Abstract
In patients with bile acid malabsorption, high concentrations of bile acids enter the colon and stimulate Cl− and fluid secretion, thereby causing diarrhoea. However, deoxycholic acid (DCA), the predominant colonic bile acid, is normally present at lower concentrations where its role in regulating transport is unclear. Thus, the current study set out to investigate the effects of physiologically relevant DCA concentrations on colonic epithelial secretory function. Cl− secretion was measured as changes in short‐circuit current across voltage‐clamped T84 cell monolayers. At high concentrations (0.5–1 mM), DCA acutely stimulated Cl− secretion but this effect was associated with cell injury, as evidenced by decreased transepithelial resistance (TER) and increased lactate dehydrogenase (LDH) release. In contrast, chronic (24 hrs) exposure to lower DCA concentrations (10–200 μM) inhibited responses to Ca2+ and cAMP‐dependent secretagogues without altering TER, LDH release, or secretagogue‐induced increases in intracellular second messengers. Other bile acids – taurodeoxycholic acid, chenodeoxycholic acid and cholic acid – had similar antisecretory effects. DCA (50 μM) rapidly stimulated phosphorylation of the epidermal growth factor receptor (EGFr) and both ERK and p38 MAPKs (mitogen‐activated protein kinases). The EGFr inhibitor, AG1478, and the protein synthesis inhibitor, cycloheximide, reversed the antisecretory effects of DCA, while the MAPK inhibitors, PD98059 and SB203580, did not. In summary, our studies suggest that, in contrast to its acute prosecretory effects at pathophysiological concentrations, lower, physiologically relevant, levels of DCA chronically down‐regulate colonic epithelial secretory function. On the basis of these data, we propose a novel role for bile acids as physiological regulators of colonic secretory capacity.
Keywords: epithelium, chloride secretion, bile acid, physiology of the colon
Introduction
Intestinal fluid transport is driven by active ion transport with absorption being driven by cations and fluid secretion being driven predominantly by Cl− secretion [1]. Normally, absorption predominates in the colon, allowing conservation of the large volume of fluid that enters the intestine each day. However, secretory processes are also ongoing and are required for appropriate hydration of the mucosal surface. This finely tuned balance between absorption and secretion can be drastically altered in disease states, leading to the clinical manifestation of diarrhoea. Cl− secretion is promoted by hormones and neuroimmune mediators, which act at cell surface G‐protein‐coupled receptors (GPCRs). Activation of GqPCRs elevates intracellular Ca2+, whereas GsPCRs are linked to increases in cytosolic cAMP. In turn, elevations in intracellular messengers activate the epithelial transport machinery to promote Cl− secretion. At the molecular level the concerted activity of basolateral Na+/K+ ATPase pumps, NKCC1 (Na+/K+/2Cl− cotransporter) and K+ channels serve to elevate Cl− within epithelial cells so that an electrochemical gradient exists for its efflux to occur when channels in the apical membrane are opened [2].
Upon ingestion of a meal, bile acids are released from the gallbladder into the small intestine where they aid in digestion and absorption of lipids. Most bile acids are reabsorbed as they pass through the distal small intestine, but with each cycle of the enterohepatic circulation, a small fraction (∼2%) enters the colon where they undergo bacterial metabolism to form secondary bile acids, the most prominent of which in human beings is deoxycholic acid (DCA). Bile acids exert a range of effects on colonic epithelial cells [3, 4, 5, 6, 7], and their role in promoting fluid and electrolyte secretion is well documented. Pathological conditions that increase bile acid delivery to the colon, such as short bowel syndrome [8, 9], defects in bile acid transporters [10], inflammatory bowel disease [11, 12], microscopic colitis [13] and irritable bowel syndrome [14], are often accompanied by diarrhoea. Furthermore, bile acid sequestrants are well‐established therapeutic tools for alleviating diarrhoea in such conditions [8, 12, 13, 15, 16]. Mechanisms underlying promotion of intestinal fluid accumulation by bile acids have been studied in animals and cultured epithelia, where they have been shown to induce Cl− secretion [17, 18, 19, 20, 21, 22, 23]. However, although there are considerable interspecies variations in concentrations of bile acids required to promote secretion, in human beings it is clear that pathophysiological levels must be attained before secretory responses occur [24].
Although links between pathophysiological concentrations of colonic bile acids and increased fluid secretion are well established, there is little known of their role in regulating transport under physiological circumstances where colonic epithelia are continuously exposed to relatively low bile acid levels. Thus, in the present study we investigated the effects of low DCA concentrations on colonic epithelial secretory function.
Materials and methods
DCA, cholic acid, carbachol (CCh) and forskolin (FSK) were purchased from Sigma Chemical Co, UK. Tyrphostin AG1478, SB203580 and PD98059 were obtained from Calbiochem (San Diego, CA). Anti‐phospho‐p38 and anti‐phospho‐ERK antibodies were obtained from New England Biolabs (Beverley, MA). Anti‐EGF receptor (mouse monoclonal IgG1) was obtained from Upstate Biotechnology Inc. (Lake Placid, NY). Anti‐phosphotyrosine (PY20; monoclonal IgG2bk) was obtained from Transduction Labs (Lexington, KY). All other reagents were of analytical grade and were obtained commercially.
Cell culture
T84 colonic epithelial cells were cultured in DMEM/Ham's F12 media (1:1) supplemented with 5% newborn calf serum (HyClone, Logan, UT) in an atmosphere of 5% CO2 at 37°C. For Ussing chamber/voltage clamp studies, approximately 5 × 105 cells were seeded onto 12 mm Millicell‐HA Transwells (Millipore, Bedford, MA). For Western blotting experiments, approximately 106 cells were seeded onto 30 mm Millicel‐HA Transwells. Cells were cultured on filters for 10–15 days prior to use. Under these conditions T84 cells develop the polarized, electrically resistant phenotype of native epithelial cells.
