Abstract
Objectives. To investigate whether less-healthy work–family life histories contribute to the higher cardiovascular disease prevalence in older American compared with European women.
Methods. We used sequence analysis to identify distinct work–family typologies for women born between 1935 and 1956 in the United States and 13 European countries. Data came from the US Health and Retirement Study (1992–2006) and the Survey of Health, Aging, and Retirement in Europe (2004–2009).
Results. Work–family typologies were similarly distributed in the United States and Europe. Being a lone working mother predicted a higher risk of heart disease, stroke, and smoking among American women, and smoking for European women. Lone working motherhood was more common and had a marginally stronger association with stroke in the United States than in Europe. Simulations indicated that the higher stroke risk among American women would only be marginally reduced if American women had experienced the same work–family trajectories as European women.
Conclusions. Combining work and lone motherhood was more common in the United States, but differences in work–family trajectories explained only a small fraction of the higher cardiovascular risk of American relative to European women.
Life expectancy is shorter in the United States than in many Western European countries. Older Americans are also more likely to report poorer health and to suffer chronic conditions, particularly American women.1,2 Explanations for this so-called US health disadvantage include differences in the prevalence of smoking and other behavioral risk factors, rates of disease and injury, financial barriers to health care access, and psychosocial stress.2–6 Although higher smoking prevalence histories among older women in the United States is one of the driving explanations,3 none of these factors fully accounts for the female US health disadvantage.
Lives of American women changed substantially in the second half of the previous century. Female labor force participation increased more in the United States than in many European countries,7 and marriage rates decreased more rapidly for US women as a result of a higher fraction of American women never marrying as well as higher divorce rates.8,9 By contrast, although fertility rates declined in all countries,10 they declined less in the United States than in many European countries, leaving more American women facing the prospect of combining work and family roles, often in the context of lone motherhood.
Women who are married, employed, and have children are generally healthier than their unmarried, nonemployed, and childless counterparts.11,12 Whereas the role accumulation theory suggests that combining family and work roles is beneficial for women’s health, the multiple role theory poses that combining these roles may increase levels of stress, which has a negative impact on health.13 These negative impacts may, however, depend on the availability of supportive policies that enable parents to combine work with family roles.
We hypothesized that work–family trajectories may be differentially related to cardiovascular health in the United States than in Europe, as a result of the different work–family policy environment in the United States and Europe. If combining family and work roles is beneficial for a woman’s health, women experiencing a more family-friendly policy environment such as that in Europe may benefit more from role accumulation, resulting in better cardiovascular health. If combining roles is detrimental for a woman’s health, American women may experience more strain from work–family stress than European women as a result of a less supportive policy environment in the United States.
The aim of this study was to assess whether less-healthy work–family life histories among American women have contributed to their cardiovascular health disadvantage in older age relative to women in 13 European countries. We used unique retrospective data for 13 European countries and the United States to construct full life histories and work–family trajectories, and linked them to stroke and heart disease outcomes in older ages. We examined the association between work–family trajectories and late-life cardiovascular outcomes and assessed whether the distribution and risks associated with these work–family trajectories explain why older American women have higher stroke and heart disease prevalence than older women in Europe.
METHODS
We used data from the Survey of Health, Aging, and Retirement in Europe (SHARE) for 13 European countries (Austria, Belgium, Czech Republic, Denmark, France, Germany, Greece, Italy, Netherlands, Poland, Spain, Sweden, and Switzerland). SHARE is a longitudinal biennial survey designed to provide insight into the lives of Europeans aged 50 years and older and their spouses.14 For SHARE, representative samples of noninstitutionalized adults aged 50 years and older in each European country were selected and interviewed in the household. The SHARE survey included approximately 30 000 respondents at each wave. The third wave of SHARE (2008–2009, “SHARELIFE”) was specifically designed to gather information on retrospective life histories.15 We used these data to construct marriage, employment, and maternal histories over the life course and to derivate work–family trajectories for each individual.16 Complete work–family trajectories were available for 14 545 European women. We restricted our sample to women born between 1935 and 1956 (n = 10 569 women). We linked the work–family typologies of these women to self-reported data on chronic diseases and risk factors measured in 2006 (n = 9456), and to data on their educational attainment from either the 2004 or the 2006 wave of SHARE (n = 9047). Because educational attainment was only asked during the baseline interview, we used educational information from the 2004 wave for those women who also participated in the earlier wave. Information on age and country of residence was available for all remaining women in our sample.
For the United States, we used data from the Health and Retirement Study (HRS), a longitudinal biennial survey of American adults aged 50 years and older.17 The HRS incorporated a representative sample of the noninstitutionalized population aged 50 years and older, and it included approximately 20 000 respondents every 2 years. We reconstructed marital, employment, and maternal histories by using retrospective reports collected at the baseline HRS interview. We then complemented these histories with the respondent’s self-reports from successive waves of HRS from 1992 to 2006. In total, complete life histories were available for 7681 American women born between 1935 and 1956. We then linked the work–family typologies to chronic diseases and risk factors measured in the HRS interview in 2006, leaving 5985 women. To avoid that our US results would be driven by racial disparities in cardiovascular risk factors and chronic diseases,18,19 we controlled for race (non-Hispanic Whites, non-Hispanic Blacks, and other races).
