Abstract
Cardiac gene delivery of parvalbumin (Parv), an EF-hand Ca2+ buffer, has been studied as a therapeutic strategy for diastolic heart failure, in which slow Ca2+ reuptake is an important contributor. A limitation of wild-type (WT) Parv is the significant trade-off between faster relaxation and blunted contraction amplitude, occurring because WT-Parv sequesters Ca2+ too early in the cardiac cycle and prematurely truncates sarcomere shortening in the facilitation of rapid relaxation. We recently demonstrated that an E → Q substitution (ParvE101Q) at amino acid 12 of the EF-hand Ca2+/Mg2+ binding loop disrupts bidentate Ca2+ binding, reducing Ca2+ affinity by 99-fold and increasing Mg2+ affinity twofold. ParvE101Q caused faster relaxation and not only preserved contractility, but unexpectedly increased it above untreated myocytes. To gain mechanistic insight into the increased contractility, we focused here on amino acid 12 of the EF-hand motif. We introduced an E → D substitution (ParvE101D) at this site, which converts bidentate Ca2+ coordination to monodentate coordination. ParvE101D decreased Ca2+ affinity by 114-fold and increased Mg2+ affinity 28-fold compared to WT-Parv. ParvE101D increased contraction amplitude compared to both untreated myocytes and myocytes with ParvE101Q, with limited improvement in relaxation. Additionally, ParvE101D increased spontaneous contractions after pacing stress. ParvE101D also increased Ca2+ transient peak height and was diffusely localized around the Z-line of the sarcomere, suggesting a Ca2+-dependent mechanism of enhanced contractility. Sarcoplasmic reticulum Ca2+ load was not changed with ParvE101D, but postpacing Ca2+ waves were increased. Together, these data show that inverted Ca2+/Mg2+ binding affinities of ParvE101D increase myocyte contractility through a Ca2+-dependent mechanism without altering sarcoplasmic reticulum Ca2+ load and by increasing unstimulated contractions and Ca2+ waves. ParvE101D provides mechanistic insight into how changes in the Ca2+/Mg2+ binding affinities of parvalbumin’s EF-hand motif alter function of cardiac myocytes. These data are informative in developing new Ca2+ buffering strategies for the failing heart.
Introduction
Parvalbumin (Parv) is a cytosolic calcium (Ca2+) buffering protein endogenously expressed in glycolytic skeletal muscle, where it facilitates fast relaxation (1). Wild-type Parv (WT-Parv) contains two functional EF-hand metal ion binding sites, the CD domain and the EF domain, that bind Ca2+ with high affinity (∼10−8 M) and Mg2+ with moderate affinity (∼10−4 M) (2). Because basal cytosolic Ca2+ and Mg2+ concentrations are ∼10–100 nM and 1 mM, respectively, WT-Parv binds Mg2+ almost exclusively at rest (2). When an action potential triggers Ca2+ release from the sarcoplasmic reticulum (SR), Mg2+ must first dissociate from Parv before Ca2+ can bind. This delay in Ca2+ binding allows for contraction to occur before Ca2+ is buffered by Parv (2).
Canonical EF-hand motifs consist of a helix-loop-helix structure, where divalent metal cations coordinate with oxygen atoms of highly conserved amino acids in the 12-residue loop region. Coordinating oxygen atoms are from loop residues 1, 3, 5, 7, 9, and 12 (3, 4). The two EF-hand binding loops in WT-Parv are able to discriminate between the chemically similar Mg2+ and Ca2+ ions because of their difference in size and preferred coordination states. Six coordinating oxygen atoms in an octahedral configuration are optimal for Mg2+ binding, whereas seven coordinating oxygen atoms in a pentagonal bipyrimidal configuration are optimal for Ca2+ (4, 5). The highly conserved glutamate at position 12 of the EF-hand loop provides monodentate coordination with one side-chain oxygen atom when Mg2+ is bound and bidentate coordination with both available oxygen atoms when Ca2+ is bound (4). Thus, EF-hand motif residue 12 is key for divalent cation selectivity (4, 6). The ability of the EF-hands in WT-Parv to accommodate either Ca2+ or Mg2+ ions is what confers its unique function as a delayed Ca2+ buffer to increase the relaxation rate in fast-twitch muscle.
The therapeutic potential of transient intracellular Ca2+ buffering for diastolic dysfunction in the heart has been investigated because delayed Ca2+ reuptake is an important contributor to slow or incomplete relaxation in diseased myocardium (7). Ca2+ buffering in the heart has been tested by adenoviral gene transfer of WT-Parv in isolated cardiac myocytes because Parv is not expressed endogenously in the heart (8). These experiments demonstrated that Parv-expressing myocytes have a significantly faster relaxation rate, but this occurs at the expense of blunted contractile function (9). Reduced contraction amplitude was attributed to the kinetics of WT-Parv, which are optimized for the short bursts of contraction/relaxation cycling that occur in fast-twitch muscle. In the context of the cardiac myocyte, WT-Parv binds Ca2+ too early in the contractile cycle, which decreases Ca2+ binding to the myofilaments and subsequently blunts contraction (10). To optimize the kinetics of Ca2+ buffering for the human heart, it was hypothesized that a modified delayed buffer, engineered with decreased Ca2+ and/or increased Mg2+ binding affinities, would be required (11, 12).
We recently demonstrated that substituting a glutamine for the highly conserved glutamate at the 12th amino acid of the EF-hand ion binding loop of carp β-Parvalbumin (ParvE101Q) decreased Ca2+ affinity by 99% and paradoxically increased Mg2+ affinity twofold compared to WT-Parv (13). This E → Q substitution eliminated the bidentate coordination required for optimal Ca2+ binding in the EF-hand loop. The combination of decreased Ca2+ affinity and increased Mg2+ affinity in ParvE101Q further delayed Ca2+ buffering relative to WT-Parv, enabling ParvE101Q to be more optimally designed for the human heart (13). One unexpected observation in these experiments was that ParvE101Q not only preserved contractile function, because of the delayed Ca2+ buffering, but also increased contraction amplitude 24% above untreated myocytes (13). This is in stark contrast to the severe diminution of contraction amplitude characteristic of WT-Parv (9). The basis for the heightened contractile function of ParvE101Q is not known. One hypothesis is that localized Mg2+ buffering, together with increased Mg2+ affinity of ParvE101Q, accounts in part for this effect (13).
To accomplish the goal of implementing a Ca2+ buffering system into diseased cardiac muscle, a deeper understanding of EF-hand motifs is required in the context of the cardiac myocyte and its unique excitation-contraction pathway. Specifically, it is imperative to understand how a Ca2+ buffering protein such as ParvE101Q could, paradoxically, increase myocyte contractility. To gain mechanistic insight into the increase in contractility found with ParvE101Q, we focused here on the 12th amino acid of the canonical EF-hand motif. Another EF-hand loop residue 12 substitution that has been studied from a structural standpoint is ParvE101D (6, 14). Although glutamate at position 12 of the metal ion binding loop of canonical EF-hands is highly conserved, ∼8% of EF-hand motifs contain an aspartate at this position (4). Substitution of glutamate for aspartate at the 12th residue of the EF-hand ion binding loop in carp β-parvalbumin resulted in monodentate Ca2+ binding and an even more drastic inversion of Ca2+ and Mg2+ binding affinities than ParvE101Q (6, 13). The effects of ParvE101D on intact cardiac myocyte mechanical function and Ca2+ handling have not been tested. If the inverted Ca2+/Mg2+ binding affinities of ParvE101Q have a mechanistic role in increasing contraction amplitude, ParvE101D should elicit similar or greater effects in terms of enhanced contractility.
