Abstract
Background and Purpose
Anatomical, biochemical and pharmacological evidence suggest the existence of a crosstalk between the orexinergic and endocannabinoid systems. While the orexin receptor 1 (OX1 receptor) modulates the reinforcing properties of cannabinoids, the participation of orexins in the acute pharmacological effects of Δ9‐tetrahydrocannabinol (THC) remains unexplored.
Experimental Approach
We assessed the possible role of orexins in THC‐induced hypolocomotion, hypothermia, antinociception, anxiolytic‐ and anxiogenic‐like effects and memory impairment. Selective OX1 and OX2 receptor antagonists and OX1 knockout (KO) mice as well as prepro‐orexin (PPO) KO mice were used as pharmacological and genetic approaches. CB1 receptor levels in control and PPO KO mice were evaluated by immunoblot analysis. The expression of c‐Fos after THC treatment was analysed in several brain areas in wild‐type mice and in mice lacking the PPO gene.
Key Results
The hypothermia, supraspinal antinociception and anxiolytic‐like effects induced by THC were modulated by orexins through OX2 receptor signalling. OX1 receptors did not seem to be involved in these THC responses. No differences in CB1 receptor levels were found between wild‐type and PPO KO mice. THC‐induced increase in c‐Fos expression was reduced in the central amygdala, medial preoptic area and lateral septum in these mutant mice.
Conclusions and Implications
Our results provide new findings to further clarify the interaction between orexins and cannabinoids. OX1 and OX2 receptors are differently implicated in the pharmacological effects of cannabinoids.
Abbreviations
- DI
discrimination index
- KO
knockout
- MPE
maximum possible effect
- PPO
prepro‐orexin
- THC
Δ9‐tetrahydrocannabinol
- WT
wild type
Tables of Links
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Δ9‐Tetrahydrocannabinol (THC), the main psychoactive constituent of Cannabis sativa, produces a wide spectrum of central and peripheral actions, including antinociception, hypothermia, immunomodulation, hypolocomotion, catalepsy, memory disruption, rewarding effects and emotional responses (Pertwee, 2008). The CNS responses to THC are mainly mediated by the cannabinoid receptor 1 (CB1 receptor), which is a member of the family of GPCRs (Pertwee et al., 2010). Thus, the antinociceptive, hypolocomotor and hypothermic effects of THC, as well as the somatic signs of THC withdrawal, were not observed in CB1 knockout (KO) mice (Ledent et al., 1999). The discovery of CB2 receptors in neurons of the CNS (Van Sickle et al., 2005) suggests that these receptors could also contribute to some central pharmacological effects of THC. Several heterologous systems, different from the endocannabinoid system, also participate in the behavioural responses induced by THC. Thus, dopamine (Sami et al., 2015), endogenous opioids (Robledo et al., 2008), monoamines (Viñals et al., 2015), GABA (Radhakrishnan et al., 2015), glutamate (Castaldo et al., 2010), ACh (Goonawardena et al., 2010), adenosine (Justinová et al., 2014) and several neuropeptides (Verty et al., 2004) have been shown to be involved in cannabinoid pharmacological responses.
Orexin‐A and orexin‐B (also known as hypocretin‐1 and hypocretin‐2) are hypothalamic neuropeptides produced by the proteolysis of a common precursor, prepro‐orexin (PPO) (de Lecea et al., 1998; Sakurai et al., 1998). The pharmacological effects of orexins are mediated by the activation of two different GPCRs (OX1 and OX2 receptors) (de Lecea et al., 1998; Sakurai et al., 1998). Orexin‐expressing neurons represent a small population exclusively located in the lateral hypothalamus, the perifornical area and the dorsomedial hypothalamus, but they have extensive projections throughout the brain (Peyron et al., 1998). Consistent with this, the orexin system modulates diverse physiological processes such as sleep/wakefulness cycle (Kilduff and Peyron, 2000), feeding behaviour (Sakurai et al., 1998), reward processing (Plaza‐Zabala et al., 2012a) and emotional responses (Sakurai, 2014).
Findings are emerging suggesting the existence of a crosstalk between the orexinergic and the endocannabinoid systems (Flores et al., 2013). Anatomical studies show an overlapping distribution of CB1 and orexin receptors in several brain areas (Marcus et al., 2001; Mackie, 2005), and the existence of heterodimers between CB1 and OX1 receptors has also been demonstrated (Jäntti et al., 2014). Moreover, activation of the OX1 receptor leads to the production of 2‐arachidonoylglycerol, suggesting that endocannabinoids could contribute to orexin effects (Ho et al., 2011). The involvement of the endocannabinoid system in some physiological functions of orexins, such as food intake (Crespo et al., 2008; Cristino et al., 2013) and nociception (Ho et al., 2011), has been established. However, few studies have evaluated whether orexins participate in the pharmacological effects of cannabinoids, although a crucial role for the OX1 receptor in the reinforcing properties of the synthetic cannabinoid WIN55,212‐2 has been recently shown (Flores et al., 2014a).
The objective of this study was to elucidate the possible involvement of orexins in the acute pharmacological effects induced by THC and the orexin receptor subtypes responsible for these effects. To achieve this, selective OX1 and OX2 antagonists and OX1 KO mice as well as PPO KO mice were used as pharmacological and genetic approaches respectively. In addition, we investigated the brain areas responsible for these effects by analysing c‐Fos expression using immunofluorescence techniques.
Methods
Animals
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Experiments were performed in male C57BL/6J mice (Charles River, L'Arbresle, France), in male PPO KO and OX1 KO mice and their wild‐type (WT) C57BL/6J littermates (12–16 weeks old). Generation of mice with a deletion of the PPO gene has been previously described (Chemelli et al., 1999). These mice do not produce either the orexin‐A or the orexin‐B peptide and have been extensively characterized at the behavioural level. PPO KO mice were backcrossed for at least nine generations into C57BL/6J and genotyped, as described elsewhere (Chemelli et al., 1999). OX1 KO (Jackson Laboratories, Sacramento, CA, USA) mice were crossed at least seven generations to C57BL/6J mice and genotyped as previously described (Flores et al., 2014a). Mice were housed from two to four per cage in a temperature (21 ± 1°C)‐controlled and humidity (55 ± 10%)‐controlled room with a 12 h/12 h light/dark cycle (light on between 08:00 and 20:00). Food and water were available ad libitum. For the immunoblot study, mice were quickly killed by cervical dislocation, and brains were immediately removed in order to avoid tissue degradation. For the immunofluorescence study, mice were deeply anaesthetized with ketamine/xylazine (100/10 mg·kg−1, i.p.) and then transcardially perfused with cold 4% paraformaldehyde. Animal procedures were carried out in accordance with the guidelines of the European Communities Directive 86/609/EEC regulating animal research and were approved by the local ethical committee (CEEA‐IMAS‐UPF).