We categorized educational attainment by using the International Standard Classification of Education (ISCED)20 scale into 4 levels: ISCED levels 0 and 1 (less than high school), ISCED level 2 (high school or equivalent), ISCED levels 3 and 4 (some college), and ISCED levels 5 and 6 (college or above). The distribution of educational attainment in the US and European sample can be found in Table A (available as a supplement to the online version of this article at http://www.ajph.org).
We defined work–family trajectories on the basis of 3 dimensions: marriage, employment, and child histories. Participants were asked about starting and ending dates of all marriages and of jobs, and birth dates of children. Questions were similar for the American and European samples. We used binary variables to keep the maximum number of combinations manageable. We defined employment status as having a paid job, maternity status as having at least 1 child younger than 18 years, and marital status as being married versus nonmarried (widowed, divorced, and never married).
We focused on 2 major chronic diseases shown to be more prevalent in the United States than in European countries: heart disease and stroke.21 Participants were asked whether they had received a doctor’s diagnosis for any of these conditions. We also incorporated diagnosed high blood pressure, whether individuals had ever smoked, and body mass index (BMI) based on self-reported weight in kilograms divided by height in meters squared. A respondent was classified as being obese if her BMI was 30 or higher. The mean and range of BMI for both samples can be found in Table A. The exact questions asked to the European and American respondents are presented in Table B (available as a supplement to the online version of this article at http://www.ajph.org).
Sequence Analysis
To identify common work–family typologies in our data, we used sequence analysis, an approach that enabled us to identify work–family typologies derived from full work–family histories.22 For each woman, we determined the work–family situation on the basis of 8 possible combinations of employment status (working for pay: yes or no), marital status (married: yes or no), and maternal status (at least 1 child younger than 18 years: yes or no) at each age between 16 and 50 years. This means that, for each woman, and on the basis of retrospective reports, we assigned a work–family combination for each year of life between the ages of 16 and 50 years. The analysis then used the timing and duration of each work–family combination to derive common trajectories. A detailed description of sequence analysis is included in Figure A (available as a supplement to the online version of this article at http://www.ajph.org). An application of sequence analysis in a similar context is also available elsewhere.23
For the analysis, we pooled US and European data. In total, we found 15 542 distinct life trajectories for the 18 250 women in our sample. To define the optimal number of typologies, we used different cluster cut-off criteria, including the Point Biserial Correlation and the Calinski–Harabasz index (Table C, available as a supplement to the online version of this article at http://www.ajph.org).24 On the basis of these criteria, we established that both 5 and 6 typologies best suited the data. The 5 typologies solution provided more (sociologically) meaningful and argumentative typologies; the 6 typologies solution had an unclear and less argumentative pattern (Figure C, available as a supplement to the online version of this article at http://www.ajph.org). We therefore focused our main interpretation on the 5 typologies solution. We conducted the sequence analysis by using the TraMineR and Weighted Cluster packages in R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).24,25
Statistical Analysis
We used logistic regression to estimate the difference in cardiovascular disease and risk factors of American compared with European women. We examined whether the distribution of the work–family typologies differed between American and European women. We used logistic regression to model cardiovascular diseases and risk factors as a function of work–family typology with control for race in the United States, age (indicated by 5-year age intervals), educational attainment, whether women resided in the United States or Europe, and European country of residence. To assess whether associations between work–family typology and cardiovascular outcomes differed between the United States and Europe, we used a Wald test for the interaction between work–family typology and region of residence. To assess the contribution of work–family typology to chronic disease and risk factors between the United States and Europe, we combined estimates from the logistic models with the observed distribution of women over the typologies in the United States and Europe. This enabled us to obtain the probabilities of each outcome for US women, under the counterfactual scenario that they had been exposed to the same distribution of and cardiovascular risk associated with each typology as European women. We have applied this approach before.26
We conducted all statistical analyses with Stata SE version 13 (StataCorp LP, College Station, TX).
RESULTS
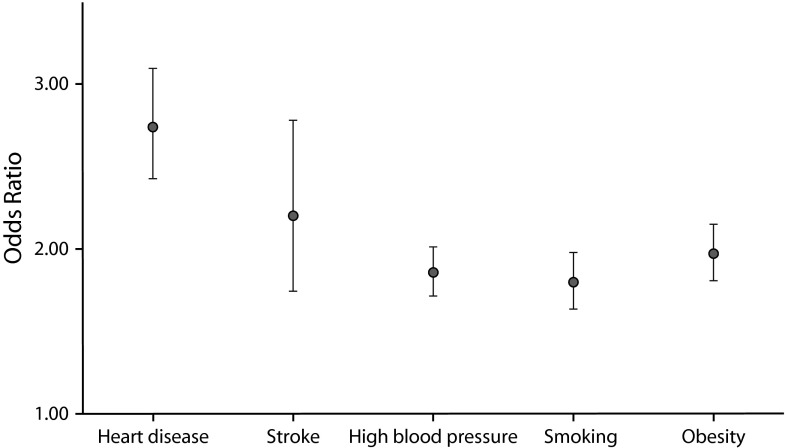
American women had poorer cardiovascular health and less-healthy risk-factor profiles than women in Europe (Figure 1), as indicated by an increased odds for all outcomes, particularly for having had a diagnosis of heart disease (odds ratio [OR] = 2.74; 95% confidence interval [CI] = 2.43, 3.10) or stroke (OR = 2.21; 95% CI = 1.75, 2.79).
