsomorphin (Table 4), this molecule represents a key advancement in the field and has served as a guide for subsequent generations of analogues with greater specificity (Table 4). Some type 1 receptor selectivity has been reported among each of these compounds and it is conceivable that, in the near future, an investigator may be able to choose the most appropriate small molecule for a given application. For instance, activating mutations in ALK2 cause both fibrodysplasia ossificans progressiva (FOP) and pediatric intrinsic diffuse glioma (PIDG) [115–119]. Four candidate molecules, LDN-212854, LDN-214117, ML-347, and 1LWY, have recently been described as having dramatically enhanced selectivity for ALK2 (and the closely related ALK1) over the other type 1 receptors [120–123]; we are unaware of data directly comparing the in vivo efficacy of these four molecules head-to-head. Similarly, Tsugawa et al. concluded that differential type 1 receptor targeting underlies the finding that LDN-193189, DMH2, and VU5350 are effective in promoting liver regeneration in a rodent model while 1LWY is not [120].
Table 4.
Small molecule inhibitors of BMP Type 1 receptors and examples of their use.
| Molecule | Comment(s) | Reference(s) |
|---|---|---|
| 1LWY | Dramatically enhanced selectivity for ALK2 versus other type 1 BMP receptors (approximate order of selectivity: ALK2 > ALK3 > ALK6); greatly reduced off-target effects compared to DM and LDN | [120] |
|
| ||
| DMH1 | Pan-type 1 BMP receptor inhibitor (approximate order of selectivity: ALK3 > ALK1 > ALK6 > ALK2); reduced off-target effects compared to DM and LDN | [121, 122, 201–205] |
|
| ||
| DMH2 | Pan-type 1 BMP receptor inhibitor (approximate order selectivity: ALK6 > ALK3 > ALK2); notable off-target effects, including BMPR2, TGFBR2, ALK4, ALK5, AMPK, and VEGFR2 | [120, 201, 206] |
|
| ||
| DMH3 | Presumed pan-type 1 BMP receptor inhibitor; reduced off-target effects compared to DM and LDN | [201] |
|
| ||
| Dorsomorphin (DM) | Pan-type 1 BMP receptor inhibitor (approximate order of selectivity: ALK2 > ALK3 > ALK1 > ALK6); notable off-target effects, including BMPR2, ACVR2A, ACVR2B, TGFBR2, ALK5, AMPK, VEGFR2, and PDGFRβ | [114, 121, 122, 124, 201, 202, 207–215] |
|
| ||
| K02288 | Modestly enhanced selectivity for ALK1 and ALK2 versus other type 1 BMP receptors (approximate order of selectivity: ALK2 > ALK1 > ALK6 > ALK3); reduced off-target effects compared to DM and LDN | [121, 216, 217] |
|
| ||
| LDN-193189 (LDN) | Pan-type 1 BMP receptor inhibitor (approximate order of selectivity: ALK1~ALK2 > ALK3 > ALK6); notable off-target effects, including BMPR2, ACVR2A, ACVR2B, TGFBR2, ALK5, AMPK, VEGFR2, and PDGFRβ | [120–122, 124, 185, 191, 207–209, 216, 218–227] |
|
| ||
| LDN-212854 | Significantly enhanced selectivity for ALK1 and ALK2 versus other type 1 BMP receptors (approximate order of selectivity: ALK2 > ALK1 > ALK3); reduced off-target effects compared to DM and LDN | [121] |
|
| ||
| LDN-214117 | Dramatically enhanced selectivity for ALK2 versus other type 1 BMP receptors (approximate order of selectivity: ALK1, ALK2 > ALK3); greatly reduced off-target effects compared to DM and LDN | [123] |
|
| ||
| ML-347 | Dramatically enhanced selectivity for ALK1 and ALK2 versus other type 1 BMP receptors (approximate order of selectivity: ALK2 > ALK1 ≫ ALK3); reduced off-target effects compared to DM and LDN | [122, 228] |
|
| ||
| VU5350 | Pan-type 1 BMP receptor inhibitor (approximate order selectivity: ALK3 > ALK2 > ALK6); notable off-target effects, including BMPR2, TGFBR2, AMPK, and VEGFR2 | [120] |
It should be noted that some of these small molecules also target type 2 BMP receptors BMPR2, ACVR2A, and ACVR2B (Table 4), which might be advantageous in some experimental designs but could be problematic in others. And, given that ACVR2A and ACVR2B are also utilized by Activin and Activin-like ligands such as Myostatin, one must also keep in mind that Dorsomorphin and LDN-193189 can effectively block SMAD2/3 activation by these ligands [124].
3.2. Regulation of Expression
As mentioned in Section 2, several miRNAs have been shown to negatively regulate the expression of BMP pathway components (Table 2). In particular, translation of the BMP effector SMAD1 is repressed by at least four distinct miRNAs. And, some miRNAs, such as miR-155, target both SMAD1 and SMAD5. This raises the possibility that gene transfer of certain miRNA sequences singly or in combination could be useful as a means to impair effectors of the canonical BMP response. Proof of principle for this approach is found in several studies that utilized viral transduction or naked DNA delivery of miRNA to impact BMP signaling (Table 2). Similarly, knockdown of BMP pathway components as a means of reducing signaling in vivo has been accomplished by gene transfer in multiple scenarios and by various methods (Tables S2, S4, and S6). Notably, one emerging gene therapy strategy uses allele-specific RNA interference (ASP-RNAi) to selectively silence a single protein isoform, such as a constitutively active (ca) mutant [125]. Two separate groups have applied ASP-RNAi to the BMP pathway in vitro to knock down disease-causing caALK2 expression [126, 127]. This strategy is particularly amenable to FOP because the same point mutation underlies the vast majority of cases, thus enabling a single set of validated siRNAs to treat most patients [128]. ASP-RNAi could potentially be applied to disease-causing dominant negative mutations as well, such as those in BMPR2 that are found in some heritable PAH patients and are associated with earlier onset and more severe disease than nonexpressed mutants [129].
In comparison to stimuli that positively modulate the BMP pathway, relatively few agents have been described to reduce expression and/or pathway activity (Table S9). Notably, the FDA-approved antianginal drug perhexiline reduces BMP signaling in vitro and decreases ossification in an ectopic assay [130]. BMP inhibition is also observed with a retinoic acid receptor-gamma agonist and a clinical trial is currently underway to examine this approach in reducing heterotopic ossification among patients with classic FOP (https://clinicaltrials.gov/).
4. Indirect Modulation of BMP Pathway Activity via Activating Other Pathways
A large body of literature describes effects on the BMP pathway when other signaling pathways are targeted. Many of these studies were designed to augment BMP signaling, especially in orthopedic and dental applications (Table S1) though other scenarios have also been evaluated (Tables S2–S7) and several ways that the cellular or tissue microenvironment can be altered to be more permissive to BMP signaling have come to light. One example of this is the synergy observed when intermittent parathyroid hormone therapy is combined with BMP2 or BMP7 in bone healing [131, 132].
Relatively little is known about how activating a different pathway can antagonize the effects of BMP signaling in vivo. One significant exception to this is the wide range of contexts in which the Activin/TGFβ and BMP pathways elicit distinctly opposing effects on the same cell type. Some examples of this includes early body patterning [133], angiogenesis [134], cell fate of type 2 alveolar epithelial cells [135], maintenance of epithelial cell polarity [136], and regulation of skeletal muscle mass [137, 138]. Also, imbalances in the ratio of TGFβ superfamily cytokines are increasingly associated with human diseases, including pulmonary and kidney fibrosis [139, 140], glaucoma [141, 142], asthma [143], and pulmonary arterial hypertension [144, 145]. This raises the intriguing possibility that the effects of Activin/TGFβ pathway inhibition, for example, on skeletal muscle mass or bone volume, could in part be due to reducing antagonism of the BMP pathway. Support for this idea comes from the fact that increasing the BMP pathway can have similar effects to inhibiting TGFβ signaling (e.g., [146–148]). While the Activin/TGFβ receptor kinase inhibitor SB431542 has been reported to increase BMP signaling in preosteoblasts [149] and BMP target gene expression in chondrocytes [150], most studies have not evaluated how modulating the BMP pathway alters transduction of the Activin/TGFβ pathway, or vice versa, so the extent to which this bidirectional antagonism impacts development and disease is not presently known. That said, in general, all cell types examined to date have the capacity to respond to BMPs, Activins, and TGFβs and these molecules are often present in the extracellular environment at the same time. Thus, how cells integrate BMP versus Activin/TGFβ information and make specific decisions is an important area for future research.