Experimental design
The observer was blind to treatment in all the experiments. The allocation of animals to the different experimental groups was randomly assessed, as well as the order in which they were treated and evaluated. Effects of THC on locomotion, body temperature and nociception were evaluated in series and in this specified order (n = 10–11 in WT/PPO KO and n = 8–9 in WT/OX1 KO experiments, n = 13–14 in VEH/SB‐334867 and n = 11–12 in VEH/TCS‐OX2‐29 experiments). Independent sets of animals were employed to evaluate anxiogenic‐like (n = 7–8 in WT/PPO KO and WT/OX1 KO experiments and n = 9–12 in VEH/SB‐334867 and VEH/TCS‐OX2‐29 experiments), anxiolytic‐like (n = 14–15 in WT/PPO KO and n = 7–8 in WT/OX1 KO experiments, n = 10–12 in VEH/SB‐334867 and n = 16–18 in VEH/TCS‐OX2‐29 experiments) and amnesic‐like effects of THC (n = 7–9 in all experiments). Biochemical studies included six to nine animals per group. A lower number of animals was employed in some experiments with WT/KO mice, due to reduced interindividual variability and limited availability.
Locomotor activity
Changes in horizontal locomotor activity after acute THC injection (5 and 10 mg·kg−1) were evaluated by using individual locomotor activity boxes (9 × 20 × 11 cm, Imetronic, Pessac, France) in a low luminosity room, as previously reported (Berrendero et al., 2005). Mice were placed in the locomotor cages 20 min after THC administration, and activity was recorded as the number of horizontal photocell counts for 15 min.
Rectal temperature
Rectal temperature before and after acute THC injection (5 and 10 mg·kg−1) was measured in each mouse by placing 3 cm of an electronic thermocouple flexible rectal probe (Panlab, Madrid, Spain) in the mice rectum. Data are expressed as the difference between basal temperature and temperature recorded 35 min after THC or vehicle injection.
Nociception
The spinal reflex was determined with the tail immersion test, by using water at 50 ± 0.5°C as the nociceptive stimulus, immediately after rectal temperature measurement. Mice were maintained in a cylinder, and their tails were immersed in the heated water, as previously reported (Berrendero et al., 2005). The latency to a rapid flick of the tail was taken as the endpoint, with a cut‐off latency of 5 s to prevent tissue damage. Next, the integrated supraspinal response was tested with the hot plate test in a glass cylinder (16 cm high and 16 cm diameter) used to keep the mice on the heated surface of the plate, which was kept at a temperature of 52 ± 0.5°C (Columbus Instruments, Columbus, OH, USA). The nociceptive threshold evaluated was the jumping response, with a 240 s cut‐off (Berrendero et al., 2005). Data are expressed as percentage of the maximum possible effect (MPE), using the following equation: (MPE %) = (individual latency − mean control latency)/(cut‐off time − mean control latency) × 100.
Elevated plus maze
The elevated plus maze was used to test the anxiety‐like effects induced by acute THC administration. THC effects on anxiety are biphasic; low doses of THC induce anxiolytic‐like effects and high doses anxiogenic‐like responses (Berrendero and Maldonado, 2002; Valjent et al., 2002). The apparatus consisted of four arms (16 × 5 cm) set in cross from a neutral central square (5 × 5 cm) (Imetronic). Two opposite arms were delimited by vertical walls (closed arms), and the two other opposite arms had unprotected edges (open arms), as previously reported (Balerio et al., 2005). Each mouse was placed in the central neutral area facing one of the open arms and observed during 5 min. Results are expressed as the percentage of time spent in the open arms. Anxiolytic‐like behaviour was evaluated 30 min after THC injection at 0.3 mg·kg−1. Anxiogenic‐like effects were evaluated 5 h after THC administration at 5 mg·kg−1 in order to avoid the hypolocomotor effects induced by this cannabinoid.
Novel object recognition test
The object recognition test was used to evaluate the amnesic‐like effects of THC, which was performed in a V‐shaped maze. Mice were habituated for 9 min in the maze. After 24 h, mice were put back into the maze for 9 min (training) where two identical objects were presented, and the time spent by the mice exploring each object was recorded. Twenty‐four hours later, mice were again placed in the maze for another 9 min (test), one of the familiar objects was replaced by a novel object and the total time spent exploring each of the two objects (novel and familiar) was computed. A discrimination index (DI) was calculated as the difference between the time spent exploring the novel (TN) and the familiar object (TF) divided by the total exploration time: DI = [(TN − TF)/(TN + TF)]. A high DI is considered to reflect greater memory retention for the familiar object. OX1 and OX2 antagonists were injected immediately after the training session, 30 min before THC (10 mg·kg−1).
Immunoblot analysis
To rule out a possible modification of CB1 receptor levels in mice lacking the PPO gene, tissues from different brain regions (hippocampus, striatum, amygdala and hypothalamus) from PPO KO mice and WT littermates were analysed in basal conditions by immunoblotting. Frozen tissue processing to obtain total solubilized fractions and Western blot analysis was performed as previously described (Ozaita et al., 2007). The antibodies used for immunoblotting were anti‐CB1 (guinea pig, 1:1000, Frontier Science,Madison, WI, USA) and anti‐GAPDH (mouse, 1:10 000, Santa Cruz Biotechnology, Dallas, TX, USA). Bound primary antibodies were detected respectively with horseradish peroxidase‐conjugated rec‐Protein A (1:5000, Zymed Laboratories Inc., San Francisco, CA, USA) and anti‐mouse antibody (1:20 000; Cell Signaling Technologies, Danvers, MA, USA) and visualized by enhanced chemiluminescence detection (West‐Femto‐SuperSignaling, Pierce, Rockford, IL, USA). The OD of the immunoreactive bands was quantified after acquisition on a ChemiDoc XRS System (Bio‐Rad, Hercules, CA, USA) controlled by The Quantity One software v4.6.3 (Bio‐Rad). For quantitative purposes, the OD values for the protein of interest were normalized to the detection of the housekeeping protein GAPDH as loading control in the same sample and expressed as a percentage of the mean value of WT group.
