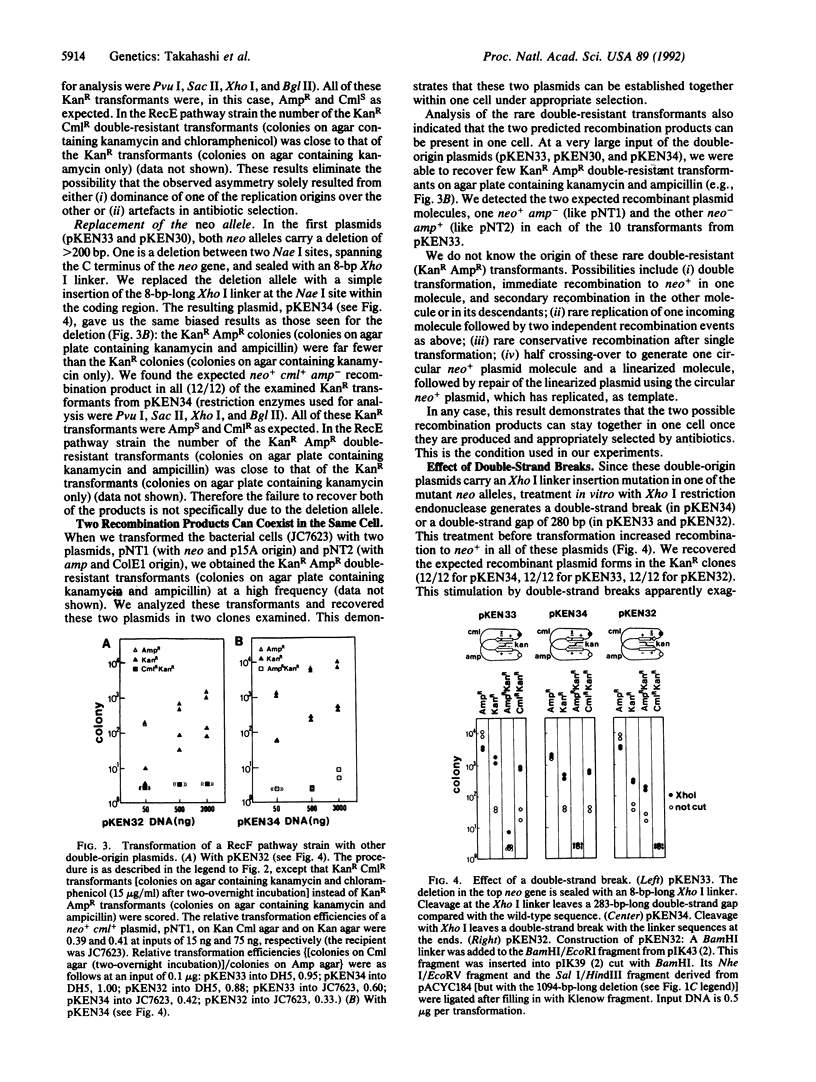
Abstract
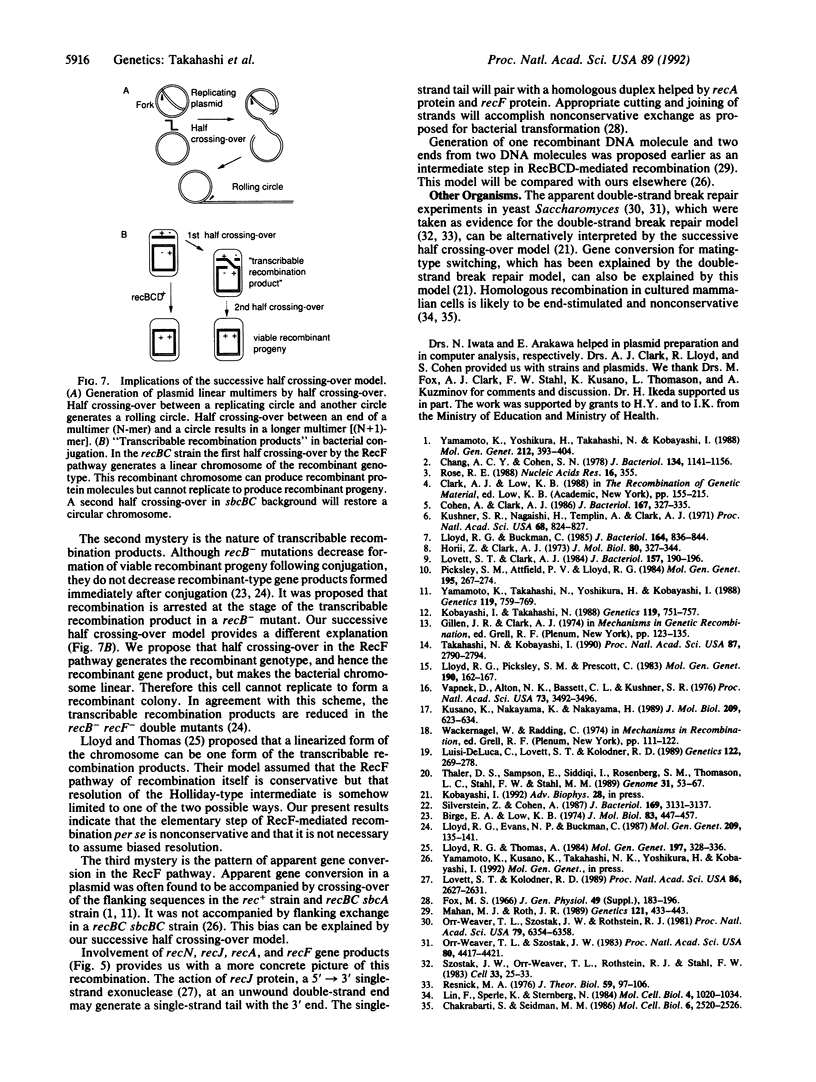
Homologous recombination between two duplex DNA molecules might result in two duplex DNA molecules (conservative) or, alternatively, it might result in only one recombinant duplex DNA molecule (nonconservative). Here we present evidence that the mode of homologous recombination is nonconservative in an Escherichia coli strain with an active RecF pathway (a recBC sbcBC mutant). We employed plasmid substrates that enable us to recover both recombination products. These plasmids carry two mutant alleles of neo gene in direct orientation, two drug-resistance marker genes, and two compatible replication origins. After their transfer to the cells followed by immediate selection for the recombination to neo+, we could recover only one recombination product. A double-strand break at the region of homology increased this nonconservative recombination. If a nonconservative exchange should leave an end, this end may stimulate another exchange. Such "successive half crossing-over events" can explain several recombination-related phenomena in E. coli, including the origin of plasmid linear multimers and of transcribable, nonreplicated recombination products, and also in yeast and mammalian cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birge E. A., Low K. B. Detection of transcribable recombination products following conjugation in rec+, reCB- and recC-strains of Escherichia coli K12. J Mol Biol. 1974 Mar 15;83(4):447–457. doi: 10.1016/0022-2836(74)90506-3. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Seidman M. M. Intramolecular recombination between transfected repeated sequences in mammalian cells is nonconservative. Mol Cell Biol. 1986 Jul;6(7):2520–2526. doi: 10.1128/mcb.6.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Clark A. J. Synthesis of linear plasmid multimers in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):327–335. doi: 10.1128/jb.167.1.327-335.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. S. On the mechanism of integration of transforming deoxyribonucleate. J Gen Physiol. 1966 Jul;49(6):183–196. doi: 10.1085/jgp.49.6.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Takahashi N. Double-stranded gap repair of DNA by gene conversion in Escherichia coli. Genetics. 