Abstract
Objective: The aim of our study is to observe the impact of cholangiocarcinoma-derived exosomes on the antitumor activities of cytokine-induced killer (CIK) cells and then demonstrate the appropriate mechanism. Methods: Tumor-derived exosomes (TEXs), which are derived from RBE cells (human cholangiocarcinoma line), were collected by ultracentrifugation. CIK cells induced from peripheral blood were stimulated by TEXs. Fluorescence-activated cell sorting (FACS) was performed to determine the phenotypes of TEX-CIK and N-CIK (normal CIK) cells. The concentrations of tumor necrosis factor-α (TNF-α) and perforin in the culture medium supernatant were examined by using an enzyme-linked immunosorbent assay (ELISA) kit. A CCK-8 kit was used to evaluate the cytotoxic activity of the CIK cells to the RBE cell line. Results: The concentrations of TNF-α and perforin of the group TEX-CIK were 138.61 pg/ml and 2.41 ng/ml, respectively, lower than those of the group N-CIK 194.08 pg/ml (P<0.01) and 3.39 ng/ml (P<0.05). The killing rate of the group TEX-CIK was 33.35%, lower than that of the group N-CIK (47.35% (P<0.01)). The population of CD3+, CD8+, NK (CD56+), and CD3+CD56+ cells decreased in the TEX-CIK group ((63.2±6.8)%, (2.5±1.0)%, (0.53±0.49)%, (0.45±0.42)%) compared with the N-CIK group ((90.3±7.3)%, (65.7±3.3)%, (4.2±1.2)%, (15.2±2.7)%), P<0.01. Conclusions: Our results suggest that RBE cells-derived exosomes inhibit the antitumor activity of CIK cells by down-regulating the population of CD3+, CD8+, NK (CD56+), and CD3+CD56+ cells and the secretion of TNF-α and perforin. TEX may play an important role in cholangiocarcinoma immune escape.
Keywords: Cholangiocarcinoma, Tumor-derived exosomes, Cytokine-induced killer cells, Immune escape
1. Introduction
Cholangiocarcinoma (CCA) is a devastating malignant disease that is proverbially difficult to diagnose and is associated with a high mortality. Although CCA accounts for only 3% of all gastrointestinal cancers, CCA represents the second most frequent primary hepatic malignant tumor after hepatocellular carcinoma, and a progressively increasing incidence rate was observed worldwide during the last decades (Khan et al., 2005; Welzel et al., 2006; Ewald et al., 2013). Adoptive cellular immunotherapy is becoming an important application for cancer therapy. In recent decades, the application of cytokine-induced killer (CIK) cells has evolved from experimental observations into early clinical studies (Morse et al., 2005; Wang et al., 2011).
CIK cells are a population of cells derived from human peripheral blood mononuclear cells (PBMCs) after ex vivo expansion with interferon-γ (IFN-γ), monoclonal antibody against cluster of differentiation 3 (CD3), and interleukin-2 (IL-2) (Lu and Negrin, 1994). CIK cells have a higher proliferation rate, increased cytolytic activities, and a non-major histocompatibility complex (MHC)-restricted killing of tumor cells by secreting tumor necrosis factor-α (TNF-α) and perforin. CIK cells-based immunotherapy has proved to be effective in patients with a variety of malignancies, including CCA. Perforin and TNF-α, which are secreted by CIK cells, play a critical role in killing tumor cells and in immunotherapy against tumors. CIK cells have a potent cytotoxicity against tumor cells in vitro (Hongeng et al., 2003), but the curative effect of immunotherapy based on CIK cells is not that satisfying (Morse et al., 2005). Thus, we suspect that some substances secreted by tumor cells inhibit the activities of CIK cells.
Exosomes are small vesicles, about 40–100 nm in size, that are secreted by a variety of cell types, such as B-cells, T-cells, dendritic cells, and tumor cells (Raposo et al., 1996; Zitvogel et al., 1998; Wolfers et al., 2001). Tumor-derived exosomes (TEXs) have been shown to play an important role in the immune system, by mediating antigen and cytokine secretions (Bretz et al., 2013; Thompson et al., 2013).