Immunofluorescence studies
c‐Fos expression studies were performed in several brain areas previously reported to become activated after acute THC administration (Valjent et al., 2002; Allen et al., 2003). The anatomical coordinates of the mouse brain atlas (Paxinos and Franklin, 2001) were used as a guide to identify the brain regions under study. The coordinates relative to bregma were as follows: core and shell subregions of the nucleus accumbens, from 1.54 to 0.98 mm; dorsal striatum, from 0.86 to 0.26 mm; lateral septum, from 1.10 to 0.38 mm; medial preoptic area of the hypothalamus, from 0.26 to −0.22 mm; paraventricular nucleus of the hypothalamus, from −0.58 to −1.06 mm; paraventricular nucleus of the thalamus, from −0.94 to −1.46 mm; central amygdala, from −1.46 to −1.94 mm; and periaqueductal grey, from −3.80 to −4.24 mm. Tissue preparation and immunofluorescence were performed as previously reported (Plaza‐Zabala et al., 2012b). Briefly, 2 h after THC (10 mg·kg−1) injection, mice were perfused with cold 4% paraformaldehyde. The brains were extracted, postfixed and then cryoprotected in a solution of 30% sucrose at 4°C. Coronal frozen sections were made at 30 μm on a freezing microtome and stored in a 5% sucrose solution. Free‐floating slices were blocked in a solution containing 3% normal goat serum and 0.3% Triton X‐100 in 0.1 M PB (NGS‐T‐PB) and incubated in the same solution with the primary antibody to c‐Fos (rabbit, 1:500, sc‐72, Santa Cruz Biotechnology). The next day, sections were incubated with the secondary antibody to rabbit (1:500, Alexa Fluor‐555, Life Technologies, Carlsbad, CA, USA) in NGS‐T‐PB. After incubation, sections were rinsed and mounted onto glass slides in Mowiol mounting media. The stained sections of the brain were analysed at 10× objective using a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany) equipped with a digital camera Leica DFC 300FX (Leica Microsystems). The images were processed using the ImageJ analysis software. c‐Fos‐positive neurons in each brain area were quantified using the automatic ‘particle counting’ option with a fixed configuration that only detected pixels that matched a certain range of size and circularity. Additionally, a fixed threshold interval was set in order to distinguish the c‐Fos positive neurons from the background. Six representative brain sections of each mouse were quantified along the rostro‐caudal trajectory of the brain nuclei, and the average number of c‐Fos‐positive neurons was calculated for each mouse. The data are expressed as the mean number of c‐Fos‐positive cells mm‐2 in seven to nine mice per group.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Values are expressed as mean ± SEM. Statistical significance was determined by two‐way ANOVA for the analysis of behavioural experiments and immunofluorescence studies, using genotype/antagonist pretreatment and treatment as between‐subject factors of variation, followed by Newman–Keuls post hoc when ANOVA revealed significant effect (P < 0.05). CB1 receptor expression detected by immunoblotting was compared between genotypes by Student's unpaired t‐test (two‐tailed). All data were analysed using Statistica (StatSoft, Tulsa, OK, USA) software. P‐values <0.05 were considered to be statistically significant.
Drugs
THC (THC‐Pharm‐GmbH, Frankfurt, Germany) was dissolved in 5% tween‐80 and physiological saline solution and administered by the i.p. route in a volume of 10 mL·kg−1 body weight. The OX1 receptor antagonist SB‐334867 (5 mg·kg−1) (Tocris, Bristol, UK) was dissolved in 1% (2‐hydroxypropyl)‐β‐cyclodextrin (Sigma‐Aldrich, Saint Louis, MO, USA) and 10% DMSO in distilled water. The OX2 receptor antagonist TCS‐OX‐229 (10 mg·kg−1) (Tocris) was dissolved in physiological saline. Both antagonists were administered by the i.p. route in a volume of 5 mL·kg−1 30 min before THC injection. Doses of orexin receptor antagonists were based on previous studies (Plaza‐Zabala et al., 2010, 2012b).
Results
Orexins modulate THC‐induced hypothermia and supraspinal antinociception through OX2 receptor signalling
The possible orexinergic modulation of the acute effects induced by THC was studied in PPO‐deficient mice, OX1 KO mice and in C57BL6/J mice pretreated with the OX1 antagonist SB‐334867 (5 mg·kg−1) or the OX2 antagonist TCS‐OX‐229 (10 mg·kg−1). Locomotion, temperature and nociception were evaluated in sequence after acute THC injection at 5 and 10 mg·kg−1. THC treatment decreased similarly spontaneous locomotor activity in PPO KO and WT mice (Figure 1A). PPO KO animals presented lower basal activity levels in comparison with WT mice (P < 0.05), as previously reported (Plaza‐Zabala et al., 2010). The decrease in locomotion induced by THC was not modified in OX1 KO and control animals (Figure 1C). In agreement with the genetic approach, pretreatment with the antagonists SB‐334867 and TCS‐OX‐229 did not change THC‐induced hypolocomotor activity (Figure 1B and 1D).
Figure 1.
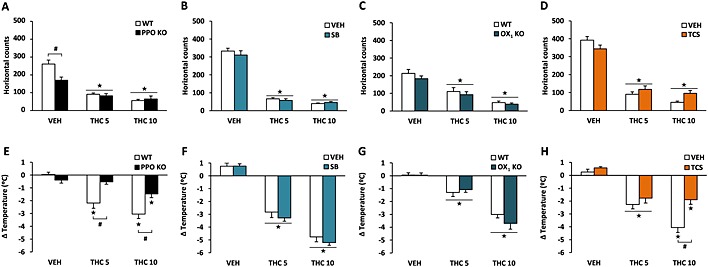
Orexinergic modulation of the hypolocomotor and hypothermic effects induced by THC. Locomotor activity (A–D) and body temperature (E–H) were measured after acute administration of THC (5 and 10 mg·kg−1) in PPO KO mice (A, E), OX1 KO mice (C, G) and in mice pretreated with the OX1 antagonist SB‐334867 (5 mg·kg−1) (B, F) or the OX2 antagonist TCS‐OX‐229 (10 mg·kg−1) (D, H). Data are expressed as mean ± SEM (n = 10–11 in A, E; 8–9 in C, G; 13–14 in B, F; and 11–12 in D, H). ★P < 0.05 when comparing with control group; #P < 0.05 comparison between genotypes or with VEH‐pretreated mice (Newman–Keuls test). VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX‐229.
Interestingly, THC‐induced hypothermia was modified in PPO KO in comparison with WT mice (Figure 1E). Post hoc comparisons showed a significant reduction of the hypothermic effects of THC in these mutant animals (P < 0.05) (Figure 1E). THC‐induced hypothermia remained unaffected by SB‐334867 pretreatment (Figure 1F) and in OX1 KO mice (Figure 1G). In contrast, TCS‐OX‐229 decreased the effect of THC in body temperature. Post hoc analysis showed that hypothermia was significantly reduced in TCS‐OX‐229‐pretreated mice at the highest dose of THC (P < 0.05) (Figure 1H), suggesting that orexins modulate the hypothermic effects of THC through the activation of OX2 receptors.
In the hot plate test, PPO KO and WT mice showed different antinociceptive responses after THC administration. Thus, THC at 5 and 10 mg·kg−1 increased the jumping latency in WT mice (P < 0.05), whereas this effect was abolished in PPO KO animals (P < 0.05) (Figure 2A). THC‐induced antinociception remained unaltered by SB‐334867 pretreatment (Figure 2B) and in mice lacking the OX1 receptor (Figure 2C). However, TCS‐OX‐229 administration altered the antinociceptive effects of THC. Post hoc comparisons revealed that THC administration increased the jumping latency in vehicle‐pretreated mice (P < 0.05), and this effect was reduced by TCS‐OX‐229 pretreatment in mice receiving THC at 5 and 10 mg·kg−1 (P < 0.05) (Figure 2D). Latency times for the jumping response are shown in the Supporting Information Fig. S1. The spontaneous latency of this response in the hot plate test was lower in PPO KO than in WT mice, as shown in the Supporting Information Fig. S1a. This result suggests a higher sensitivity of PPO‐deficient mice to painful stimuli under these experimental conditions. In the tail immersion test, THC‐induced antinociception remained unaffected in PPO KO mice (Figure 2E) and in mice lacking the OX1 receptor (Figure 2G). In agreement, the pretreatment with the antagonists SB‐334867 (Figure 2F) or TCS‐OX‐229 (Figure 2H) did not modify the antinociceptive effects of THC. Latency times for the tail‐flick response are shown in the Supporting Information Fig. S1. These results suggest that THC‐induced supraspinal antinociception is regulated by orexins through OX2 receptor signalling.
