Abstract
Background and Purpose
Exposure–response (ER) modelling (concentration–QTc analysis) is gaining as much acceptance as the traditional by‐time analysis of the placebo‐adjusted change from baseline in the QTc interval (ΔΔQTcF). It has been postulated that intensive ECG analysis and ER modelling during early‐phase drug development could be a cost‐effective approach of estimating QT liability of a new drug, in a small number of subjects.
Experimental Approach
We used a highly automated analysis of ECGs from 46 subjects from a crossover thorough QT/QTc study to detect ΔΔQTcF with moxifloxacin. Using these data, we also simulated (bootstrapped) 1000 datasets of a parallel study with eight subjects receiving moxifloxacin and eight others receiving placebo.
Key Results
The slope from the concentration–QTc analysis for moxifloxacin in 46 subjects was 4.12 ms of ΔΔQTcF per μg‐1 mL‐1; at mean C max of 2.95 μg·mL−1, estimated ΔΔQTcF was 13.4 ms (90% confidence interval 11.3, 15.4 ms). In the 1000 simulated datasets, in 996 datasets, ER modelling showed that the upper bound of the 90% confidence interval for ΔΔQTcF at geometric mean C max exceeded 10 ms. In 895 of these 996 datasets, the slope of the ER relationship was statistically significantly positive. Thus, with a small sample size (eight subjects on active drug and eight on placebo), moxifloxacin‐induced QTc prolongation was demonstrated using ER analysis with statistical power of >80%.
Conclusions and Implications
Our study adds to the growing body of data supporting intensive ECG collection and analysis in early‐phase studies to estimate QT liability.
Abbreviations
- ICH
International Conference on Harmonization
- LOQ
- SAD
single ascending dose
- TQT
thorough QT/QTc
Tables of Links
| TARGETS |
|---|
| Voltage‐gated ion channels |
| HERG, Kv11.1 channels |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
The International Conference on Harmonization (ICH) E14 guidance requires that all new drugs with systemic bioavailability be studied for their off‐target effect on cardiac repolarization by a ‘thorough QT/QTc’ (TQT) study (ICH Harmonized Tripartite Guideline E14, 2005). Over 400 drugs have undergone TQT evaluation since 2005 (Stockbridge et al., 2013). With experience on exposure–response modelling on data from many of these TQT studies, results obtained from concentration–QTc analysis are gaining as much acceptance as findings from by‐time analysis of the placebo‐adjusted change from baseline in the QTc interval after administration of a study drug (Stockbridge et al., 2013). Given the high cost of performing a TQT study, which is usually done late in phase II of clinical drug development, there has been a move towards performing intensive ECG analysis in single ascending dose (SAD) studies (Valentin, 2010; Stockbridge et al., 2013; Kothari et al., 2015). While the sample size in these early‐phase studies is statistically underpowered to demonstrate a placebo‐adjusted change in mean QTc as described in the ICH E14 guidance, one could arguably obtain the same information by exposure–response modelling on early‐phase data (Salvi et al., 2010). A recent study, the International Consortium for Innovation and Quality in Pharmaceutical Development and the Cardiac Safety Research Consortium (IQ‐CSRC) study, has shown that it is possible to detect drug‐induced QTc prolongation with a limited number of healthy subjects using exposure–response modelling (Darpo et al., 2015a).
A TQT study typically involves measurement of the QT interval in as many as 30 000 ECGs performed manually by trained readers or by a semi‐automated process where annotations placed by a computer algorithm in each ECG are overread and corrected where necessary by an experienced cardiologist (Green et al., 2012; ICH Harmonized Tripartite Guideline E14 Questions and Answers, 2014). The ICH E14 Implementation Working Group has clarified in 2008 that fully automated analysis is not encouraged in TQT studies as they can yield misleading results in the presence of noisy ECG signals or when dealing with abnormal ECG rhythms, low amplitude P or T waves, or overlapping U waves (ICH Harmonized Tripartite Guideline E14 Questions and Answers, 2012). With improvement in performance of computer algorithms over the years, some recent studies have used a ‘highly automated’ process, where ECGs exceeding pre‐specified cut‐offs for confidence scores based on the quality of the ECG waveform that would limit the performance of the algorithm or those with outlier interval measurements are reviewed by a cardiologist, and ECG annotations are either retained if acceptable or rejected if unacceptable; this hybrid process offers the advantage of higher throughput and less variability in QT measurements of the automated analysis with manual confirmation of automated annotations in those ECGs where they could have been inappropriately positioned (Kligfield et al., 2010; Green et al., 2012; ICH Harmonized Tripartite Guideline E14 Questions and Answers, 2014).