FIGURE 1—
Odds Ratios of Heart Disease, Stroke, High Blood Pressure, Smoking, and Obesity for American Women Compared With European Women, Aged 50–72 Years
Note. Models controlled for race (United States only), age, and education.
Source. US Health and Retirement Study (1992–2006) and the Survey of Health, Aging, and Retirement in Europe (2004–2009).
Work–Family Typologies Over the Life Course
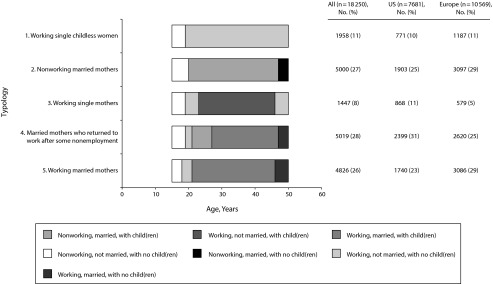
The most common work–family typology comprised married mothers who returned to work after a few years of nonemployment (28%), and the least common typology comprised working single mothers (8%; Figure 2). In both the United States and Europe, about 10% of women were single, working, childless women (typology 1, 10% in the United States, 11% in Europe). Twenty-five percent of American women were stay-home married mothers (typology 2), compared with 29% of European women. A larger proportion of American (11%) than European (5%) women was classified as working single mothers (typology 3). More American (31%) than European women (25%) were married mothers that returned to work after a few years of nonemployment (typology 4). More European (29%) than American (23%) women were working married mothers (typology 5). Distributions of age, educational attainment, and reported cardiovascular risk factors and chronic diseases by typology are presented in Table D (available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 2—
Work–Family Typologies Between the Ages of 16 and 50 Years for US and European Women Aged 50–72 Years
Source. US Health and Retirement Study (1992–2006) and the Survey of Health, Aging, and Retirement in Europe (2004–2009).
Cardiovascular Disease and Risk Factors
Working, single, childless women had lower odds of having high blood pressure than working married mothers (Table 1; OR = 0.84; 95% CI = 0.74, 0.96). Compared with working married mothers, nonworking married mothers had significantly lower odds of having ever smoked (OR = 0.85; 95% CI = 0.76, 0.96), but higher odds of being obese (OR = 1.12; 95% CI = 1.01, 1.25). Working single mothers had higher odds of heart disease (OR = 1.40; 95% CI = 1.14, 1.71), stroke (OR = 1.74; 95% CI = 1.22, 2.47), and smoking (OR = 1.77; 95% CI = 1.50, 2.09). Married mothers who left work and subsequently returned to work after a few years had higher odds of stroke (OR = 1.35; 95% CI = 1.03, 1.77) compared with working married mothers.
TABLE 1—
Odds Ratios of Combinations of Work, Marital Status, and Having Children on Risk Factors and Chronic Diseases for Women Aged 50–72 Years in the Pooled Data and Separately for the United States and Europe
| Chronic Diseases and Risk Factors | Sample Size, No. | Typology 1: Working Single Childless Women, OR (95% CI) | P | Typology 2: Nonworking Married Mothers, OR (95% CI) | P | Typology 3: Working Single Mothers, OR (95% CI) | P | Typology 4: Married Mothers Who Returned to Work After Some Nonemployment, OR (95% CI) | P | Typology 5: Working Married Mothers, OR (Ref) |
| Heart disease | .48 | .82 | .87 | .07 | ||||||
| Pooled | 15 026 | 1.04 (0.84, 1.28) | 0.98 (0.84, 1.15) | 1.40 (1.14, 1.71) | 1.05 (0.91, 1.21) | 1 | ||||
| US | 5 979 | 0.96 (0.72, 1.27) | 0.96 (0.79, 1.18) | 1.34 (1.05, 1.70) | 0.94 (0.78, 1.14) | 1 | ||||
| Europe | 9 047 | 1.11 (0.82, 1.52) | 1.00 (0.78, 1.28) | 1.39 (0.95, 2.02) | 1.24 (0.99, 1.55) | 1 | ||||
| Stroke | .24 | .57 | .06 | .95 | ||||||
| Pooled | 15 028 | 1.23 (0.83, 1.83) | 1.01 (0.75, 1.38) | 1.74 (1.22, 2.47) | 1.35 (1.03, 1.77) | 1 | ||||
| US | 5 981 | 1.53 (0.91, 2.57) | 1.09 (0.72, 1.64) | 2.09 (1.36, 3.21) | 1.38 (0.96, 2.00) | 1 | ||||
| Europe | 9 047 | 0.94 (0.51, 1.73) | 0.90 (0.56, 1.46) | 0.88 (0.39, 1.97) | 1.36 (0.91, 2.03) | 1 | ||||
| High blood pressure | .17 | .45 | .96 | .14 | ||||||
| Pooled | 15 027 | 0.84 (0.74, 0.96) | 1.05 (0.95, 1.16) | 0.96 (0.83, 1.12) | 1.05 (0.96, 1.15) | 1 | ||||