5. Methods
Studies germane to this topic were identified in http://pubmed.com/ by combining the following search terms: antagonism; antagonist; bmp; bone morphogenetic protein; gene therapy; inhibition; inhibitor; siRNA. Articles retrieved were indexed to MEDLINE prior to January 6, 2016. Clinical trials were identified on https://clinicaltrials.gov/ and https://www.clinicaltrialsregister.eu/ prior to January 21, 2016. Specific applications highlighted are meant to be representative rather than exhaustive of the field and no endorsement by the authors of any particular application should be inferred.
Supplementary Material
Specific reports of BMP pathway modulation and related applications are provided in the supplemental material. Specific applications highlighted are meant to be representative rather than exhaustive of the field and no endorsement by the authors of any particular application should be inferred.
Competing Interests
The authors declare no competing interests.
References
- 1.Urist M. R. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Urist M. R., Strates B. S. Bone morphogenetic protein. Journal of Dental Research. 1971;50(6):1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 3.Itoh F., Watabe T., Miyazono K. Roles of TGF-β family signals in the fate determination of pluripotent stem cells. Seminars in Cell and Developmental Biology. 2014;32:98–106. doi: 10.1016/j.semcdb.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Fei T., Chen Y.-G. Regulation of embryonic stem cell self-renewal and differentiation by TGF-β family signaling. Science China Life Sciences. 2010;53(4):497–503. doi: 10.1007/s11427-010-0096-2. [DOI] [PubMed] [Google Scholar]
- 5.Scarfì S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World Journal of Stem Cells. 2016;8(1):1–12. doi: 10.4252/wjsc.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi Z., Chen Y.-G. Regulation of intestinal stem cell fate specification. Science China Life Sciences. 2015;58(6):570–578. doi: 10.1007/s11427-015-4859-7. [DOI] [PubMed] [Google Scholar]
- 7.Gunesdogan U., Magnusdottir E., Surani M. A. Primordial germ cell specification: a context-dependent cellular differentiation event. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369(1657) doi: 10.1098/rstb.2013.0543.20130543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oxburgh L., Brown A. C., Fetting J., Hill B. BMP signaling in the nephron progenitor niche. Pediatric Nephrology. 2011;26(9):1491–1497. doi: 10.1007/s00467-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rishikaysh P., Dev K., Diaz D., Shaikh Qureshi W. M., Filip S., Mokry J. Signaling involved in hair follicle morphogenesis and development. International Journal of Molecular Sciences. 2014;15(1):1647–1670. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z., Chen Y.-G. Functions of BMP signaling in embryonic stem cell fate determination. Experimental Cell Research. 2013;319(2):113–119. doi: 10.1016/j.yexcr.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Lowery J. W., Pazin D., Intini G., et al. The role of BMP2 signaling in the skeleton. Critical Reviews in Eukaryotic Gene Expression. 2011;21(2):177–185. doi: 10.1615/CritRevEukarGeneExpr.v21.i2.60. [DOI] [PubMed] [Google Scholar]
- 12.Wagner D. O., Sieber C., Bhushan R., Börgermann J. H., Graf D., Knaus P. BMPs: from bone to body morphogenetic proteins. Science Signaling. 2010;3(107, article mr1) doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- 13.Salazar V. S., Gamer L. W., Rosen V. BMP signalling in skeletal development, disease and repair. Nature Reviews Endocrinology. 2016;12(4):203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 14.Morrell N. W., Bloch D. B., Ten Dijke P., et al. Targeting BMP signalling in cardiovascular disease and anaemia. Nature Reviews Cardiology. 2016;13(2):106–120. doi: 10.1038/nrcardio.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowery J. W., de Caestecker M. P. BMP signaling in vascular development and disease. Cytokine and Growth Factor Reviews. 2010;21(4):287–298. doi: 10.1016/j.cytogfr.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandyopadhyay A., Yadav P. S., Prashar P. BMP signaling in development and diseases: a pharmacological perspective. Biochemical Pharmacology. 2013;85(7):857–864. doi: 10.1016/j.bcp.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Wozney J. M., Rosen V., Celeste A. J., et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 18.Garrison K. R., Donell S., Ryder J., et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technology Assessment. 2007;11(30):1–150. doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 19.Alt V., Heissel A. Economic considerations for the use of recombinant human bone morphogenetic protein-2 in open tibial fractures in Europe: the German model. Current Medical Research and Opinion. 2006;22(supplement 1):S19–S22. doi: 10.1185/030079906x80602. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y., Shuang Y., Fu H., et al. Characterization of a shorter recombinant polypeptide chain of bone morphogenetic protein 2 on osteoblast behaviour. BMC Oral Health. 2015;15(1, article 171) doi: 10.1186/s12903-015-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suárez-González D., Lee J. S., Diggs A., et al. Controlled multiple growth factor delivery from bone tissue engineering scaffolds via designed affinity. Tissue Engineering—Part A. 2014;20(15-16):2077–2087. doi: 10.1089/ten.tea.2013.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.-Y., Choo J.-E., Choi Y.-S., et al. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials. 2009;30(21):3532–3541. doi: 10.1016/j.biomaterials.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.-Y., Choo J.-E., Park H.-J., et al. Synthetic peptide-coated bone mineral for enhanced osteoblastic activation in vitro and in vivo. Journal of Biomedical Materials Research Part A. 2008;87(3):688–697. doi: 10.1002/jbm.a.31721. [DOI] [PubMed] [Google Scholar]
- 24.Tang S., Zhao J., Xu S., et al. Bone induction through controlled release of novel BMP-2-related peptide from PTMC11-F127-PTMC11 hydrogels. Biomedical Materials. 2012;7(1) doi: 10.1088/1748-6041/7/1/015008.015008 [DOI] [PubMed] [Google Scholar]
- 25.Lin Z.-Y., Duan Z.-X., Guo X.-D., et al. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. Journal of Controlled Release. 2010;144(2):190–195. doi: 10.1016/j.jconrel.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Kang E.-J., Kim S.-K., Eom T.-G., Choi K.-O., Lee T.-H. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. The International Journal of Oral & Maxillofacial Implants. 2013;28(3):749–756. doi: 10.11607/jomi.2825. [DOI] [PubMed] [Google Scholar]
- 27.Zouani O. F., Chollet C., Guillotin B., Durrieu M.-C. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials. 2010;31(32):8245–8253. doi: 10.1016/j.biomaterials.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 28.Seol Y.-J., Park Y.-J., Lee S.-C., et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. Journal of Biomedical Materials Research—Part A. 2006;77(3):599–607. doi: 10.1002/jbm.a.30639. [DOI] [PubMed] [Google Scholar]
- 29.Saito A., Suzuki Y., Ogata S.-I., Ohtsuki C., Tanihara M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochimica et Biophysica Acta. 2003;1651(1-2):60–67. doi: 10.1016/s1570-9639(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 30.He X., Ma J., Jabbari E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir. 2008;24(21):12508–12516. doi: 10.1021/la802447v. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y., Zhang Z., Liu Y., et al. Nanotubes functionalized with BMP2 knuckle peptide improve the osseointegration of titanium implants in Rabbits. Journal of Biomedical Nanotechnology. 2015;11(2):236–244. doi: 10.1166/jbn.2015.2006. [DOI] [PubMed] [Google Scholar]
- 32.Falcigno L., D'Auria G., Calvanese L., et al. Osteogenic properties of a short BMP-2 chimera peptide. Journal of Peptide Science. 2015;21(9):700–709. doi: 10.1002/psc.2793. [DOI] [PubMed] [Google Scholar]
