Abstract
Adult stem cell treatment of complex disorders is a promising therapeutic approach and multipotent marrow stromal cells (MSCs) have been shown to be effective in various animal models of diseases. Acute kidney injury (AKI) is a common and serious problem in hospitalized patients and bone marrow derived multipotent MSCs have been shown to be effective in different models of AKI. The mechanism of action of MSCs is complex but involves paracrine actions including growth factor secretion. Knockdown of vascular enthothelial growth factor (VEGF) by siRNA reduced effectiveness of MSCs in the treatment of ischemic AKI in a rat model. Animals treated with MSCs had increased renal microvessel density compared to VEGF knockdown MSC‐treated and vehicle‐treated animals. These results show that VEGF is an important mediator of the early and late phase of renoprotective action after AKI in the context of stem cell treatment.
Keywords: paracrine, apoptosis, vascularity, VEGF, MSC, bone marrow derived stem cells, AKI, treatment
Introduction
Stem cell therapy offers a promising new option for the treatment of complex diseases. While there are ethical and tumourigenic concerns with embryonic stem cells, adult stem cells are already used successfully and safely to treat patients. Multipotent marrow stromal cells (MSCs) are bone marrow derived adherent fibroblast‐like cells that differentiate into a large number of cell types, have immunomodulatory properties and secrete cytokines and growth factors [1], together making them potential ideal candidates for therapies of various disorders [2]. MSCs have been used successfully to treat a number of diseases in animal models and are currently used in clinical trials to treat different diseases including myocardial infarction, graft versus host disease, Crohn’s disease and others [3].
The specific mechanism of action of MSCs in organoprotection and repair does not involve, as initially thought, differentiation into resident parenchymal cells but rather paracrine actions, including growth factor secretion, as well as immunomodulatory functions [4, 5].
MSCs are effective in reducing renal injury and enhancing recovery of renal function in animal models of acute kidney injury (AKI), including an ischemia/reperfusion as well as a cisplatinum toxicity model, but do not or only rarely contribute to differentiated renal cell types, e.g. tubular cells or endothelial cells [6]. Growth factors including IGF‐1 [7], EGF and vasculotropic factors [8] have been shown to be mediators of renal repair and this effect can be reproduced using MSC conditioned medium [9]. Paracrine factors secreted by MSCs also have been shown to be important in the process of wound healing by recruiting macrophages and thereby enhancing repair [10]. VEGF is the major factor that regulates vessel growth in normal as well as injured tissues and is highly secreted by MSCs [11, 12, 13]. Here we show, in vitro and in vivo, using VEGF specific siRNA knockdown technology, that VEGF is an important mediator of the paracrine actions of MSCs in AKI.
Materials and methods
Animals and cells
The Institutional Animal Use and Care Committees (IACUC) of the Veterans Affairs Medical Center (Salt Lake City, UT, USA) approved all procedures involving animals. MSCs were generated from F344 rats as described before [14]. In brief, femurs of sacked animals were flushed with phosphate buffered saline (PBS) and cells cultured in alpha‐minimal essential medium (MEM) containing 10% fetal bovine serum (FBS). Adherent cells were removed after 3 days and MSCs passaged at subconfluence. Fluorescence activated cell sorting (FACS) staining for CD45, CD90 and CD105 and differentiation into adipocytes, osteocytes and chondrocytes characterized MSCs.
Surgical procedures and MSC treatment
Ischemia/reperfusion AKI was induced in anesthetized female Sprague Dawley (SD) rats as described before [14]. In brief, renal pedicles of adult female SD rats weighting 200–250 g were clamped for 48 min. and animals were infused immediately after reflow and via the left carotid artery with 2 × 106/kg body weight MSCs derived from F344 rats (wild type or VEGF siRNA treated) in 1 ml of PBS. All controls with identical AKI were infused, via the left carotid artery, with 1 ml of PBS. This constitutes an allogeneic MSC protocol.
Kidney function
Serum creatinine was determined using the Dimension RxL Max Clinical Chemistry System (Dade Behring, Deerfield, IL, USA) from a plasma sample of heparinized blood.