Electrophysiological measurements
T84 cell monolayers were washed in serum‐free medium and treated with bile acids as described in the figure legends. Cells were then mounted in Ussing chambers (aperture 0.6 cm2), voltage‐clamped to zero potential difference, and monitored for changes in short‐circuit current (ΔIsc) using a VCC MC8 Voltage Clamp (Physiological Instruments, San Diego, CA). Under such conditions secretagogue‐induced ΔIsc across T84 monolayers is wholly reflective of changes in electrogenic Cl− secretion [25]. Isc measurements were carried out in Ringer's solution containing (in mM) 140 Na+, 5.2 K+, 1.2 Ca2+, 0.8 Mg2+, 119.8 Cl−, 25 HCO3 −, 2.4 H2PO4 2− and 10 glucose. Results were normalized and expressed as ΔIsc (μA/cm2). Measurements of transepithelial resistance (TER) were made using an EvOhm apparatus (World Precision Instruments, Sarasota, FL) and results were expressed as Ω·cm2. For examining DCA effects on rat colonic ion transport male Sprague‐Dawley rats (200–250 g) were used. Animals were kept on a 12‐hr light/dark cycle and were given food and water ad libitum. Following anaesthesia (2% isofluorane gas + 2L O2/min) rats were killed by cervical dislocation and the distal colon was removed, stripped of its smooth muscle layers, mounted in Ussing chambers and Isc measurements performed. Approval for these studies was obtained from the Beaumont Hospital Ethics Committee.
Western blotting
After treatment T84 monolayers were lysed in ice‐cold lysis buffer (1% Triton‐X‐100, 1 mM NaVO4, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 μg/ml antipain, 1 mM NaF, 1 mM EDTA and 100 μg/ml PMSF in PBS). Lysates were collected, centrifuged at 15,300 g for 10 min and the pellet discarded. Supernatants were normalized for protein content, mixed with 2X gel loading buffer (50 mM Tris, pH 6.8, 2% SDS, 100 mM dithiothreitol, 0.2% bromophenol blue, 20% glycerol), boiled for 3 min, separated by SDS‐PAGE and transferred to polyvinylidene fluoride (PVDF) membranes. Membranes were preblocked in 1% blocking buffer for 30 min, followed by incubation with primary antibody in 1% blocking buffer for 60 min. After washing (×5) in Tris buffered saline with 1% Tween (TBST), membranes were incubated with horseradish peroxidase (HRP)‐conjugated secondary antibodies in 1% blocking buffer for 30 min. After further washing in TBST, immunoreactive proteins were detected by enhanced chemiluminescence. Protein phosphorylation was quantified using Scion image software. Western blots for phosphorylated proteins were routinely stripped and re‐probed for total protein to ensure equal loading.
Intracellular Ca2+ imaging
T84 cells grown on coverslips were loaded with 5 μM Fura‐2/AM (dissolved in 0.01% Pluronic F‐127 plus 0.1% DMSO) in physiological salt solution (PSS; 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 10 mM D‐glucose and 10 mM HEPES‐trimethylamine, pH 7.4) at room temperature for 30 min. Coverslips were washed and mounted in a perfusion chamber on a Nikon microscope stage. Cells were perfused with normal PSS for 5 min before adding CCh to the perfusing solution. The ratio of Fura‐2 fluorescence with excitation at 340 or 380 nm (F340/380) was measured every 3 sec, and images were captured with an intensified CCD camera (ICCD200) and a MetaFluor Imaging System (Universal Imaging, Corporation, Downingtown, PA).
cAMP assay
Monolayers of T84 cells grown on 30 mm Millicell‐HA Transwells were treated with DCA for 24 hours. Cells were then exposed to FSK (10 μM; 5 min) and lysed with 0.1 M HCl. Lysates were centrifuged (15,300 g; 10 min) and cAMP levels in the supernatants were quantified with a commercially available kit (Sigma‐Aldrich, UK). Results were expressed as pmol cAMP/mg protein.
Statistical analysis
All data are expressed as mean ± SEM for a series of n experiments. Where applicable EC50 values were calculated by extrapolation of data from concentration‐response curves generated in separate experiments. Paired Student's t‐tests were used to compare paired data. One‐way anova with the Student Newman‐Keuls post‐test was used when three or more groups of data were compared. P‐values <0.05 were considered statistically significant.
Results
High concentrations of DCA acutely stimulate colonic epithelial Cl− secretion
We first analysed acute effects of DCA on Cl− secretion across voltage‐clamped T84 cells. Bilateral addition of 1 mM DCA stimulated a transient increase in Isc that rapidly returned towards baseline over the course of 20 min (Fig. 1). The threshold concentration for this prosecretory effect of DCA was greater than 200 μM with an EC50 of 488.8 ± 45.6 μM (Fig. 1B). At concentrations greater than 500 μM, DCA reduced TER (EC50= 982.5 ± 194.2 μM; Fig. 1C), whereas at concentrations greater than 1 mM, DCA induced cell lysis and lactate dehydrogenase (LDH) release (EC50= 1.99 ± 9.5 mM; Fig. 1D). Figure 1E shows that within 1 hr after addition, DCA (500 μM) almost abolished TER across T84 monolayers. However, if the bile acids was washed from the cells after 40 min, there was a gradual restoration of TER. Twenty‐four hours after DCA exposure, the TER across monolayers from which DCA was removed had returned to 54.9 ± 7.3% of that in control cells although continued exposure to the bile acid obliterated TER (Figs. 1E and 2E). Thus, upon removal of DCA the barrier function of colonic epithelial cells can, at least partially, be restored.
Figure 1.