The purpose of this study was therefore to test the hypothesis that inverted Ca2+/Mg2+ binding affinities in ParvE101D increase contractile function in cardiac myocytes. The results of these experiments provide evidence that the increased Mg2+ affinity and decreased Ca2+ affinity of ParvE101D increases contractile function and gives new mechanistic insight into enhanced contractility at the subcellular level. Additionally, the data presented here increase knowledge of how specific structural changes in EF-hand motifs underlie functional effects in cardiac myocyte physiology, particularly related to changes in coordination spheres for divalent metal ions. This work provides the critical framework to optimize this protein-based transient Ca2+ buffering strategy for human heart failure.
Materials and Methods
Ca2+/Mg2+ affinity and dissociation rates
The Ca2+ and Mg2+ affinities and dissociation rates were measured at 35°C in bacterially expressed and purified proteins as previously described (12, 13). Briefly, all steady-state fluorescence measurements were performed using a Perkin-Elmer LS55B Spectrofluorometer (Waltham, MA) by adding microliter amounts of CaCl2 or MgCl2 to 2 ml of the proteins (1 μM) in 200 mM 3-(N-morpholino)propanesulfonic acid, 150 mM KCl, 4 mM EGTA, 1 mM DTT, pH 7.0. Ca2+ and Mg2+ dissociation rates were measured using an Applied Photophysics (Leatherhead, UK) model SX.18 MV stopped-flow instrument. The buffer used for the stopped-flow experiments was 10 mM 3-(N-morpholino)propanesulfonic acid, 150 mM KCl, 1 mM DTT, at pH 7.0.
Adenovirus production
Human α-parvalbumin recombinant DNA was subcloned into the TOPO TA vector system (Life Technologies, Grand Island, NY), transformed into One Shot TOP10 chemically competent Escherichia coli (Life Technologies), and ligated into the pDC316 plasmid (Microbix Biosystems, Mississauga, Ontario, Canada). Amino acid substitutions were incorporated using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) and DNA transformed into XL1 Blue Supercompetent Cells (Agilent). Sequence-verified clones were used for making adenovirus.
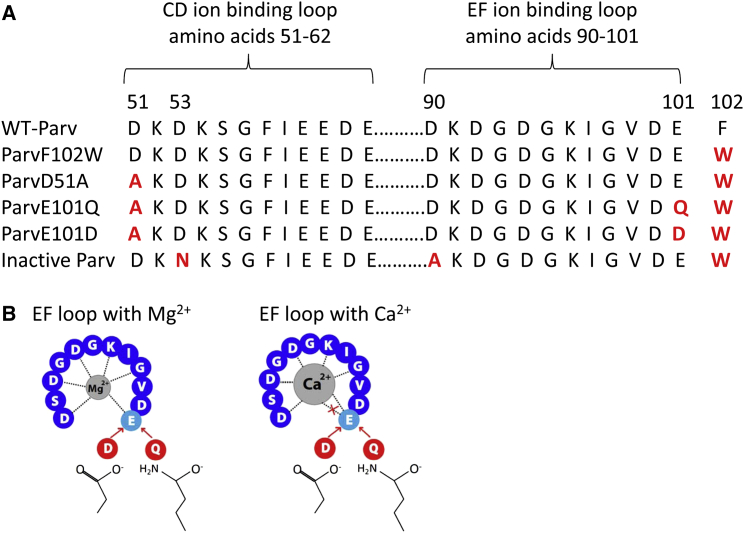
The F102W substitution was incorporated into all parvalbumins to conduct in vitro fluorescence binding assays. This substitution does not significantly alter Ca2+/Mg2+ binding affinities compared to WT-Parv (13), so ParvF102W was used as a positive control for our experiments. Another substitution, D51A was incorporated to disable ion binding in the CD site, as shown previously (13, 14), to simplify Parv structure-function analysis. ParvE101D and ParvE101Q contain all three substitutions. As a negative control, an inactive Parv with disabled cation binding in both the CD and EF hands was developed by incorporating three substitutions—D53N, D90A, and F102W. These three substitutions were previously shown to eliminate Ca2+ and Mg2+ binding (13). A FLAG tag (DYKDDDDK) was inserted onto the N-terminal end of all Parvs because the F102W substitution prevents Western blot and IF detection by commercially available antibodies. Fig. 1 shows the amino acid substitutions included in each construct (Fig. 1 A) and illustrates the loss of the seventh coordinating oxygen for Ca2+ binding in the EF ion binding site with ParvE101D and ParvE101Q (Fig. 1 B).
Figure 1.
Amino acid substitutions in human α-parvalbumin. (A) Amino acid sequences of the CD and EF metal ion binding loop of human α-Parv. Red letters represent substituted amino acids for each of the modified proteins. (B) Schematic representation of the EF-hand Ca2+/Mg2+ binding loop. Dotted lines represent amino acids providing coordinating oxygen atoms. The six coordinating oxygen atoms preferred by Mg2+ are available with E, D, or Q at residue 101. The seven oxygen atoms preferred by Ca2+ can only occur with bidentate coordination of E at residue 101. Substituting D or Q eliminates the seventh coordinating oxygen when Ca2+ is bound.
Adenovirus was made using the Ad-Max Adenoviral Vector System (Microbix Biosystems). 293 cells underwent calcium phosphate transfection (15) with pDC316-Parv and pBHGloxΔE1,E3, followed the next day by noble agar overlay. Plaques were harvested, expanded, and adenovirus purified using the AdenoX Maxi Purification Kit (Clontech Laboratories, Mountain View, CA), or high-titer purified virus was made by the University of Michigan Vector Core.
Adult rat cardiac myocyte isolation and culture
Cardiac myocytes from adult female Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were isolated and cultured as previously described (16). Myocytes were plated on laminin coated glass coverslips at a density of 20,000 cells/coverslip. 1 h after plating, myocytes were treated with adenovirus in modified M199 medium (Gibco, Life Technologies, Grand Island, NY) containing 26 mM NaHCO3, 25 mM Hepes, 0.2% (w/v) bovine serum albumin, 1% (v/v) ITS (insulin, transferrin, sodium selenite, Sigma Aldrich, St. Louis, MO), and 1× Penicillin/Streptomycin. Myocytes were given fresh media the next morning. Experiments were done 3 days postgene transfer. Myocytes were maintained in culture at 37°C and 5% CO2.