Figure 2.
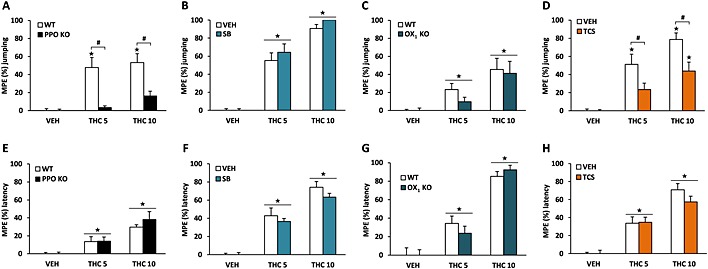
Orexinergic modulation of the antinociceptive effects induced by THC. The supraspinal component of THC‐induced antinociception was evaluated in the hot plate test (A–D) and the spinal component in the tail immersion test (E–H). The nociceptive thresholds evaluated were the jumping response and the latency to tail flick, respectively, after the acute administration of THC (5 and 10 mg·kg−1) in PPO KO mice (A, E), OX1 KO mice (C, G) and in mice pretreated with the OX1 antagonist SB‐334867 (5 mg·kg−1) (B, F) or the OX2 antagonist TCS‐OX‐229 (10 mg·kg−1) (D, H). Data are expressed as mean ± SEM of the maximum possible effect (MPE %), calculated as MPE (%) = (individual latency − mean control latency)/(cut‐off time − mean control latency) × 100 (n = 10–11 in A, E; 8–9 in C, G; 13–14 in B, F; and 11–12 in D, H). ★P < 0.05 when comparing with control group; #P < 0.05 comparison between genotypes or with VEH‐pretreated mice (Newman–Keuls test). VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX‐229.
THC‐induced anxiolytic‐like effects, but not anxiogenic‐ or amnesic‐like effects, are modulated by orexins through OX2 receptor signalling
Low doses of cannabinoids induce anxiolytic‐like effects, whereas high doses produce anxiogenic‐like responses (Berrendero and Maldonado, 2002; Valjent et al., 2002). The involvement of orexins in the anxiolytic‐like and anxiogenic‐like effects of THC was studied by using the elevated plus maze. After exposure to an anxiogenic dose of THC (5 mg·kg−1), both PPO KO and WT mice spent similarly less time in the open arms than vehicle‐treated animals (Figure 3A). A similar result was found in OX1 KO animals (Figure 3C). Moreover, neither OX1 nor OX2 receptor blockade altered the reduction of time spent in the open arms induced by THC at 5 mg·kg−1 (Figure 3B and 3D). On the contrary, injection of an anxiolytic dose of THC (0.3 mg·kg−1) increased the percentage of time spent in the open arms in WT animals (P < 0.05) but not in PPO‐deficient mice (P < 0.05) (Figure 3E). In addition, the anxiolytic‐like effects of THC at 0.3 mg·kg−1 were maintained in SB‐334867‐pretreated animals (Figure 3F) and in OX1 KO mice (Figure 3G) but not in TCSOX229‐pretreated mice (Figure 3H). No significant differences in the total number of entries were observed between groups in any of the experiments performed (Supporting Information Fig. S2). These data point to an orexinergic modulation of THC‐induced anxiolytic‐like effects, but not anxiogenic‐like effects, through OX2 receptor signalling.
Figure 3.
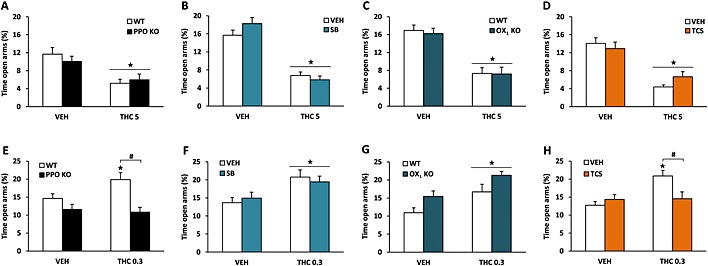
Orexinergic modulation of the anxiogenic‐like or anxiolytic‐like effects induced by THC. Anxiogenic‐like effects (A–D) were evaluated 5 h after the acute injection of THC (5 mg·kg−1), whereas anxiolytic‐like effects (E–H) were measured 30 min after THC administration (0.3 mg·kg−1). The time spent in the open and the closed arms was recorded for 5 min in PPO KO mice (A, E), OX1 KO mice (C, G) and in mice pretreated with the OX1 antagonist SB‐334867 (5 mg·kg−1) (B, F) or the OX2 antagonist TCS‐OX‐229 (10 mg·kg−1) (D, H). Data are expressed as mean ± SEM of the percentage of time spent in the open arms (n = 7–8 in A, C, G; 9–12 in B, D; 14–15 in E; 10–12 in F; and 16–18 in H). ★P < 0.05 when comparing with control group; #P < 0.05 comparison between genotypes or with VEH‐pretreated mice (Newman–Keuls test). VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX‐229.
We evaluated the amnesic‐like effects of THC in the novel object recognition task, as previously reported (Puighermanal et al., 2009). THC (10 mg·kg−1) after the training session reduced similarly the DI in PPO KO and WT mice (Figure 4A). A similar response was observed in OX1 KO animals (Figure 4C). THC‐induced amnesic‐like effects also remained unaffected by SB‐334867 and TCS‐OX‐229 administration (Figure 4B and 4D), confirming that under these experimental conditions, the orexin system is not involved in this THC pharmacological response. No significant differences in the total time of exploration were observed between groups (Supporting Information Fig. S3).
Figure 4.
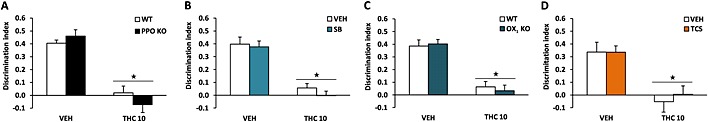
Orexinergic modulation of the amnesic‐like effects induced by THC. Amnesic‐like effects (A–D) were evaluated in the novel object recognition task. THC (10 mg·kg−1) was administered 20 min after the training session in PPO KO mice (A), OX1 KO mice (C) and in mice pretreated with the OX1 antagonist SB‐334867 (5 mg·kg−1) (B) or the OX2 antagonist TCS‐OX‐229 (10 mg·kg−1) (D) immediately after the training trial. Data are expressed as mean ± SEM of the discrimination index (n = 7–9 for all experiments). ★P < 0.05 when comparing with control group. VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX‐229.