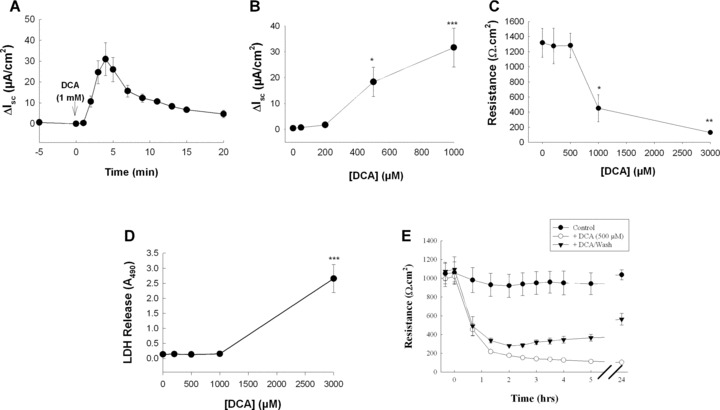
Acute exposure to high concentrations of DCA stimulates Cl− secretion across T84 cells. (A) Voltage‐clamped monolayers of T84 cells were bilaterally exposed to DCA (1 mM) and Isc responses were recorded (n= 5). (B) Voltage‐clamped T84 cells were exposed to increasing concentrations of bilateral DCA and maximal Isc responses were measured (n= 5). (C) Monolayers of T84 cells were exposed to increasing concentrations of DCA for 20 min after which TER was measured (n= 3). (D) Aliquots of supernatants from cells exposed to DCA at varying concentrations for 20 min were analysed for LDH release. Data are expressed as sample absorbance at 490 nm (n= 3). (E) TER was measured across T84 cell monolayers exposed to 500 μM DCA (bilateral). In one set of monolayers DCA was removed after 40 min (n= 4). *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2.
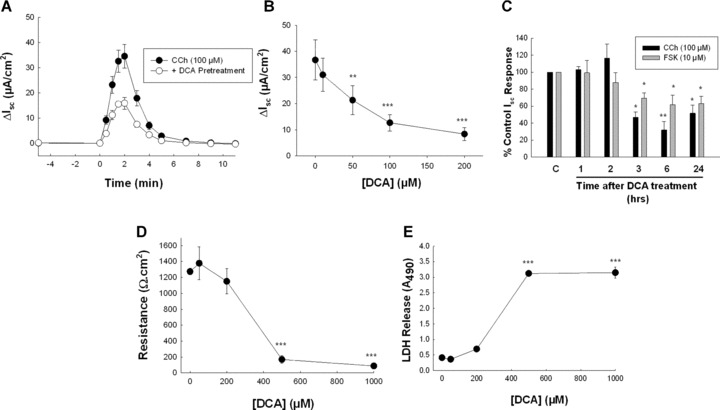
Chronic exposure to low concentrations of DCA inhibits Cl− secretion across T84 cells. (A) Monolayers of T84 cells were exposed to bilateral DCA (50 μM) for 24 hrs and subsequent Isc responses to CCh (100 μM) were measured. (B) T84 cell monolayers were exposed to increasing concentrations of DCA for 24 hrs after which Isc responses to CCh (100 μM) were measured (n= 5). (C) Cells were exposed to DCA (50 μM) for varying periods after which Isc responses to CCh (100 μM; n= 4) or FSK (10 μM; n= 4) were measured. (D) Monolayers of T84 cells were exposed to increasing concentrations of DCA for 24 hrs after which TER was measured (n= 5). (E) Aliquots of supernatants from cells exposed to DCA at varying concentrations for 24 hrs were analysed for LDH. Data are expressed as sample absorbance at 490 nM (n= 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Low concentrations of DCA chronically inhibit colonic epithelial Cl− secretion
Because human colonic epithelial cells in vivo are normally exposed to DCA at concentrations that do not induce secretion [26, 27], we examined the effects of prolonged exposure to low concentrations of the bile acid on secretagogue‐induced responses. In cells exposed to DCA (50 μM) for 24 hrs subsequent responses to a prototypical GqPCR agonist, CCh (100 μM), were attenuated to 49.5 ± 3.6% of those in control cells (Fig. 2A; n= 26; P < 0.001). The EC50 for this antisecretory effect was 44.3 ± 3.8 μM (n= 5) (Fig. 2B) and the effects of DCA were maximal between 3 and 6 hrs (Fig. 2C). Chronic treatment with DCA (50 μM; 24 hrs) also reduced responses to thapsigargin (2 μM), which increases intracellular Ca2+ in a receptor‐independent fashion, and to the cAMP‐dependent secretagogue, FSK (10 μM), to 50.6 ± 5.3 (n= 3; P < 0.05) and 82.1 ± 3.7% (n= 7; P < 0.05) of those in control cells, respectively. Interestingly, cAMP‐dependent secretory responses were less sensitive to pretreatment with DCA than were Ca2+‐dependent responses (Fig. 2C). Prolonged exposure of T84 cells to DCA did not alter TER or LDH release until concentrations more than 200 μM were achieved (Fig. 2D and E). Together, these data indicate that chronic exposure to low levels of DCA decreases epithelial secretory capacity without causing apparent cell damage.
We next analysed DCA effects on Cl− secretion across sections of rat colon. In these experiments DCA was added apically in order to more closely mimic in vivo situations where bile acids are found in the lumen. As expected, high concentrations of DCA (1 mM) stimulated a rapid and transient ΔIsc response (Fig. 3A). The effects of DCA were concentration‐dependent with levels more than 200 μM being required for a prosecretory effect to occur (Fig. 3B). However, when tissues were exposed to relatively low concentrations of DCA (200 μM) on the apical side for prolonged periods (3 hrs), subsequent secretory responses to CCh (100 μM) or FSK (10 μM) were significantly attenuated (Fig. 3C). This effect of DCA was not associated with alterations in tissue viability or integrity as indicated by transmucosal conductance measurements. The conductance across control tissues was 26.5 ± 1.8 mS/cm2 compared to 29.7 ± 3.7 mS/cm2 in DCA‐pretreated tissues (n= 5). Thus, similar to its effects in cultured human colonic epithelia, prolonged exposure to relatively low concentrations of DCA exerts antisecretory effects in rat colonic mucosa.
Figure 3.