In this study, in order to confirm the impact of TEX to CIK cells, we used the RBE cell line (human CCA cell line) to generate TEX. CIK cells were stimulated by TEX to evaluate the antitumor activities to the RBE cell line and to probe into their mechanisms, which caused CIK therapy to be dissatisfactory.
2. Materials and methods
2.1. Cell lines and culture
The RBE cell line, which is one of the human CCA cell lines, was obtained from the Committee of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). RBE cells were grown in the Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, USA) and antibiotics (50 μg/ml each of penicillin, streptomycin, and gentamicin; Gibco, USA) at 37 °C in a humidified 5% CO2 and 95% of air atmosphere.
2.2. Preparation of CIK cells
We collected 15 ml of peripheral blood in evacuated tubes that contained heparin. PBMCs were isolated by using Ficoll density-gradient centrifugation and incubated in tissue culture flasks at 37 °C for 2 h, supplemented with an RPMI-1640 medium. On Day 0, for the non-adherent PBMCs we added 1000 U/ml recombinant human IFN-γ (Genentech, USA). After 24 h, 500 U/ml IL-2 (Chiron, USA), and 50 μg/ml CD3 monoclonal antibody (OKT3; Ortho Biotech, USA) were added and then these cells were cultured for another 7 d. Half of the old medium was replaced every 2 d with fresh CIK medium and 500 U/ml IL-2, respectively.
2.3. Preparation and identification of TEX
2.3.1. Cell culture
The RBE cells were cultured in an RPMI-1640 medium, supplemented with 10% FBS and antibiotics, as described previously, until the culture flask was almost full. Then, the culture media were discarded, and the cells were washed three times with phosphate-buffered saline (PBS; Gibco, USA). Next, new media supplemented with RPMI-1640 medium, without FBS, and the antibiotics mentioned above were added to the media, and the cells were cultured for another 72 h.
2.3.2. Isolation of TEX
After that, the cell culture media were collected and sequential centrifugations were performed. The cell culture media were centrifuged at 300g for 3 min at 4 °C to remove floating cells. These supernatants were then centrifuged at 2000g for 15 min at 4 °C and at 12 000g for 35 min at 4 °C to remove all cell debris. These supernatants were then passed through a 0.22-μm filter (Millipore, USA). The filtrates were ultracentrifuged at 120 000g for 3 h at 4 °C to collect exosomes using an Optima TLX Ultracentrifuge (Beckman Coulter, CA, USA). Exosome pellets were resuspended in PBS and were further ultracentrifuged at 120 000g for 3 h at 4 °C. Exosome protein quantification was performed using a BCA protein assay kit (KeyGen Biotechnologies, China). The final exosome pellets were stored at −80 °C until use (Chiba et al., 2012).
2.3.3. Nanoparticle tracking analysis
Isolated TEX from RBE cell lines were analyzed using the nanoparticle tracking analysis (NTA) Version 2.3 Build 0034. Analysis of the data was done using the software supplied with the machine. Graphical analysis shows the size distribution of the TEX.
2.3.4. Western blot analysis
TEX was isolated by the procedure mentioned above from the RBE cell lines. A total of 20 μg TEX and RBE cell samples were solubilized in NP40 buffer (Boston BioProducts, MA, USA) and were run on a Western blot according to standard protocols. The following antibodies were used: mouse anti-CD63 (1:500, Abcam, UK), mouse anti-tumor susceptibility gene 101 (TSG101) (1:500, Abcam, UK), mouse anti-ALIX (1:500, Abcam, UK), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam, UK) as the loading control.