Here we have used the highly automated ECG analysis approach using the FAT‐QT system (version 1.3.0, AMPS LLC, New York, NY, USA), a relatively new computerized ECG analysis system, to detect moxifloxacin‐induced QTc prolongation using ECGs from a TQT study. Using the highly automated ECG measurement values from the TQT study, we also performed a simulation study to assess the statistical power of a single‐dose study with eight subjects each in the moxifloxacin and placebo arms, to further investigate whether intensive ECG recording and analysis in SAD studies in early‐phase drug development can provide an estimate of the QT liability of a drug that is robust enough to match the results obtained from a TQT study.
Methods
Group sizes
This evaluation was performed using ECGs from the moxifloxacin and placebo treatment arms of a previously published TQT study (Natekar et al., 2011). The study protocol was approved by the Institutional Review Board of the sponsor as well as the study site. All subjects signed an informed consent document prior to enrollment in the study.
Part 1 – the TQT study
In the original study, 124 subjects were randomized to receive a single oral dose of moxifloxacin (400 mg) or placebo (n = 62 in each group) in a crossover design. The number of subjects in the original study was relatively large, because the TQT study design requires these subjects to be subsequently randomized to three parallel arms to receive either multiple doses of placebo or two doses of the investigational drug, as the investigational drug had a relatively long half‐life. Most crossover thorough QT studies now include only 35–50 subjects, due to improvement in variability in QT interval measurements. Assuming a within‐subject variability of 8 ms for the placebo‐adjusted change from baseline in QTcF with moxifloxacin, 46 subjects were required to demonstrate a significant QTcF prolongation with an upper bound of 10 ms with a one‐sided α of 95% and power of 80% in a crossover study. We therefore randomly selected 46 subjects from the original 124 subjects such that there were an equal number (23) of males and females.
Part 2 – single‐dose phase I simulation study
In part 2 of the study, we assessed the ability to detect moxifloxacin‐induced QT prolongation in an early‐phase study‐like scenario using the highly automated ECG analysis approach. Early‐phase studies typically have a smaller sample size with 6–9 subjects in each SAD arm and 6–10 subjects in the placebo arm. We, therefore, performed a simulation study using the ECG and pharmacokinetic data from the 46 subjects described in part 1. Each simulated dataset had eight subjects in the placebo group and eight different subjects in the moxifloxacin treatment group to mimic a phase I single‐dose study with a parallel design. One thousand such datasets were simulated. Although we could have used ECG data from all 124 subjects for the simulation study, we performed the simulation study using data from only the 46 subjects from part 1 so as to enable comparison of results from part 2 (phase I simulation) with those from part 1 (a formal TQT study).
Randomization
Part 1 – the TQT study
In the original TQT study, 124 subjects were randomized such that 62 received moxifloxacin on day 1 and placebo on day 3 and the other 62 received placebo first followed by moxifloxacin. For part 1 of our study, we randomly selected 46 subjects from the original 124 such that 23 were men and 23 were women. Of the 46 subjects, 21 had been randomized to receive moxifloxacin on day 1 and placebo on day 3, and 25 received placebo first followed by moxifloxacin.
Part 2 – single‐dose phase I simulation study
In part 2, from the complete dataset of 46 subjects, a subset of eight subjects in the moxifloxacin treatment group were selected by random sampling with replacement using sas (SAS Institute, Cary, NC, USA ). After excluding subjects who were selected in the moxifloxacin group, another eight subjects were selected by random sampling with replacement. Thus, from the original dataset of 46 subjects who received moxifloxacin and placebo in a crossover design, a parallel design dataset was simulated such that no subject appeared in the moxifloxacin as well as the placebo groups. One thousand such data subsets with eight subjects in the moxifloxacin arm and eight subjects in the placebo arm were created.
Blinding
In the original study, the treatments were open labelled, but the ECG analysis was by readers in a central ECG laboratory who were blinded to the identity of the subjects, treatment allocation and the sequence of ECG recordings.
In the highly automated ECG analysis process employed in the present study, the cardiologist who performed the manual overread of the ECGs was blinded to the identity of the subjects, treatment allocation and the sequence of ECG recordings.
Statistical methods
Analyses were performed using sas version 9.2.
Part 1 – the TQT study
The mean and 90% confidence intervals (CIs) of placebo‐adjusted change from baseline for QTcF (ΔΔQTcF) at each of the post‐dose timepoints were estimated using repeated measures analysis of covariance with baseline QTcF as a covariate, gender and timepoint as factors and subject as random effect (Florian et al., 2011). Concentration–QTc modelling was performed using a linear mixed‐effects model with ΔΔQTcF as the dependent variable and drug plasma concentration as a continuous covariate (Garnett et al., 2008).
Part 2 – single‐dose phase I simulation study
Geometric mean C max values were obtained, and concentration–response modelling was performed on each of the 1000 datasets using a linear mixed‐effects model with ΔQTcF as the dependent variable and plasma concentration of moxifloxacin and timepoint as a covariate, treatment (moxifloxacin or placebo) as a factor and a random intercept per subject. Model‐based estimates for the mean value of ∆∆QTcF and its 90% confidence bounds at the corresponding geometric mean of the maximum concentration (C max) of moxifloxacin for each of the 1000 datasets were also calculated.