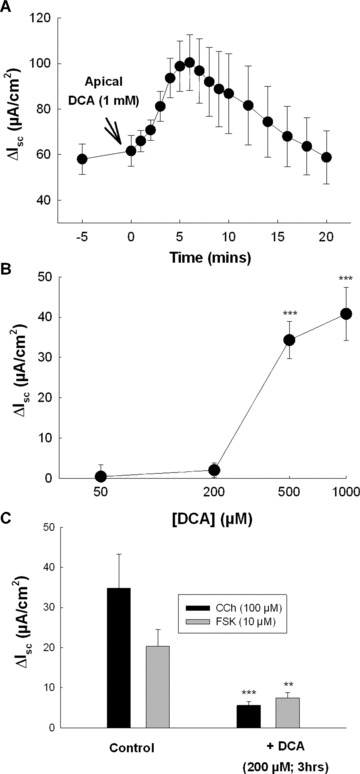
Prolonged exposure to physiological concentrations of DCA inhibits Cl− secretion across rat colon. (A) Voltage‐clamped segments of stripped rat colonic mucosa were apically exposed to DCA (1 mM) and Isc responses were recorded (n= 5). (B) Voltage‐clamped sections of rat colon were exposed to increasing concentrations of apical DCA and maximal Isc responses were measured (n= 4–7 for each concentration tested). (C) Voltage‐clamped rat colonic mucosa was exposed to 200 μM apical DCA and after 3 hrs incubation subsequent Isc responses to CCh (100 μM; n= 4) and FSK (10 μM; n= 5) were measured. ***P < 0.005 when compared to control cells.
Structure–activity relationships for antisecretory effects of bile acids
The secretory and antisecretory effects of other naturally occurring bile acids were also examined. Taurodeoxycholic acid (TDCA, the taurine conjugate of DCA) and cholic acid (CA, the trihydroxy, primary bile acid from which DCA is formed) were selected for study. Similar to DCA, and as previously reported [19], TDCA stimulated Cl− secretion across T84 cells at relatively high concentrations with an EC50 of 427.5 ± 80.2 μM (n= 5) (Fig. 4A). In contrast, CA did not alter basal Isc. However, similar to DCA, we found that prolonged exposure to relatively low concentrations of both TDCA and CA inhibited secretory responses to CCh (Fig. 4B). In further analyses we examined the ability of naturally occurring isomers of DCA to alter agonist‐stimulated Isc responses and found that both isodeoxycholic acid (iso‐DCA) and lagodeoxycholic (lago‐DCA) acid were approximately equipotent to DCA in exerting antisecretory effects (Table 1). Similarly, the dihydroxy primary bile acid, chenodeoxycholic acid (CDCA), was also found to be equipotent with DCA in inhibiting agonist‐stimulated secretory responses. Finally, we examined the sidedness by which conjugated and unconjugated bile acids exert their antisecretory effects. Although DCA was equally effective when applied either apically or basolaterally, TDCA was active only on the basolateral side (Fig. 4C).
Figure 4.
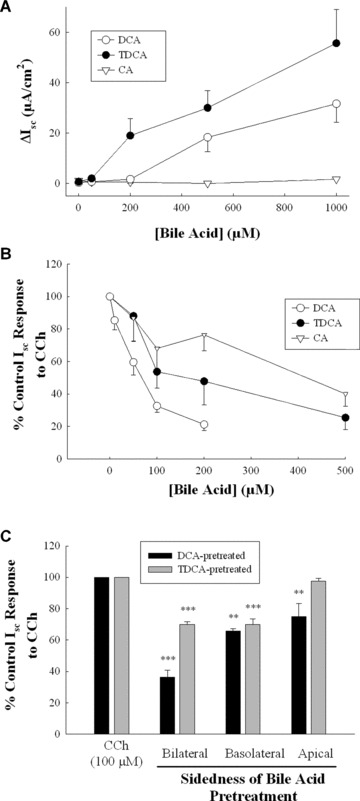
Structure–activity relationships for bile acids in regulating epithelial secretory function. (A) Monolayers of T84 cells were exposed bilaterally to increasing concentrations of DCA (n= 5), TDCA (n= 5), or cholic acid (n= 3) and maximal Isc responses were measured. (B) T84 cell monolayers were exposed to increasing concentrations of bilateral DCA (n= 5), TDCA (n= 4), and cholic acid (n= 5) for 24 hrs after which secretory responses to CCh (100 μM) were measured. (C) Monolayers of T84 cells were exposed to DCA (50 μM; n= 4) or TDCA (200 μM; n= 4) on the apical, basolateral, or both sides for 24 hrs after which cells were voltage‐clamped and subsequent secretory responses to CCh (100 μM) were measured. **P < 0.01; ***P < 0.001.
Table 1.
Antisecretory effects of dihydroxy bile acids in T84 cells
| Bile acid (50 μM) | Hydroxyl group positions | % Control Isc response to CCh (100 μM) |
|---|---|---|
| Deoxycholic acid | 3α‐12α‐OH | 52.5 ± 6.0 (n= 9; P < 0.001) |
| Isodeoxycholic acid | 3β‐12α‐OH | 58.5 ± 7.8 (n= 3; P < 0.05) |
| Lagodeoxycholic acid | 3α‐12β‐OH | 57.5 ± 7.3 (n= 6; P < 0.001) |
| Chenodeoxycholic acid | 3α‐7α‐OH | 51.4 ± 7.4 (n= 6; P < 0.001) |
The antisecretory effects of DCA are not mediated by muscarinic receptor activation or by alterations in the second messenger levels
Previous studies suggest bile acids can exert biological effects through activation of muscarinic M3 receptors [28]. However, we found that although it abolished responses to CCh (data not shown), the muscarinic receptor antagonist, atropine (1 μM), did not prevent the antisecretory effect of DCA (Fig. 5A). We also examined the possibility that DCA exerts antisecretory effects by altering secretagogue‐induced mobilization of the second messengers. However, in cells pretreated with DCA (50 μM, 24 hrs), neither CCh‐stimulated mobilization of intracellular Ca2+ nor FSK‐induced accumulation of cAMP was altered (Fig. 5B and C). These data suggest that DCA exerts its antisecretory effects downstream from agonist‐induced mobilization of prosecretory second messengers.