- 33.Zhou X., Feng W., Qiu K., et al. BMP-2 derived peptide and dexamethasone incorporated mesoporous silica nanoparticles for enhanced osteogenic differentiation of bone mesenchymal stem cells. ACS Applied Materials and Interfaces. 2015;7(29):15777–15789. doi: 10.1021/acsami.5b02636. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z., Tang Y., Kang T., et al. Synergistic effect of HA and BMP-2 mimicking peptide on the bioactivity of HA/PMMA bone cement. Colloids and Surfaces B: Biointerfaces. 2015;131:39–46. doi: 10.1016/j.colsurfb.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Allendorph G. P., Read J. D., Kawakami Y., Kelber J. A., Isaacs M. J., Choe S. Designer TGFβ superfamily ligands with diversified functionality. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0026402.e26402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto H., LeBleu V. S., Bosukonda D., et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nature Medicine. 2012;18(3):396–404. doi: 10.1038/nm.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo M. M.-C., Nguyen P. H., Jeon Y.-H., Kim S., Yoon S.-M., Choe S. MB109 as bioactive human bone morphogenetic protein-9 refolded and purified from E. coli inclusion bodies. Microbial Cell Factories. 2014;13(1, article 29) doi: 10.1186/1475-2859-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauzon M.-A., Marcos B., Faucheux N. Effect of initial pBMP-9 loading and collagen concentration on the kinetics of peptide release and a mathematical model of the delivery system. Journal of Controlled Release. 2014;182(1):73–82. doi: 10.1016/j.jconrel.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Bergeron E., Senta H., Mailloux A., Park H., Lord E., Faucheux N. Murine preosteoblast differentiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Engineering—Part A. 2009;15(11):3341–3349. doi: 10.1089/ten.tea.2009.0189. [DOI] [PubMed] [Google Scholar]
- 40.Beauvais S., Drevelle O., Lauzon M.-A., Daviau A., Faucheux N. Modulation of MAPK signalling by immobilized adhesive peptides: effect on stem cell response to BMP-9-derived peptides. Acta Biomaterialia. 2016;31:241–251. doi: 10.1016/j.actbio.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Yuvaraj S., Al-Lahham S. H., Somasundaram R., Figaroa P. A., Peppelenbosch M. P., Bos N. A. E. coli-produced BMP-2 as a chemopreventive strategy for colon cancer: a proof-of-concept study. Gastroenterology Research and Practice. 2012;2012:6. doi: 10.1155/2012/895462.895462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon B.-H., Esquivies L., Ahn C., et al. An activin A/BMP2 chimera, AB204, displays bone-healing properties superior to those of BMP2. Journal of Bone and Mineral Research. 2014;29(9):1950–1959. doi: 10.1002/jbmr.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn C., Maslennikov I., Choi J. Y., Oh H., Cheong C., Choe S. Characterization of activin/BMP2 chimera, AB204, formulated for preclinical studies. Protein and Peptide Letters. 2014;21(5):426–433. doi: 10.2174/092986652105140218110302. [DOI] [PubMed] [Google Scholar]
- 44.Yoon B.-H., Lee J. H., Na K., et al. The effects of a single intravenous injection of novel activin A/BMP-2 (AB204) on toxicity and the respiratory and central nervous systems. Drug and Chemical Toxicology. 2015;39(3):284–289. doi: 10.3109/01480545.2015.1092548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon B.-H., Lee J. H., Na K., Cho J., Choe S. The toxicological evaluation of repetitive 2- and 4-week intravenous injection of Activin A/BMP-2 chimera (AB204) into rats. Regulatory Toxicology and Pharmacology. 2015;73(1):1–8. doi: 10.1016/j.yrtph.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Kim M., Kim J. I., Kim J. B., Choe S. The activin-βA/BMP-2 chimera AB204 is a strong stimulator of adipogenesis. Journal of Tissue Engineering and Regenerative Medicine. 2015 doi: 10.1002/term.2050. [DOI] [PubMed] [Google Scholar]
- 47.Jung J. W., Ahn C., Shim S. Y., Gray P. C., Kwiatkowski W., Choe S. Regulation of FSHβ induction in LβT2 cells by BMP2 and an Activin A/BMP2 chimera, AB215. Journal of Endocrinology. 2014;223(1):35–45. doi: 10.1530/joe-14-0317. [DOI] [PubMed] [Google Scholar]
- 48.Schwaerzer G. K., Hiepen C., Schrewe H., et al. New insights into the molecular mechanism of multiple synostoses syndrome (SYNS): mutation within the GDF5 knuckle epitope causes noggin-resistance. Journal of Bone and Mineral Research. 2012;27(2):429–442. doi: 10.1002/jbmr.532. [DOI] [PubMed] [Google Scholar]
- 49.Seemann P., Brehm A., König J., et al. Mutations in GDF5 reveal a key residue mediating BMP inhibition by NOGGIN. PLoS Genetics. 2009;5(11) doi: 10.1371/journal.pgen.1000747.e1000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Degenkolbe E., Schwarz C., Ott C.-E., et al. Improved bone defect healing by a superagonistic GDF5 variant derived from a patient with multiple synostoses syndrome. Bone. 2015;73:111–119. doi: 10.1016/j.bone.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 51.Sebald H.-J., Klenke F. M., Siegrist M., Albers C. E., Sebald W., Hofstetter W. Inhibition of endogenous antagonists with an engineered BMP-2 variant increases BMP-2 efficacy in rat femoral defect healing. Acta Biomaterialia. 2012;8(10):3816–3820. doi: 10.1016/j.actbio.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 52.Albers C. E., Hofstetter W., Sebald H.-J., Sebald W., Siebenrock K. A., Klenke F. M. L51P—a BMP2 variant with osteoinductive activity via inhibition of Noggin. Bone. 2012;51(3):401–406. doi: 10.1016/j.bone.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 53.Khattab H. M., Ono M., Sonoyama W., et al. The BMP2 antagonist inhibitor L51P enhances the osteogenic potential of BMP2 by simultaneous and delayed synergism. Bone. 2014;69:165–173. doi: 10.1016/j.bone.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Lin X., Elliot J., Carnes D., et al. Augmentation of osseous phenotypes in vivo with a synthetic peptide. Journal of Orthopaedic Research. 2007;25(4):531–539. doi: 10.1002/jor.20303. [DOI] [PubMed] [Google Scholar]
- 55.Lin X., Guo H., Takahashi K., Liu Y., Zamora P. O. B2A as a positive BMP receptor modulator. Growth Factors. 2012;30(3):149–157. doi: 10.3109/08977194.2012.671310. [DOI] [PubMed] [Google Scholar]
- 56.Liu Y., Lin X., Takahashi K., Zamora P. O. B2A, a receptor modulator, increases the growth of pluripotent and preosteoblast cells through bone morphogenetic protein receptors. Growth Factors. 2012;30(6):410–417. doi: 10.3109/08977194.2012.745520. [DOI] [PubMed] [Google Scholar]
- 57.Smucker J. D., Bobst J. A., Petersen E. B., Nepola J. V., Fredericks D. C. B2A peptide on ceramic granules enhance posterolateral spinal fusion in rabbits compared with autograft. Spine. 2008;33(12):1324–1329. doi: 10.1097/BRS.0b013e3181732a74. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham B. W., Atkinson B. L., Hu N., et al. Ceramic granules enhanced with B2A peptide for lumbar interbody spine fusion: an experimental study using an instrumented model in sheep: laboratory investigation. Journal of Neurosurgery: Spine. 2009;10(4):300–307. doi: 10.3171/2009.1.spine08565. [DOI] [PubMed] [Google Scholar]
- 59.Lin X., Zamora P. O., Albright S., Glass J. D., Peña L. A. Multidomain synthetic peptide B2A2 synergistically enhances BMP-2 in vitro. Journal of Bone and Mineral Research. 2005;20(4):693–703. doi: 10.1359/jbmr.041104. [DOI] [PubMed] [Google Scholar]
- 60.Valera E., Isaacs M. J., Kawakami Y., Belmonte J. C. I., Choe S. BMP-2/6 heterodimer is more effective than BMP-2 or BMP-6 homodimers as inductor of differentiation of human embryonic stem cells. PLoS ONE. 2010;5(6) doi: 10.1371/journal.pone.0011167.e11167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isaacs M. J., Kawakami Y., Allendorph G. P., Yoon B.-H., Izpisua Belmonte J. C., Choe S. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Molecular Endocrinology. 2010;24(7):1469–1477. doi: 10.1210/me.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buijs J. T., Van Der Horst G., Van Den Hoogen C., et al. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene. 2012;31(17):2164–2174. doi: 10.1038/onc.2011.400. [DOI] [PubMed] [Google Scholar]
- 63.Bi W., Gu Z., Zheng Y., Zhang X., Guo J., Wu G. Heterodimeric BMP-2/7 antagonizes the inhibition of all-trans retinoic acid and promotes the osteoblastogenesis. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0078198.e78198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng Y., Wang L., Zhang X., Zhang X., Gu Z., Wu G. BMP2/7 heterodimer can modulate all cellular events of the in vitro RANKL-mediated osteoclastogenesis, respectively, in different dose patterns. Tissue Engineering Part A. 2012;18(5-6):621–627. doi: 10.1089/ten.tea.2011.0366. [DOI] [PubMed] [Google Scholar]