| US | 5 980 | 0.94 (0.77, 1.15) | 1.10 (0.93, 1.28) | 0.98 (0.80, 1.20) | 1.14 (0.99, 1.31) | 1 | ||||
| Europe | 9 047 | 0.78 (0.66, 0.92) | 1.01 (0.89, 1.15) | 0.97 (0.78, 1.21) | 0.99 (0.87, 1.12) | 1 | ||||
| Smoking | .20 | .90 | .14 | .73 | ||||||
| Pooled | 9 806 | 1.12 (0.97, 1.31) | 0.85 (0.76, 0.96) | 1.77 (1.50, 2.09) | 1.08 (0.97, 1.21) | 1 | ||||
| US | 5 984 | 1.11 (0.91, 1.35) | 0.85 (0.73, 0.99) | 1.67 (1.38, 2.03) | 1.08 (0.94, 1.24) | 1 | ||||
| Europe | 3 822 | 1.37 (1.07, 1.75) | 0.87 (0.70, 1.07) | 2.20 (1.62, 2.99) | 1.12 (0.94, 1.34) | 1 | ||||
| Obesity | .13 | .39 | .37 | .13 | ||||||
| Pooled | 14 915 | 0.99 (0.86, 1.14) | 1.12 (1.01, 1.25) | 1.06 (0.91, 1.24) | 1.03 (0.93, 1.14) | 1 | ||||
| US | 5 984 | 1.08 (0.88, 1.33) | 1.17 (1.00, 1.37) | 1.01 (0.83, 1.23) | 0.95 (0.82, 1.09) | 1 | ||||
| Europe | 8 931 | 0.87 (0.71, 1.06) | 1.06 (0.92, 1.24) | 1.17 (0.91, 1.49) | 1.11 (0.96, 1.28) | 1 |
Note. CI = confidence interval; OR = odds ratio. The analyses controlled for race (United States only), age, education, and country of residence. The P value of the Wald test shows whether the estimated odds ratio for the United States differs significantly from the odds ratio in Europe. P values of the Wald test of less than .05 show significant differences.
Source. US Health and Retirement Study (1992–2006) and the Survey of Health, Aging, and Retirement in Europe (2004–2009).
There was no consistent pattern suggesting that certain typologies were systematically more harmful for American than European women. In Europe, working, single, childless women were less likely to have high blood pressure (OR = 0.78; 95% CI = 0.66, 0.92) but more likely to have smoked (OR = 1.37; 95% CI = 1.07, 1.75) than working married mothers. These associations were not significant in the United States (OR = 0.94; 95% CI = 0.77, 1.15 and OR = 1.11; 95% CI = 0.91, 1.35 for high blood pressure and smoking, respectively). The odds of stroke among working single mothers compared with married working mothers were higher in the United States (OR = 2.09; 95% CI = 1.36, 1.64) than in Europe (OR = 0.88; 95% CI = 0.39, 1.97). The odds for smoking among working single mothers compared with married working mothers were higher in Europe (OR = 2.20; 95% CI = 1.62, 2.99) than in the United States (OR = 1.67; 95% CI = 1.38, 2.03). Although some typologies were associated with cardiovascular risk factors and chronic diseases within Europe and the United States, no statistically significant differences were found in the odds of any of the cardiovascular risk factors and chronic diseases between American and European women in all work–family typologies.
US–Europe Differences in Cardiovascular Health
Table 2 shows the observed probabilities of each cardiovascular outcome, alongside the counterfactual probabilities if US women had experienced the same distribution of and risks associated with work–family typologies as European women. American women would have had a marginal lower probability of chronic diseases and risk factors except for obesity if they had been exposed to the same distribution of work–family typologies as European women. For example, the original probability of stroke for US women was 4.4% (95% CI = 3.9%, 4.9%); however, if US women would experience the same work–family typology distribution as European women, their counterfactual probability would be 4.1% (95% CI = 3.6%, 4.6%). These decreases in the probabilities for US women reflected the fact that European women had slightly “healthier” work–family typologies (e.g., working married mothers) and fewer were working single mothers than were American women. If US women would have had the same health risks associated with each work–family typology as European women, the probability for stroke and high blood pressure would decrease, but the probability for heart disease, smoking, and obesity would increase.