1988 Aug;119(4):751–757. doi: 10.1093/genetics/119.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Nakayama K., Nakayama H. Plasmid-mediated lethality and plasmid multimer formation in an Escherichia coli recBC sbcBC mutant. Involvement of RecF recombination pathway genes. J Mol Biol. 1989 Oct 20;209(4):623–634. doi: 10.1016/0022-2836(89)90000-4. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Model for homologous recombination during transfer of DNA into mouse L cells: role for DNA ends in the recombination process. Mol Cell Biol. 1984 Jun;4(6):1020–1034. doi: 10.1128/mcb.4.6.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Buckman C. Identification and genetic analysis of sbcC mutations in commonly used recBC sbcB strains of Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):836–844. doi: 10.1128/jb.164.2.836-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Evans N. P., Buckman C. Formation of recombinant lacZ+ DNA in conjugational crosses with a recB mutant of Escherichia coli K12 depends on recF, recJ, and recO. Mol Gen Genet. 1987 Aug;209(1):135–141. doi: 10.1007/BF00329848. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Picksley S. M., Prescott C. Inducible expression of a gene specific to the RecF pathway for recombination in Escherichia coli K12. Mol Gen Genet. 1983;190(1):162–167. doi: 10.1007/BF00330340. [DOI] [PubMed] [Google Scholar]
- Lloyd R. G., Thomas A. A molecular model for conjugational recombination in Escherichia coli K12. Mol Gen Genet. 1984;197(2):328–336. doi: 10.1007/BF00330981. [DOI] [PubMed] [Google Scholar]
- Lovett S. T., Clark A. J. Genetic analysis of the recJ gene of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):190–196. doi: 10.1128/jb.157.1.190-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett S. T., Kolodner R. D. Identification and purification of a single-stranded-DNA-specific exonuclease encoded by the recJ gene of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2627–2631. doi: 10.1073/pnas.86.8.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi-DeLuca C., Lovett S. T., Kolodner R. D. Genetic and physical analysis of plasmid recombination in recB recC sbcB and recB recC sbcA Escherichia coli K-12 mutants. Genetics. 1989 Jun;122(2):269–278. doi: 10.1093/genetics/122.