Prepro‐orexin knockout and wild‐type mice show similar CB1 receptor protein levels
We next evaluated whether the behavioural modifications observed in PPO‐deficient mice could be a consequence of altered basal levels of CB1 receptors in KO animals. Western blot analysis revealed no differences in CB1 receptor expression levels in the striatum, hippocampus, amygdala and hypothalamus of PPO‐deficient mice (Figure 5). These results suggest that the reduction in THC‐induced hypothermic, antinociceptive and anxiolytic‐like effects observed in KO mice is not due to a lower expression of CB1 receptors in these mutant animals.
Figure 5.
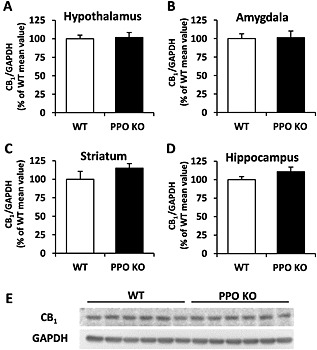
CB1 receptor levels in WT and PPO KO mice. Immunoblot analysis was used to determine total CB1 receptor levels in the hypothalamus (A), amygdala (B), striatum (C) and hippocampus (D) of PPO KO and WT mice under basal conditions. Data are expressed as mean ± SEM of the densitometric value for CB1 receptors related to GAPDH, as a percentage of the control (WT) group (n = 6 mice per group). Displayed are representative blots showing CB1 receptor expression in the hypothalamus of six WT and six KO mice, as well as GAPDH levels as a loading control for each corresponding sample (E).
THC‐induced c‐Fos expression is reduced in the central amygdala, lateral septum and the preoptic area of prepro‐orexin knockout mice
Acute administration of THC induces c‐Fos expression, a marker of neuronal activity, in several brain regions (Valjent et al., 2002; Allen et al., 2003). We investigated the possible modulation of this neuronal activation induced by THC (10 mg·kg−1) in WT and PPO‐deficient mice in different brain structures. Immunofluorescence analysis showed an increase in c‐Fos expression in the shell and core of the nucleus accumbens, dorsal striatum, lateral septum area, paraventricular thalamic nucleus, medial preoptic area, paraventricular hypothalamic nucleus, central amygdala and periaqueductal grey (Table 1; Figure 6) in WT and KO mice. Interestingly, a reduction in c‐Fos expression was observed in the central amygdala of PPO‐deficient mice (P < 0.05) in comparison with WT animals (Table 1). Moreover, a similar decrease in c‐Fos expression was found in the preoptic area and lateral septum of KO mice, although no significant genotype × treatment interaction was revealed in these two brain areas (Table 1). These results suggest that the central amygdala, lateral septum and medial preoptic area could be involved in the altered responses induced by THC in PPO‐deficient mice.
Table 1.
Number of c‐Fos + cells mm−2 in WT and PPO KO animals after THC administration (10 mg·kg−1)
| WT‐VEH | PPO KO‐VEH | WT‐THC | PPO KO‐THC | ANOVA | |
|---|---|---|---|---|---|
| Nucleus accumbens (shell) | 54 ± 10 | 52 ± 9 | 163 ± 12 | 131 ± 19 | t |
| Nucleus accumbens (core) | 84 ± 14 | 57 ± 14 | 150 ± 22 | 149 ± 14 | t |
| Dorsal striatum | 20 ± 4 | 10 ± 3 | 80 ± 12 | 86 ± 16 | t |
| Lateral septum | 149 ± 29 | 88 ± 21 | 325 ± 34 | 242 ± 27 | t **, g * |
| Preoptic area | 165 ± 17 | 143 ± 22 | 298 ± 17 | 219 ± 26 | t **, g * |
| Paraventricular nucleus of the hypothalamus | 248 ± 39 | 336 ± 81 | 877 ± 84 | 726 ± 146 | t |
| Paraventricular nucleus of the thalamus | 259 ± 59 | 243 ± 47 | 822 ± 52 | 722 ± 63 | t |
| Central amygdala | 39 ± 5 | 40 ± 5 | 825 ± 79 | 503 ± 109 | t **, g *, t × g ** |
| Periaqueductal grey | 176 ± 27 | 116 ± 11 | 229 ± 30 | 216 ± 25 | t |
Results are expressed as mean ± SEM. Displayed are significant effects (t = treatment, g = genotype) and interactions (t × g = treatment × genotype) revealed by two‐way ANOVA.
P < 0.05.
P < 0.01.
Figure 6.
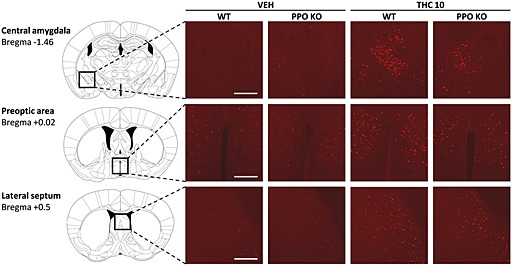
Representative images obtained by fluorescence microscopy showing c‐Fos immunoreactivity in the central amygdala, the medial preoptic area and the lateral septum of PPO KO and WT mice receiving acute injections of vehicle or THC (10 mg·kg−1) (n = 7–9 mice per group). The scale bar represents 200 μm.
Discussion
This study reveals that orexins modulate THC‐induced hypothermia, supraspinal antinociception and anxiolytic‐like effects through OX2 receptor signalling. In contrast, these neuropeptides are not involved in the hypolocomotion, spinal antinociception, amnesic‐ and anxiogenic‐like effects produced by this cannabinoid. Immunoblot analyses show similar levels of CB1 receptors in WT and PPO KO mice ruling out a possible bias due to different expressions of these receptors in mutant animals. Furthermore, THC administration induced c‐Fos immunoreactivity in WT and PPO KO mice in several brain areas, and this effect was significantly reduced in the central amygdala, medial preoptic area and lateral septum in mutant mice.
The hypolocomotion induced by THC was not modified in mice lacking the PPO gene. However, a lower basal locomotor activity was observed in PPO KO mice in comparison with WT animals. This effect is consistent with previous studies (España et al., 2007; Anaclet et al., 2009; Plaza‐Zabala et al., 2010) and suggests that orexins contribute to the maintenance of wakefulness by enhancing locomotion (Anaclet et al., 2009). Indeed, the i.c.v. administration of orexin‐A has been shown to increase arousal and locomotor activity in rats (Hagan et al., 1999; Mang et al., 2012). In contrast to the effects observed in locomotion, THC‐induced hypothermia was abolished in PPO KO mice. This effect seems to be mediated by OX2 receptor activation because the OX2 antagonist TCS‐OX2‐29, but not the OX1 antagonist SB‐334867, reduced this cannabinoid response. The hypothermic effects of THC were also maintained in OX1 KO mice. Orexins are involved in thermoregulation (Messina et al., 2014), although hypothermic (Jászberényi et al., 2002) and hyperthermic (Monda et al., 2004, 2007) effects have been described following orexin‐A administration. Interestingly, the enhancement in c‐Fos expression induced by THC in the medial preoptic area was reduced in PPO KO mice, indicating that this hypothalamic region could be important to explain this cannabinoid/orexin interaction. Indeed, the preoptic area is an important thermoregulatory centre (McKinley et al., 2015) containing a dense projection of orexin‐positive nerve terminals (Peyron et al., 1998) and high density of OX2 receptor mRNA (Marcus et al., 2001). Therefore, our results suggest that cannabinoid exposure could induce the release of orexins in the medial preoptic area. The subsequent activation of OX2 receptors by orexins in this hypothalamic region would be important for the modulation of the hypothermic effects of THC.