Further methods
Part 1 – the TQT study
We randomly selected 46 subjects from the original 124 subjects such that there were an equal number of males and females (23 of each).
Each subject had ECG recording at seven timepoints (one pre‐dose and 0.5, 1, 1.5, 2, 4 and 6 h post‐dose timepoints) during each treatment period. Six replicate ECGs were recorded at each timepoint in the original study using a digital electrocardiograph (Eli 250, Mortara Instrument Inc., Milwaukee, WI, USA) with a sampling rate of 1000 Hz. The replicates were recorded 1 min apart starting approximately 3 min before the specified timepoint and ending approximately 3 min after the timepoint. These ECGs had been electronically transmitted to the central laboratory of Quintiles Cardiac Safety Services, in Mumbai, India, and converted into the FDA compliant HL7 XML files.
Moxifloxacin plasma concentration was determined at each of the six post‐dose timepoints at which ECGs were recorded and blood samples were drawn after the ECG recording. Blood samples of 10 mL were collected at each timepoint by separate venepuncture in Vacutainer®, (Becton Dickinson, Franklin Lakes, NJ, USA) tubes containing heparin for the determination of plasma concentrations of moxifloxacin. Pharmacokinetic parameters were calculated using non‐compartmental analysis to obtain C max and T max of moxifloxacin after single p.o. dose of 400 mg moxifloxacin. Concentrations below the limit of quantitation (LOQ) were calculated as zero.
ECG analysis
Of the six replicate ECGs recorded at each timepoint, only the first three ECGs were included in this analysis because most TQT studies use triplicate ECGs. The ECGs were analysed by a highly automated approach using the FAT‐QT software (version 1.3.0, 2015; AMPS LLC), which embeds the Bravo algorithm: for each of the 12 ECG leads, a representative ‘median’ beat is mathematically computed, and then automated annotations are computed on the global median beat, that is, on the Euclidean vector magnitude of the 12 median beats. On the global median beats, fiducial markers are placed with criteria based on adaptive thresholds techniques combining first and second derivatives of the ECG signal (Badilini and Sarapa, 2006).
Subsequently, quality scores were generated by the FAT‐QT system based on 10 quality parameters for the ECG waveforms that included high‐frequency noise and low‐frequency noise of waveforms, QRS regularity, T wave characteristics and outlier values for ECG parameters. All ECGs where the quality score exceeded a pre‐specified cut‐off, customized for the central ECG laboratory, were then reviewed by a single trained cardiologist who was allowed to accept or reject the automated annotations; no manual change in placement of the annotations was permitted.
Statistical analysis
For this part of the evaluation, the ECG data of all 46 subjects recorded on days 1 and 3 were used. The first three replicate ECGs recorded at the pre‐dose timepoint on days 1 and 3 were used as the baseline for the post‐dose ECGs on the corresponding days. QTcF was calculated using RR, QT intervals from each ECG, and the average QTcF value from the triplicate ECGs was considered as the QTc interval at the particular timepoint.
The mean and 90% confidence intervals of placebo‐adjusted change from baseline for QTcF (ΔΔQTcF) at each of the post‐dose timepoints were estimated using repeated measures analysis of covariance (Florian et al., 2011). Concentration–QTc modelling was performed using a linear mixed‐effects model with ΔΔQTcF as the dependent variable and drug plasma concentration as a continuous covariate (Garnett et al., 2008).
Part 2 – single‐dose phase I simulation study
In part 2 of the study, we assessed the ability to detect moxifloxacin‐induced QT prolongation in an early‐phase study‐like scenario by performing a simulation study using the original dataset of 46 subjects from the TQT study described in part 1.
From the original dataset of 46 subjects who received moxifloxacin and placebo in a crossover design, a parallel design dataset was simulated. A total of 1000 such data subsets with eight subjects in the moxifloxacin arm and eight subjects in the placebo arm were created.
In this part of the study, the ECG data from all six replicate ECGs recorded at each timepoint were included in order to reduce the within‐subject and between‐subject variability in ∆QTcF and the confidence limits of its estimate (Natekar et al., 2011; Darpo et al., 2015b). Thus, each data subset consisted of ECG data from 672 ECGs, with 336 ECGs from eight subjects in the moxifloxacin arm and 336 ECGs from eight subjects in the placebo arm.
Statistical analysis
For each of the 1000 datasets, concentration–response modelling was performed using a linear mixed‐effects model with ΔQTcF as the dependent variable and plasma concentration of moxifloxacin as a covariate. Model‐based estimates for the mean value of ∆∆QTcF and its 90% confidence bounds at the geometric mean of the maximum concentration (C max) of moxifloxacin for each dataset were also calculated.