- 65.Xu J., Li X., Lian J. B., Ayers D. C., Song J. Sustained and localized in vitro release of BMP-2/7, RANKL, and tetracycline from FlexBone, an elastomeric osteoconductive bone substitute. Journal of Orthopaedic Research. 2009;27(10):1306–1311. doi: 10.1002/jor.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morimoto T., Kaito T., Matsuo Y., et al. The bone morphogenetic protein-2/7 heterodimer is a stronger inducer of bone regeneration than the individual homodimers in a rat spinal fusion model. Spine Journal. 2015;15(6):1379–1390. doi: 10.1016/j.spinee.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 67.Dang J., Jing L., Shi W., Qin P., Li Y., Diao A. Expression and purification of active recombinant human bone morphogenetic 7-2 dimer fusion protein. Protein Expression and Purification. 2015;115:61–68. doi: 10.1016/j.pep.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 68.Aono A., Hazama M., Notoya K., et al. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochemical and Biophysical Research Communications. 1995;210(3):670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 69.Krase A., Abedian R., Steck E., Hurschler C., Richter W. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthritis and Cartilage. 2014;22(2):284–292. doi: 10.1016/j.joca.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Neugebauer J. M., Kwon S., Kim H.-S., et al. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(18):E2307–E2316. doi: 10.1073/pnas.1501449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walsh D. W., Godson C., Brazil D. P., Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends in Cell Biology. 2010;20(5):244–256. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Ciuclan L., Sheppard K., Dong L., et al. Treatment with anti-gremlin 1 antibody ameliorates chronic hypoxia/SU5416-induced pulmonary arterial hypertension in mice. American Journal of Pathology. 2013;183(5):1461–1473. doi: 10.1016/j.ajpath.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hampton D. W., Asher R. A., Kondo T., Steeves J. D., Ramer M. S., Fawcett J. W. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo . European Journal of Neuroscience. 2007;26(11):3024–3035. doi: 10.1111/j.1460-9568.2007.05940.x. [DOI] [PubMed] [Google Scholar]
- 74.Hampton D. W., Steeves J. D., Fawcett J. W., Ramer M. S. Spinally upregulated noggin suppresses axonal and dendritic plasticity following dorsal rhizotomy. Experimental Neurology. 2007;204(1):366–379. doi: 10.1016/j.expneurol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 75.Akkiraju H., Bonor J., Olli K., et al. Systemic injection of CK2.3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. Journal of Orthopaedic Research. 2015;33(2):208–215. doi: 10.1002/jor.22752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed S., Metpally R. P. R., Sangadala S., Reddy B. V. B. Virtual screening and selection of drug-like compounds to block noggin interaction with bone morphogenetic proteins. Journal of Molecular Graphics and Modelling. 2010;28(7):670–682. doi: 10.1016/j.jmgm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 77.Cao Y., Wang C., Zhang X., et al. Selective small molecule compounds increase BMP-2 responsiveness by inhibiting Smurf1-mediated Smad1/5 degradation. Scientific Reports. 2014;4, article 4965 doi: 10.1038/srep04965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada M., Sangadala S., Liu Y., et al. Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity. Cell Biochemistry and Function. 2009;27(8):526–534. doi: 10.1002/cbf.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kato S., Sangadala S., Tomita K., Titus L., Boden S. D. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Molecular and Cellular Biochemistry. 2011;349(1-2):97–106. doi: 10.1007/s11010-010-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okada M., Kim J. H., Hutton W. C., Yoon S. T. Upregulation of intervertebral disc-cell matrix synthesis by pulsed electromagnetic field is mediated by bone morphogenetic proteins. Journal of Spinal Disorders and Techniques. 2013;26(3):167–173. doi: 10.1097/BSD.0b013e31823d36cf. [DOI] [PubMed] [Google Scholar]
- 81.Jansen J. H. W., Van Der Jagt O. P., Punt B. J., et al. Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study. BMC Musculoskeletal Disorders. 2010;11, article 188 doi: 10.1186/1471-2474-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwartz Z., Simon B. J., Duran M. A., Barabino G., Chaudhri R., Boyan B. D. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. Journal of Orthopaedic Research. 2008;26(9):1250–1255. doi: 10.1002/jor.20591. [DOI] [PubMed] [Google Scholar]
- 83.Nam J., Perera P., Rath B., Agarwal S. Dynamic regulation of bone morphogenetic proteins in engineered osteochondral constructs by biomechanical stimulation. Tissue Engineering—Part A. 2013;19(5-6):783–792. doi: 10.1089/ten.tea.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rath B., Rath B., Deschner J., et al. Biomechanical forces exert anabolic effects on osteoblasts by activation of SMAD 1/5/8 through type 1 BMP receptor. Biorheology. 2011;48(1):37–48. doi: 10.3233/BIR-2011-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balachandran K., Sucosky P., Jo H., Yoganathan A. P. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. The American Journal of Pathology. 2010;177(1):49–57. doi: 10.2353/ajpath.2010.090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Z., Ren L., Deng F., Wang Z., Song J. Low-intensity pulsed ultrasound induces osteogenic differentiation of human periodontal ligament cells through activation of bone morphogenetic protein-smad signaling. Journal of Ultrasound in Medicine. 2014;33(5):865–873. doi: 10.7863/ultra.33.5.865. [DOI] [PubMed] [Google Scholar]
- 87.Angle S. R., Sena K., Sumner D. R., Virkus W. W., Virdi A. S. Combined use of low-intensity pulsed ultrasound and rhBMP-2 to enhance bone formation in a rat model of critical size defect. Journal of Orthopaedic Trauma. 2014;28(10):605–611. doi: 10.1097/BOT.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xue H., Zheng J., Cui Z., et al. Low-intensity pulsed ultrasound accelerates tooth movement via activation of the BMP-2 signaling pathway. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0068926.e68926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou C.-H., Hou S.-M., Tang C.-H. Ultrasound increased BMP-2 expression via PI3K, Akt, c-Fos/c-Jun, and AP-1 pathways in cultured osteoblasts. Journal of Cellular Biochemistry. 2009;106(1):7–15. doi: 10.1002/jcb.21934. [DOI] [PubMed] [Google Scholar]
- 90.Zhang H., Lin C.-Y. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine. 2008;33(16):E525–E531. doi: 10.1097/BRS.0b013e31817c561b. [DOI] [PubMed] [Google Scholar]
- 91.Kodach L. L., Bleuming S. A., Peppelenbosch M. P., Hommes D. W., van den Brink G. R., Hardwick J. C. H. The effect of statins in colorectal cancer is mediated through the bone morphogenetic protein pathway. Gastroenterology. 2007;133(4):1272–1281. doi: 10.1053/j.gastro.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 92.Bradley J. D., Cleverly D. G., Burns A. M., et al. Cyclooxygenase-2 inhibitor reduces simvastatin-induced bone morphogenetic protein-2 and bone formation in vivo. Journal of Periodontal Research. 2007;42(3):267–273. doi: 10.1111/j.1600-0765.2006.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song C., Guo Z., Ma Q., et al. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochemical and Biophysical Research Communications. 2003;308(3):458–462. doi: 10.1016/s0006-291x(03)01408-6. [DOI] [PubMed] [Google Scholar]
- 94.Maeda T., Matsunuma A., Kawane T., Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochemical and Biophysical Research Communications. 2001;280(3):874–877. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- 95.Sugiyama M., Kodama T., Konishi K., Abe K., Asami S., Oikawa S. Compactin and simvastatin, but not pravastatin, induce sone morphogenetic protein-2 in human osteosarcoma cells. Biochemical and Biophysical Research Communications. 2000;271(3):688–692. doi: 10.1006/bbrc.2000.2697. [DOI] [PubMed] [Google Scholar]
- 96.Kanazawa I., Yamaguchi T., Yano S., Yamauchi M., Sugimoto T. Fasudil hydrochloride induces osteoblastic differentiation of stromal cell lines, C3H10T1/2 and ST2, via bone morphogenetic protein-2 expression. Endocrine Journal. 2010;57(5):415–421. doi: 10.1507/endocrj.k09e-328. [DOI] [PubMed] [Google Scholar]
- 97.Kanazawa I., Yamaguchi T., Yano S., Yamauchi M., Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. American Journal of Physiology—Endocrinology and Metabolism. 2009;296(1):E139–E146. doi: 10.1152/ajpendo.90677.2008. [DOI] [PubMed] [Google Scholar]