TABLE 2—
Counterfactual Probability of Chronic Diseases and Risk Factors for US Women Aged 50–72 Years if They Would Have Had the Same Distribution of and Health Risks Associated With Each Work-Family Typology as European Women
| Variable | Heart Disease, Probability (95% CI) | Stroke, Probability (95% CI) | High Blood Pressure, Probability (95% CI) | Smoking, Probability (95% CI) | Obesity, Probability (95% CI) |
| Observed original probabilities | |||||
| US womena | 0.172 (0.162, 0.181) | 0.044 (0.039, 0.049) | 0.533 (0.521, 0.546) | 0.511 (0.498, 0.523) | 0.371 (0.358, 0.383) |
| European womenb | 0.068 (0.063, 0.074) | 0.018 (0.015, 0.021) | 0.324 (0.314, 0.334) | 0.403 (0.387, 0.418) | 0.211 (0.203, 0.220) |
| Difference between the US and Europec | 0.103 (−0.067, 0.273) | 0.026 (−0.099, 0.151) | 0.209 (0.000, 0.418) | 0.108 (−0.127, 0.343) | 0.159 (−0.042, 0.361) |
| US counterfactual probabilities if European work–family typology distributions | |||||
| US counterfactual probabilityd | 0.169 (0.160, 0.179) | 0.041 (0.036, 0.046) | 0.531 (0.519, 0.545) | 0.503 (0.490, 0.515) | 0.374 (0.361, 0.386) |
| Difference with original US probabilitye | −0.002 (–0.196, 0.191) | −0.003 (−0.144, 0.139) | −0.001 (−0.224, 0.221) | −0.008 (−0.231, 0.215) | 0.003 (−0.216, 0.222) |
| Difference with European probabilityf | 0.101 (−0.069, 0.271) | 0.023 (−0.100, 0.147) | 0.208 (−0.001, 0.417) | 0.100 (−0.135, 0.335) | 0.162 (−0.039, 0.364) |
| US counterfactual probabilities if European health risks associated with each work–family typology | |||||
| US counterfactual probabilityg | 0.188 (0.179, 0.198) | 0.036 (0.032, 0.041) | 0.514 (0.501, 0.527) | 0.526 (0.514, 0.539) | 0.376 (0.364, 0.389) |
| Difference with original US probabilitye | 0.017 (−0.179, 0.212) | −0.008 (−0.147, 0.132) | −0.019 (−0.242, 0.203) | 0.016 (−0.207, 0.238) | 0.006 (−0.213, 0.225) |
| Difference with European probabilityf | 0.120 (−0.052, 0.292) | 0.018 (−0.103, 0.139) | 0.190 (−0.019, 0.399) | 0.124 (−0.111, 0.359) | 0.165 (−0.037, 0.366) |
| US counterfactual probabilities if European work–family typology distributions and European health risks associated with each typology | |||||
| US counterfactual probabilityh | 0.183 (0.173, 0.193) | 0.035 (0.031, 0.040) | 0.514 (0.501, 0.526) | 0.515 (0.502, 0.527) | 0.373 (0.360, 0.385) |
| Difference with original US probabilitye | 0.012 (−0.183, 0.206) | −0.008 (−0.148, 0.131) | −0.020 (−0.242, 0.203) | 0.004 (−0.207, 0.238) | 0.002 (−0.217, 0.221) |
| Difference with European probabilityf | 0.115 (−0.057, 0.286) | 0.017 (−0.103, 0.138) | 0.190 (−0.020, 0.399) | 0.112 (−0.123, 0.347) | 0.161 (−0.040, 0.363) |
Note. CI = confidence interval. The analyses controlled for race (United States only), age, education, and country of residence.
Source. US Health and Retirement Study (1992–2006) and the Survey of Health, Aging, and Retirement in Europe (2004–2009).
The probability of risk factors and chronic diseases for US women.
The probability of risk factors and chronic diseases for European women.
The difference in probability for US and European women.
The probability of risk factors and chronic diseases for US women if the distribution of work–family typologies in the United States was substituted by the European distribution.
The difference between the original probability for US women and the estimated one.
The difference between the estimated probability for US women and the original probability for European women.
The probability of risk factors and chronic diseases for US women if the odds ratios related to the work–family typologies in the United States were substituted by those seen in Europe.
The probability of risk factors and chronic diseases for US women if the distribution of work–family typologies in the United States and the odds ratios related to the work–family typologies in the United States were substituted by those seen in Europe.
When we combine all information, results show that American women would have had a marginally lower probability of stroke and high blood pressure if they had been exposed to both the European distribution as well as the European health risks associated with each typology. The reduction in the probability of high blood pressure would be around 2 percentage points (from 53.3% to 51.4%), and the reduction in the probability of stroke would be about 1 percentage point (from 4.4% to 3.5%). These findings suggest that differences in work–family typologies explained only a small fraction of the higher prevalence of cardiovascular disease of American relative to European women.
DISCUSSION
Overall, distributions of work–family typologies and their associated cardiovascular health risks were relatively similar between the United States and Europe. American women were more likely to have had a history of working single motherhood than European women. Single working motherhood was consistently associated with worse cardiovascular health outcomes, but we found no evidence that this association was stronger for American than for European women. This larger probability of stroke among American women, however, would only be marginally reduced if American women had experienced similar work–family trajectories and risks as European women. Our findings suggest that work–family typologies contributed little to the higher prevalence of cardiovascular disease and risk factors of American relative to European women.