Figure 5.
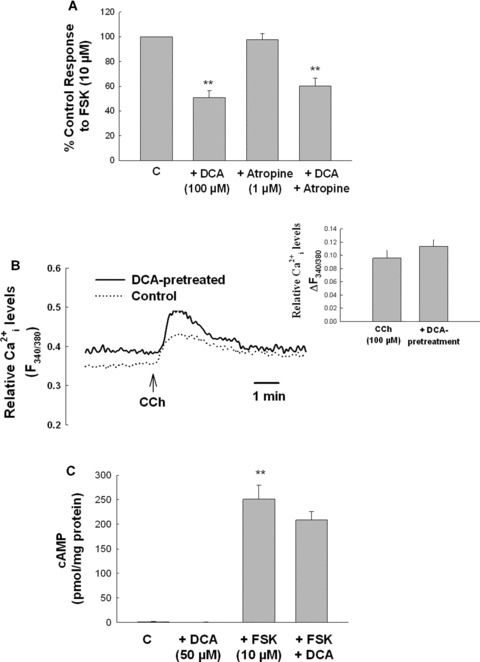
The antisecretory effect of DCA is not mediated by muscarinic receptors or by alterations in agonist‐induced generation of the second messengers. (A) Cells were pretreated with atropine (1 μM) for 30 min prior to DCA (100 μM). After a further 24 hrs incubation, cells were washed and Isc responses to FSK (10 μM) were measured (n= 5). (B) T84 cells grown on glass coverslips were pretreated for 24 hrs with DCA (50 μM) after which CCh‐induced mobilization of intracellular Ca2+ was measured by Fura‐2 fluorescence. Data are expressed as mean fluorescence ratio at 340 and 380 nM (n= 3 coverslips for each condition). The inset shows the net change in mean fluorescence ratio (ΔF340/380) for these experiments. (C) Monolayers of T84 cells were treated with DCA (50 μM) for 24 hrs and then stimulated with FSK (10 μM) for 5 min, lysed, and intracellular cAMP was measured (n= 4). **P < 0.01.
EGFr transactivation and de novo protein synthesis mediate the antisecretory effects of DCA
Our previous work has identified the epidermal growth factor receptor (EGFr) and mitogen‐activated protein kinases (MAPKs) as important regulators of epithelial secretory responses [29, 30]. Furthermore, others have shown that bile acids induce EGFr and extracellular signal‐regulated kinase (ERK) activation in colonic epithelia [5, 31, 32]. Thus, we examined a potential role for the EGFr and MAPKs in mediating antisecretory effects of DCA. As shown in Fig. 6A and B, DCA (50 μM) rapidly stimulated EGFr phosphorylation and pretreatment with the EGFr inhibitor, tyrphostin AG1478 (100 nM), reversed the antisecretory effects of the bile acid (Fig. 6C). DCA also activated both ERK and p38 MAPKs (Fig. 7A). However, although PD98059 (20 μM) and SB203580 (10 μM) effectively prevented DCA‐induced ERK and p38 phosphorylation, respectively, neither inhibitor prevented the antisecretory actions of DCA (Fig. 7B and C). However, inhibition of protein translation with cycloheximide (CHX; 100 ng/ml), was found to attenuate the antisecretory actions of DCA (Fig. 7D).
Figure 6.
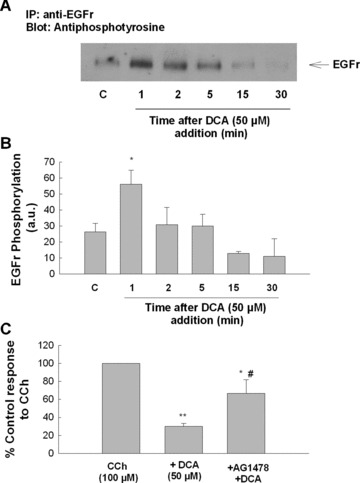
EGFr activation mediates the antisecretory effect of DCA in colonic epithelial cells. (A) Cells were treated with DCA (50 μM; bilateral) for the times indicated after which EGFr phosphorylation was measured by immunoprecipitation and Western blotting. (B) Densitometric analysis of several similar experiments (n= 3). (C) Cells were pretreated with tyrphostin AG1478 (100 nM) for 30 min prior to DCA (50 μM). After a further 24 hrs incubation, cells were mounted in Ussing chambers and Isc responses to CCh (100 μM) were measured. Responses to CCh in DCA‐treated cells were expressed as percentage of vehicle or AG1478‐treated controls, as appropriate. Single asterisk ‘*’ denotes significant differences from cells stimulated with CCh alone (n= 5; *P < 0.05; **P < 0.01). Symbol ‘#’ denotes significant difference from cells pretreated with DCA.
Figure 7.
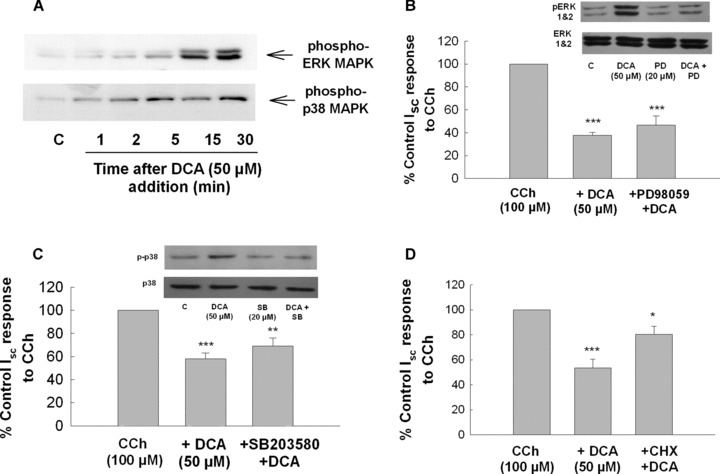
Inhibition of protein synthesis but not ERK or p38 MAPK blocks the antisecretory effect of DCA in colonic epithelial cells. (A) Cells were treated bilaterally with DCA (50 μM) for the times indicated after which cell lysates were analysed by Western blotting with anti‐phospho‐ERK or anti‐phospho‐p38 antibodies. Each blot is representative of three similar experiments. (B–D) Cells were pretreated with either (B) PD98059 (20 μM), (C) SB203580 (10 μM) or (D) cycloheximide (100 ng/ml) for 30 min prior to DCA (50 μM). After a further 24 hrs incubation, cells were mounted in Ussing chambers and Isc responses to CCh (100 μM) were measured. The insets to Fig. 7B and D show that PD98059 and SB203580 inhibited DCA‐stimulated ERK and p38 phosphorylation, respectively (these blots are representative of three similar experiments). Responses to CCh in DCA‐treated cells were expressed as percentage of vehicle or inhibitor‐treated controls, as appropriate (n= 5; *P < 0.05; **P < 0.01; ***P < 0.001 compared to cells stimulated with CCh alone).