Western blotting and immunofluorescence
Relative Parv expression was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting of protein from virus-treated myocytes. Briefly, protein was boiled in radio-immunoprecipitation assay buffer with 2× Laemmli buffer and protease inhibitors for 5 min and then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 4–12% bis-tris gel (NuPAGE, Life Technologies). Protein was transferred to a PVDF membrane, blocked with 5% nonfat dry milk, and blotted with FLAG M2 mouse monoclonal antibody (1:1,500; Sigma F1804) and actin N-term rabbit polyclonal antibody (1:15,000; Sigma A2013) overnight at 4°C. The next day, membranes were incubated with secondary antibodies (goat antimouse IRDye 680, 1:10,000, LiCOR, Lincoln NE; and goat antirabbit IRDye 800, 1:10,000, Rockland Immunochemicals, Limerick, PA) for 1 h at room temperature. Membranes were imaged on the LiCOR Odyssey Infrared Imaging System.
Immunofluorescence detection of ParvE101D was done to determine subcellular localization. Myocytes were fixed in 4% paraformaldehyde, permeabilized with Triton X-100, and blocked with 20% normal goat serum (NGS). Myocytes were probed with FLAG M2 (1:500) and actin N-term (1:1000) for 1 h in 2% NGS. Myocytes were washed, reblocked in 2% NGS and probed with antimouse AF488 (1:500) and antirabbit 594 (1:1000). Coverslips were mounted onto slides with Permafluor mounting medium (Thermo Scientific, Waltham, MA) and imaged on a Zeiss Axioskop LSM 510 laser scanning confocal microscope (Thornwood, NY).
Sarcomere length measurements
Contraction and relaxation kinetics were measured with the Myocyte Calcium and Contractility System by IonOptix (Milton, MA) 3 days after adenovirus treatment. Myocytes were bathed in Tyrode’s solution and stimulated at 0.2 or 1 Hz and 25 V. For the pacing stress experiment, myocytes were paced for 20 s each at 0.2, 0.5, 1.0, and 2.0 Hz, after which pacing was stopped and spontaneous contractions were recorded for 20 s. To test acute changes in Mg2+, untreated myocytes 1 day postisolation were used. Myocytes were paced for 2 min at 0.2 Hz in Tyrode’s containing 0.2, 0.8, or 5.0 mM Mg2+, after which sarcomere length measurements were recorded for 10 min. Myocytes were visualized using an inverted microscope (Nikon Eclipse TE2000-U, Melville, NY) with a 40× objective. Sarcomere length data were acquired at 1000 Hz. Contractions were averaged and analyzed with IonWizard software (IonOptix) using fast Fourier transform. All experiments were carried out at 30°C.
Calcium measurements
Calcium transients
Myocytes were loaded with 2 μM Fura-2AM (Life Technologies) for 10 min, followed by deesterification for 20 min at room temperature. Calcium transient data were collected with the IonOptix Myocyte Calcium and Contractility System, using the μ-Stepper Switch to measure 360 nm:380 nm, which gives the relative change in cytosolic Ca2+. 360 nm measures both Ca2+ bound and Ca2+-free Fura, and 380 nm measures Ca2+-free Fura. The 380 nm wavelength is measured at 1000 Hz, and the 360 nm wavelength measured once every 10 s to account for any photobleaching or loss of Fura. Cells were paced at 0.2 or 1.0 Hz and 25 V at 30°C, transients were averaged, and kinetics calculated using IonWizard Software.
SR calcium load
SR Ca2+ load was measured by treating cells with caffeine and measuring the 340:380 nm ratio using the Ionoptix system fitted with the HyperSwitch, which measures Ca2+ bound (340 nm) and Ca2+-free (380 nm) Fura at 250 Hz in real time (no extrapolation of wavelengths is required). Myocytes were loaded with Fura-2 AM for 5 min, followed by 10 min deesterification, and then mounted onto a heated perfusion chamber. Myocytes were first perfused with normal Tyrode’s and paced for ∼10 contractions. The perfusate was then switched to Ca2+-free, Na+-free Tyrode’s for ∼20 s, followed by perfusion with Ca2+-, Na+-free Tyrode’s with 20 mM caffeine for 8 s. Caffeine was washed out with Ca2+-free, Na+-free Tyrode’s and finally, cells were paced again with normal Tyrode’s at the end of the experiment to ensure cells were still viable and Ca2+ cycling was intact. Li+ was used to replace Na+ in the Ca2+/Na+-free solution. One myocyte was analyzed per coverslip.
Calcium sparks
Myocytes were loaded with 10 μM Fluo-4AM (Life Technologies) for 20 min and deesterification proceeded for another 20 min at room temp. Myocytes were bathed in Tyrode’s solution and viewed with a 63× oil objective on a Zeiss Axioskop LSM 510 laser scanning confocal microscope, and sparks were measured with the line scanning feature using an argon laser with excitation at 488 nm and fluorescence measured at >505 nm. A single line of 512 pixels was scanned 1000 times, with a 1.61 μsec/pixel dwell time, pixel size of 0.3 μm, and 3.07 msec/line scanning speed, at 30°C after 20 s of pacing at 1 Hz. Sparks were analyzed using the SparkMaster plugin (17) for Image J (National Institutes of Health, Bethesda, MD). The criteria were set at 3.8 for all myocytes.
Statistical analysis
Data were analyzed using GraphPad Prism 6 (La Jolla, CA). For most experiments, a two-tailed t-test was used to measure differences between two groups and one-way analysis of variance with Tukey’s test for multiple comparisons was used to compare more than two groups. A Mann-Whitney test was used to analyze Ca2+ sparks because the data were not normally distributed. Spontaneous contractions were analyzed with a Chi-square test, and Ca2+ waves were analyzed with a Fisher’s exact test. P < 0.05 was considered statistically significant.
Results
Ca2+/Mg2+ binding affinities and dissociation rates
In vitro experiments with purified ParvF102W and ParvE101D were conducted to determine the Ca2+ and Mg2+ binding affinities and dissociation rates in the context of the human α-parvalbumin isoform. This isoform was studied to allow for more relevant translation in potential future clinical applications. These experiments revealed a 114-fold decrease in Ca2+ affinity and a 28-fold increase in Mg2+ affinity for ParvE101D compared to ParvF102W. Dissociation rates were 95% faster for Ca2+ and 95% slower for Mg2+ in ParvE101D compared to ParvF102W (Table 1). Compared to published data for carp β-ParvE101Q, human α-ParvE101D has a slightly lower Ca2+ affinity and a notably larger increase in Mg2+ affinity, twofold versus 28-fold over ParvF102W (13).
Table 1.
Ca2+/Mg2+ Binding Affinities and Dissociation Rates for Human α-ParvF102W and E101D
| ParvF102W | ParvE101D | |
|---|---|---|
| Binding Sites | 2a | 1a |
| KaCa2+ (M−1) | 5 × 108 ± 1 × 108 | 4.4 × 106 ± 0.1 × 106 |
| KaMg2+ (M−1) | 3.243 × 104 ± 0.008 × 104 | 9 × 105 ± 2 × 105 |
| KdCa2+ (nM) | 2.4 ± 0.5 | 226 ± 7 |
| KdMg2+ (μM) | 30.84 ± 0.08 | 1.4 ± 0.3 |
| KoffCa2+ (S−1) | 1.81 ± 0.06 | 31.5 ± 0.8 |
| KoffMg2+ (S−1) | 13.4 ± 0.4 | 1.36 ± 0.01 |
| KonCa2+ (M-1S−1) | 8 × 108 ± 2 × 108 | 1.40 × 108 ± 0.05 × 108 |
| KonMg2+ (M-1S−1) | 4.4 × 105 ± 0.1 × 105 | 10 × 105 ± 2 × 105 |
Not directly tested in this experiment, but inferred from data in (13).