The study was considered as positive (i.e. it could detect the moxifloxacin‐induced QT prolongation) for a dataset if both of the following criteria were satisfied:
the plasma concentration–ΔΔQTcF relationship showed a statistically significant (P < 0.05) positive slope, and
the upper bound of the 90% two‐sided confidence limit of the ∆∆QTcF at geometric mean C max for the dataset exceeded 10 ms.
Datasets where the slope of the plasma concentration–ΔΔQTcF relationship was not statistically significant or the upper 90% confidence limit for mean ∆∆QTcF at moxifloxacin concentration corresponding to the mean C max was <10 ms were considered as ‘false negative’ studies. The overall statistical power of the entire study methodology was given by the percentage of datasets that showed a positive moxifloxacin effect using concentration–ΔΔQTc modelling.
Results
A total of 3857 ECGs recorded from 46 healthy adult subjects (23 women and 23 men) that included six replicates at each of seven timepoints (one pre‐dose and six post‐dose) were analysed. The annotations were initially placed by the automated ECG analysis algorithm, which also provided a quality score of each ECG. Based on the quality score, 491 (12.73%) ECGs were selected for cardiologist's review; 194 of these ECGs (5.02% of 3857 ECGs and 39.1% of ECGs with a poor quality score) were rejected after review by the cardiologist for incorrect placement of annotations.
Part 1 – the TQT study
Although six replicate ECGs were recorded at each timepoint, in part 1 of the study, only the first three replicates were considered at each timepoint as typical TQT studies are now performed with three or four replicate ECGs. A total of 1932 ECGs were included, of which 231 (12%) ECGs required cardiologist's review and 98 (5.1%) ECGs were rejected after review.
Assay sensitivity
The demonstration of moxifloxacin‐induced QT prolongation by the highly automated approach for QTcF is shown in Figure 1.There was a statistically significant prolongation of placebo‐adjusted change from baseline in the QTcF interval (ΔΔQTcF) with moxifloxacin at all six post‐dose timepoints (Figure 1). Maximum ΔΔQTcF was seen at 4 h post‐dose (16.7 ms). The lower bound of the 90% two‐sided confidence limits exceeded 5 ms, and the upper bound exceeded 10 ms at the 1, 1.5, 2, 4 and 6 h post‐dose timepoints. Treatment × period interaction was not statistically significant (P = 0.23).
Figure 1.
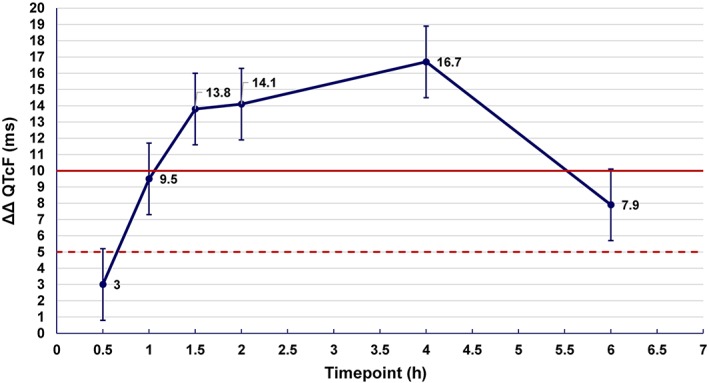
Mean placebo‐adjusted change from baseline in QTcF (ΔΔQTcF) and its 90% two‐sided confidence limits by a highly automated analysis approach using the Bravo algorithm in 46 healthy subjects after a single oral dose of 400 mg moxifloxacin. The important thresholds for ΔΔQTcF and its 90% confidence limits are shown at 5 ms (dotted line) and 10 ms (bold line).
Concentration–ΔΔQTcF evaluation
PK–PD evaluation was performed using a linear mixed‐effects model with ΔΔQTcF as the dependent variable, drug plasma concentration as a continuous covariate and intercept as a random effect using data from 46 healthy subjects after a single 400 mg oral dose of moxifloxacin. The slope of the concentration–ΔΔQTcF relationship was 4.12 ms·μg−1·mL−1 (SE = 0.61; P < 0.001) (Figure 2). Using this model, the estimated mean ΔΔQTcF at the geometric mean concentration of moxifloxacin (2.9478 μg·mL−1) was 13.4 ms (90% two‐sided confidence limits 11.3, 15.4 ms).
Figure 2.