- 98.Horiuchi H., Saito N., Kinoshita T., Wakabayashi S., Tsutsumimoto T., Takaoka K. Enhancement of bone morphogenetic protein-2-induced new bone formation in mice by the phosphodiesterase inhibitor pentoxifylline. Bone. 2001;28(3):290–294. doi: 10.1016/S8756-3282(00)00450-6. [DOI] [PubMed] [Google Scholar]
- 99.Munisso M. C., Kang J.-H., Tsurufuji M., Yamaoka T. Cilomilast enhances osteoblast differentiation of mesenchymal stem cells and bone formation induced by bone morphogenetic protein 2. Biochimie. 2012;94(11):2360–2365. doi: 10.1016/j.biochi.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 100.Tokuhara Y., Wakitani S., Imai Y., et al. Local delivery of rolipram, a phosphodiesterase-4-specific inhibitor, augments bone morphogenetic protein-induced bone formation. Journal of Bone and Mineral Metabolism. 2010;28(1):17–24. doi: 10.1007/s00774-009-0103-5. [DOI] [PubMed] [Google Scholar]
- 101.Horiuchi H., Saito N., Kinoshita T., Wakabayashi S., Yotsumoto N., Takaoka K. Effect of phosphodiesterase inhibitor-4, rolipram, on new bone formations by recombinant human bone morphogenetic protein-2. Bone. 2002;30(4):589–593. doi: 10.1016/s8756-3282(02)00681-6. [DOI] [PubMed] [Google Scholar]
- 102.Yang J., Li X., Al-Lamki R. S., et al. Sildenafil potentiates bone morphogenetic protein signaling in pulmonary arterial smooth muscle cells and in experimental pulmonary hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology. 2013;33(1):34–42. doi: 10.1161/ATVBAHA.112.300121. [DOI] [PubMed] [Google Scholar]
- 103.Rondelet B., Dewachter L., Kerbaul F., et al. Sildenafil added to sitaxsentan in overcirculation-induced pulmonary arterial hypertension. American Journal of Physiology—Heart and Circulatory Physiology. 2010;299(4):H1118–H1123. doi: 10.1152/ajpheart.00418.2010. [DOI] [PubMed] [Google Scholar]
- 104.Yen C.-H., Leu S., Lin Y.-C., et al. Sildenafil limits monocrotaline-induced pulmonary hypertension in rats through suppression of pulmonary vascular remodeling. Journal of Cardiovascular Pharmacology. 2010;55(6):574–584. doi: 10.1097/FJC.0b013e3181d9f5f4. [DOI] [PubMed] [Google Scholar]
- 105.Sayed D., Abdellatif M. Micrornas in development and disease. Physiological Reviews. 2011;91(3):827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 106.Cunha S. I., Pardali E., Thorikay M., et al. Genetic and pharmacological targeting of activin receptor-like kinase 1 impairs tumor growth and angiogenesis. The Journal of Experimental Medicine. 2010;207(1):85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bendell J. C., Gordon M. S., Hurwitz H. I., et al. Safety, pharmacokinetics, pharmacodynamics, and antitumor activity of dalantercept, an activin receptor-like kinase-1 ligand trap, in patients with advanced cancer. Clinical Cancer Research. 2014;20(2):480–489. doi: 10.1158/1078-0432.CCR-13-1840. [DOI] [PubMed] [Google Scholar]
- 108.Mitchell D., Pobre E. G., Mulivor A. W., et al. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Molecular Cancer Therapeutics. 2010;9(2):379–388. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 109.Larrivée B., Prahst C., Gordon E., et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Developmental Cell. 2012;22(3):489–500. doi: 10.1016/j.devcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hawinkels L. J. A. C., De Vinuesa A. G., Paauwe M., et al. Activin receptor-like kinase 1 ligand trap reduces microvascular density and improves chemotherapy efficiency to various solid tumors. Clinical Cancer Research. 2016;22(1):96–106. doi: 10.1158/1078-0432.CCR-15-0743. [DOI] [PubMed] [Google Scholar]
- 111.Ricard N., Ciais D., Levet S., et al. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood. 2012;119(25):6162–6171. doi: 10.1182/blood-2012-01-407593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrison C. A., Al-Musawi S. L., Walton K. L. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors. 2011;29(5):174–186. doi: 10.3109/08977194.2011.608666. [DOI] [PubMed] [Google Scholar]
- 113.Lin J. S., Kauff A., Diao Y., Yang H., Lawrence S., Juengel J. L. Creation of DNA aptamers against recombinant bone morphogenetic protein 15. Reproduction, Fertility and Development. 2015 doi: 10.1071/rd14409. [DOI] [PubMed] [Google Scholar]
- 114.Yu P. B., Hong C. C., Sachidanandan C., et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nature Chemical Biology. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shore E. M., Xu M., Feldman G. J., Fenstermacher D. A., Brown M. A., Kaplan F. S. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nature Genetics. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 116.Buczkowicz P., Hoeman C., Rakopoulos P., et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nature Genetics. 2014;46(5):451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taylor K. R., Mackay A., Truffaux N., et al. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nature Genetics. 2014;46(5):457–461. doi: 10.1038/ng.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu G., Diaz A. K., Paugh B. S., et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nature Genetics. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fontebasso A. M., Papillon-Cavanagh S., Schwartzentruber J., et al. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nature Genetics. 2014;46(5):462–466. doi: 10.1038/ng.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsugawa D., Oya Y., Masuzaki R., et al. Specific activin receptor-like kinase 3 inhibitors enhance liver regeneration. Journal of Pharmacology and Experimental Therapeutics. 2014;351(3):549–558. doi: 10.1124/jpet.114.216903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohedas A. H., Xing X., Armstrong K. A., Bullock A. N., Cuny G. D., Yu P. B. Development of an ALK2-biased BMP type I receptor kinase inhibitor. ACS Chemical Biology. 2013;8(6):1291–1302. doi: 10.1021/cb300655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Engers D. W., Frist A. Y., Lindsley C. W., Hong C. C., Hopkins C. R. Synthesis and structure-activity relationships of a novel and selective bone morphogenetic protein receptor (BMP) inhibitor derived from the pyrazolo[1.5-a]pyrimidine scaffold of Dorsomorphin: the discovery of ML347 as an ALK2 versus ALK3 selective MLPCN probe. Bioorganic and Medicinal Chemistry Letters. 2013;23(11):3248–3252. doi: 10.1016/j.bmcl.2013.03.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohedas A. H., Wang Y., Sanvitale C. E., et al. Structure-activity relationship of 3,5-diaryl-2-aminopyridine ALK2 inhibitors reveals unaltered binding affinity for fibrodysplasia ossificans progressiva causing mutants. Journal of Medicinal Chemistry. 2014;57(19):7900–7915. doi: 10.1021/jm501177w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Horbelt D., Boergermann J. H., Chaikuad A., et al. Small molecules dorsomorphin and LDN-193189 inhibit myostatin/GDF8 signaling and promote functional myoblast differentiation. The Journal of Biological Chemistry. 2015;290(6):3390–3404. doi: 10.1074/jbc.m114.604397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hohjoh H. Disease-causing allele-specific silencing by RNA interference. Pharmaceuticals. 2013;6(4):522–535. doi: 10.3390/ph6040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaplan J., Kaplan F. S., Shore E. M. Restoration of normal BMP signaling levels and osteogenic differentiation in FOP mesenchymal progenitor cells by mutant allele-specific targeting. Gene Therapy. 2012;19(7):786–790. doi: 10.1038/gt.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takahashi M., Katagiri T., Furuya H., Hohjoh H. Disease-causing allele-specific silencing against the ALK2 mutants, R206H and G356D, in fibrodysplasia ossificans progressiva. Gene Therapy. 2012;19(7):781–785. doi: 10.1038/gt.2011.193. [DOI] [PubMed] [Google Scholar]
- 128.Lowery J. W., Rosen V. Allele-specific RNA interference in FOP silencing the FOP gene. Gene Therapy. 2012;19(7):701–702. doi: 10.1038/gt.2011.190. [DOI] [PubMed] [Google Scholar]
- 129.Austin E. D., Phillips J. A., Cogan J. D., et al. Truncating and missense BMPR2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respiratory Research. 2009;10, article 87 doi: 10.1186/1465-9921-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamamoto R., Matsushita M., Kitoh H., et al. Clinically applicable antianginal agents suppress osteoblastic transformation of myogenic cells and heterotopic ossifications in mice. Journal of Bone and Mineral Metabolism. 2013;31(1):26–33. doi: 10.1007/s00774-012-0380-2. [DOI] [PubMed] [Google Scholar]