Methodological Considerations
We pooled data to derive work–family typologies to ensure that both American and European women were clustered in the same way. In sensitivity analysis, we conducted sequence analyses for women in the US and Europe separately (results available upon request), but this revealed no substantial differences in typologies between the United States and Europe. The only exception was for single working mothers, whereby US women were often clustered in a new typology of working married mothers who became divorced or widowed. Some of the differences in risk associated with this typology may thus reflect the fact that we were comparing 2 partly different groups of women in the United States and Europe.
Sequence analysis requires complete life trajectories. We used partial imputations and made some assumptions on the basis of the retrospective data when information was incomplete. For SHARE, we assumed no gap between 2 spells of employment if information was incomplete.27 For HRS, we inferred information on work and family life from information on children’s birth dates, wedding and divorce dates, and starting and ending dates of jobs.23 If these inferences were insufficient, we applied partial imputations to minimize the amount of missing data; this was true for about 3% of marriage histories, about 24% of work histories, and 0% of parental histories. For HRS, we validated work history imputations by using Social Security data. These Social Security records were available from 1951 onward. Women born in 1935 would have been aged 16 years in 1951, which is the first age of our work–life trajectory, and therefore we only included women born in 1935 or later in our sample. This resulted in women aged 50 to 72 years being selected for all samples.
When defining the work–family variables, we decided to use dichotomized variables to represent whether each woman was an employee, a wife, or a mother. It could be argued that our analysis might have benefitted from more detailed information on employment, marital status, and child histories. In particular, employed women might have been working part-time or full-time, nonemployment may have been a choice, women who were not married might have been cohabitating or may have had strong support from family or friends, and mothers might have had 1 or more children. However, this additional information would also increase the number of manageable work–family combinations, potentially compromising comparability across regions. For this analysis, we felt that a dichotomous version of exposure variables was appropriate to provide a sense of the overall contribution of work–family typologies to health differences between American and European women.
We used self-reported measures of cardiovascular disease because no objective measures of cardiovascular disease endpoints were available in HRS and SHARE. However, Banks et al.1 have shown large consistency between data from biomarkers and self-reports with data from HRS (United States) and the English Longitudinal Study of Aging (England). In addition, Glymour and Avendano28 found that incidence rates of stroke based on self-reports in HRS compare well to stroke incidence estimates from clinically verified studies. Therefore, although we acknowledge this limitation, we believe self-reported data provide an overall good estimate of the prevalence of broad categories of cardiovascular disease in a population. Another possible limitation of our measures is that both heart disease and stroke comprise broad categories of cardiovascular disease, so that we are unable to derive conclusions on the prevalence of subtypes of cardiovascular disease. In addition, differences in how the risk factors and chronic diseases were measured in HRS and SHARE (Table B) remain a potential source of bias. However, it is unclear whether this discrepancy would change the general conclusion regarding the association between work–family typologies and the cardiovascular outcomes, particularly smoking.
Interpretation
Consistent with findings from previous studies, we found that women who were consistently working, married, and had children were in general healthier than women following a different work–family life trajectory.23,29 This is consistent with the role accumulation hypothesis, which suggests that combining these roles is beneficial for a woman’s health.13 As an alternative, however, this finding is likely to reflect at least some selection: healthier women are more likely to marry, have children, and work. We also found that married mothers who returned to paid employment after some years of nonemployment had elevated odds of stroke and that working single mothers were worse off than working married mothers. This suggests that combining work and parenthood while having little (spousal) support may be detrimental for a woman’s cardiovascular health in the long run.30
Lone motherhood was more common in the United States than in Europe and was more strongly related to stroke among American women. Lone mothers are at higher risk of poverty and unemployment.31,32 Therefore, more generous family policies in Europe may relieve poverty and related stress, for example, by providing maternity leave, offering the possibility to work part-time, and providing better child care and support.33 As a result, single working mothers in the United States may have had more often difficulties to make ends meet, and they may have experienced more stressful lives than European mothers under more supportive policy regimes. Single nonworking mothers in the United States who lacked (employment-related) health insurance may have experienced larger inequalities in health care access than European mothers because health care is more universally accessible in Europe than the United States.34
The prevalence of lone motherhood increased in recent decades in both Europe and the United States.35 Our findings, therefore, highlight the need to develop wider policies to support single mothers in both regions. First, policies that target cardiovascular disease prevention to single working mothers may prove important to obtain gains in population health. Second, programs that support poor, working, single mothers such as the Earned Income Tax Credit or child allowances for working parents may contribute to reducing their health disadvantage.