VEGF siRNA knockdown
Cultured MSCs from F344 rats were treated with siRNA targeted at three different exons of the VEGF gene that are common to all splice variants (exons 2–6) and NeoFx transfection agent (Ambion, Austin, TX, USA). Silencer® pre‐designed siRNAs (siRNA ID #192613, 192614 and 192615) were purchased (Ambion) and tested at three different concentrations (5, 10, 30 nm) in regular culture medium. Cells were incubated for 24 hrs with siRNA and washed with PBS afterwards. A concentration of 10 nm proved to be most effective and was therefore used for all subsequent experiments. Controls consisted of cells treated with Silencer® negative control siRNA (Ambion), NeoFx transfection agent only and untreated cells. Gene expression measured by real‐time quantitative RT‐PCR with a SmartCycler (Cepheid, Sunnyvale, CA, USA) was tested 24 and 48 hrs after knockdown (VEGF forward primer: gcactggaccctggcttt; reverse primer: cggggtactcctggaagatg), and VEGF protein secretion by ELISA in medium conditioned for 24 hrs (RnD Systems, Minneapolis, MN, USA). VEGF‐receptor primers used were: flt‐1: forward – agcaacaggtgcaggaaacca; reverse – tgcaccgaatagcgagcaga; flt‐4 forward – ctccaacttcttgcgtgtca; reverse – acaaggtcctccatggtcag; flk‐1: caggggagggttggcataga; reverse – caccccagatcggtgagaaag.
In vitro studies
Rat proximal tubular cells (NRK, ATCC, Manassas, VA) were seeded in 96‐well plates at a density of 15,000 cells/well and subjected to 48 hrs of stimulation either with conditioned medium from MSCs or serum‐free control medium or medium containing 10% FBS (Hyclone, Logan, UT, USA). Conditioned medium was generated from 1 × 106 MSCs seeded in a well of a 6‐well plate over 24 hrs. Proliferative activity was determined using a colorimetric tetrazolium based MTT assay.
Microvessel density determination
Kidney sections of SD rats 4 weeks after induction of AKI were immunostained with mouse monoclonal CD34 antibody (Santa Cruz, Santa Cruz, CA, USA) to visualize microvessels. No nuclear counterstaining was applied. The percentage area of stained microvessel was determined with ImageJ (National Institutes of Health) using the following image processing steps: (i) a binary image was created from the raw image; (ii) a threshold level was set (the same level for all sections); (iii) ImageJ ‘Measure’ function was used to determine the percentage area of CD34 staining. Three random areas from the cortex were analysed for five animals from each group (normal MSC treatment, VEGF knockdown MSC treatment, and control vehicle treatment). Each random area included 10 high power fields that were analysed in the described stepwise, standardized fashion.
Statistical analyses
Data are presented as means ± S.D., unless otherwise stated. Statistical analyses were performed with GraphPad Prism 4 for Macintosh (GraphPad Software, San Diego, CA, USA). anova, t‐test and Kaplan–Meier analysis were used to assess differences between data means as appropriate. All groups consisted of at least six animals. A P‐value of <0.05 was considered significant.
Results
VEGF knockdown efficiency with siRNA
Adherent MSCs in culture flasks were treated for 24 hrs with VEGF siRNA and NeoFX transfection agent in regular growth medium in order to knockdown VEGF expression. Knockdown efficiency was determined at RNA and protein levels 24 hrs and 48 hrs after the end of the transfection period. Efficiency of the knockdown approach was tested before each experiment and adjusted if necessary (combination of siRNAs or different concentrations). Initially, combination of three VEGF siRNA yielded a greater than 80% knockdown of VEGF at the RNA and protein levels. In later experiments, 10 nm of siRNA ID #192613 was used and yielded a greater than 60% knockdown at the protein level. Results are shown in Fig. 1. Knockdown was verified at the mRNA as well as the protein level.
Figure 1.
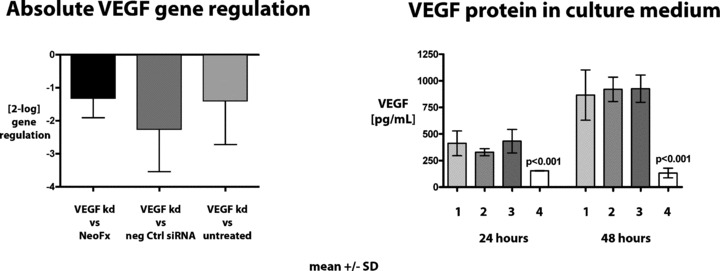
Knockdown efficiency of VEGF on the mRNA and protein level. Left panel: Absolute gene regulation determined by real time quantitative RT‐PCR. Right panel: VEGF protein concentrations determined by ELISA in tissue culture supernatant of cells treated with VEGF siRNA after 24 and 48 hrs after the end of the siRNA incubation period (24 hrs). VEGF knockdown on mRNA and protein level was highly significant (P < 0.01, t‐test).