Discussion
In the present study we demonstrate for the first time that bile acids exert directly opposing actions on colonic epithelial cells when they are present at high or low concentrations. These data are likely to be important when considering the role that bile acids play in regulating colonic transport both in normal conditions and in disease states. Increased bile acid delivery to the colon is a feature of many pathological conditions and it is likely that diarrhoea associated with such conditions is primarily because of the prosecretory actions of dihydroxy bile acids [9, 18]. We observed secretory responses to DCA in cultured human colonic epithelial cells and rat colon only to relatively high (i.e. >200 μM) concentrations and as its levels increased beyond this threshold, DCA became cytotoxic. These data are reminiscent of previous studies in animal models and human beings [24, 33, 34], and on the basis of these observations we propose that increased Cl− and fluid secretion occurring in response to high levels of bile acids likely serves as a protective mechanism that dilutes the luminal contents before epithelial damage can occur. This hypothesis is supported by our findings that epithelial barrier function, as measured by changes in TER, was partially restored upon removal of DCA.
Normally, however DCA is present in human colon at levels lower than those that promote secretion and we found that at such concentrations (i.e. <200 μM), prolonged exposure to DCA reduced responses to both Ca2+‐ and cAMP‐dependent secretagogues. The EC50 for this antisecretory effect of DCA was approximately 75 μM, which is expected to be well within the physiological range in human proximal colon in vivo[26, 27]. Our findings in cultured epithelial cells were supported by those obtained in rat colon, in that apical addition of DCA at a concentration that did not acutely promote secretion, chronically inhibited subsequent agonist‐induced responses. Thus, our data suggest that, in direct contrast to its prosecretory actions at high concentrations, DCA at more physiological levels exerts long‐term antisecretory effects on colonic epithelia.
Other bile acids, including CDCA and TDCA, were found to share the antisecretory properties of DCA. However, in contrast to DCA, a relatively lipophilic molecule that is effective both apically and basolaterally, TDCA was effective only from the basolateral side. We have previously shown a similar polarity to TDCA actions in promoting secretion at high concentrations [18], and our findings are consistent with the view that basolateral transporters are necessary for uptake of hydrophilic, conjugated bile acids into colonic epithelia [23, 25, 35]. We also examined the effects of the naturally occurring isomers of DCA, isodeoxycolic acid and lagodeoycholic acid. We have previously shown that, similar to DCA, lago‐DCA acutely stimulates Cl− secretion across colonic epithelial cells at high concentrations, whereas iso‐DCA was without effect [18]. However, in the present study we found that prolonged exposure to low concentrations of either iso‐ or lago‐DCA exerted antisecretory actions with a similar potency to DCA. In contrast to DCA and its isomers, we found that the primary bile acid, cholic acid, was devoid of prosecretory actions. This is in agreement with previous studies demonstrating that only bile acids with two α‐hydroxyl groups exert prosecretory activity [34, 36]. However, similar to the dihydroxy bile acids, CA exerted antisecretory actions upon prolonged exposure to epithelial cells. Although it is unlikely that such high concentration of CA would occur in the colonic lumen under normal conditions, these data demonstrate a marked difference in structure–activity relationships for pro‐ and antisecretory actions of bile acids and suggest that these actions are likely to be mediated by distinct mechanisms.
Similar to our previous studies showing that prosecretory actions of DCA do not involve muscarinic receptor activation [18], we found that the antisecretory effect of DCA was also independent of muscarinic receptor activation. Thus, mechanisms by which bile acids regulate epithelial transport appear to be distinct from the M3 receptor‐dependent pathway that has been reported to mediate their effects on cell growth [5]. Furthermore, because low concentrations of DCA did not alter secretagogue‐induced mobilization of prosecretory second messengers, our data suggest that DCA exerts its antisecretory actions at a point distal to generation of prosecretory second messengers.
The EGFr is emerging as an important regulator of epithelial transport [29, 37, 38] and because bile acids can induce EGFr activation in some systems [5, 31, 32, 39], we hypothesized that it might mediate the antisecretory actions of DCA in colonic epithelia. This appears to be the case because DCA rapidly induced EGFr phosphorylation, and a specific EGFr inhibitor, tyrphostin AG1478, at least partially reversed the antisecretory effects of DCA. However, although DCA also stimulated activation of both ERK and p38, inhibition of either of these MAPKs did not alter the actions of the bile acid. In light of the previous studies showing that EGFr/MAPK signalling mediates cell growth in response to bile acids [5], these data are intriguing in that they suggest that the EGFr transduces independent signals to differentially regulate cell growth and ion transport in response to bile acids. Although DCA‐induced antisecretory signalling mechanisms downstream of the EGFr remain to be identified, it is likely that de novo protein synthesis is involved because these actions are slow in onset and are sensitive to the inhibitor of protein translation, cycloheximide. Furthermore, because DCA induces a generalized and sustained down‐regulation in epithelial secretory capacity, it is likely that it ultimately acts by altering the expression and/or activity of a transport protein involved in the Cl− secretory pathway. Experiments are currently underway in our laboratory to investigate this hypothesis.