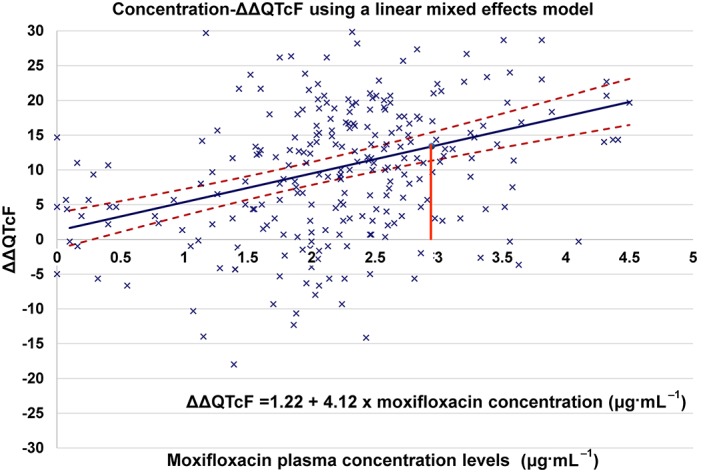
Moxifloxacin plasma concentration versus placebo‐adjusted change from baseline in QTcF (ΔΔQTcF) in 46 healthy subjects after administration of 400 mg moxifloxacin. The mean predicted ΔΔQTcF at various concentrations of moxifloxacin is shown as a solid line, and its two‐sided 90% confidence limits are shown by the broken lines. At the mean geometric C max value of 2.9478 μg·mL−1 (indicated by vertical line), the model‐based value of mean ΔΔQTcF was 13.4 ms (90% two‐sided confidence limits 11.3, 15.4 ms).
Part 2 – single‐dose phase I simulation study
In part 2 of the study, a total of 1000 datasets, each with eight subjects on moxifloxacin and eight on placebo, were created from the TQT ECG data by bootstrapping so as to simulate early‐phase studies; all six replicate ECGs recorded at each timepoint were included in the simulation study. The mean of the observed peak plasma concentration of moxifloxacin (C max) in each of the 1000 datasets ranged from 2.22 to 3.70μg·mL−1 (Figure 3) with the median value being 2.96μg·mL−1. A linear mixed‐effects model similar to that used in part 1 of the study was used to determine the slope of the relationship between the QTc effect and drug plasma levels in each of the 1000 datasets; treatment was used as a fixed effect as has been carried out in several previous studies (Badilini and Sarapa, 2006; Florian et al., 2011). The concentration–∆∆QTcF relationship for each of the 1000 datasets, its P‐value and the model‐derived estimate of the ∆∆QTcF at mean C max of moxifloxacin for each dataset were calculated. The mean slope of the concentration–∆∆QTcF relationship for the 1000 datasets was 5.9 ms·μg−1·mL−1 (90% confidence interval 2.9, 9.3), while the mean estimated ∆∆QTcF at mean C max was 16.5 ms (90% confidence interval 9.5, 24.1 ms); the upper bound of the 90% confidence interval for ∆∆QTcF at mean C max was 23.9 ms (mean; 90% confidence interval 14.9, 34.1 ms).
Figure 3.
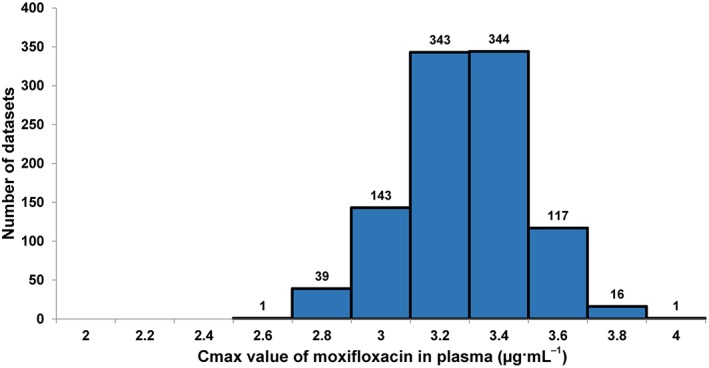
Distribution of peak plasma concentration (geometric mean C max) values for moxifloxacin in the 1000 datasets.
In 895 of the 1000 datasets, both criteria for a positive study were met, and moxifloxacin‐induced QT prolongation could be demonstrated. Of the remaining 105 datasets, in 101 datasets, the estimated 90% two‐sided upper confidence bound for moxifloxacin was >10 ms but the P‐value for the slope was ≥0.05, and in four datasets, the 90% two‐sided upper confidence bound for ∆∆QTcF was <10 ms and the P‐value for the slope was ≥0.05 (Figures 4, 5).
Figure 4.
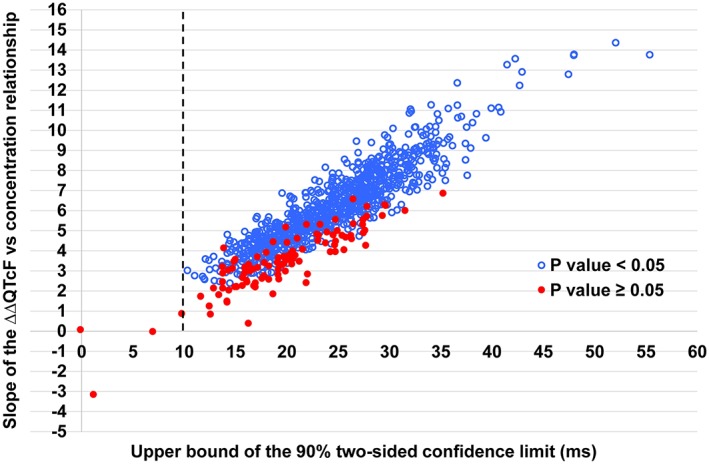
Distribution of the slope of the placebo‐adjusted change from baseline for QTcF (ΔΔQTcF) versus concentration relationship for moxifloxacin and the upper confidence limit for ΔΔQTcF at mean C max for the 1000 simulated datasets. Only four datasets had an upper confidence limit for ΔΔQTcF that was less than 10 ms (seen as falling to the left of the vertical line). For 105 datasets, the slope of the ΔΔQTcF versus concentration relationship for moxifloxacin did not reach statistical significance (P≥ 0.05).