- 131.Kempen D. H. R., Lu L., Hefferan T. E., et al. Enhanced bone morphogenetic protein-2-induced ectopic and orthotopic bone formation by intermittent parathyroid hormone (1-34) administration. Tissue Engineering—Part A. 2010;16(12):3769–3777. doi: 10.1089/ten.tea.2010.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morgan E. F., Mason Z. D., Bishop G., et al. Combined effects of recombinant human BMP-7 (rhBMP-7) and parathyroid hormone (1–34) in metaphyseal bone healing. Bone. 2008;43(6):1031–1038. doi: 10.1016/j.bone.2008.07.251. [DOI] [PubMed] [Google Scholar]
- 133.Yamamoto M., Beppu H., Takaoka K., et al. Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. The Journal of Cell Biology. 2009;184(2):323–334. doi: 10.1083/jcb.200808044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goumans M.-J., Lebrin F., Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends in Cardiovascular Medicine. 2003;13(7):301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 135.Zhao L., Yee M., O'Reilly M. A. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-β and BMP signaling. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2013;305(6):L409–L418. doi: 10.1152/ajplung.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saitoh M., Shirakihara T., Fukasawa A., et al. basolateral BMP signaling in polarized epithelial cells. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0062659.e62659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Winbanks C. E., Chen J. L., Qian H., et al. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. The Journal of Cell Biology. 2013;203(2):345–357. doi: 10.1083/jcb.201211134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sartori R., Schirwis E., Blaauw B., et al. BMP signaling controls muscle mass. Nature Genetics. 2013;45(11):1309–1321. doi: 10.1038/ng.2772. [DOI] [PubMed] [Google Scholar]
- 139.Nguyen T. Q., Goldschmeding R. Bone morphogenetic protein-7 and connective tissue growth factor: novel targets for treatment of renal fibrosis? Pharmaceutical Research. 2008;25(10):2416–2426. doi: 10.1007/s11095-008-9548-9. [DOI] [PubMed] [Google Scholar]
- 140.Izumi N., Mizuguchi S., Inagaki Y., et al. BMP-7 opposes TGF-β1-mediated collagen induction in mouse pulmonary myofibroblasts through Id2. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2006;290(1):L120–L126. doi: 10.1152/ajplung.00171.2005. [DOI] [PubMed] [Google Scholar]
- 141.Wordinger R. J., Fleenor D. L., Hellberg P. E., et al. Effects of TGF-β2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Investigative Ophthalmology & Visual Science. 2007;48(3):1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 142.Zode G. S., Clark A. F., Wordinger R. J. Bone morphogenetic protein 4 inhibits TGF-β2 stimulation of extracellular matrix proteins in optic nerve head cells: role of gremlin in ECM modulation. GLIA. 2009;57(7):755–766. doi: 10.1002/glia.20803. [DOI] [PubMed] [Google Scholar]
- 143.Stumm C. L., Halcsik E., Landgraf R. G., Camara N. O. S., Sogayar M. C., Jancar S. Lung remodeling in a mouse model of asthma involves a balance between TGF-β1 and BMP-7. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0095959.e95959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han C., Hong K.-H., Kim Y. H., et al. SMAD1 deficiency in either endothelial or smooth muscle cells can predispose mice to pulmonary hypertension. Hypertension. 2013;61(5):1044–1052. doi: 10.1161/HYPERTENSIONAHA.111.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morrell N. W., Yang X., Upton P. D., et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β 1 and bone morphogenetic proteins. Circulation. 2001;104(7):790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 146.Reynolds A. M., Holmes M. D., Danilov S. M., Reynolds P. N. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. European Respiratory Journal. 2012;39(2):329–343. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 147.Thomas M., Docx C., Holmes A. M., et al. Activin-like kinase 5 (ALK5) mediates abnormal proliferation of vascular smooth muscle cells from patients with familial pulmonary arterial hypertension and is involved in the progression of experimental pulmonary arterial hypertension induced by monocrotaline. The American Journal of Pathology. 2009;174(2):380–389. doi: 10.2353/ajpath.2009.080565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Long L., Crosby A., Yang X., et al. Altered bone morphogenetic protein and transforming growth factor-β signaling in rat models of pulmonary hypertension. Potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119(4):566–576. doi: 10.1161/circulationaha.108.821504. [DOI] [PubMed] [Google Scholar]
- 149.Schindeler A., Morse A., Peacock L., et al. Rapid cell culture and pre-clinical screening of a transforming growth factor-Β (TGF-β) inhibitor for orthopaedics. BMC Musculoskeletal Disorders. 2010;11, article 105 doi: 10.1186/1471-2474-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kawamura I., Maeda S., Imamura K., et al. SnoN suppresses maturation of chondrocytes by mediating signal cross-talk between transforming growth factor-β and bone morphogenetic protein pathways. The Journal of Biological Chemistry. 2012;287(34):29101–29113. doi: 10.1074/jbc.m112.349415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sardar Z., Alexander D., Oxner W., et al. Twelve-month results of a multicenter, blinded, pilot study of a novel peptide (B2A) in promoting lumbar spine fusion. Journal of Neurosurgery: Spine. 2015;22(4):358–366. doi: 10.3171/2013.11.spine121106. [DOI] [PubMed] [Google Scholar]
- 152.Kasten P., Beyen I., Bormann D., Luginbühl R., Plöger F., Richter W. The effect of two point mutations in GDF-5 on ectopic bone formation in a β-tricalciumphosphate scaffold. Biomaterials. 2010;31(14):3878–3884. doi: 10.1016/j.biomaterials.2010.01.109. [DOI] [PubMed] [Google Scholar]
- 153.Kleinschmidt K., Wagner-Ecker M., Bartek B., Holschbach J., Richter W. Superior angiogenic potential of GDF-5 and GDF-5V453/V456 compared with BMP-2 in a rabbit long-bone defect model. The Journal of Bone and Joint Surgery—American Volume. 2014;96(20):1699–1707. doi: 10.2106/jbjs.m.01462. [DOI] [PubMed] [Google Scholar]
- 154.Brock M., Trenkmann M., Gay R. E., et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circulation Research. 2009;104(10):1184–1191. doi: 10.1161/circresaha.109.197491. [DOI] [PubMed] [Google Scholar]
- 155.Jia J., Feng X., Xu W., et al. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Experimental and Molecular Medicine. 2014;46(7, article e107) doi: 10.1038/emm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang J.-F., Fu W.-M., He M.-L., et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA biology. 2011;8(5):829–838. doi: 10.4161/rna.8.5.16043. [DOI] [PubMed] [Google Scholar]
- 157.Brock M., Samillan V. J., Trenkmann M., et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. European Heart Journal. 2014;35(45):3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 158.Rogler C. E., LeVoci L., Ader T., et al. MicroRNA-23b cluster microRNAs regulate transforming growth factor-beta/bone morphogenetic protein signaling and liver stem cell differentiation by targeting Smads. Hepatology. 2009;50(2):575–584. doi: 10.1002/hep.22982. [DOI] [PubMed] [Google Scholar]