2.4. Identification of CIK cells
An amount of 1×106 CIK cells was harvested and washed twice with PBS. These cells were resuspended in 150 μl of PBS, labeled with 20 μl of antibodies against CD3/4/8 (PerCP-conjugated anti-CD3, FITC-conjugated anti-CD4, PE-conjugated anti-CD8; BD Biosciences, USA) and 10 μl of anti-CD56 antibody (APC-conjugated anti-CD56; BD Biosciences, USA), and placed in the dark for 30 min at 4 °C, and then washed twice with PBS. Fluorescence-activated cell sorting (FACS) was applied by using a FACSCalibur flow cytometer (BD Biosciences, USA) and CellQuest software (BD Biosciences, USA).
2.5. Detection of TNF-α and perforin
An amount of 1×106 CIK cells was added to a new 6-well flat-bottomed plate. These CIK cells, labeled as group TEX-CIK, were then treated with TEX at a ratio of 1×106 CIK cells:20 μg of TEX for 24 h. Another batch of 1×106 CIK cells, labeled as group N-CIK, was added to a new 6-well flat-bottomed plate for 24 h. After 24 h, the concentrations of TNF-α and perforin in the culture medium supernatant, with or without TEX, were examined by using an enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences, USA). Each group was tested in triplicate.
2.6. Cytotoxic examination of CIK cells
The RBE cells in the logarithmic growth phase were added to a 96-well plate (10 000 cells/well) as a target group. On the second day, TEX-CIK and N-CIK cells were added as an effector to the target ratio of 10:1 and tested in triplicate. Mixed incubation was done for 24 h. The supernatant was removed and all cells were washed twice with PBS. Then, CCK-8 was added, followed by incubation for another 3 h. The blank control was set with only PBS and CCK-8. The absorbance (optical density (OD)) was measured at 450 nm using a microplate reader. The killing rate was calculated as follows: killing rate=(ODexperiment−ODblank)/(ODnegative−ODblank)×100%.
2.7. Statistical analysis
Data were expressed as the mean±standard deviation (SD). A t-test was used for analysis of comparisons, correlation, and variance using SPSS 19.0 software (IBM, USA). P<0.05 was regarded as statistically significant.
3. Results
3.1. Characterization of TEX
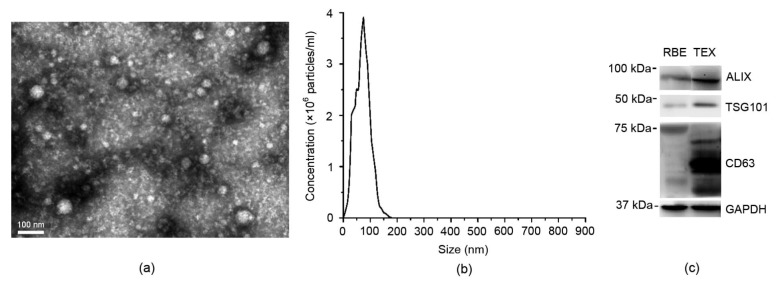
A Philips 208 transmission electron microscope (FEI Company, the Netherlands) was utilized to analyze our TEX preparation for the RBE cell supernatants from a morphological point of view. The electron microscopy indicated that TEX was accorded with the characteristics of the exosomes (Fig. 1a). The size distribution of TEX was confirmed by using the Nanoparticle tracking analysis (Version 2.3 Build 0034), which was consistent with the exosomes (Fig. 1b). The exosome identity markers ALIX, CD63, and TSG101 were confirmed by using a Western blot analysis (Fig. 1c).
Fig. 1.