The antinociceptive responses induced by THC in the tail immersion test were not modified in PPO KO and OX1 KO mice or after OX1 or OX2 antagonist pretreatment. In contrast, THC‐induced antinociception in the hot plate test was reduced in PPO KO animals as well as in TCS‐OX‐229‐pretreated mice. These results suggest that orexins, through OX2 receptor signaling, are involved in the supraspinal but not in the spinal antinociceptive effects of THC. Interestingly, the endocannabinoid 2‐arachidonoylglycerol also participates in the supraspinal antinociception induced by orexin‐A (Ho et al., 2011). Several studies support an important role for orexins in the control of nociception (Chiou et al., 2010). Thus, orexins induced antinociceptive effects in different assays for thermal, mechanical and chemical stimuli (Mobarakeh et al., 2005). OX1 receptor activation has been classically implicated in the antinociceptive effects of orexins (Jeong and Holden, 2009; Ho et al., 2011; Heidari‐Oranjaghi et al., 2012), although a role for OX2 receptors has also been recently suggested (Azhdari‐Zarmehri et al., 2013). The central amygdala could participate in the regulation by orexins of THC‐induced antinociception because a reduction in c‐Fos expression was observed in PPO KO mice following THC administration. This brain area receives ascending nociceptive signals, has efferent projections to areas that are involved in pain modulation and is crucial in controlling pain threshold and its emotional component (Palazzo et al., 2011). Although the expression of OX2 receptors in the central amygdala is low, a recent study has related an increased orexin transmission at OX2 receptors in this brain region with compulsive‐like heroin self‐administration behaviour (Schmeichel et al., 2015). Other brain areas could also participate in the modulation of this nociceptive interaction, although no changes in c‐Fos expression between WT and PPO KO mice were observed in important regions for pain regulation, such as the periaqueductal grey matter or the nucleus accumbens.
The anxiogenic‐like effects of THC were not modified in mice lacking the PPO gene, the OX1 receptor or after pharmacological blockade of OX1 or OX2 receptors. However, orexin peptides and particularly OX1 receptors have been shown to be involved in anxiety and panic states (Pich and Melotto, 2014), as well as in the anxiogenic‐like effects of other drugs of abuse, such as nicotine (Plaza‐Zabala et al., 2010). In contrast, THC‐induced anxiolytic‐like effects were abolished in PPO KO animals and in mice pretreated with the OX2 antagonist TCS‐OX‐229. So far, few studies have evaluated the possible role of OX2 receptors in anxiety. Recently, an interesting study suggests that orexinergic activity in the basolateral amygdala may be responsible for bidirectional modulation of anxious behaviour (Arendt et al., 2014). Knocking down the OX2, but not the OX1 receptor, in this brain area increased anxiety as measured by reduced social preference and reduced time spent in the centre of an open field (Arendt et al., 2014). These data indicate that OX2 receptor activation in the basolateral amygdala could have the potential to alleviate anxiety. In agreement, our results show that the OX2 receptor mediates the anxiolytic‐like effects of THC, which could have important therapeutic implications. The role played by orexins in the basolateral amygdala was not evaluated because THC did not induce c‐Fos expression in this brain region, as previously reported (Valjent et al., 2002). Future experiments will be necessary to investigate whether these receptors also participate in the anxiolytic‐like effects of endocannabinoids (Busquets‐Garcia et al., 2011) and the brain areas responsible for this interaction.
The amnesic‐like effects induced by THC in the object recognition test were not mediated by orexins. These hypothalamic neuropeptides facilitate memory processing because they are critically involved in hippocampus‐dependent social recognition memory (Yang et al., 2013), emotional memories (Sears et al., 2013; Soya et al., 2013; Flores et al., 2014b) and improvement of cognitive performance in sleep‐deprived nonhuman primates (Deadwyler et al., 2007). The detrimental effects of THC on learning and memory could be aggravated in mice lacking the PPO gene, although we did not observe this effect probably due to a ceiling effect in the object recognition test.
In conclusion, this study reveals a novel role for orexins and OX2 receptors in specific acute pharmacological effects of THC, providing interesting data of the interaction between orexins and cannabinoids. The activation of OX1 and OX2 receptors modulates the effects induced by cannabinoids in a different way. Therefore, the blockade of OX1 receptors could abolish the reinforcing properties of cannabinoids (Flores et al., 2014a) without affecting other pharmacological responses of these compounds.
Author contributions
A.F. conducted the behavioural, biochemical and immunohistochemical experiments and wrote the manuscript. M.J‐H. conducted the behavioural and immunohistochemical experiments. R.M. funded the project and wrote the manuscript. F.B. conceptualized, supervised and funded the project, participated in experimental design and wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Nociceptive thresholds evaluated in the hot plate test (A, B, C, D) and the tail immersion test (E, F, G, H), expressed in seconds. The jumping response and the latency to tail‐flick, respectively, were determined after the acute administration of THC (5 and 10 mg kg‐1) in PPO KO mice (A, E), in OX1 KO mice (C, G), and in mice pre‐treated with the OX1 antagonist SB‐334867 (5 mg kg‐1) (B, F) or the OX2 antagonist TCS‐OX‐229 (10 mg kg‐1) (D, H). Data are expressed as mean ± SEM (n = 9‐14 mice for each group). PPO, prepro‐orexin; VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX‐229. ★P < 0.01 when comparing with control group; #P < 0.05 comparison between genotypes or with VEH pre‐treated mice (Newman–Keuls test).Figure S2. No significant differences in exploration parameters were observed between groups during the evaluation of anxiety‐like behaviour in the elevated plus maze. Anxiogenic‐like effects (A, B, C, D) were evaluated 5 hours after the acute injection of THC at 5 mg kg‐1, whereas anxiolytic‐like effects (E, F, G, H) were measured 30 minutes after the administration of THC at 0.3 mg kg‐1. The total number of entries was recorded for 5 minutes in PPO KO mice (A, E), OX1 KO mice (C,G), and in mice pre‐treated with the OX1 antagonist SB‐334867 (5 mg kg‐1) (B, F) or the OX2 antagonist TCS‐OX2‐29 (10 mg kg‐1) (D, H). Data are expressed as mean ± s.e.m. (n = 7‐12 mice for each group in anxiogenic‐like approach, n = 8‐18 in anxiolytic‐like approach). PPO, prepro‐orexin; VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX2‐29.Figure S3. No significant differences in the total exploration time was observed between groups during the evaluation of amnesic‐like behaviour. Amnesic‐like effects (A, B, C, D) were evaluated in the novel object recognition task. THC (10 mg kg‐1) was administered 20 minutes after the training session to PPO KO mice (A), OX1 KO mice (C), and to mice receiving the OX1 antagonist SB‐334867 (5 mg kg‐1) (B) or the OX2 antagonist TCS‐OX2‐29 (10 mg kg‐1) (D) immediately after the training trial. Data are expressed as mean ± s.e.m. (n = 7‐9 mice for each group). PPO, prepro‐orexin; VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX2‐29.