- 159.Dey B. K., Gagan J., Yan Z., Dutta A. miR-26a is required for skeletal muscle differentiation and regeneration in mice. Genes and Development. 2012;26(19):2180–2191. doi: 10.1101/gad.198085.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Icli B., Wara A. K. M., Moslehi J., et al. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circulation Research. 2013;113(11):1231–1241. doi: 10.1161/CIRCRESAHA.113.301780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li Y., Fan L., Hu J., et al. MiR-26a rescues bone regeneration deficiency of mesenchymal stem cells derived from osteoporotic mice. Molecular Therapy. 2015;23(8):1349–1357. doi: 10.1038/mt.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fuchs H., Theuser M., Wruck W., Adjaye J. miR-27 negatively regulates pluripotency-associated genes in human embryonal carcinoma cells. PloS ONE. 2014;9(11) doi: 10.1371/journal.pone.0111637.e111637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu T., Zhou H., Hong Y., Li J., Jiang X., Huang H. miR-30 family members negatively regulate osteoblast differentiation. The Journal of Biological Chemistry. 2012;287(10):7503–7511. doi: 10.1074/jbc.m111.292722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu H., Zhang N., Tian D. MiR-30b is involved in methylglyoxal-induced epithelial-mesenchymal transition of peritoneal mesothelial cells in rats. Cellular and Molecular Biology Letters. 2014;19(2):315–329. doi: 10.2478/s11658-014-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zeng Y., Qu X., Li H., et al. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Letters. 2012;586(16):2375–2381. doi: 10.1016/j.febslet.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 166.Castoldi M., Spasic M. V., Altamura S., et al. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. The Journal of Clinical Investigation. 2011;121(4):1386–1396. doi: 10.1172/jci44883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huber L. C., Ulrich S., Leuenberger C., et al. Featured Article: microRNA-125a in pulmonary hypertension: regulator of a proliferative phenotype of endothelial cells. Experimental Biology and Medicine. 2015;240(12):1580–1589. doi: 10.1177/1535370215579018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zumbrennen-Bullough K. B., Wu Q., Core A. B., et al. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. The Journal of Biological Chemistry. 2014;289(34):23796–23808. doi: 10.1074/jbc.m114.577387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Z., Hassan M. Q., Volinia S., et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(37):13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bhinge A., Poschmann J., Namboori S. C., et al. MiR-135b is a direct PAX6 target and specifies human neuroectoderm by inhibiting TGF-β/BMP signaling. The EMBO Journal. 2014;33(11):1271–1283. doi: 10.1002/embj.201387215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nicolas F. E., Pais H., Schwach F., et al. mRNA expression profiling reveals conserved and non-conserved miR-140 targets. RNA Biology. 2011;8(4):607–615. doi: 10.4161/rna.8.4.15390. [DOI] [PubMed] [Google Scholar]
- 172.van Baal J. W. P. M., Verbeek R. E., Bus P., et al. MicroRNA-145 in Barrett's oesophagus: regulating BMP4 signalling via GATA6. Gut. 2013;62(5):664–675. doi: 10.1136/gutjnl-2011-301061. [DOI] [PubMed] [Google Scholar]
- 173.Song H., Wang Q., Wen J., et al. ACVR1, a therapeutic target of fibrodysplasia ossificans progressiva, is negatively regulated by miR-148a. International Journal of Molecular Sciences. 2012;13(2):2063–2077. doi: 10.3390/ijms13022063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cao Y., Lv Q., Lv C. MicroRNA-153 suppresses the osteogenic differentiation of human mesenchymal stem cells by targeting bone morphogenetic protein receptor type II. International Journal of Molecular Medicine. 2015;36(3):760–766. doi: 10.3892/ijmm.2015.2275. [DOI] [PubMed] [Google Scholar]
- 175.Rai D., Kim S.-W., McKeller M. R., Dahia P. L. M., Aguiar R. C. T. Targeting of SMAD5 links microRNA-155 to the TGF-β pathway and lymphomagenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(7):3111–3116. doi: 10.1073/pnas.0910667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yin Q., Wang X., Fewell C., et al. MicroRNA miR-155 inhibits Bone Morphogenetic Protein (BMP) signaling and BMP-mediated Epstein-Barr virus reactivation. Journal of Virology. 2010;84(13):6318–6327. doi: 10.1128/JVI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lin E. A., Kong L., Bai X.-H., Luan Y., Liu C.-J. miR-199a∗, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. The Journal of Biological Chemistry. 2009;284(17):11326–11335. doi: 10.1074/jbc.m807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim J. S., Kurie J. M., Ahn Y.-H. BMP4 depletion by miR-200 inhibits tumorigenesis and metastasis of lung adenocarcinoma cells. Molecular Cancer. 2015;14(1, article 173) doi: 10.1186/s12943-015-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tabruyn S. P., Hansen S., Ojeda-Fernández M.-L., et al. MiR-205 is downregulated in hereditary hemorrhagic telangiectasia and impairs TGF-beta signaling pathways in endothelial cells. Angiogenesis. 2013;16(4):877–887. doi: 10.1007/s10456-013-9362-9. [DOI] [PubMed] [Google Scholar]
- 180.Kang H., Louie J., Weisman A., et al. Inhibition of microRNA-302 (miR-302) by Bone Morphogenetic Protein 4 (BMP4) facilitates the BMP signaling pathway. The Journal of Biological Chemistry. 2012;287(46):38656–38664. doi: 10.1074/jbc.m112.390898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Z., Zhou Y., Yuan Y., et al. MiR542-3p regulates the epithelial-mesenchymal transition by directly targeting BMP7 in NRK52e. International Journal of Molecular Sciences. 2015;16(11):27945–27955. doi: 10.3390/ijms161126075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Souza T. A., Chen X., Guo Y., et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Molecular Endocrinology. 2008;22(12):2689–2702. doi: 10.1210/me.2008-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lowery J. W., Intini G., Gamer L., et al. Loss of BMPR2 leads to high bone mass due to increased osteoblast activity. Journal of Cell Science. 2015;128(7):1308–1315. doi: 10.1242/jcs.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Meeteren L. A., Thorikay M., Bergqvist S., et al. Anti-human activin receptor-like kinase 1 (ALK1) antibody attenuates bone morphogenetic protein 9 (BMP9)-induced ALK1 signaling and interferes with endothelial cell sprouting. The Journal of Biological Chemistry. 2012;287(22):18551–18561. doi: 10.1074/jbc.m111.338103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Steinbicker A. U., Sachidanandan C., Vonner A. J., et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117(18):4915–4923. doi: 10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Derwall M., Malhotra R., Lai C. S., et al. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(3):613–622. doi: 10.1161/ATVBAHA.111.242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yoon B.-H., Jeon Y.-H., Hwang B., Kwon H., Choe S., Yang Z. Anti-wrinkle effect of bone morphogenetic protein receptor 1a-extracellular domain (BMPR1a-ECD) BMB Reports. 2013;46(9):465–470. doi: 10.5483/BMBRep.2013.46.9.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baud'huin M., Solban N., Cornwall-Brady M., et al. A soluble bone morphogenetic protein type IA receptor increases bone mass and bone strength. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(30):12207–12212. doi: 10.1073/pnas.1204929109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jian C.-X., Liu X.-F., Hu J., et al. 20-hydroxyecdysone-induced bone morphogenetic protein-2-dependent osteogenic differentiation through the ERK pathway in human periodontal ligament stem cells. European Journal of Pharmacology. 2013;698(1–3):48–56. doi: 10.1016/j.ejphar.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 190.Tanaka K.-I., Inoue Y., Hendy G. N., et al. Interaction of Tmem119 and the bone morphogenetic protein pathway in the commitment of myoblastic into osteoblastic cells. Bone. 2012;51(1):158–167. doi: 10.1016/j.bone.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 191.Lee Y.-C., Cheng C.-J., Bilen M. A., et al. BMP4 promotes prostate tumor growth in bone through osteogenesis. Cancer Research. 2011;71(15):5194–5203. doi: 10.1158/0008-5472.CAN-10-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kwak Y.-D., Hendrix B. J., Sugaya K. Secreted type of amyloid precursor protein induces glial differentiation by stimulating the BMP/Smad signaling pathway. Biochemical and Biophysical Research Communications. 2014;447(3):394–399. doi: 10.1016/j.bbrc.2014.03.139. [DOI] [PubMed] [Google Scholar]
- 193.Wang L., Trebicka E., Fu Y., et al. The bone morphogenetic protein-hepcidin axis as a therapeutic target in inflammatory bowel disease. Inflammatory Bowel Diseases. 2012;18(1):112–119. doi: 10.1002/ibd.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Andriopoulos B., Jr., Corradini E., Xia Y., et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nature Genetics. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Matsumoto Y., Otsuka F., Inagaki K., et al. An in vivo role of bone morphogenetic protein-6 in aldosterone production by rat adrenal gland. Journal of Steroid Biochemistry and Molecular Biology. 2012;132(1-2):8–14. doi: 10.1016/j.jsbmb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 196.Yanagita M., Okuda T., Endo S., et al. Uterine sensitization-associated gene-1 (USAG-1), a novel BMP antagonist expressed in the kidney, accelerates tubular injury. The Journal of Clinical Investigation. 2006;116(1):70–79. doi: 10.1172/jci25445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lv S., Liu G., Sun A., et al. Mesenchymal stem cells ameliorate diabetic glomerular fibrosis in vivo and in vitro by inhibiting TGF-β signalling via secretion of bone morphogenetic protein 7. Diabetes and Vascular Disease Research. 2014;11(4):251–261. doi: 10.1177/1479164114531300. [DOI] [PubMed] [Google Scholar]