Figure 5.
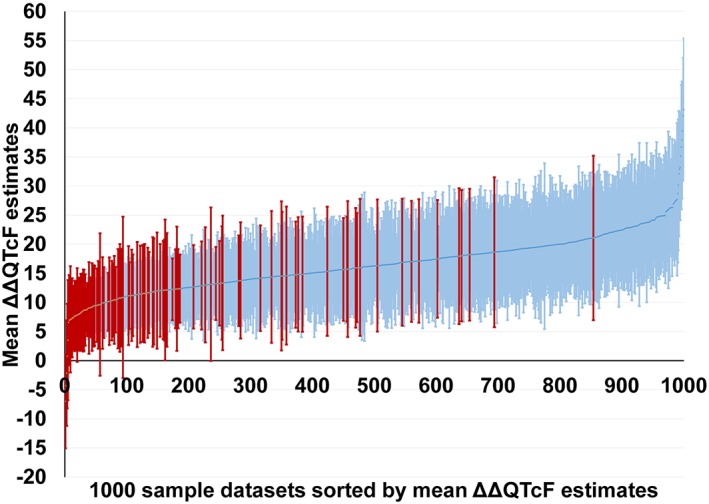
Estimated mean ΔΔQTcF and its two‐sided 90% confidence limits at mean C max of moxifloxacin for each of the 1000 datasets from the phase I simulation study. Data are sorted in ascending order of the mean ΔΔQTcF. Results from 895 datasets with a positive result are shown in blue (lighter bars), while results from the remaining 105 datasets that could not demonstrate a drug‐induced ΔΔQTcF prolongation are shown in red (darker bars).
Discussion
This study used a highly automated QT/QTc measurement approach using the Bravo algorithm in the FAT‐QT system, which measures the QT interval on the global median beat derived from all 12 ECG leads. In part 1 of the analysis, the highly automated approach was able to demonstrate assay sensitivity with a statistically significant placebo‐adjusted prolongation of QTcF with moxifloxacin. The ICH has stated that assay sensitivity with moxifloxacin is demonstrated if the 90% two‐sided lower confidence bound of mean ∆∆QTcF exceeds >5 ms (ICH Harmonized Tripartite Guideline E14 Questions and Answers, 2014). This criterion was satisfied at five post‐dose timepoints in our study with 46 subjects and triplicate ECGs at each timepoint, with maximum mean ∆∆QTcF of 16.7 ms at 4 h post‐dose timepoint; by concentration–effect modelling, the mean ΔΔQTcF at mean C max was 13.4 ms (90% two‐sided confidence limits 11.3, 15.4 ms). These observations are consistent with those reported by Florian et al. from an analysis of 20 TQT studies. They found that the mean ∆∆QTcF by by‐time analysis ranged from 7.7 to 16.7 ms and by concentration–effect modelling ranged from 6.4 to 12.9 ms. For reasons not clear, they too observed lower estimate of mean ∆∆QTcF by the concentration–effect model than with the by‐time analysis (Florian et al., 2011). The slope of the concentration response in our study (4.12 ms·μg−1·mL−1 of moxifloxacin) was also within the range of 1.6–4.8ms·μg−1·mL−1 reported by Florian et al. (2011).
In the last few years, there has been a move to investigate if drug‐induced QT prolongation of the extent defined in the ICH E14 guidance can also be detected by intensive ECG recordings in SAD studies during early‐phase development (Salvi et al., 2010). However, evidence to support this concept is still emerging. Using a highly automated approach performed with the COMPAS system (iCardiac Technologies Inc., Rochester, NY, USA), Darpo et al. (the IQ‐CSRC study) could demonstrate moxifloxacin‐induced QTcF prolongation using 10 replicate ECGs recorded in nine subjects receiving moxifloxacin and six receiving placebo in a parallel study (Darpo et al., 2015b). In another simulation study, Ferber et al. used subsampling from three conventional TQT studies to simulate a single‐dose early‐phase study with small sample sizes (Ferber et al., 2015). Using a concentration–effect modelling similar to that in the IQ‐CSRC study, they found that to detect a drug‐induced QTc effect of 12–14 ms with good consistency required a sample size of nine or more subjects on drug and six on placebo.