The physiological/pathophysiological significance of opposing actions of bile acids at low and high concentrations is as yet unclear. However, based on data from our own and previous studies we put forward the following hypothetical model. Normally, colonic DCA levels are relatively low and it exerts antisecretory actions, which, in vivo, would serve to promote normal colonic absorptive function. However, as absorption proceeds and the luminal contents become dehydrated, bile acid concentrations would increase. When a certain threshold concentration is reached, DCA becomes acutely prosecretory, which in vivo would serve to rehydrate the luminal contents, thereby preventing bile acid concentrations from achieving sufficient levels to exert cytotoxic actions. In turn, as it becomes diluted DCA would again become antisecretory. In this way, we propose a novel role for bile acids as colonic ‘osmosignals’ that dynamically regulate the fluidity of the luminal contents. However, it must be acknowledged that the situation in vivo is more complex than in the models employed here and factors such as mucosal blood flow, the unstirred mucous layer, luminal bacteria and fluctuations in pH are all likely to alter the bioavailability of bile acids to epithelial cells.
In summary, we have identified novel antisecretory actions of DCA, and other bile acids, on colonic epithelial cells when they are present at relatively low concentrations. This effect appears to be mediated by an EGFr‐dependent signalling pathway, requires de novo protein synthesis and interferes with the secretory mech anism at a point distal to the second messenger generation. Our data suggest a novel role for bile acids as colonic osmosignals, and future studies will aim to more fully elucidate the molecular mechanisms involved in transducing their effects both in physiological and pathophysiological conditions.
Supporting information
Fig. S1 Time course of LDH release in response to DCA in T84 cells. Cells were treated bilaterally with DCA (500 M) and at the times indicated aliquots were removed from the bathing solution and assayed for LDH release. Results were expressed as percentage of total cell lysis in response to Triton‐X‐100‐containing lysis buffer. Although there was no change in LDH release after 20 min, 16.8 ± 3.0% of cells were lysed after 40 min (n = 6).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgements
This work was supported by a Senior Investigator Award from the CCFA and by an Investigator Programme Award from the Science Foundation of Ireland (to S.J.K) and by NIH Grant DK 64891 (to A.F.H.).
References
- 1. Montrose MM, Keely SJ, Barrett KE. Secretion and absorption: small intestine and colon. In: Yamada T, Alpers D, Kaplowitz N, Laine L, Owyang C, Powell D, editors. Textbook of gastroenterology, 4th ed. Philadelphia : Lippencott‐Raven, 2003; pp. 308–39. [Google Scholar]
- 2. Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Ann Rev Physiol. 2000; 62: 535–72. [DOI] [PubMed] [Google Scholar]
- 3. Münch A, Ström M, Söderholm JD. Dihydroxy bile acids increase mucosal permeability and bacterial uptake in human colon biopsies. Scand J Gasteoenterol. 2007; 42: 1167–74. [DOI] [PubMed] [Google Scholar]
- 4. Muhlbauer M, Allard B, Bosserhoff AK, et al . Differential effects of deoxycholic acid and taurodeoxycholic acid on NF‐{kappa}B signal transduction and IL‐8 gene expression in colonic epithelial cells. Am J Physiol. 2004; 286: G1000–8. [DOI] [PubMed] [Google Scholar]
- 5. Cheng K, Raufman JP. Bile acid‐induced proliferation of a human colon cancer cell line is mediated by transactivation of epidermal growth factor receptors. Biochem Pharmacol. 2005; 70: 1035–47. [DOI] [PubMed] [Google Scholar]
- 6. Milovic V, Teller IC, Murphy GM, et al . Deoxycholic acid stimulates migration in colon cancer cells. Eur J Gastroenterol Hepatol. 2001, 13: 945–9. [DOI] [PubMed] [Google Scholar]
- 7. Barcelo A, Claustre J, Toumi F, et al . Effect of bile salts on colonic mucus secretion in isolated vascularly perfused rat colon. Dig Dis Sci. 2000; 46: 1223–31. [DOI] [PubMed] [Google Scholar]
- 8. Hofmann AF, Poley JR. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology. 1972; 62: 918–34. [PubMed] [Google Scholar]
- 9. Potter GD. Bile acid diarrhea. Dig Dis Sci. 1998; 16: 118–24. [DOI] [PubMed] [Google Scholar]
- 10. Dawson PA, Haywood J, Craddock AL, et al . Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003; 278: 33920–7. [DOI] [PubMed] [Google Scholar]
- 11. Kruis W, Kalek HD, Stellaard F, et al . Altered fecal bile acid pattern in patients with inflammatory bowel disease. Digestion. 1986; 35: 189–98. [DOI] [PubMed] [Google Scholar]
- 12. Nyhlin H, Merrick MV, Eastwood MA. Bile acid malabsorption in Crohn's disease and indications for its assessment using SeHCAT. Gut. 1994; 35: 90–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ung K‐A, Gillberg R, Kilander A, et al . Role of bile acids and bile acid binding agents in patients with collagenous colitis. Gut. 2000; 46: 170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalia N, Hardcastle J, Grasa L, et al . Intestinal secretory and absorptive function in Trichinella spiralis mouse model of post‐infective gut dysfunction role of bile acids. Gut. 2008; 57: 41–9. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez‐Banares F, Salas A, Esteve M, et al . Collagenous and lymphocytic colitis: evaluation of clinical and histological features, response to treatment, and long‐term follow‐up. Am J Gastroenterol. 2003; 98: 340–7. [DOI] [PubMed] [Google Scholar]
- 16. Smith MJ, Cherian P, Raju GS, et al . Bile acid malabsorption in persistent diarrhoea. J R Coll Physicians Lond. 2000; 34: 448–51. [PMC free article] [PubMed] [Google Scholar]