Characteristics of TEX
(a) Electron microscopy of TEX (bar=100 nm). The culture supernatants yielded pellets consisting of a similar population of vesicles 30–120 nm in diameter. (b) The size distribution of TEX. (c) Western plot showing the expressions of exosomal markers ALIX, TSG101, and CD63 in TEX, with GAPDH as the loading control
3.2. Phenotypic analysis of CIK cells
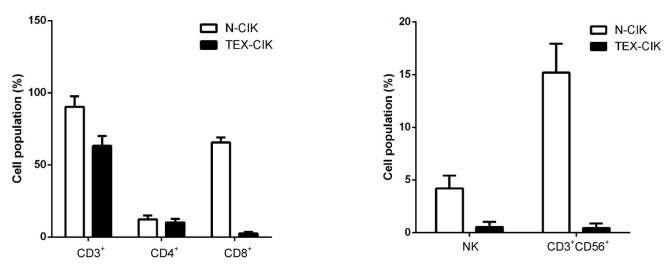
To determine the immunological effects of TEX-CIK and N-CIK cells, the phenotypes were characterized. The population of CD3+, CD8+, NK (CD56+) and CD3+CD56+ cells decreased in the TEX-CIK group ((63.2±6.8)%, (2.5±1.0)%, (0.53±0.49)%, (0.45±0.42)%) compared with the N-CIK group ((90.3±7.3)%, (65.7±3.3)%, (4.2±1.2)%, (15.2±2.7)%) (P<0.01; Fig. 2). The population of CD4+ cells in the TEX-CIK group was (10.1±2.5)%, not statistically significant when compared with that (12.3±2.7)% in the N-CIK group (P>0.05).
Fig. 2.
Characteristics of cell phenotypes
The populations of CD3+, CD8+, NK (CD56+), and CD3+CD56+ cells decreased in the TEX-CIK group compared with the N-CIK group (P<0.01). However, the population change of CD4+ cells was not statistically significant (P>0.05)
3.3. TEX down-regulates the secretion of TNF-α and perforin
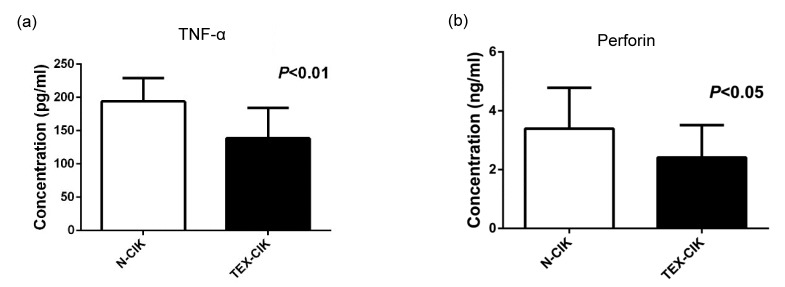
The concentrations of TNF-α and perforin of the group TEX-CIK were (138.61±46.01) pg/ml and (2.41±1.10) ng/ml, respectively, lower than those of the group N-CIK (194.08±35.14) pg/ml and (3.39±1.39) ng/ml (P<0.05; Fig. 3).
Fig. 3.
Concentrations of TNF-α and perforin
(a) The concentration of TNF-α of the groups TEX-CIK and N-CIK were (138.61±46.01) pg/ml, (194.08±35.14) pg/ml, respectively. (b) The concentration of perforin of the groups TEX-CIK and N-CIK were (2.41±1.10) ng/ml, (3.39±1.39) ng/ml, respectively. The secretion of TNF-α (P<0.01) and perforin (P<0.05) was down-regulated by TEX in the CIK cells
3.4. TEX inhibits the antitumor activity of the CIK cells
The killing rate of the group TEX-CIK was (33.35±10.05)%, significantly lower than that of the group N-CIK (47.35±12.74)% (P<0.01; Fig. 4).
Fig. 4.