In vitro studies with MSC conditioned medium
NRK cells express VEGF receptors Flt‐1, flt‐4 and flk‐1 as determined by PCR and showed proliferative activity when VEGF was added to the medium (data not shown). VEGF knockdown with siRNA reduced VEGF protein levels in MSC conditioned medium (Fig. 1, right panel). Conditioned medium from VEGF knockdown MSC exerted less proliferative activity on proximal tubular cells compared to regular conditioned medium from MSCs treated with irrelevant siRNAs (P= 0.029) or control medium (S.F. [serum‐free medium], P= 0.038) (Fig. 2), thereby demonstrating the mitogenic activity of VEGF in tubular cells. Addition of 10 ng/ml VEGF restored proliferative activity of the conditioned medium.
Figure 2.
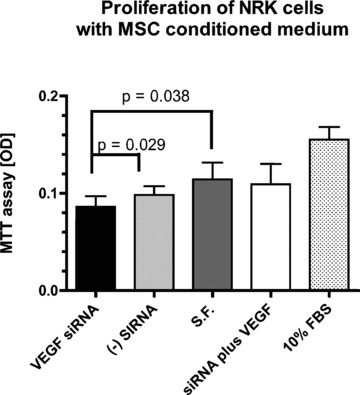
In vitro study to determine the effect of VEGF knockdown on proliferation of MSC‐conditioned medium (MSC CM) on NRK cells using the MTT assay. Data are shown as mean + S.D. (n= 6 per group). S.F. = serum free medium. (−) siRNA = negative control (irrelevant) siRNA. MSC CM after knockdown of VEGF significantly reduced proliferation of NRK cells compared to negative control siRNA (P= 0.029), and control MSC CM (P= 0.038). Addition of 10 ng/ml VEGF (S.F. + VEGF) brought proliferation back to baseline. 10% FBS as positive control showed the highest proliferative activity.
VEGF knockdown reduces renoprotection of MSCs
In order to investigate the applicability of our in vitro results to the in vivo situation, we studied the comparative renoprotective effects of wild type MSCs and to VEGF knockdown MSCs in the standard model of ischemia/reperfusion AKI. Female SD rats were subjected to 48 min. of bilateral renal pedicle clamping to induce severe AKI. Regular MSCs were renoprotective as shown by lower serum creatinine values on days 1 and 7 compared to vehicle injection (Fig. 3A). VEGF knockdown rendered MSCs less effective in exerting renoprotection and recovery, which was highly significant at day 7 (P= 0.0004). Survival of animals treated with VEGF knockdown MSCs during the first three days after clamping was lower compared to MSC treated animals (Fig. 2B).
Figure 3.
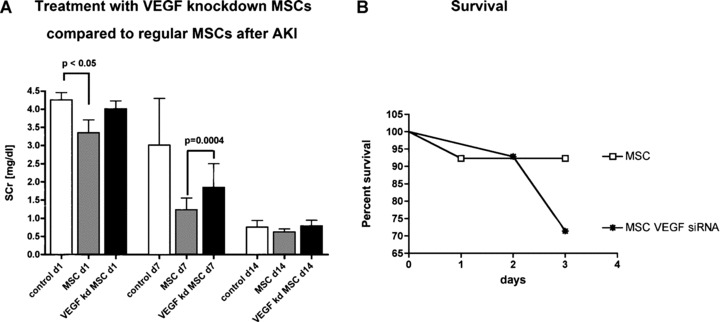
In vivo study to determine the effect of VEGF knockdown on therapeutic effectiveness in an ischemia/reperfusion model of AKI in rats. Left panel: Regular MSCs (grey bars) were renoprotective and enhance recovery from AKI in rats compared to VEGF knockdown MSCs (black bars). Right panel: Survival was increased in animals treated with wild type MSCs compared to VEGF knockdown MSCs (P < 0.05; n= 8).