In part 2 of the present study, we used the bootstrapping technique to create 1000 data subsets by repeated subsampling of data from the TQT study to simulate a smaller early‐phase clinical trial with intensive ECG assessment. Concentration–effect modelling was used to estimate moxifloxacin‐induced QT prolongation in 1000 datasets created from the TQT study data. With eight subjects in the placebo group and eight subjects in the moxifloxacin group, we could detect a significant drug‐induced QTc prolongation in 895 of the 1000 datasets, indicating a statistical power of around 89%. If we consider studies where the upper bound of the 90% CI of the estimated ∆∆QTcF at mean C max exceeded 10 ms but the P‐value of the slope of the concentration–effect model was ≥0.05 as having an indeterminate outcome, a further 101 studies fell in this category.
In a simulation study using data from three TQT studies, Ferber et al. found that if six subjects were included in the active treatment group and six in the placebo group, only 68% of samples had a statistically significant positive slope of the plasma concentration/ΔΔ QTcF relationship (Darpo et al., 2015b; Ferber et al., 2015). This increased to 76% with 9 subjects and 82% with 12 subjects. Our results are similar and show that with eight subjects on active treatment and eight subjects on placebo, a significant QTcF prolongation can be detected with >80% power.
Before extrapolating our results to a SAD study, one should note that while only a single therapeutic dose of moxifloxacin was used in our study, in a typical SAD study, there would be several dose groups for the active compound using ascending doses. It is therefore likely that there may be one or more dose groups receiving smaller doses and some receiving larger (supra‐therapeutic) doses. This would considerably enhance the power of the study to detect the QT effect using concentration–effect modelling by providing more data points at higher concentrations, thereby providing better estimates of the slope and mean ∆∆QTc and thereby lowering the false negative rate (Kothari et al., 2015). Including subjects from different dose groups would also increase the overall sample size and thus reduce the confidence limits for the estimated ∆∆QTc, thus reducing the false positive rate (Kothari et al., 2015).
Most previous studies with an early‐phase design have had 10 replicate ECGs recorded at each timepoint in each subject in an attempt to reduce within‐subject and between‐subject variability (Sparve et al., 2014; Darpo et al., 2015a). This is because the between‐subject and within‐subject variability decreases with increasing replicates (Beasley et al., 2005; Natekar et al., 2011), but the optimum number of ECGs needed in early‐phase studies needs to be further investigated. The present study suggests that recording six replicate ECGs may be adequate if consistency in QT interval measurement can be ensured.
Our study has some limitations. Firstly, we only studied the ability of a single‐dose phase I‐like study design to detect a significant QTc prolongation with moxifloxacin, a drug with a known QT liability. It would also be important to ensure that this study design also had the ability to exclude the absence of a QT liability with a drug proven to be negative in a TQT study (i.e. to exclude false positive results). Secondly, the half‐life of moxifloxacin is 12 h, and therefore, the washout period between the two treatments in the crossover study should have been a minimum of 60 h (five half‐lives) while it was 48 h in the present study. However, when treatment × period interaction was studied, this was not statistically significant, suggesting the absence of a carry‐over effect.
In conclusion, the present study shows that moxifloxacin‐induced QTc prolongation could be demonstrated in a thorough QT study, using a highly automated ECG analysis method and the Bravo algorithm. More importantly, we also studied the performance of the highly automated approach in detecting QTc changes in simulated early‐phase clinical trials, by subsampling subjects to create 1000 datasets from the TQT study data. Using concentration–effect analysis, we found that QTcF prolongation could be reliably detected with a small sample size (eight subjects on active drug and eight on placebo) with a power of more than 80%. Our study therefore adds to the growing body of data supporting intensive ECG collection and analysis in early‐phase studies that, when interpreted along with preclinical data from the proposed Comprehensive in vitro Pro‐arrhythmia Assessment, may provide a good estimate of the QT liability of a new drug (Cavero and Holzgrefe, 2014).
Author contributions
G.K.P., D.K., and A.D. performed the research. G.K.P., D.K., and S.K. designed the research study. F.B. contributed essential tools. G.K.P., D.K., and P.K. analysed the data. G.K.P., D.K., and P.K. wrote the paper. G.K.P., D.K., F.B., P.K., S.K. drafted the manuscript.
Conflict of interest
G.K.P, P.K. and S.K. are employees of Quintiles Cardiac Safety Services. F.B. is a member of AMPS, a limited liability company from New York that owns the software used for the ECG analysis of the study. D.K. and A.D. are consultants to Quintiles Cardiac Safety Services.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The authors are grateful to Saikat Chakraborty and Dr Khushboo Chaudhari from Quintiles Cardiac Safety Services for coordinating the ECG analysis workflow for the study.