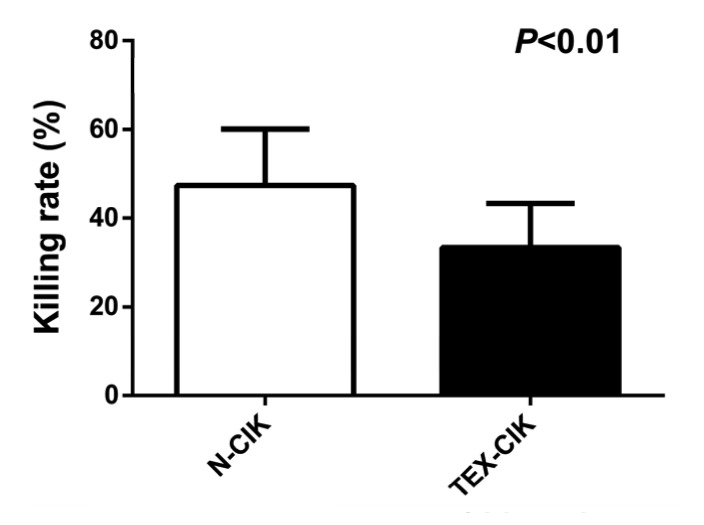
Killing rate of CIK cells
The killing rate of the group TEX-CIK was (33.35±10.05)%, significantly lower than that of the group N-CIK (47.35±12.74)% (P<0.01)
4. Discussion
CCA was first reported by Durand-Fardel in 1840 (Olnes and Erlich, 2004). The disease is notoriously difficult to diagnose and is usually fatal because of its late clinical identification and the lack of effective non-surgical therapeutic modalities (Khan et al., 2005). Over the past two decades, the development of CIK cells for the treatment of carcinoma has received considerable attention. CIK cells have a major histocompatibility complex (MHC)-independent antitumor ability in both solid and hematologic malignancies. The antitumor activity is primarily related to a high percentage of the CD3+CD56+ subset (Ma et al., 2012; Nakano et al., 2012). The exact mechanism is still unclear, but primarily relates to the secretion of cytokines to inhibit the growth of tumor cells (Pievani et al., 2011; Wang et al., 2011; Yang et al., 2012). A large number of in vitro studies had confirmed the ability of CIK cells to kill various types of solid tumors. The CD3+CD56+ fraction of CIK cells is considered the crucial effector for the antitumor activities. Proofs of antitumor activities were clearly provided but the clinical responses were much lower than expected (Mesiano et al., 2012).
Exosomes are microvesicles released from various cells into the extracellular space, including tumor cell lines (Thompson et al., 2013). TEXs have been shown to play an important role in the immune system (Sun et al., 2012) by mediating apoptosis of the T lymphocytes and down-regulating the cytokine’s presentation (Wolfers et al., 2001). CD3 as a T-cell co-receptor is an important part of the T-cell receptor (TCR) complex. CD8 also serves as a co-receptor for the TCR and binds it to an MHC class I molecule (Garcia et al., 1996). The importance of the MHC class and TCR in cancer immunology is greater than expected when compared with previous studies (Slansky et al., 2000; Xue et al., 2013).
The results in this study showed the imporatance of TEX in down-regulating the population of CD3+, CD8+, NK (CD56+), and CD3+CD56+ cells in the TEX-CIK group when compared with the N-CIK group (P<0.01). The concentrations of TNF-α and perforin in the group TEX-CIK were lower than those in the group N-CIK (P<0.05). The down-regulation of CD3 and CD8 may attenuate the ability of antigen in presenting, thus meditating the tumor immune escape. Taylor et al. (2003) suggested that FasL associated with TEXs was responsible for apoptosis of the T lymphocytes and a decrease of CD3+ cells in patients with ovarian carcinoma. Wieckowski et al. (2009) showed that tumor-derived microvesicles induce apoptosis in CD8+ T lymphocytes. The subset of the CD3+CD56+ cells, which expresses both the T-cell marker CD3 and the natural killer cell marker CD56, is termed non-MHC-restricted T cells. These cells are capable of killing both autologous and allogeneic tumor targets, and show the greatest cytotoxicity (Schmidt-Wolf et al., 1993). CD3+CD56+ lymphocytes play a critical role in the antitumor process by secreting TNF-α and perforin. TNF-α is a cytokine able to induce hemorrhagic necrosis of tumors and is regarded as a promising approach for cancer therapeutics (Carswell et al., 1975). TNF-α is able to induce fever, apoptotic cell death, cachexia, and inflammation, and to inhibit tumorigenesis and viral replication. Perforin is a cytolytic mediator produced by killer lymphocytes. The protein is partially homologous to the terminal components of the membrane attack complex, which complements and produces pores of up to 20 nm in diameter in the target membranes (Liu et al., 1995). Upon degranulation, perforin inserts itself into the target cell’s plasma membrane, forming pores (Tschopp et al., 1986). This protein creates transmembrane tubules and is capable of lysing non-specifically a variety of target cells. This protein is one of the main cytolytic proteins of cytolytic granules, and it is also known to be a key effector molecule for T-cell- and natural killer-cell-mediated cytolysis. The data above showed that TEX down-regulated the population of CD3+, CD8+, NK (CD56+), and CD3+CD56+ cells, especially the population of CD3+CD56+ cells, resulting in the secretion decreases of TNF-α and perforin, thus the antitumor ability of the TEX-CIK group was lower than that of the N-CIK group. An increasing number of literature show that transforming growth factor-β (TGF-β), Wnt signaling components, phosphatase and tensin homolog (PTEN), epidermal growth factor receptor (EGFR), and Fas ligand may play important roles in exosome-meditated tumor immunosuppression (Apte et al., 2013). Thus, the further mechanism underlying the depletion of CD3+CD56+ CIK cells upon exosome treatment needs additional research.