Decreased microvessel density after treatment with VEGF knockdown MSCs
VEGF is the major mediator of vascular growth and repair and microvascular injury is an important pathophysiological component of AKI [15, 16]. Therefore, we determined renal microvessel density at 4 weeks after ischemia/reperfusion AKI in animals treated with regular MSCs and VEGF knockdown MSCs, using an immunostaining approach. Paraffin sections of kidneys were immunostained with CD34 to visualize the renal vasculature (Fig. 4A). No counterstaining was applied and all sections were examined the same way with ImageJ. Area percentage of staining from binary images (Fig. 4B) was determined with the ‘measure’ function. Animals treated with regular MSCs had a significantly higher percentage area of vasculature compared to animals treated with VEGF knockdown MSCs and vehicle treated animals (Fig. 4C; P < 0.001). Tissue injury (apoptosis/necrosis) was not determined at this time point since surviving animals had recovered from the acute phase of AKI and these data would not have added additional information.
Figure 4.
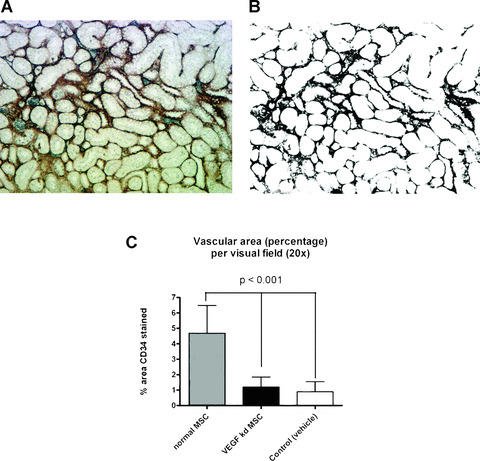
Assessment of microvessel density in renal cortex sections of rats 4 weeks after AKI. (A) CD34 staining of renal vasculature without nuclear counterstaining. (B) Binary image of (A) made with ImageJ in order to automatically determine the area percentage of the stained vessels. (C) Calculated mean vascular area (percent of section) per visual field in the renal cortex. Three 20× field per section from every group (n= 5) were randomly chosen and averages are plotted. Animals treated with regular MSCs have a significantly higher vascular area compared to VEGF knockdown MSC treated animals and controls (vehicle treated).
Discussion
MSCs are bone marrow derived stem cells and, together with haematopoietic stem cells, are already in clinical use to treat patients with various diseases [3]. Their effectiveness has been shown in a number of diseases, but the mechanism of action is incompletely defined and likely includes a multitude of actions, e.g. paracrine growth factor secretion, immunomodulation and anti‐apoptotic properties. Since AKI is caused by multi‐factorial pathophysiological mechanisms, including inflammation and vascular injury, MSCs appear to be suitable candidates for a cell based therapy of this common disease that is associated with high hospital mortality [17].
Vascular damage is an early and important mediator of AKI [15], and also leads to long‐term damage and progressive loss of renal function [18]. VEGF is the major angiogenic factor that is important for vascular maintenance after AKI. Renal ischemia inhibits VEGF expression by multiple mechanisms, shifting the balance from a pro‐angiogenic to an anti‐angiogenic milieu, thereby inhibiting renal repair and paving the way to long‐term progressive loss of renal function [19]. MSCs express VEGF [8] amongst other growth factors and have been shown to exert paracrine actions that are renoprotective and enhance recovery from AKI [14]. Recently, IGF‐1 has been implicated as a paracrine mediator of renoprotection in a cisplatinum model of AKI [7]. Because a single factor is unlikely to be the sole mediator of renoprotection, we examined the potential significance of VEGF as a renoprotective mediator of MSCs in AKI. Accordingly, VEGF was knocked down in MSCs and their organ protection activity in AKI was compared to that of wild type MSCs. Our data show that knocking down VEGF in MSCs decreases their proliferative effects in rat proximal tubular cells in vitro and decreases their effectiveness after AKI in vivo. Rats treated with VEGF knockdown MSCs had a higher mortality and slower recovery of renal function after AKI. These data clearly demonstrate the importance of VEGF mediating renoprotection of MSCs after AKI. It cannot be excluded, that other trophic factors are affected by the VEGF knockdown. Interplay between trophic factors is complicated and multifactorial, and in vitro assays are different from the in vivo situation. However, our data clearly indicate an important role of VEGF. Furthermore, microvessel density was significantly higher in animals treated with regular MSCs compared to VEGF knockdown MSCs and controls, demonstrating the importance of early VEGF administration via MSCs for the long‐term outcome after AKI.