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan M. J., Roth J. R. Role of recBC function in formation of chromosomal rearrangements: a two-step model for recombination. Genetics. 1989 Mar;121(3):433–443. doi: 10.1093/genetics/121.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picksley S. M., Attfield P. V., Lloyd R. G. Repair of DNA double-strand breaks in Escherichia coli K12 requires a functional recN product. Mol Gen Genet. 1984;195(1-2):267–274. doi: 10.1007/BF00332758. [DOI] [PubMed] [Google Scholar]
- Resnick M. A. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol. 1976 Jun;59(1):97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Rose R. E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988 Jan 11;16(1):355–355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein Z., Cohen A. Synthesis of linear multimers of OriC and pBR322 derivatives in Escherichia coli K-12: role of recombination and replication functions. J Bacteriol. 1987 Jul;169(7):3131–3137. doi: 10.1128/jb.169.7.3131-3137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kobayashi I. Evidence for the double-strand break repair model of bacteriophage lambda recombination. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2790–2794. doi: 10.1073/pnas.87.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler D. S., Sampson E., Siddiqi I., Rosenberg S. M., Thomason L. C., Stahl F. W., Stahl M. M. Recombination of bacteriophage lambda in recD mutants of Escherichia coli. Genome. 1989;31(1):53–67. doi: 10.1139/g89-013. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Alton N. K., Bassett C. L., Kushner S. R. Amplification in Escherichia coli of enzymes involved in genetic recombination: construction of hybrid ColE1 plasmids carrying the structural gene for exonuclease I. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3492–3496. doi: 10.1073/pnas.73.10.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Takahashi N., Yoshikura H., Kobayashi I. Homologous recombination involving a large heterology in Escherichia coli. Genetics. 1988 Aug;119(4):759–769. doi: 10.1093/genetics/119.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Yoshikura H., Takahashi N., Kobayashi I. Apparent gene conversion in an Escherichia coli rec+ strain is explained by multiple rounds of reciprocal crossing-over. Mol Gen Genet. 1988 Jun;212(3):393–404. doi: 10.1007/BF00330842. [DOI] [PubMed] [Google Scholar]