Overall, we found only small differences in the composition of and cardiovascular risks associated with work–family typologies over the lifetime of American and European women. This suggests that the dominant mechanisms linking work, marital, and maternal status (including financial security and social support) to cardiovascular risk, and the buffering role of social policies for women in these typologies, are relatively similar and do not result in different associations with cardiovascular outcomes. Instead, other factors may explain the higher prevalence of cardiovascular disease among US women. For example, smoking, obesity, and other proximal risk factors have been put forward in the literature as partial explanations for the US female health disadvantage.2–6 Also, larger educational disparities in mortality in the United States than in Europe partly explain why mortality in the United States is higher than that in many European countries.26 Overall, we found that differences in work–family typologies explained only a small fraction of the excess risk of stroke and high blood pressure of American women compared with European women.
Conclusions and Implications
Working single motherhood was more common in the United States than in Europe, but differences in work–family trajectories explained only a small fraction of the excess cardiovascular risk of American relative to European women. Policies and interventions that support women combining work and family roles may improve women’s cardiovascular health, but may only marginally contribute to reducing the health disadvantage of American compared with European women. Further research should examine whether other aspects of women’s life trajectories may be more important in understanding the health disadvantage of US relative to European women.
ACKNOWLEDGMENTS
This study was funded in part by grant 1 R01 AG040248-01 (principal investigator: L. F. B), “Social Protection, Work, and Family Strain: Cumulative Disadvantage Effects in the US and Europe.” M. Avendaño is additionally supported by a Starting Researcher grant from the European Research Council (ERC grant 263684), the MacArthur Foundation Research Network on an Aging Society, and the European Union's Horizon2020 research and innovation programme under grant agreement 633666 (Lifepath). In this study, we used data from SHARE waves 1 and 2 (release 2.5.0, May 24, 2011), and wave 3 (SHARELIFE, release 1.0.0, November 24, 2010, DOI: 10.6103/SHARE.w3.100). The SHARE data collection has been primarily funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812), and FP7 (SHARE-PREP: No. 211909, SHARE-LEAP: No. 227822, SHARE M4: No. 261982). Additional funding from the German Ministry of Education and Research, the US National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064), and from various national funding sources is gratefully acknowledged (see http://www.share-project.org).
In this study, we used data from the generated Job Episodes Panel (DOI: 10.6103/SHARE.jep.200). The Job Episodes Panel release 2.0.0 is based on SHARE waves 1 and 2 (release 2.5.0, May 24, 2011) and SHARELIFE (DOI: 10.6103/SHARE.w3.100).
Note. The funding sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
HUMAN PARTICIPANT PROTECTION
Human participant protection was not required because we used only secondary, de-identified data.
REFERENCES
- 1.Banks J, Marmot M, Oldfield Z, Smith J. Disease and disadvantage in the United States and in England. JAMA. 2006;295(17):2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- 2.National Research Council and Institute of Medicine. US Health in International Perspective: Shorter Lives, Poorer Health. In: Woolf SH, Aron L, editors. Panel on Understanding Cross-National Health Differences Among High-Income Countries. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 3.National Research Council. International Differences in mortality at Older Ages: Dimensions and Sources. In: Crimmins EM, Preston SH, Cohen B, editors. Panel on Understanding Divergent Trends in Longevity in High-Income Countries. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 4.National Research Council. Explaining Divergent Levels of Longevity in High Income Countries. In: Crimmins EM, Preston SH, Cohen B, editors. Panel on Understanding Divergent Trends in Longevity in High-Income Countries. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 5.Preston SH, Glei DA, Wilmoth JR. A new method for estimating smoking-attributable mortality in high-income countries. Int J Epidemiol. 2010;39(2):430–438. doi: 10.1093/ije/dyp360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston SH, Stokes A. Contribution of obesity to international differences in life expectancy. Am J Public Health. 2011;101(11):2137–2143. doi: 10.2105/AJPH.2011.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaumotte F. Female labour force participation: past trends and main determinants in OECD countries. Paris, France: Organisation for Economic Co-operation and Development, Economics Department; 2003. Working paper no. 376.
- 8.The Organisation for Economic Co-operation and Development. OECD family database. SF3.1: Marriage and divorce rates. Available at: https://www.oecd.org/els/family/SF3_1_Marriage_and_divorce_rate_Jan2014.pdf. Accessed January 21, 2015.
- 9. Eurostat. Crude marriage rate, selected years, 1960–2012 (per 1 000 inhabitants). 2014. Available at: http://ec.europa.eu/eurostat/statistics-explained/index.php/File:Crude_marriage_rate,_selected_years,_1960%E2%80%932012_(per_1_000_inhabitants)_YB14.png#file. Accessed January 21, 2015.
- 10. United Nations Department of Economic and Social Affairs, Population Division. World population prospects: the 2015 revision. Total fertility by major area, region and country, 1950-2100. 2015. Available at: https://esa.un.org/unpd/wpp/DVD/Files/1_Indicators%20(Standard)/EXCEL_FILES/2_Fertility/WPP2015_FERT_F04_TOTAL_FERTILITY.XLS. Accessed May 23, 2016.