Basile has demonstrated that VEGF is down regulated after AKI and a long‐term consequence of AKI is decreased microvessel density and impaired renal concentrating ability [18, 19]. MSC treatment early in the course of AKI might appear thus beneficial for the long‐term outcome after AKI.
In conclusion, we have identified VEGF as an important mediator of the paracrine actions of MSCs in the treatment of AKI in vitro and in vivo.
Acknowledgements
This work was supported in part by funds from the National Kidney Foundation (Utah, Idaho), the Merit Review Program of the Dept. of Veterans Affairs, Washington, DC, the National Institute of Diabetes and Digestive and Kidney Diseases, and Nephrogen, LLC. Parts of this work were presented at the Renal Week 2007 in San Francisco, CA, and published in abstract form (J Am Soc Nephrol 18: [SU‐FC174], 2007).
References
- 1. Schinkothe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev . 2008; 17: 199–206. [DOI] [PubMed] [Google Scholar]
- 2. Porada CD, Zanjani ED, Almeida‐Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther . 2006; 1: 365–9. [DOI] [PubMed] [Google Scholar]
- 3. Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol . 2007; 211: 27–35. [DOI] [PubMed] [Google Scholar]
- 4. Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovasc Med . 2007; 4: S21–6. [DOI] [PubMed] [Google Scholar]
- 5. Mishra PK. Bone marrow‐derived mesenchymal stem cells for treatment of heart failure: is it all paracrine actions and immunomodulation J Cardiovasc Med . 2008; 9: 122–8. [DOI] [PubMed] [Google Scholar]
- 6. Humphreys BD, Duffield JS, Bonventre JV. Renal stem cells in recovery from acute kidney injury. Minerva Urol Nefrol . 2006; 58: 329–37. [PubMed] [Google Scholar]
- 7. Imberti B, Morigi M, Tomasoni S, et al . Insulin‐like growth factor‐1 sustains stem cell mediated renal repair. J Am Soc Nephrol . 2007; 18: 2921–8. [DOI] [PubMed] [Google Scholar]
- 8. Togel F, Weiss K, Yang Y, et al . Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol . 2007; 292: F1626–35. [DOI] [PubMed] [Google Scholar]
- 9. Bi B, Schmitt R, Israilova M, et al . Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol . 2007; 18: 2486–96. [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Tredget EE, Wu PY, et al . Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008; 3: e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mayer H, Bertram H, Lindenmaier W, et al . Vascular endothelial growth factor (VEGF‐A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem . 2005; 95: 827–39. [DOI] [PubMed] [Google Scholar]
- 12. Geiger F, Lorenz H, Xu W, et al . VEGF producing bone marrow stromal cells (BMSC) enhance vascularization and resorption of a natural coral bone substitute. Bone . 2007; 41: 516–22. [DOI] [PubMed] [Google Scholar]
- 13. Hung SC, Pochampally RR, Chen SC, et al . Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K‐Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells . 2007; 25: 2363–70. [DOI] [PubMed] [Google Scholar]
- 14. Togel F, Hu Z, Weiss K, et al . Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation‐independent mechanisms. Am J Physiol Renal Physiol . 2005; 289: F31–42. [DOI] [PubMed] [Google Scholar]
- 15. Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int . 2004; 66: 496–9. [DOI] [PubMed] [Google Scholar]
- 16. Sutton TA, Mang HE, Campos SB et al. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol . 2003; 285: F191–8. [DOI] [PubMed] [Google Scholar]
- 17. Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med . 2008; 59: 311–25. [DOI] [PubMed] [Google Scholar]
- 18. Basile DP, Donohoe D, Roethe K, et al . Renal ischemic injury results in permanent damage to peritubular capillaries and influences long‐term function. Am J Physiol Renal Physiol . 2001; 281: F887–99. [DOI] [PubMed] [Google Scholar]
- 19. Basile DP, Fredrich K, Chelladurai B, et al . Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS‐1, a novel VEGF inhibitor. Am J Physiol Renal Physiol . 2008; 294: F928–36. [DOI] [PubMed] [Google Scholar]