Myocyte contraction studies
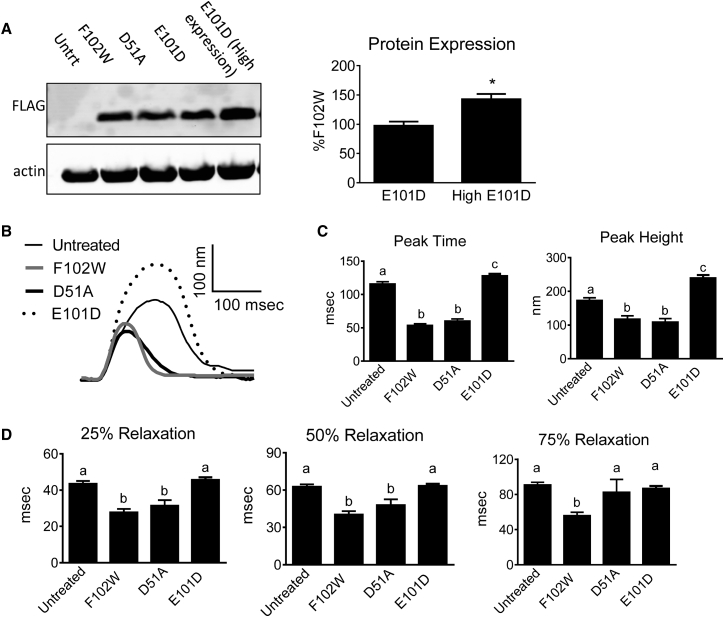
To determine the functional effects of modified Parvs, adult rat cardiac myocytes underwent adenoviral gene transfer to induce Parv expression and then sarcomere length measurements were made. Protein expression is illustrated in Fig. 2 A. ParvF102W was used as a positive control because it has been shown to have similar Ca2+/Mg2+ binding affinities and contractility/relaxation effects as WT-Parv (13). ParvD51A was tested to determine the effect of disabling ion binding in the Parv CD loop. Compared to untreated myocytes, both ParvF102W and ParvD51A exhibited diminished contraction amplitude (Fig. 2, B and C), and had faster times from peak to 25% and 50% relaxation (Fig. 2, B and D). The 75% relaxation time for ParvD51A was not different from untreated myocytes (Fig. 2, B and D), indicating a decreased buffering capacity in the late relaxation phase by disabling the CD loop. Together, these data demonstrate that ParvF102W has a similar effect compared to previously published data on WT-Parv (9), and disabling the CD site does not appreciably alter contraction or early relaxation compared to ParvF102W at the expression levels in this experiment.
Figure 2.
Protein expression and contractile function of ParvF102W, ParvD51A, and ParvE101D. (A) Left: Western blot showing relative expression levels of ParvF102W, D51A, and E101D. Untrt, untreated myocytes. Right: Quantification of band density for low and high ParvE101D expression, normalized to actin and expressed as a percentage of ParvF102W on the same blot. Bars are mean ± SE of bands from 11 to 12 samples from seven rats. ∗P < 0.05. (B) Representative traces normalized to baseline, and (C) contraction and (D) relaxation function of untreated myocytes and myocytes expressing equal protein expression of ParvF102W, D51A, and E101D. Relaxation was measured as time from peak height. Myocytes were paced at 0.2 Hz and 30°C. Data are presented as mean ± SE, N = 27–55 myocytes/group from four rats. Groups with different letters have P < 0.05.
In contrast to ParvF102W and ParvD51A, equal protein expression of ParvE101D (Fig. 2 A) resulted in significantly increased contraction amplitude (Fig. 2, B and C), and had no significant effect on relaxation (Fig. 2, B and D). Additionally, time from baseline to peak height (peak time) was increased with ParvE101D (Fig. 2 B). The absence of an effect on relaxation suggests minimal Ca2+ buffering occurs with ParvE101D at this level of protein expression.
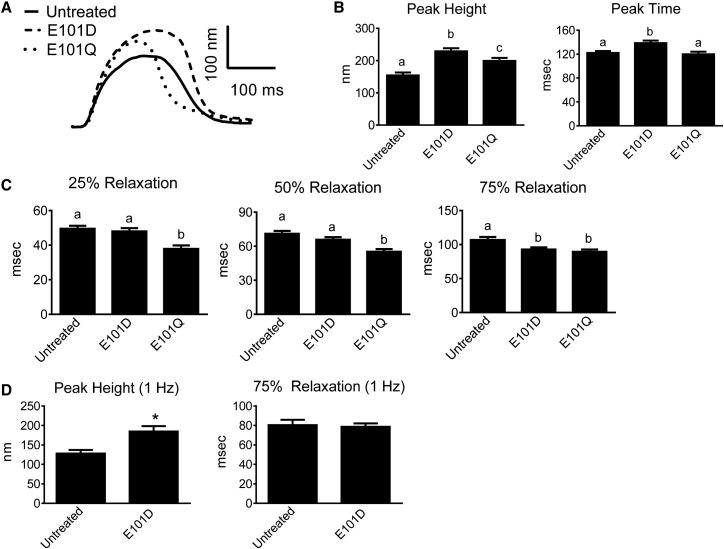
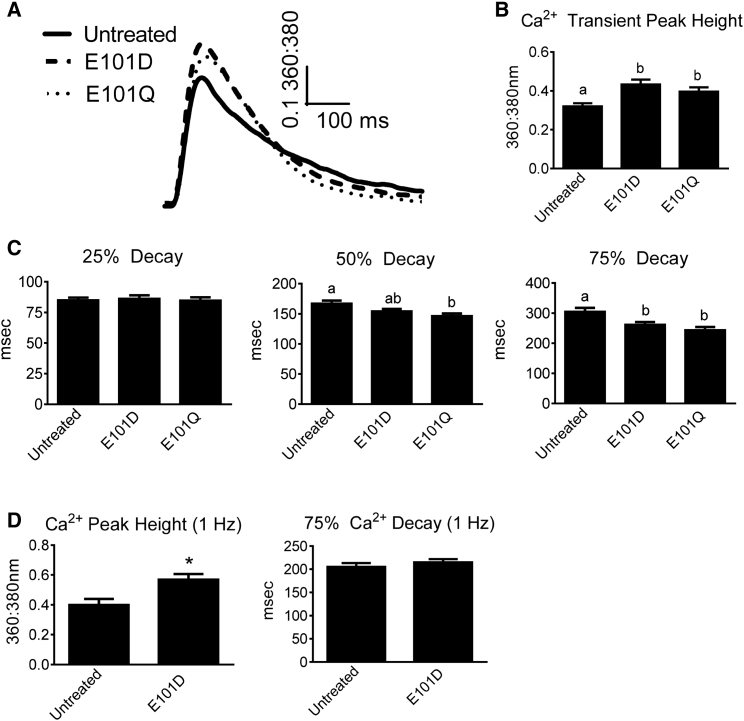
Because increasing intracellular Parv concentration increases Ca2+ buffering capacity (18), the effect of increased expression of ParvE101D was tested. A relative 1.5-fold increase in ParvE101D protein expression (Fig. 2 A, high expression) caused a significant increase in contraction amplitude compared to untreated myocytes (Fig. 3, A and B), and also exhibited decreased time from peak to 75% relaxation (Fig. 3, A and C). These data suggest the presence of Ca2+ buffering in the late relaxation phase with high expression of ParvE101D, although this did not translate to a shorter duration of the entire contraction/relaxation cycle because of the increased peak time (Fig. 3, A and B). When compared to human α-ParvE101Q, ParvE101D had significantly greater contraction amplitude and slower relaxation. The effects of human α-ParvE101Q closely mirror the effects of carp β-ParvE101Q previously published (13). The functional differences between ParvE101D and ParvE101Q suggest that higher Mg2+ affinity and slower Mg2+ dissociation from ParvE101D may have an important role in both the peak height of contraction and the timing of Ca2+ buffering. Corroborating this finding, increasing the pacing rate from 0.2 to 1 Hz preserved the increased contraction amplitude but abrogated the relaxation effect in ParvE101D-treated myocytes (Fig. 3 D).