Supporting Information
Acknowledgements
This work was supported by the Instituto de Salud Carlos III grants [#PI13/00042 and #RD12/0028/0023 (RTA‐RETICS)], by the Spanish Ministry of Science (#SAF2011‐29864 and #SAF2014‐59648‐P), National Plan on Drugs (#2014I019) and the Catalan Government (SGR2014‐1547). We thank Marta Linares for invaluable technical assistance.
Flores, Áf. , Julià‐Hernández, M. , Maldonado, R. , and Berrendero, F. (2016) Involvement of the orexin/hypocretin system in the pharmacological effects induced by Δ9‐tetrahydrocannabinol. British Journal of Pharmacology, 173: 1381–1392. doi: 10.1111/bph.13440.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen KV, McGregor IS, Hunt GE, Singh ME, Mallet PE (2003). Regional differences in naloxone modulation of Delta(9)‐THC induced Fos expression in rat brain. Neuropharmacology 44: 264–274. [DOI] [PubMed] [Google Scholar]
- Anaclet C, Parmentier R, Ouk K, Guidon G, Buda C, Sastre JP, et al. (2009). Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock‐out mouse models. J Neurosci 29: 14423–14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, et al. (2014). Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhdari‐Zarmehri H, Reisi Z, Vaziri A, Haghparast A, Shaigani P, Haghparast A (2013). Involvement of orexin‐2 receptors in the ventral tegmental area and nucleus accumbens in the antinociception induced by the lateral hypothalamus stimulation in rats. Peptides 47: 94–98. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R (2005). Involvement of the opioid system in the effects induced by nicotine on anxiety‐like behaviour in mice. Psychopharmacology (Berl) 181: 260–269. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Maldonado R (2002). Involvement of the opioid system in the anxiolytic‐like effects induced by Delta(9)‐tetrahydrocannabinol. Psychopharmacology (Berl) 163: 111–117. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Mendizábal V, Robledo P, Galeote L, Bilkei‐Gorzo A, Zimmer A, et al. (2005). Nicotine‐induced antinociception, rewarding effects, and physical dependence are decreased in mice lacking the preproenkephalin gene. J Neurosci 25: 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets‐Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A (2011). Differential role of anandamide and 2‐arachidonoylglycerol in memory and anxiety‐like responses. Biol Psychiatry 70: 479–486. [DOI] [PubMed] [Google Scholar]
- Castaldo P, Magi S, Cataldi M, Arcangeli S, Lariccia V, Nasti AA, et al. (2010). Altered regulation of glutamate release and decreased functional activity and expression of GLT1 and GLAST glutamate transporters in the hippocampus of adolescent rats perinatally exposed to Delta(9)‐THC. Pharmacol Res 61: 334–341. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437–451. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Lee HJ, Ho YC, Chen SP, Liao YY, Ma CH, et al. (2010). Orexins/hypocretins: pain regulation and cellular actions. Curr Pharm Des 16: 3089–3100. [DOI] [PubMed] [Google Scholar]
- Crespo I, Gómez de Heras R, Rodríguez de Fonseca F, Navarro M (2008). Pretreatment with subeffective doses of Rimonabant attenuates orexigenic actions of orexin A‐hypocretin 1. Neuropharmacology 54: 219–225. [DOI] [PubMed] [Google Scholar]
- Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C, et al. (2013). Obesity‐driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci U S A 110: E2229–E2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA, et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Porrino L, Siegel JM, Hampson RE (2007). Systemic and nasal delivery of orexin‐A (hypocretin‐1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci 27: 14239–14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. (1998). The hypocretins: hypothalamus‐specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95: 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, McCormack SL, Mochizuki T, Scammell TE (2007). Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep 30: 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Maldonado R, Berrendero F (2013). Cannabinoid–hypocretin cross‐talk in the central nervous system: what we know so far. Front Neurosci 7: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores Á, Maldonado R, Berrendero F (2014a). The hypocretin/orexin receptor‐1 as a novel target to modulate cannabinoid reward. Biol Psychiatry 75: 499–507. [DOI] [PubMed] [Google Scholar]
- Flores Á, Valls‐Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F (2014b). The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology 39: 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonawardena AV, Robinson L, Hampson RE, Riedel G (2010). Cannabinoid and cholinergic systems interact during performance of a short‐term memory task in the rat. Learn Mem 17: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. (1999). Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A 96: 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari‐Oranjaghi N, Azhdari‐Zarmehri H, Erami E, Haghparast A (2012). Antagonism of orexin‐1 receptors attenuates swim‐ and restraint stress‐induced antinociceptive behaviors in formalin test. Pharmacol Biochem Behav 103: 299–307. [DOI] [PubMed] [Google Scholar]
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. (2011). Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2‐arachidonoylglycerol)‐induced disinhibition. J Neurosci 31: 14600–14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäntti MH, Mandrika I, Kukkonen JP (2014). Human orexin/hypocretin receptors form constitutive homo‐ and heteromeric complexes with each other and with human CB1 cannabinoid receptors. Biochem Biophys Res Commun 445: 486–490. [DOI] [PubMed] [Google Scholar]
- Jászberényi M, Bujdosó E, Kiss E, Pataki I, Telegdy G (2002). The role of NPY in the mediation of orexin‐induced hypothermia. Regul Pept 104: 55–59. [DOI] [PubMed] [Google Scholar]
- Jeong Y, Holden JE (2009). The role of spinal orexin‐1 receptors in posterior hypothalamic modulation of neuropathic pain. Neuroscience 159: 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinová Z, Redhi GH, Goldberg SR, Ferré S (2014). Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on Δ9‐tetrahydrocannabinol (THC) self‐administration in squirrel monkeys. J Neurosci 34: 6480–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C (2000). The hypocretin/orexin ligand‐receptor system: implications for sleep and sleep disorders. Trends Neurosci 23: 359–365. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. (1999). Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283: 401–404. [DOI] [PubMed] [Google Scholar]
- Mackie K (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol 168: 299–325. [DOI] [PubMed] [Google Scholar]
- Mang GM, Dürst T, Bürki H, Imobersteg S, Abramowski D, Schuepbach E, et al. (2012). The dual orexin receptor antagonist almorexant induces sleep and decreases orexin‐induced locomotion by blocking orexin 2 receptors. Sleep 35: 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6–25. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D (2015). The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 214: 8–32. [DOI] [PubMed] [Google Scholar]