5. Conclusions
From this discussion, one may conclude that RBE cells-derived exosomes (TEXs) inhibit the antitumor activity of CIK cells by down-regulating the population of CD3+, CD8+, NK (CD56+), and CD3+CD56+ cells and the secretion of TNF-α and perforin. TEX may play an important role in CCA immune escape.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81272671) and the Foundation of Health and Family Planning Commission of Zhejiang Province (No. 2015KYB218), China
Compliance with ethics guidelines: Jiong-huang CHEN, Jian-yang XIANG, Guo-ping DING, and Li-ping CAO declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. All participants or their guardians gave written consent for their blood samples to be used for scientific research. The research protocol was reviewed and approved by the Research Ethics Committee of Zhejiang University, Hangzhou, China.
References
- 1.Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144(6):1210–1219. doi: 10.1053/j.gastro.2012.11.037. (Available from: http://dx.doi.org/10.1053/j.gastro.2012.11.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretz NP, Ridinger J, Rupp AK, et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288(51):36691–36702. doi: 10.1074/jbc.M113.512806. (Available from: http://dx.doi.org/10.1074/jbc.M113.512806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. PNAS. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. (Available from: http://dx.doi.org/10.1073/pnas.72.9.3666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiba M, Kimura M, Asari S. Exosomes secreted from human colorectal cancer cell lines contain mRNAs, microRNAs and natural antisense RNAs, that can transfer into the human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep. 2012;28(5):1551–1558. doi: 10.3892/or.2012.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewald F, Grabinski N, Grottke A, et al. Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma. Int J Cancer. 2013;133(9):2065–2076. doi: 10.1002/ijc.28214. (Available from: http://dx.doi.org/10.1002/ijc.28214) [DOI] [PubMed] [Google Scholar]
- 6.Garcia KC, Scott CA, Brunmark A, et al. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384(6609):577–581. doi: 10.1038/384577a0. (Available from: http://dx.doi.org/10.1038/384577a0) [DOI] [PubMed] [Google Scholar]
- 7.Hongeng S, Petvises S, Worapongpaiboon S, et al. Generation of CD3+CD56+ cytokine-induced killer cells and their in vitro cytotoxicity against pediatric cancer cells. Int J Hematol. 2003;77(2):175–179. doi: 10.1007/BF02983217. (Available from: http://dx.doi.org/10.1007/BF02983217) [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 9.Liu CC, Walsh CM, Young JD. Perforin: structure and function. Immunol Today. 1995;16(4):194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 10.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153(4):1687–1696. [PubMed] [Google Scholar]
- 11.Ma Y, Xu YC, Tang L, et al. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1(1):11. doi: 10.1186/2162-3619-1-11. (Available from: http://dx.doi.org/10.1186/2162-3619-1-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesiano G, Todorovic M, Gammaitoni L, et al. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12(6):673–684. doi: 10.1517/14712598.2012.675323. (Available from: http://dx.doi.org/10.1517/14712598.2012.675323) [DOI] [PubMed] [Google Scholar]