Figure 3.
Function of ParvE101D compared with ParvE101Q. (A) Representative traces normalized to baseline, and (B) contraction and (C) relaxation function of untreated myocytes and myocytes treated with ParvE101D and ParvE101Q. Relaxation is measured as time from peak height. Data were collected at 0.2 Hz and 30°C. N = 56–57 myocytes/group from four rats. Different letters have P < 0.05. (D) Peak height and 75% relaxation time for untreated myocytes and myocytes with ParvE101D paced at 1 Hz and 30°C. N = 52–56 myocytes per group from three rats, ∗P < 0.05. All data are presented as mean ± SE.
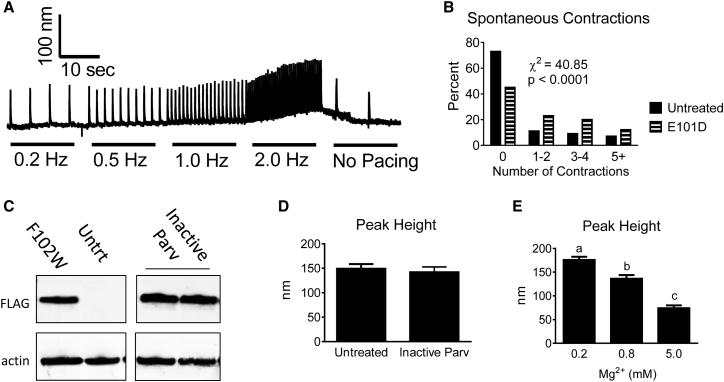
Several additional experiments were performed to mechanistically dissect the increased contraction amplitude caused by ParvE101D. First, it was noted that ParvE101D sensitized myocytes to spontaneous contractions in unstimulated conditions. To quantify this effect, myocytes underwent pacing stress after which spontaneous contractions were counted (Fig. 4 A). In the 20 s immediately following pacing, myocytes expressing ParvE101D had a significantly higher number of spontaneous contractions (Fig. 4 B). Second, to determine the necessity of Ca2+/Mg2+ binding for the increased contractility of ParvE101D, myocytes expressing an inactive Parv (both CD and EF cation binding loops disabled) were tested. At expression levels causing robust effects in myocytes expressing ParvF102W and ParvE101D (Fig. 4 C), the inactive Parv had no effect on contraction amplitude (Fig. 4 D), indicating that cation binding is necessary for the increased contractile function of ParvE101D. Finally, myocytes acutely treated with buffer containing high and low Mg2+ concentrations exhibited decreased and increased contraction amplitude, respectively (Fig. 4 E). The Mg2+ dependence on contractile function in this experiment provides indirect evidence that the Mg2+ buffering capacity of ParvE101D may have a mechanistic role in increasing contraction.
Figure 4.
Spontaneous contractions with ParvE101D, Inactive Parv contractile function, and function of myocytes with acute changes in Mg2+. (A) Representative trace and (B) histogram depicting the percentage of myocytes having unstimulated contractions after a pacing stress protocol. N = 56–60 myocytes/group from six rats. Untreated and ParvE101D groups are significantly different from each other. (C) Protein expression of Inactive Parv in cardiac myocytes 3 days after viral gene transfer. All bands are on the same blot, but intervening lanes were omitted. Untrt, untreated myocytes. (D) Peak height of cardiac myocytes expressing Inactive Parv. Data were collected at 0.2 Hz, and N = 36–41 myocytes per group from three rats. (E) Peak height was measured in cardiac myocytes acutely treated with varying concentrations of extracellular Mg2+ at 0.2 Hz. N = 51–53 myocytes from three rats. Different letters have P < 0.05. All experiments were done at 30°C.
Calcium transients
To further probe the mechanism of increased contractility in ParvE101D, Ca2+ transients were measured in myocytes loaded with Fura-2 AM. Compared to untreated myocytes, ParvE101D-expressing myocytes had a significant increase in Ca2+ transient amplitude and a faster time from peak to 75% Ca2+ transient decay (Fig. 5, A–C). ParvE101Q also caused increased peak Ca2+ transient amplitude and faster times from peak to 50% and 75% decay (Fig. 5, A–C), indicating ParvE101Q buffers Ca2+ earlier in the relaxation phase than ParvE101D. When ParvE101D-treated myocytes were paced at 1 Hz, peak Ca2+ transient amplitude remained significantly increased, but faster decay was no longer evident (Fig. 5 D), indicating that Ca2+ binding by ParvE101D is too delayed to buffer Ca2+ at a higher pacing rate. Taken together, the Ca2+ transient data closely mirror the contractility data, suggesting that both the contraction and relaxation effects of human α-ParvE101D and α-ParvE101Q are occurring through a Ca2+-dependent mechanism.
Figure 5.
Ca2+ transients from myocytes with ParvE101D and ParvE101Q. Myocytes were loaded with Fura-2 AM and 360:380 nm was measured to determine the relative amount of intracellular Ca2+. (A) Representative traces, and (B) transient peak, and (C) decay kinetics for untreated myocytes and myocytes with ParvE101D or ParvE101Q. Myocytes were paced at 0.2 Hz and 30°C. N = 56–57 myocytes/group from four rats. Different letters have P < 0.05. (D) Transient peak and decay for untreated myocytes and myocytes with ParvE101D paced at 1 Hz and 30°C. N = 35–39 myocytes per group from three rats. Data are presented as mean ± SE, ∗P < 0.05 compared to untreated cells.