- Messina G, Dalia C, Tafuri D, Monda V, Palmieri F, Dato A, et al. (2014). Orexin‐A controls sympathetic activity and eating behavior. Front Psychol 5: Article 997, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarakeh JI, Takahashi K, Sakurada S, Nishino S, Watanabe H, Kato M, et al. (2005). Enhanced antinociception by intracerebroventricularly and intrathecally‐administered orexin A and B (hypocretin‐1 and ‐2) in mice. Peptides 26: 767–777. [DOI] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano A, Fuccio F, De Luca V (2004). Injection of orexin A into the diagonal band of Broca induces sympathetic and hyperthermic reactions. Brain Res 1018: 265–271. [DOI] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D, et al. (2007). Sympathetic and hyperthermic reactions by orexin A: role of cerebral catecholaminergic neurons. Regul Pept 139: 39–44. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R (2007). Regulation of PI3K/Akt/GSK‐3 pathway by cannabinoids in the brain. J Neurochem 102: 1105–1114. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Marabese I, Soukupova M, Luongo L, Boccella S, Giordano C, et al. (2011). Metabotropic glutamate receptor subtype 8 in the amygdala modulates thermal threshold, neurotransmitter release, and rostral ventromedial medulla cell activity in inflammatory pain. J Neurosci 31: 4687–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. (2014). The IUPHAR/BPS guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucleic Acids Res 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2008). Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol 13: 147–159. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev 62: 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich EM, Melotto S (2014). Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Front Neurosci 8: Article 26, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza‐Zabala A, Martín‐García E, de Lecea L, Maldonado R, Berrendero F (2010). Hypocretins regulate the anxiogenic‐like effects of nicotine and induce reinstatement of nicotine‐seeking behavior. J Neurosci 30: 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza‐Zabala A, Maldonado R, Berrendero F (2012a). The hypocretin/orexin system: implications for drug reward and relapse. Mol Neurobiol 45: 424–439. [DOI] [PubMed] [Google Scholar]
- Plaza‐Zabala A, Flores Á, Maldonado R, Berrendero F (2012b). Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry 71: 214–223. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets‐Garcia A, Lutz B, Maldonado R, Ozaita A (2009). Cannabinoid modulation of hippocampal long‐term memory is mediated by mTOR signaling. Nat Neurosci 12: 1152–1158. [DOI] [PubMed] [Google Scholar]
- Robledo P, Berrendero F, Ozaita A, Maldonado R (2008). Advances in the field of cannabinoid–opioid cross‐talk. Addict Biol 13: 213–224. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Skosnik PD, Cortes‐Briones J, Sewell RA, Carbuto M, Schnakenberg A, et al. (2015). GABA deficits enhance the psychotomimetic effects of Δ(9)‐THC. Neuropsychopharmacology 40: 2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein‐coupled receptors that regulate feeding behavior. Cell 92: 573–585. [DOI] [PubMed] [Google Scholar]
- Sakurai T (2014). The role of orexin in motivated behaviours. Nat Rev Neurosci 15: 719–731. [DOI] [PubMed] [Google Scholar]
- Sami MB, Rabiner EA, Bhattacharyya S (2015). Does cannabis affect dopaminergic signaling in the human brain? A systematic review of evidence to date. Eur Neuropsychopharmacol 2015 Mar 30. pii: S0924‐977X(15)00088‐7. doi:10.1016/j.euroneuro.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, et al. (2015). Hypocretin receptor 2 antagonism dose‐dependently reduces escalated heroin self‐administration in rats. Neuropsychopharmacology 40: 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE (2013). Orexin/hypocretin system modulates amygdala‐dependent threat learning through the locus coeruleus. Proc Natl Acad Sci U S A 110: 20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, et al. (2013). Orexin receptor‐1 in the locus coeruleus plays an important role in cue‐dependent fear memory consolidation. J Neurosci 33: 14549–14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310: 329–332. [DOI] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R (2002). Behavioural and biochemical evidence for interactions between Delta 9‐tetrahydrocannabinol and nicotine. Br J Pharmacol 135: 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty AN, McFarlane JR, McGregor IS, Mallet PE (2004). Evidence for an interaction between CB1 cannabinoid and oxytocin receptors in food and water intake. Neuropharmacology 47: 593–603. [DOI] [PubMed] [Google Scholar]
- Viñals X, Moreno E, Lanfumey L, Cordomí A, Pastor A, de La Torre R, et al. (2015). Cognitive impairment induced by Delta9‐tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5‐HT2A receptors. PLoS Biol 13 e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, et al. (2013). Hypocretin/orexin neurons contribute to hippocampus‐dependent social memory and synaptic plasticity in mice. J Neurosci 33: 5275–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Nociceptive thresholds evaluated in the hot plate test (A, B, C, D) and the tail immersion test (E, F, G, H), expressed in seconds. The jumping response and the latency to tail‐flick, respectively, were determined after the acute administration of THC (5 and 10 mg kg‐1) in PPO KO mice (A, E), in OX1 KO mice (C, G), and in mice pre‐treated with the OX1 antagonist SB‐334867 (5 mg kg‐1) (B, F) or the OX2 antagonist TCS‐OX‐229 (10 mg kg‐1) (D, H). Data are expressed as mean ± SEM (n = 9‐14 mice for each group). PPO, prepro‐orexin; VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX‐229. ★P < 0.01 when comparing with control group; #P < 0.05 comparison between genotypes or with VEH pre‐treated mice (Newman–Keuls test).Figure S2. No significant differences in exploration parameters were observed between groups during the evaluation of anxiety‐like behaviour in the elevated plus maze. Anxiogenic‐like effects (A, B, C, D) were evaluated 5 hours after the acute injection of THC at 5 mg kg‐1, whereas anxiolytic‐like effects (E, F, G, H) were measured 30 minutes after the administration of THC at 0.3 mg kg‐1. The total number of entries was recorded for 5 minutes in PPO KO mice (A, E), OX1 KO mice (C,G), and in mice pre‐treated with the OX1 antagonist SB‐334867 (5 mg kg‐1) (B, F) or the OX2 antagonist TCS‐OX2‐29 (10 mg kg‐1) (D, H). Data are expressed as mean ± s.e.m. (n = 7‐12 mice for each group in anxiogenic‐like approach, n = 8‐18 in anxiolytic‐like approach). PPO, prepro‐orexin; VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX2‐29.Figure S3. No significant differences in the total exploration time was observed between groups during the evaluation of amnesic‐like behaviour. Amnesic‐like effects (A, B, C, D) were evaluated in the novel object recognition task. THC (10 mg kg‐1) was administered 20 minutes after the training session to PPO KO mice (A), OX1 KO mice (C), and to mice receiving the OX1 antagonist SB‐334867 (5 mg kg‐1) (B) or the OX2 antagonist TCS‐OX2‐29 (10 mg kg‐1) (D) immediately after the training trial. Data are expressed as mean ± s.e.m. (n = 7‐9 mice for each group). PPO, prepro‐orexin; VEH, vehicle; SB, SB‐334867; TCS, TCS‐OX2‐29.
Supporting Information