- 17. Dharmsathaphorn K, Huott PA, Vongkovit P, et al . Cl− secretion induced by bile salts. A study of the mechanism of action based on a cultured colonic epithelial cell line. J Clin Invest. 1989; 84: 945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keely SJ, Scharl MM, Bertelsen LS, et al . Bile acid‐induced secretion in polarized monolayers of T84 colonic epithelial cells: structure‐activity relationships. Am J Physiol. 2007; 292: G290–7. [DOI] [PubMed] [Google Scholar]
- 19. Devor DC, Sekar MC, Frizzell RA, et al . Taurodeoxycholate activates potassium and chloride conductances via an IP3‐mediated release of calcium from intracellular stores in a colonic cell line (T84). J Clin Invest. 1993; 92: 2173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mauricio AC, Slawik M, Heitzmann D, et al . Deoxycholic acid (DOC) affects the transport properties of distal colon. Eur J Physiol. 2000; 439: 532–40. [DOI] [PubMed] [Google Scholar]
- 21. Gelbmann CM, Schteingart CD, Thompson SM, et al . Mast cells and histamine contribute to bile acid‐stimulated secretion in the mouse colon. J Clin Invest. 1995; 95: 2831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Venkatasubramanian J, Selvaraj N, Carlos M, et al . Differences in Ca2+ signaling underlie age‐specific effects of secretagogues on colonic Cl− transport. Am J Physiol. 2001; 280: C646–58. [DOI] [PubMed] [Google Scholar]
- 23. Weihrauch D, Kanchanapoo J, Ao M, et al . Weanling, but not adult, rabbit colon absorbs bile acids: flux is linked to expression of putative bile acid transporters. Am J Physiol. 2006; 290: G439–50. [DOI] [PubMed] [Google Scholar]
- 24. Mekjian HS, Phillips SF, Hofmann AF. Colonic secretion of water and electrolytes induced by bile acids: perfusion studies in man. J Clin Invest. 1971; 50: 1569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cartwright CA, McRoberts JA, Mandel KG, et al . Synergistic action of cyclic adenosine monophosphate‐ and calcium‐mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985; 76: 1837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kok TM, Van Faassen A, Glinghammar B, et al . Bile acid concentrations, cytotoxicity, and pH of fecal water from patients with colorectal adenomas. Dig Dis Sci. 1999; 44: 2218–25. [DOI] [PubMed] [Google Scholar]
- 27. Hamilton JP, Xie G, Raufman J‐P, et al . Human cecal bile acids: concentration and spectrum. Am J Physiol. 2007; 293: G256–63. [DOI] [PubMed] [Google Scholar]
- 28. J‐P Raufman, Cheng K, Zimniak P. Activation of muscarinic receptor signaling by bile acids: physiological and medical implications. Dig Dis Sci. 2003; 48: 1431–44. [DOI] [PubMed] [Google Scholar]
- 29. Keely SJ, Uribe JM, Barrett KE. Carbachol stimulates transactivation of epidermal growth factor receptor and mitogen‐activated protein kinase in T84 cells. Implications for carbachol‐stimulated chloride secretion. J Biol Chem. 1998; 273: 27111–7. [DOI] [PubMed] [Google Scholar]
- 30. Keely SJ, Barrett KE. p38 mitogen‐activated protein kinase inhibits calcium‐dependent chloride secretion in T84 colonic epithelial cells. Am J Physiol. 2003; 284: C339–48. [DOI] [PubMed] [Google Scholar]
- 31. Raimondi F, Santoro P, Barone MV, et al . Bile acids modulate tight junction structure and barrier function of Caco‐2 monolayers via EGFR activation. Am J Physiol. 2008; 294: G906–13. [DOI] [PubMed] [Google Scholar]
- 32. Cheng K, Xie G, Raufman J‐P. Matrix metalloproteinase‐7‐catalyzed release of HB‐EGF mediates deoxycholyltaurine‐induced proliferation of a human colon cancer cell line. Biochem Pharmacol. 2007; 73: 1001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teem MV, Phillips SF. Perfusion of the hamster jejunum with conjugated and unconjugated bile acids: inhibition of water absorption and effects on morphology. Gastroenterology 1972; 62: 261–7. [PubMed] [Google Scholar]
- 34. Chadwick VS, Gaginella TS, Carlson GL, et al . Effect of molecular structure on bile acid‐induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979; 94: 661–74. [PubMed] [Google Scholar]
- 35. Dawson PA, Hubbert M, Haywood J, et al . The heteromeric organic solute transporter {alpha}‐{beta}, Ost{alpha}‐Ost{beta}, is an ileal basolateral bile acid transporter. J Biol Chem. 2005; 280: 6960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon SJ, Kinsey MD, Magen JS, et al . Structure of bile acids associated with secretion in the rat cecum. Gastroenterology. 1979; 77: 38–44. [PubMed] [Google Scholar]
- 37. Bertelsen LS, Barrett KE, Keely SJ. Gs protein‐coupled receptor agonists induce transactivation of the EGFr in T84 cells: implications for cAMP‐dependent chloride secretion. J Biol Chem. 2004; 279: 6271–9. [DOI] [PubMed] [Google Scholar]
- 38. Khurana S, Nath SK, Levine SA, et al . Brush border phosphatidylinositol 3‐kinase mediates epidermal growth factor stimulation of intestinal NaCl absorption and Na+/H+ exchange. J Biol Chem. 1996; 271: 9919–27. [DOI] [PubMed] [Google Scholar]
- 39. Merchant NB, Rogers CM, Trivedi B, et al . Ligand‐dependent activation of the epidermal growth factor receptor by secondary bile acids in polarizing colon cancer cells. Surgery. 2005; 138: 415–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Time course of LDH release in response to DCA in T84 cells. Cells were treated bilaterally with DCA (500 M) and at the times indicated aliquots were removed from the bathing solution and assayed for LDH release. Results were expressed as percentage of total cell lysis in response to Triton‐X‐100‐containing lysis buffer. Although there was no change in LDH release after 20 min, 16.8 ± 3.0% of cells were lysed after 40 min (n = 6).
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