Subcellular localization
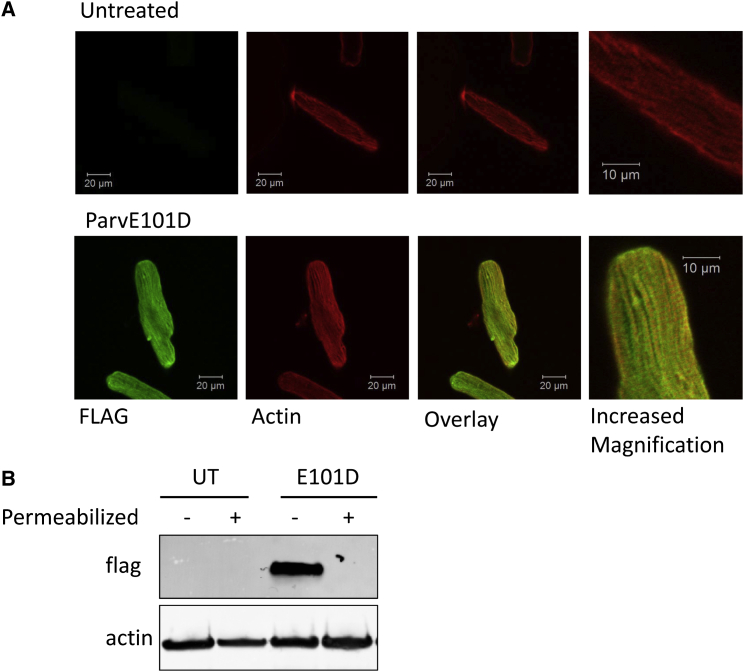
Immunofluorescence imaging of ParvE101D expression in cardiac myocytes was done to determine if subcellular localization could provide insight into the increased contractile function and Ca2+ transient amplitude. Myocytes were fixed and then permeabilized before treating with antibodies against FLAG (for ParvE101D detection) and actin. Confocal microscopy revealed general diffuse ParvE101D staining throughout myocytes, and a striated pattern that colocalized between the actin striated pattern (Fig. 6 A). When myocytes were permeabilized, washed, and then probed for ParvE101D via Western blot, there was no detectable band (Fig. 6 B), indicating that ParvE101D is cytosolic and weakly associated with the Z-line of the sarcomere.
Figure 6.
Subcellular localization of ParvE101D. (A) Representative immunofluorescence images of untreated myocytes and myocytes treated with ParvE101D. Images were obtained using confocal microscopy and a 63× oil objective. (B) Myocytes with and without ParvE101D were permeabilized with 0.1% Triton-X 100, washed, and then protein was probed for the presence of ParvE101D. UT, untreated myocytes.
SR Ca2+ load and Ca2+ leak
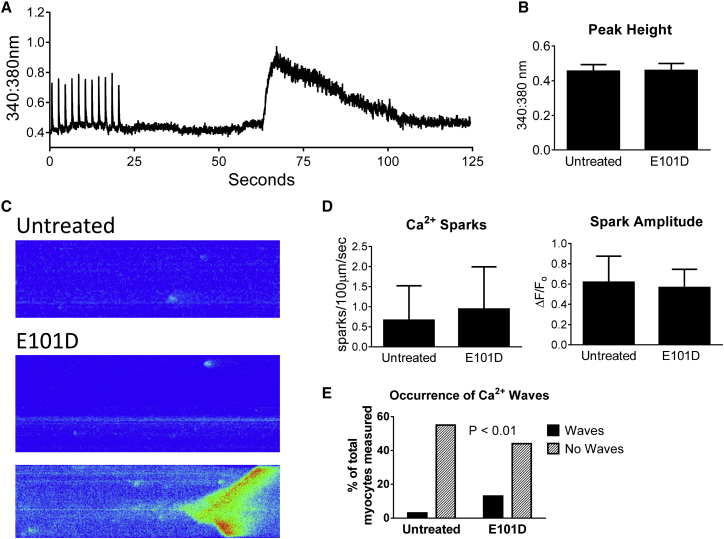
Increased Ca2+ transients caused by ParvE101D and its weak localization with the Z-line suggested that ParvE101D may affect the process of calcium-induced calcium release (CICR). Increased Ca2+ transients can occur as a result of increased SR Ca2+ load and/or increasing the percentage of total SR Ca2+ released through RyR2 during CICR (19). Thus, functional SR Ca2+ load was measured by caffeine treatment of myocytes, without Ca2+ and Na+ to eliminate potential confounding effects of NCX activity (20) (Fig. 7 A). Compared to untreated myocytes, ParvE101D-expressing myocytes had no increase in the peak height of the Ca2+ transient with caffeine (Fig. 7 B). Because of the increase in spontaneous contractions with ParvE101D (Fig. 4 B), Ca2+ sparks were measured to determine whether ParvE101D affects RyR2 open probability (Fig. 7 C). In myocytes expressing ParvE101D, there was no difference in spark frequency or amplitude (Fig. 7 D). Interestingly, the number of Ca2+ waves was increased with ParvE101D (Fig. 7, C and E), providing mechanistic insight into the spontaneous contractions measured in Fig. 4. Taken together, these data suggest that increased Ca2+ transients caused by ParvE101D are occurring independently of increased SR Ca2+ load. Despite no difference in Ca2+ spark frequency, increased Ca2+ waves suggests a potential role for ParvE101D to induce alterations in Ca2+ cycling.
Figure 7.
SR Ca2+ load, Ca2+ sparks, and waves with ParvE101D. (A) Representative trace and (B) peak height of caffeine-induced Ca2+ transients. Myocytes were tested at 30°C. Data are mean ± SE, N = 16 myocytes/group from four rats. (C) Representative images, (D) frequency and amplitude of Ca2+ sparks, and (E) occurrence of Ca2+ waves. Myocytes were tested at 30°C after pacing at 1 Hz for 20 s. Data are presented as median ± interquartile range in (D), N = 159–167 sparks and 57–58 myocytes from three rats.
Discussion
This study provides new, to our knowledge, mechanistic insights into the biophysical effects of parvalbumin-based modified EF-hand motifs. We tested the hypothesis that the inverted Ca2+/Mg2+ binding affinities of ParvE101D will increase contractility in cardiac myocytes. The main new findings from this study are 1) the 114-fold lower Ca2+ affinity and 28-fold higher Mg2+ affinity of ParvE101D compared with ParvF102W led to significant increases in Ca2+ transient amplitude and decreased time to 75% transient decay, 2) Ca2+ transient alterations can explain the increased contraction amplitude and decreased time to 75% relaxation, 3) increased contractility by ParvE101D was greater than ParvE101Q, and 4) altered global Ca2+ handling by ParvE101D caused an increase in spontaneous Ca2+ waves. The knowledge gained from these experiments provides new insight into the complexities of altered Ca2+ and Mg2+ binding affinities and dissociation rates of modified parvalbumins in the regulation of contractile function in living cardiac myocytes. These data further highlight the importance of parvalbumin’s EF-hand loop residue 12 in conferring Ca2+ and Mg2+ binding affinities, and thus determining its functional effects in cardiac myocytes. Specifically, compared to previously published studies, these experiments increase our understanding of how a Ca2+ buffering protein is able to increase contractility and highlight the importance of species/isoform-dependent effects in determining subcellular localization and effects on the Ca2+ transient. Taken together, the knowledge gained from this study contributes to the overall framework needed for strategic development of physiologically relevant Ca2+ buffers for the failing heart.