Panicker, G. K. , Karnad, D. R. , Kadam, P. , Badilini, F. , Damle, A. , and Kothari, S. (2016) Detecting moxifloxacin‐induced QTc prolongation in thorough QT and early clinical phase studies using a highly automated ECG analysis approach. British Journal of Pharmacology, 173: 1373–1380. doi: 10.1111/bph.13436.
References
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE, et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badilini F, Sarapa N (2006). Implications of methodological differences in digital electrocardiogram interval measurement. J Electrocardiol 39: S152–S156. [DOI] [PubMed] [Google Scholar]
- Beasley CM Jr, Mitchell MI, Dmitrienko AA, Emmick JT, Shen W, Costigan TM, et al. (2005). The combined use of ibutilide as an active control with intensive electrocardiographic sampling and signal averaging as a sensitive method to assess the effects of tadalafil on the human QT interval. J Am Coll Cardiol 46: 678–687. [DOI] [PubMed] [Google Scholar]
- Cavero I, Holzgrefe H (2014). Comprehensive in vitro Proarrhythmia Assay, a novel in vitro/in silico paradigm to detect ventricular proarrhythmic liability: a visionary 21st century initiative. Expert Opin Drug Saf 13: 745–758. [DOI] [PubMed] [Google Scholar]
- Darpo B, Benson C, Dota C, et al. (2015a). Results from the IQ‐CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin Pharmacol Ther 97: 326–335. [DOI] [PubMed] [Google Scholar]
- Darpo B, Garnett C, Keirns J, Stockbridge N (2015b). Implications of the IQ‐CSRC prospective study: time to revise ICH E14. Drug Saf 38: 773–780. [DOI] [PubMed] [Google Scholar]
- Ferber G, Zhou M, Darpo B (2015). Detection of QTc effects in small studies – implications for replacing the thorough QT study. Ann Noninvasive Electrocardiol 20: 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian JA, Tornoe CW, Brundage R, Parekh A, Garnett CE (2011). Population pharmacokinetic and concentration–QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol 51: 1152–1162. [DOI] [PubMed] [Google Scholar]
- Garnett CE, Beasley N, Bhattaram VA, Jadhav PR, Madabushi R, Stockbridge N, et al. (2008). Concentration–QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 48: 13–18. [DOI] [PubMed] [Google Scholar]
- Green CL, Kligfield P, George S, et al. (2012). Detection of QT prolongation using a novel ECG analysis algorithm applying intelligent automation: prospective blinded evaluation using the Cardiac Safety Research Consortium ECG database. Am Heart J 163: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH Harmonized Tripartite Guideline E14 (2005). The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073153.pdf
- ICH Harmonized Tripartite Guideline E14 Questions and Answers (2012). The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs questions and answers http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002878.pdf
- ICH Harmonized Tripartite Guideline E14 Questions and Answers (2014). The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs questions and answers (R2). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_QAs_R2_Step4.pdf
- Kligfield P, Green CL, Mortara J, et al. (2010). The Cardiac Safety Research Consortium electrocardiogram warehouse: thorough QT database specifications and principles of use for algorithm development and testing. Am Heart J 160: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Kothari S, Karnad D, Panicker GK, Turner JR (2015). Cardiac safety investigations 10 years after ICH guidance E14: evolving industry and regulatory viewpoints on evaluation of proarrhythmic risk during new drug development. J Clin Stud 7: 22–30. http://www.jforcs.com/cardiac‐safety‐investigations‐10‐years‐after‐ich‐guidance‐e14‐evolving‐industry‐and‐regulatory‐viewpoints‐on‐evaluation‐of‐proarrhythmic‐risk‐during‐new‐drug‐development. [Google Scholar]
- Natekar M, Hingorani P, Gupta P, et al. (2011). Effect of number of replicate electrocardiograms recorded at each time point in a thorough QT study on sample size and study cost. J Clin Pharmacol 51: 908–914. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SPH, Buneman OP, et al NC‐IUPHAR(2014). The IUPHAR/BPS Guide to PHARMACOLOGY: an expert‐driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 42 (Database Issue): D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi V, Karnad DR, Panicker GK, Kothari S (2010). Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development. Br J Pharmacol 159: 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparve E, Quartino AL, Lüttgen M, Tunblad K, Gårdlund AT, Fälting J, et al. (2014). Prediction and modeling of effects on the QTc interval for clinical safety margin assessment, based on single‐ascending‐dose study data with AZD3839. J Pharmacol Exp Ther 350: 469–478. [DOI] [PubMed] [Google Scholar]
- Stockbridge N, Morganroth J, Shah RR, Garnett C (2013). Dealing with global safety issues: was the response to QT‐liability of non‐cardiac drugs well‐coordinated? Drug Saf 36: 167–182. [DOI] [PubMed] [Google Scholar]
- Valentin JP (2010). Reducing QT liability and proarrhythmic risk in drug discovery and development. Br J Pharmacol 159: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