- 13.Morse MA, Garst J, Osada T, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3(1):9. doi: 10.1186/1479-5876-3-9. (Available from: http://dx.doi.org/10.1186/1479-5876-3-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano M, Saeki C, Takahashi H, et al. Activated natural killer T cells producing interferon-gamma elicit promoting activity to murine dendritic cell-based autoimmune hepatic inflammation. Clin Exp Immunol. 2012;170(3):274–282. doi: 10.1111/j.1365-2249.2012.04664.x. (Available from: http://dx.doi.org/10.1111/j.1365-2249.2012.04664.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66(3):167–179. doi: 10.1159/000077991. (Available from: http://dx.doi.org/10.1159/000077991) [DOI] [PubMed] [Google Scholar]
- 16.Pievani A, Borleri G, Pende D, et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118(12):3301–3310. doi: 10.1182/blood-2011-02-336321. (Available from: http://dx.doi.org/10.1182/blood-2011-02-336321) [DOI] [PubMed] [Google Scholar]
- 17.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi: 10.1084/jem.183.3.1161. (Available from: http://dx.doi.org/10.1084/jem.183.3.1161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt-Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21(13):1673–1679. [PubMed] [Google Scholar]
- 19.Slansky JE, Rattis FM, Boyd LF, et al. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13(4):529–538. doi: 10.1016/s1074-7613(00)00052-2. (Available from: http://dx.doi.org/10.1016/S1074-7613(00)00052-2) [DOI] [PubMed] [Google Scholar]
- 20.Sun D, Zhuang X, Zhang S, et al. Exosomes are endogenous nanoparticles that can deliver biological information between cells. Adv Drug Deliv Rev. 2012;65(3):342–347. doi: 10.1016/j.addr.2012.07.002. (Available from: http://dx.doi.org/10.1016/j.addr.2012.07.002) [DOI] [PubMed] [Google Scholar]
- 21.Taylor DD, Gerçel-Taylor C, Lyons KS, et al. T-cell apoptosis and suppression of T-cell receptor CD3-ζ by Fas ligand containing membrane vesicles shed from ovarian tumors. Clin Cancer Res. 2003;9(14):5113–5119. [PubMed] [Google Scholar]
- 22.Thompson CA, Purushothaman A, Ramani VC, et al. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288(14):10093–10099. doi: 10.1074/jbc.C112.444562. (Available from: http://dx.doi.org/10.1074/jbc.C112.444562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tschopp J, Masson D, Stanley KK. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature. 1986;322(6082):831–834. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Dai H, Li H, et al. Growth of human colorectal cancer SW1116 cells is inhibited by cytokine-induced killer cells. Clin Dev Immunol. 2011;2011:621414. doi: 10.1155/2011/621414. (Available from: http://dx.doi.org/10.1155/2011/621414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98(12):873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 26.Wieckowski EU, Visus C, Szajnik M, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. (Available from: http://dx.doi.org/10.4049/jimmunol.0900970) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. (Available from: http://dx.doi.org/10.1038/85438) [DOI] [PubMed] [Google Scholar]
- 28.Xue SA, Gao L, Ahmadi M, et al. Human MHC class I-restricted high avidity CD4+ T cells generated by co-transfer of TCR and CD8 mediate efficient tumor rejection in vivo. Oncoimmunology. 2013;2(1):e22590. doi: 10.4161/onci.22590. (Available from: http://dx.doi.org/10.4161/onci.22590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Zhang Q, Xu K, et al. Combined therapy with cytokine-induced killer cells and oncolytic adenovirus expressing IL-12 induce enhanced antitumor activity in liver tumor model. PLoS ONE. 2012;7(9):e44802. doi: 10.1371/journal.pone.0044802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. (Available from: http://dx.doi.org/10.1038/nm0598-594) [DOI] [PubMed] [Google Scholar]