Mechanism of increased contractility with ParvE101D
The hypercontractile effect of ParvE101D is at least partially Ca2+ dependent, as evidenced by the corresponding increase in Ca2+ transient amplitude in paced myocytes. Free intracellular Ca2+ and Mg2+ have been implicated in the regulation of multiple Ca2+ handling proteins, including RyR2 (21, 22, 23), KATP (24), LTCC (25, 26), and NCX (27). Additionally, altered Mg2+ concentration can affect myofilament function (28). The significantly greater increase in contraction amplitude with ParvE101D compared to ParvE101Q suggests that the greater Mg2+ affinity of ParvE101D contributes mechanistically to increased contractility. We speculate ParvE101D causes small, transient changes in localized Ca2+ and Mg2+ concentrations through Mg2+ release/Ca2+ binding during the relaxation phase and Ca2+ release/Mg2+ binding when the Ca2+ transient has terminated. These small changes in localized Ca2+ and Mg2+ may lead to altered function of proteins involved in CICR. The increase in spontaneous contractions in myocytes with ParvE101D led us to analyze Ca2+ sparks and Ca2+ waves as live cell functional measures of global intracellular Ca2+ handling. In our experiments, Ca2+ spark frequency was not changed, but spontaneous Ca2+ waves were significantly increased. The mechanism of increased Ca2+ waves in the absence of increased sparks is not known.
Species- and isoform-specific effects of parvalbumin function
To increase the translatability of Ca2+ buffering for the human heart, this study importantly made use of the human α-Parv isoform, whereas previously the carp β-Parv isoform was used (13). Although similar in tertiary structure, the two proteins contain only 58% sequence homology. We found species/isoform-specific differences in subcellular localization patterns and Ca2+ transient effects. The increased Ca2+ transient amplitude caused by human α-ParvE101D and ParvE101Q in this study is in contrast to our previously published study on carp β-ParvE101Q, which reported a Ca2+-independent increase in contractility (13). It is possible that different subcellular localization patterns between the two isoforms contributes to the mechanistic differences of enhanced contractile function. The different localization patterns are presumably the result of sequence differences between the isoforms, although this needs to be more thoroughly investigated in future studies.
In addition to the species/isoform-specific effects of Parv on Ca2+/Mg2+ binding kinetics and buffering capacity, species differences in cardiac myocyte contraction/relaxation kinetics and Ca2+ cycling also likely has an effect on the functional effects of Parv expression. Although ParvE101D significantly increased contractility and modestly decreased relaxation time in the context of rat cardiac myocytes, the slower contraction/relaxation kinetics in the human heart may be more optimal for this ultra-delayed Ca2+ buffering protein. ParvE101D may buffer Ca2+ at a different stage of the human heart contraction/relaxation cycle, and, in this case, have a more robust effect on relaxation. These translational experiments in human heart or other large mammalian models with more human-like Ca2+ cycling kinetics will be important in the development of Ca2+ buffering as a therapeutic for human heart failure.
Mg2+ buffering
Total intracellular free Mg2+ concentrations are generally quite stable and highly buffered, making changes in Mg2+ difficult to study in the context of the intact cell. Total intracellular Mg2+ concentrations are between 17 and 20 mM and free Mg2+ is ∼1 mM (29). Based upon estimated concentrations of virally transduced human α-Parv in cardiac myocytes from previous studies (9, 18, 30), the concentration of the high dose of ParvE101D used for most experiments in this study is estimated at 0.15–0.20 mM. This relatively small increase in Mg2+ buffering capacity in cardiac myocytes appears negligible in comparison to total cellular Mg2+, but may be an important contributor to transient buffering of free Mg2+ during myocyte pacing in our experiments. In addition, the diffuse subcellular localization of ParvE101D around the Z-line may allow the formation of functionally relevant microdomains where ParvE101D is more concentrated and able to significantly buffer Mg2+ on a beat-to-beat basis during pacing. Indeed, restricted diffusion of cytosolic ions and proteins within cardiac myocytes, particularly near the sarcolemma, is an important contributor to spatiotemporal signaling (31). Indirect evidence for Mg2+ buffering as a mechanism of increased contractility in ParvE101D was shown by the contractility changes that occurred acutely in untreated myocytes exposed to solutions with different Mg2+ concentrations. Although changes in localized intracellular Mg2+ concentration were not measured directly in our study, to our knowledge, this is the first study to provide evidence of the contractile effects of Mg2+ buffering in intact cardiac myocytes.
Structure-function of ParvE101D
Eliminating EF-hand loop residue 12 bidentate coordination of Ca2+ with a single E → D substitution elicited dramatic and opposing functional effects in cardiac myocytes. Canonical EF-hand binding loops, such as WT-Parv, bind Ca2+ with high affinity because of the ability of loop residue 12 to contribute both side-chain oxygen atoms to form the pentagonal bipyrimidal coordination sphere preferred by Ca2+. Bidentate Ca2+ coordination by glutamate in ParvF102W and ParvD51A hastened relaxation at the expense of contractile function. Monodentate coordination by aspartate in ParvE101D decreased Ca2+ affinity and increased Mg2+ affinity, subsequently increasing contraction amplitude nearly 50% above that of untreated myocytes while minimally affecting relaxation. ∼8% of EF-hand proteins in nature contain aspartate at position 12. These EF-hands are generally smaller and more compact in structure than parvalbumin so that the shorter aspartate side chain can form both monodentate and bidentate coordination (32, 33). Crystallization studies and molecular dynamics simulations of Parv with an E → D substitution at loop residue 12 revealed that monodentate rather than bidentate coordination of Ca2+ occurred in the context of the larger binding loop. This preference occurred because of the rigidity of the tertiary structure and the required distance the F-helix would need to move for both side-chain oxygen atoms to be in coordinating distance to the Ca2+ ion (6, 14).
Conclusions
In conclusion, we provide evidence that the inverted Ca2+/Mg2+ binding affinities of human α-ParvE101D increase contraction amplitude in cardiac myocytes, and affect relaxation to a lesser extent. This study demonstrates that eliminating bidentate binding at EF-hand loop position 12 can elicit dramatic changes in myocyte contractile function. Additionally, these data demonstrate there is a delicate balance between Ca2+/Mg2+ binding affinities and dissociation rates that is required in designing a temporally optimized Ca2+ buffering system for human heart failure that both preserves contractile function and increases the rate of relaxation. Therefore, engineered ParvE101D provides mechanistic insight into the effects of Mg2+ buffering on rodent cardiac myocyte contractility. It will be important in future works to fully assess this and other EF-hand motifs in myocytes and hearts from larger mammals with Ca2+ handling properties more comparable to human heart.
Author Contributions
M.L.A. designed and performed most of the experiments, analyzed data, and wrote the article; F.V.S. performed myocyte isolations, made viruses, and contributed to manuscript preparation; J.S. and J.D. made purified protein, performed in vitro binding assays, and contributed to manuscript preparation; J.M.M. directed and oversaw all aspects of the project, and contributed to writing the article.
Acknowledgments
The authors thank Jenny Seong for her assistance with cloning, Lillehei Heart Institute for use of the confocal microscope, and Wang Wang and members of the J.M.M. lab for insightful discussions about the data.
This research was supported by funds from National Institutes of Health (NIH) to J.M.M. and by NIH National Heart, Lung, and Blood Institute (NHLBI) award 5F32HL115876 to M.L.A.