- 11.Jaffe DH, Manor O, Eisenbach Z, Neumark YD. The protective effect of marriage on mortality in a dynamic society. Ann Epidemiol. 2007;17(7):540–547. doi: 10.1016/j.annepidem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Ross CE, Mirowsky J. Does employment affect health? J Health Soc Behav. 1995;36(3):230–243. [PubMed] [Google Scholar]
- 13.Martikainen P. Women’s employment, marriage, motherhood and mortality: a test of the multiple role and role accumulation hypotheses. Soc Sci Med. 1995;40(2):199–212. doi: 10.1016/0277-9536(94)e0065-z. [DOI] [PubMed] [Google Scholar]
- 14.Börsch-Supan A, Brugiavini A, Jürges H, editors. First results from the Survey of Health, Ageing and Retirement in Europe (2004–2007). Starting the longitudinal dimension. Mannheim, Germany: Mannheim Research Institute for the Economics of Aging; 2008.
- 15.Börsch-Supan A. Survey of Health, Ageing and Retirement in Europe (SHARE) wave 3 - SHARELIFE. Release version 1.0.0. SHARE-ERIC data set. 2010.
- 16.Antonova L, Aranda L, Brugiavini A, Cavapozzi D, Pasini G, Trevisan E. SHARE job episodes panel, release version: 2.0.0. SHARE-ERIC data set. 2014.
- 17.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30(suppl):S7–S56. [Google Scholar]
- 18.Kurian AK, Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17(1):143–152. [PubMed] [Google Scholar]
- 19. Heart disease and stroke statistics—2009 update. Dallas, TX: American Heart Association; 2009.
- 20.United Nations Educational; Scientific, and Cultural Organization. International Standard Classification of Education: ISCED 1997. 1997. Available at: http://www.unesco.org/education/information/nfsunesco/doc/isced_1997.htm. Accessed July 22, 2013.
- 21.Avendano M, Glymour MM, Banks J, Mackenbach JP. Health disadvantage in US adults aged 50 to 74 years: a comparison of the health of rich and poor Americans with that of Europeans. Am J Public Health. 2009;99(3):540–548. doi: 10.2105/AJPH.2008.139469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbott A. Sequence analysis: new methods for old ideas. Annu Rev Sociol. 1995;21:93–113. [Google Scholar]
- 23.Sabbath EL, Mejía Guevara I, Glymour MM, Berkman LF. Use of life course work-family profiles to predict mortality risk among US women. Am J Public Health. 2015;105(4):e96–e102. doi: 10.2105/AJPH.2014.302471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Studer M. WeightedCluster library manual. A practical guide to creating typologies of trajectories in the social sciences with R. LIVES Working Papers. 2013;24:1–32. [Google Scholar]
- 25.Gabadinho A, Ritschard G, Müller NS, Studer M. Analyzing and visualizing state sequences in R with TraMineR. J Stat Softw. 2011;40:1–37. [Google Scholar]
- 26.van Hedel K, Avendano M, Berkman LF et al. The contribution of national disparities to international differences in mortality between the United States and 7 European countries. Am J Public Health. 2015;105(4):e112–e119. doi: 10.2105/AJPH.2014.302344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brugiavini A, Cavapozzi D, Pasini G, Trevisan E. Working life histories from SHARELIFE: a retrospective panel. 2013. Survey of Health, Aging, and Retirement in Europe working paper no. 11.
- 28.Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors? Evidence from the Health and Retirement Study. Stroke. 2009;40(3):873–879. doi: 10.1161/STROKEAHA.108.529479. [DOI] [PubMed] [Google Scholar]
- 29.Weatherall R, Joshi H, Macran S. Double burden or double blessing? Employment, motherhood and mortality in the longitudinal study of England and Wales. Soc Sci Med. 1994;38(2):285–297. doi: 10.1016/0277-9536(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 30.Berkman LF, Zheng Y, Glymour MM, Avendano M, Börsch-Supan A, Sabbath EL. Mothering alone: cross-national comparisons of later-life disability and health among women who were single mothers. J Epidemiol Community Health. 2015;69(9):865–872. doi: 10.1136/jech-2014-205149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gornick J, Meyers M. More alike than different: revisiting the long-term prospect for developing “European-style” work/family policies in the United States. J Comp Policy Anal. 2004;6(3):251–273. [Google Scholar]
- 32.Whitehead M, Burström B, Diderichsen F. Social policies and the pathways to inequalities in health: a comparative analysis of lone mothers in Britain and Sweden. Soc Sci Med. 2000;50(2):255–270. doi: 10.1016/s0277-9536(99)00280-4. [DOI] [PubMed] [Google Scholar]
- 33.Ruhm CJ, Teague JL. Parental leave policies in Europe and North America. Cambridge, MA: National Bureau of Economic Research; 1995. Working paper no. 5065.
- 34.Adams PF, Dey AN, Vickerie JL. Summary health statistics for the US population: National Health Interview Survey, 2005. Vital Health Stat. 2007;10(233):1–104. [PubMed] [Google Scholar]
- 35.Chapple S. Child well-being and sole-parent family structure in the OECD: an analysis. 2009. Organisation for Economic Co-operation and Development Social, Employment and Migration working paper no. 82.