- 198.Myllymaa S., Pasternack A., Mottershead D. G., et al. Inhibition of oocyte growth factors in vivo modulates ovarian folliculogenesis in neonatal and immature mice. Reproduction. 2010;139(3):587–598. doi: 10.1530/rep-09-0391. [DOI] [PubMed] [Google Scholar]
- 199.Theurl I., Schroll A., Sonnweber T., et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118(18):4977–4984. doi: 10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Babitt J. L., Huang F. W., Xia Y., Sidis Y., Andrews N. C., Lin H. Y. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. The Journal of Clinical Investigation. 2007;117(7):1933–1939. doi: 10.1172/jci31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hao J., Ho J. N., Lewis J. A., et al. In vivo structure—activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chemical Biology. 2010;5(2):245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ao A., Hao J., Hopkins C. R., Hong C. C. DMH1, a novel BMP small molecule inhibitor, increases cardiomyocyte progenitors and promotes cardiac differentiation in mouse embryonic stem cells. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041627.e41627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Alsamarah A., LaCuran A. E., Oelschlaeger P., Hao J., Luo Y. Uncovering molecular bases underlying bone morphogenetic protein receptor inhibitor selectivity. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0132221.e0132221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Owens P., Pickup M. W., Novitskiy S. V., et al. Inhibition of BMP signaling suppresses metastasis in mammary cancer. Oncogene. 2015;34(19):2437–2449. doi: 10.1038/onc.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sheng Y., Sun B., Guo W.-T., et al. (4-[6-(4-Isopropoxyphenyl)pyrazolo [1,5-a]pyrimidin-3-yl] quinoline) is a novel inhibitor of autophagy. British Journal of Pharmacology. 2014;171(21):4970–4980. doi: 10.1111/bph.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Langenfeld E., Hong C. C., Lanke G., Langenfeld J. Bone morphogenetic protein type I receptor antagonists decrease growth and induce cell death of lung cancer cell lines. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061256.e61256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hamasaki M., Hashizume Y., Yamada Y., et al. Pathogenic mutation of ALK2 inhibits induced pluripotent stem cell reprogramming and maintenance: mechanisms of reprogramming and strategy for drug identification. Stem Cells. 2012;30(11):2437–2449. doi: 10.1002/stem.1221. [DOI] [PubMed] [Google Scholar]
- 208.Vogt J., Traynor R., Sapkota G. P. The specificities of small molecule inhibitors of the TGFß and BMP pathways. Cellular Signalling. 2011;23(11):1831–1842. doi: 10.1016/j.cellsig.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 209.Boergermann J. H., Kopf J., Yu P. B., Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. International Journal of Biochemistry and Cell Biology. 2010;42(11):1802–1807. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Garulli C., Kalogris C., Pietrella L., et al. Dorsomorphin reverses the mesenchymal phenotype of breast cancer initiating cells by inhibition of bone morphogenetic protein signaling. Cellular Signalling. 2014;26(2):352–362. doi: 10.1016/j.cellsig.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 211.Kim D.-S., Lee J. S., Leem J. W., et al. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Reviews and Reports. 2010;6(2):270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- 212.Chang H.-M., Cheng J.-C., Taylor E., Leung P. C. K. Oocyte-derived BMP15 but not GDF9 down-regulates connexin43 expression and decreases gap junction intercellular communication activity inimmortalized human granulosa cells. Molecular Human Reproduction. 2014;20(5):373–383. doi: 10.1093/molehr/gau001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hao J., Daleo M. A., Murphy C. K., et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0002904.e2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bai H., Gao Y., Arzigian M., Wojchowski D. M., Wu W.-S., Wang Z. Z. BMP4 regulates vascular progenitor development in human embryonic stem cells through a Smad-dependent pathway. Journal of Cellular Biochemistry. 2010;109(2):363–374. doi: 10.1002/jcb.22410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shanmugam N. K. N., Cherayil B. J. Serum-induced up-regulation of hepcidin expression involves the bone morphogenetic protein signaling pathway. Biochemical and Biophysical Research Communications. 2013;441(2):383–386. doi: 10.1016/j.bbrc.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sanvitale C. E., Kerr G., Chaikuad A., et al. A new class of small molecule inhibitor of BMP signaling. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0062721.e62721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kerr G., Sheldon H., Chaikuad A., et al. A small molecule targeting ALK1 prevents Notch cooperativity and inhibits functional angiogenesis. Angiogenesis. 2015;18(2):209–217. doi: 10.1007/s10456-014-9457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cuny G. D., Yu P. B., Laha J. K., et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorganic and Medicinal Chemistry Letters. 2008;18(15):4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Balboni A. L., Hutchinson J. A., DeCastro A. J., et al. Δnp63α-mediated activation of bone morphogenetic protein signaling governs stem cell activity and plasticity in normal and malignant mammary epithelial cells. Cancer Research. 2013;73(2):1020–1030. doi: 10.1158/0008-5472.can-12-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yu P. B., Deng D. Y., Lai C. S., et al. BMP type I receptor inhibition reduces heterotopic ossification. Nature Medicine. 2008;14(12):1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Saeed O., Otsuka F., Polavarapu R., et al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(2):299–307. doi: 10.1161/ATVBAHA.111.240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Helbing T., Herold E.-M., Hornstein A., et al. Inhibition of BMP activity protects epithelial barrier function in lung injury. Journal of Pathology. 2013;231(1):105–116. doi: 10.1002/path.4215. [DOI] [PubMed] [Google Scholar]
- 223.Komatsu Y., Yu P. B., Kamiya N., et al. Augmentation of Smad-dependent BMP signaling in neural crest cells causes craniosynostosis in mice. Journal of Bone and Mineral Research. 2013;28(6):1422–1433. doi: 10.1002/jbmr.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mayeur C., Kolodziej S. A., Wang A., et al. Oral administration of a bone morphogenetic protein type I receptor inhibitor prevents the development of anemia of inflammation. Haematologica. 2015;100(2):e68–e71. doi: 10.3324/haematol.2014.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Peterson J. R., Rosa S. D. L., Eboda O., et al. Treatment of heterotopic ossification through remote ATP hydrolysis. Science Translational Medicine. 2014;6(255) doi: 10.1126/scitranslmed.3008810.255ra132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kajimoto H., Kai H., Aoki H., et al. BMP type I receptor inhibition attenuates endothelial dysfunction in mice with chronic kidney disease. Kidney International. 2015;87(1):128–136. doi: 10.1038/ki.2014.223. [DOI] [PubMed] [Google Scholar]
- 227.Malhotra R., Burke M. F., Martyn T., et al. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix gla protein deficiency. PLoS ONE. 2015;10(1) doi: 10.1371/journal.pone.0117098.e0117098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Engers D. W., Frist A. Y., Lindsley C. W., Hong C. H., Hopkins C. R. Probe Reports from the NIH Molecular Libraries Program. Bethesda, Md, USA: National Center for Biotechnology Information; 2010. Development of a potent and ALK2 selective bone morphogenetic protein receptor (BMP) inhibitor. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specific reports of BMP pathway modulation and related applications are provided in the supplemental material. Specific applications highlighted are meant to be representative rather than exhaustive of the field and no endorsement by the authors of any particular application should be inferred.