Editor: David Warshaw.
References
- 1.Schwaller B. The continuing disappearance of “pure” Ca2+ buffers. Cell. Mol. Life Sci. 2009;66:275–300. doi: 10.1007/s00018-008-8564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauls T.L., Cox J.A., Berchtold M.W. The Ca2+(−)binding proteins parvalbumin and oncomodulin and their genes: new structural and functional findings. Biochim. Biophys. Acta. 1996;1306:39–54. doi: 10.1016/0167-4781(95)00221-9. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V.D., Lee L., Edwards B.F.P. Refined crystal structure of calcium-liganded carp parvalbumin 4.25 at 1.5-A resolution. Biochemistry. 1990;29:1404–1412. doi: 10.1021/bi00458a010. [DOI] [PubMed] [Google Scholar]
- 4.Gifford J.L., Walsh M.P., Vogel H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 5.Permyakov E.A. Nova Science Publishers; New York: 2007. Parvalbumin. [Google Scholar]
- 6.Cates M.S., Berry M.B., Phillips G.N., Jr. Metal-ion affinity and specificity in EF-hand proteins: coordination geometry and domain plasticity in parvalbumin. Structure. 1999;7:1269–1278. doi: 10.1016/s0969-2126(00)80060-x. [DOI] [PubMed] [Google Scholar]
- 7.Beuckelmann D.J., Näbauer M., Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 8.Szatkowski M.L., Westfall M.V., Metzger J.M. In vivo acceleration of heart relaxation performance by parvalbumin gene delivery. J. Clin. Invest. 2001;107:191–198. doi: 10.1172/JCI9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutu P., Metzger J.M. Genetic manipulation of calcium-handling proteins in cardiac myocytes. I. Experimental studies. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H601–H612. doi: 10.1152/ajpheart.00424.2004. [DOI] [PubMed] [Google Scholar]
- 10.Asp M.L., Martindale J.J., Metzger J.M. Calcium mishandling in diastolic dysfunction: mechanisms and potential therapies. Biochim. Biophys. Acta. 2013;1833:895–900. doi: 10.1016/j.bbamcr.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coutu P., Metzger J.M. Genetic manipulation of calcium-handling proteins in cardiac myocytes. II. Mathematical modeling studies. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H613–H631. doi: 10.1152/ajpheart.00425.2004. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., Shettigar V., Davis J.P. Engineering parvalbumin for the heart: optimizing the Mg binding properties of rat β-parvalbumin. Front. Physiol. 2011;2:77–85. doi: 10.3389/fphys.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W., Barnabei M.S., Metzger J.M. Noncanonical EF-hand motif strategically delays Ca2+ buffering to enhance cardiac performance. Nat. Med. 2013;19:305–312. doi: 10.1038/nm.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cates M.S., Teodoro M.L., Phillips G.N., Jr. Molecular mechanisms of calcium and magnesium binding to parvalbumin. Biophys. J. 2002;82:1133–1146. doi: 10.1016/S0006-3495(02)75472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2005. Calcium phosphate - mediated transfection of eukaryotic cells. Nat. Methods. 2:319–320.
- 16.Asp M.L., Martindale J.J., Metzger J.M. Direct, differential effects of tamoxifen, 4-hydroxytamoxifen, and raloxifene on cardiac myocyte contractility and calcium handling. PLoS One. 2013;8:e78768. doi: 10.1371/journal.pone.0078768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picht E., Zima A.V., Bers D.M. SparkMaster: automated calcium spark analysis with ImageJ. Am. J. Physiol. Cell Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 18.Coutu P., Metzger J.M. Optimal range for parvalbumin as relaxing agent in adult cardiac myocytes: gene transfer and mathematical modeling. Biophys. J. 2002;82:2565–2579. doi: 10.1016/S0006-3495(02)75599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassani J.W., Yuan W., Bers D.M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am. J. Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 20.Bassani J.W., Bassani R.A., Bers D.M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusev K., Niggli E. Modulation of the local SR Ca2+ release by intracellular Mg2+ in cardiac myocytes. J. Gen. Physiol. 2008;132:721–730. doi: 10.1085/jgp.200810119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laver D.R., Honen B.N. Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: cytoplasmic and luminal regulation modeled in a tetrameric channel. J. Gen. Physiol. 2008;132:429–446. doi: 10.1085/jgp.200810001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahradníková A., Valent I., Zahradník I. Frequency and release flux of calcium sparks in rat cardiac myocytes: a relation to RYR gating. J. Gen. Physiol. 2010;136:101–116. doi: 10.1085/jgp.200910380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michailova A., Saucerman J., McCulloch A.D. Modeling regulation of cardiac KATP and L-type Ca2+ currents by ATP, ADP, and Mg2+ Biophys. J. 2005;88:2234–2249. doi: 10.1529/biophysj.104.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunet S., Scheuer T., Catterall W.A. Cooperative regulation of Ca(v)1.2 channels by intracellular Mg(2+), the proximal C-terminal EF-hand, and the distal C-terminal domain. J. Gen. Physiol. 2009;134:81–94. doi: 10.1085/jgp.200910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M., Tashiro M., Berlin J.R. Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J. Physiol. 2004;555:383–396. doi: 10.1113/jphysiol.2003.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei S.K., Quigley J.F., Haigney M.C.P. Cytosolic free magnesium modulates Na/Ca exchange currents in pig myocytes. Cardiovasc. Res. 2002;53:334–340. doi: 10.1016/s0008-6363(01)00501-6. [DOI] [PubMed] [Google Scholar]
- 28.Smith G.A., Vandenberg J.I., Freestone N.S., Dixon H.B.F. The effect of Mg 2 + on cardiac muscle function : is CaATP the substrate for priming myofibril cross-bridge formation and Ca 2 + reuptake by the sarcoplasmic reticulum? Biochem. J. 2001;551:539–551. doi: 10.1042/0264-6021:3540539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani A.M.P. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011;512:1–23. doi: 10.1016/j.abb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodenbaugh D.W., Wang W., Metzger J.M. Parvalbumin isoforms differentially accelerate cardiac myocyte relaxation kinetics in an animal model of diastolic dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1705–H1713. doi: 10.1152/ajpheart.00232.2007. [DOI] [PubMed] [Google Scholar]
- 31.Alekseev A.E., Reyes S., Terzic A. Compartmentation of membrane processes and nucleotide dynamics in diffusion-restricted cardiac cell microenvironment. J. Mol. Cell. Cardiol. 2012;52:401–409. doi: 10.1016/j.yjmcc.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook W.J., Jeffrey L.C., Vijay-Kumar S. Structure of a sarcoplasmic calcium-binding protein from amphioxus refined at 2.4 A resolution. J. Mol. Biol. 1993;229:461–471. doi: 10.1006/jmbi.1993.1046. [DOI] [PubMed] [Google Scholar]
- 33.Vijay-Kumar S., Cook W.J. Structure of a sarcoplasmic calcium-binding protein from Nereis diversicolor refined at 2.0 A resolution. J. Mol. Biol. 1992;224:413–426. doi: 10.1016/0022-2836(92)91004-9. [DOI] [PubMed] [Google Scholar]